Abstract
Unlike autosomal dominant polycystic kidney disease (ADPKD), autosomal recessive polycystic kidney disease (ARPKD) is not generally known to be associated with vascular abnormalities. Only 4 cases of ARPKD patients with intracranial aneurysms have been reported previously. We present 2 ARPKD patients with extracranial vascular abnormalities: a young man with infrarenal aortic and iliac artery aneurysms complicated by dissection and a teenage girl with multiple splenic and gastric artery aneurysms and arterial vascular malformations. These cases raise the question of whether vascular integrity and development may be impaired in ARPKD, perhaps through molecular mechanisms overlapping with ADPKD. This possibility is supported by studies in mice that show ARPKD gene expression in the walls of large blood vessels.
Keywords: Autosomal recessive polycystic kidney disease, Autosomal dominant polycystic kidney disease, Congenital hepatic fibrosis, Aneurysms, Arterial vascular malformations, Extracranial aneurysm, Portal hypertension
Introduction
Clinical manifestations of autosomal recessive polycystic kidney disease (ARPKD) include enlarged kidneys with innumerable collecting duct cysts, progressive chronic kidney disease (CKD), and liver disease with dilated intrahepatic bile ducts, congenital hepatic fibrosis (CHF), and portal hypertension [1]. In contrast to autosomal dominant polycystic kidney disease (ADPKD), in which intracranial aneurysms are reported in 5–10% of patients [2,3,4], there is no strong association of vascular abnormalities with ARPKD. To date, there have been 4 reported cases of ARPKD patients with intracranial aneurysms [5,6,7,8]. To our knowledge, there have been no prior reports of ARPKD patients with extracranial aneurysms. Here, we describe 2 ARPKD patients who presented with unusual extracranial vascular abnormalities.
Patient 1
A 36-year-old male with ARPKD, stage 4 CKD, Caroli syndrome, CHF, and portal hypertension presented with 2 weeks of intermittent, cramping mid-lower abdominal pain radiating to the bilateral flanks. His past medical history was notable for hypersplenism with thrombocytopenia and esophageal varices without bleeding, previously treated with banding for primary prophylaxis. He had hepatocellular carcinoma at age 24 years and underwent hepatic wedge resection. He had no significant history of systemic hypertension.
Initial exam revealed a blood pressure of 135/77 mm Hg with normal heart rate. He was not in acute distress. He had splenomegaly, minimal lower abdominal tenderness, hypoactive bowel sounds, and no abdominal bruits. He had no edema, normal capillary refill, and normal femoral and pedal pulses. Laboratory studies showed normal hemoglobin, platelets 90,000/µL, serum creatinine 2.3 mg/dL, INR 1.3, normal liver enzymes, total bilirubin 1.5 mg/dL, and serum albumin 3.1 g/dL.
Noncontrast abdominal computed tomography (CT) showed stranding along the infrarenal aorta and hypodensity in the left common iliac artery, suspicious for thrombus. Aorto-iliac duplex study revealed a 3.2-cm fusiform infrarenal abdominal aortic aneurysm (AAA) and 1.9-cm aneurysms of the bilateral common iliac arteries, with possible dissection of the right common iliac artery. CT angiogram confirmed fusiform dilation of the infrarenal abdominal aorta (3.1 cm in diameter), with complex dissection extending from the level of the second lumbar vertebra to the right common iliac artery (Fig. 1). There was also fusiform dilation of the bilateral common iliac arteries (1.9 cm in diameter) and of the celiac artery (1.2 cm in diameter), with linear hypodensity in the celiac artery that was concerning for dissection.
Fig. 1.
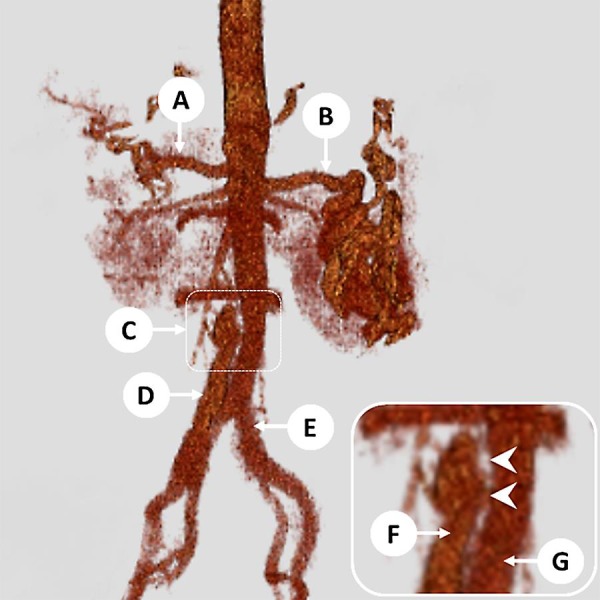
Patient 1: 3-D reconstruction images of the aorta from the CT angiogram, demonstrating an abdominal aortic aneurysm with complex dissection extending into the right common iliac artery. A, common hepatic artery; B, splenic artery (tortuous); C, top of the aortic aneurysm and dissection (magnified in inset); D, false lumen extending into the right common iliac artery; E, true lumen extending into the left common iliac artery; F, false lumen of the aorta; G, true lumen of the aorta. Inset Arrowheads indicate dissection flap in the aorta.
He received anticoagulation with a heparin infusion followed by transition to oral warfarin and was started on oral propranolol for primary prophylaxis against esophageal variceal bleeding.
Patient 2
A 17-year-old female with ARPKD, stage 3 CKD, CHF, and severe portal hypertension with a history of multiple esophageal variceal bleeds (with portocaval shunt placement at age 3 years and multiple prior sclerotherapy treatments) presented with massive upper gastrointestinal (GI) tract bleeding with abdominal pain, hematemesis, and grossly bloody stool. She was tachycardic and hypotensive to 80/40 mm Hg. Gastric lavage showed gross blood. Laboratory studies showed hemoglobin 9.3 g/dL, platelets 75,000/µL, serum potassium 5.5 mmol/L, serum creatinine 1.3 mg/dL, INR 1.6, and serum albumin concentration 1.8 g/dL. She had significant blood loss requiring numerous transfusions of packed red cells, platelets, plasma, and cryoprecipitate. Additional supportive therapy included octreotide infusion, pantoprazole, ranitidine, vitamin K, vasopressors, intubation, and sedation.
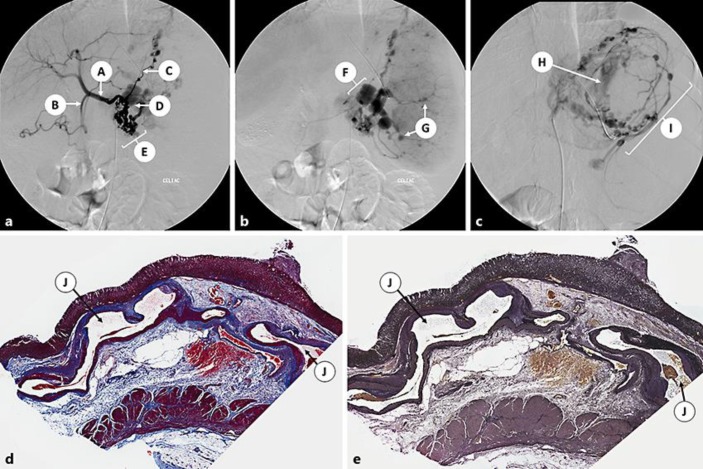
Emergency endoscopy could not localize the source of bleeding due to a large amount of blood in the stomach. Emergency celiac angiography showed multiple aneurysms involving the celiac trunk, including multiple short gastric arteries, the splenic artery and its branches, and the left gastric artery (Fig. 2a–c). She developed massive hemorrhage from the left gastric artery during the procedure, and coil embolization was performed with cessation of bleeding. Recurrent GI bleeding 2 days later necessitated exploratory laparotomy and oversewing of the bleeding vessel. Over the following 2 years, she had recurrent episodes of GI hemorrhage. At age 19 years, she presented with massive GI bleeding refractory to medical and endoscopic therapies. She underwent emergent subtotal gastrectomy, splenectomy, and transverse colectomy but died the following day. Gastric pathology revealed multiple large submucosal arterial vascular malformations (up to 3.5 cm in diameter; Fig. 2d, e), some with ulceration of the overlying mucosa (Dieulafoy-like lesions).
Fig. 2.
Patient 2: Celiac angiography (a–c) and stomach pathology (d, e). a–c Celiac angiography demonstrates multiple aneurysms including multiple short gastric arteries, the splenic artery and its branches, and the left gastric artery. A, common hepatic artery; B, gastroduodenal artery; C, left gastric artery with multiple aneurysms; D, absent proximal splenic artery, occluded at origin; E, multiple aneurysms in presumed short gastric arteries; F, collaterals reconstituting distally into markedly deformed aneurysmal splenic artery stump; G, multiple intrasplenic aneurysms; H, active extravasation from the left gastric artery; I, multiple intragastric arterial aneurysms. d Stomach, trichrome stain. e Stomach, elastic stain. J, large submucosal arterial vascular malformations.
Discussion
There have been 4 prior reports of intracranial aneurysms in ARPKD patients [5,6,7,8], but to our knowledge, these are the first reported cases of ARPKD patients with extracranial vascular abnormalities. Patient 1 presented in his 4th decade with an AAA and common iliac artery aneurysms with dissection. Patient 2 presented as a teenager with multiple aneurysms and arterial vascular malformations arising from branches of the celiac artery, including the splenic artery and its branches, and the gastric arteries. Pathology showed large submucosal arterial vascular malformations, suggestive of a developmental vascular defect.
It is unlikely that these vascular abnormalities represent a simple coincidence with ARPKD. AAA and iliac artery aneurysms are the first and second most common intra-abdominal arterial aneurysms in the general population [9]. However, AAA is exceedingly rare in younger age groups; in a population-based study of over 6,000 individuals aged 25–84 years, no one aged less than 48 years had AAA [10]. Patient 1 had no other risk factors for AAA, such as hypertension, smoking, or hypercholesterolemia [11]. However, CKD is also a risk factor for AAA [12] and may have been a predisposing factor in this patient.
Splenic artery aneurysms (SAA) are the third most common intra-abdominal arterial aneurysms [9] but are rare, with an estimated population prevalence of 0.78% [13]. However, SAA is much more common in patients with portal hypertension, with a prevalence as high as 8.8% in patients with cirrhotic portal hypertension [14]. SAA is also more common in females than in males [9]. Patient 2 had both of these risk factors. However, aneurysms of the gastric arteries are much rarer, representing only about 2% of true splanchnic artery aneurysms [15]. In addition, the gastric histopathology in patient 2 was more consistent with a primary developmental arterial vascular defect rather than a secondary aneurysmal weakening of the vessel walls.
It is possible that these patients had a coexisting primary vascular disorder, such as fibromuscular dysplasia, which has been associated with aneurysms and dissection in aortic, iliac, and celiac artery distributions [16]. Although we cannot disprove this possibility, we note that any characteristic histopathologic findings of fibromuscular dysplasia, such as medial fibroplasia, medial hyperplasia, intimal fibroplasia, and perimedial dysplasia [17], were absent in patient 2.
Given our patients’ other risk factors for intra-abdominal aneurysms, including CKD and portal hypertension [14, 18], it is possible that the aneurysms resulted from nonspecific secondary effects of other pathological factors rather than a direct association with ARPKD. However, these cases, along with the prior reports of intracranial aneurysms in ARPKD patients [5,6,7,8], raise the question of whether there could be disease-specific effects of ARPKD on vascular integrity or development. The association of ADPKD with intracranial aneurysms is well established [2,3,4], and since the pathways involved in ARPKD and ADPKD are linked, it is possible that there is a previously underrecognized overlap in the vascular phenotype.
ARPKD is caused by mutations in PKHD1 (6p21.1-p12) [19, 20], a large gene whose protein product, fibrocystin/polyductin (FPC), is a single membrane-spanning protein that is expressed most highly in tubular epithelia of the kidney, bile ducts, and pancreas [20, 21]. Mouse studies have also shown strong gene expression in the muscular wall of large blood vessels, including the thoracic and abdominal aorta [22].
ADPKD is caused by mutations in 1 of 2 genes, PKD1 (16p13.3), accounting for ∼85% of patients [23], or PKD2 (4q21). The PKD1 product, polycystin-1, is a large integral membrane protein, and the PKD2 product, polycystin-2, is a calcium-transporting channel in the transient receptor potential (TRP) family [24]. Polycystin-1 is widely expressed in adult tissues, particularly in renal tubular epithelia [25]. Mouse studies have also shown high levels of Pkd1 expression in the heart, aorta, intracranial arteries, and other major blood vessels. Polycystins-1 and −2 interact to form a complex [26] that localizes to the primary cilium [27], an organelle with essential signaling and sensory roles in numerous mammalian cell types, including renal tubules [28]. The ARPKD protein, FPC, also interacts with polycystin-2 and regulates its expression and function [29,30,31]. Thus, common signaling pathways appear to underlie the pathophysiology of ARPKD and ADPKD.
ADPKD is strongly associated with vascular abnormalities, particularly intracranial aneurysms, which occur in 5–10% of patients [2,3,4]. Other vascular abnormalities have also been described in ADPKD, including intracranial dolichoectasia [32, 33], ascending aortic aneurysms [34], and dissections of the coronary [35, 36] and vertebral [37] arteries. It has also been suggested that ADPKD patients are at higher risk for AAA [38,39,40,41], although a systematic study found no increased risk of AAA in ADPKD patients compared to healthy family members [42].
As reviewed recently by Perrone et al. [43], there is evidence to suggest that polycystins are involved in maintaining vascular integrity [44]. ADPKD has been associated with endothelial dysfunction and defects in angiogenesis [45]. Possible mechanisms for these defects include abnormal mechanosensation by vascular endothelial primary cilia [46], abnormal endothelial cell migration [47], or dysregulation of VEGF pathways [48] or TGF-β signaling [49].
Data from animal models also suggest a role for FPC in maintaining vascular integrity. Abnormal microvascular structure has been described in the PCK rat model of ARPKD [50]. In this model, endothelial dysfunction occurs even before the onset of hypertension or CKD, suggesting a primary effect of ARPKD [51].
These 2 patients, along with the biological evidence that polycystins and FPC have a role in maintaining vascular integrity, highlight the need for further studies to elucidate whether ARPKD patients may be at higher risk for vascular complications. In the meantime, increased vigilance for these serious complications seems warranted in ARPKD patients.
Statement of Ethics
Informed consent was obtained as appropriate. The Children's Hospital of Philadelphia Institutional Review Board has determined that this case report does not meet the definition of human subjects research and does not require review.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
There are no funding sources to report.
Acknowledgements
The authors are grateful to patient 1 and his mother for providing updated information. We are also grateful to Colleen Zak, RN, for encouraging us to report these cases and for everything that she has done to further the investigation of ARPKD.
References
- 1.Hartung EA, Guay-Woodford LM. Autosomal recessive polycystic kidney disease: a hepatorenal fibrocystic disorder with pleiotropic effects. Pediatrics. 2014;134:e833–e845. doi: 10.1542/peds.2013-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graf S, Schischma A, Eberhardt KE, Istel R, Stiasny B, Schulze BD. Intracranial aneurysms and dolichoectasia in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2002;17:819–823. doi: 10.1093/ndt/17.5.819. [DOI] [PubMed] [Google Scholar]
- 3.Chapman AB, Rubinstein D, Hughes R, et al. Intracranial aneurysms in autosomal dominant polycystic kidney disease. N Engl J Med. 1992;327:916–920. doi: 10.1056/NEJM199209243271303. [DOI] [PubMed] [Google Scholar]
- 4.Huston J, Torres VE, Sulivan PP, Offord KP, Wiebers DO. Value of magnetic resonance angiography for the detection of intracranial aneurysms in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1993;3:1871–1877. doi: 10.1681/ASN.V3121871. [DOI] [PubMed] [Google Scholar]
- 5.Chalhoub V, Abi-Rafeh L, Hachem K, Ayoub E, Yazbeck P. Intracranial aneurysm and recessive polycystic kidney disease: the third reported case. JAMA Neurol. 2013;70:114–116. doi: 10.1001/jamaneurol.2013.584. [DOI] [PubMed] [Google Scholar]
- 6.Lilova MI, Petkov DL. Intracranial aneurysms in a child with autosomal recessive polycystic kidney disease. Pediatr Nephrol. 2001;16:1030–1032. doi: 10.1007/s004670100019. [DOI] [PubMed] [Google Scholar]
- 7.Neumann HP, Krumme B, van Velthoven V, Orszagh M, Zerres K. Multiple intracranial aneurysms in a patient with autosomal recessive polycystic kidney disease. Nephrol Dial Transplant. 1999;14:936–939. doi: 10.1093/ndt/14.4.936. [DOI] [PubMed] [Google Scholar]
- 8.De Blasi R, Lasjaunias P, Rodesch G, Alvarez H. Endovascular treatment of a ruptured intracranial arterial aneurysm in a 12-year-old child with recessive polycystic kidney disease. Interv Neuroradiol. 1997;3:333–336. doi: 10.1177/159101999700300410. [DOI] [PubMed] [Google Scholar]
- 9.Al-Habbal Y, Christophi C, Muralidharan V. Aneurysms of the splenic artery – a review. Surgeon. 2010;8:223–231. doi: 10.1016/j.surge.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Singh K, Bønaa KH, Jacobsen BK, Bjørk L, Solberg S. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study: the Tromsø Study. Am J Epidemiol. 2001;154:236–244. doi: 10.1093/aje/154.3.236. [DOI] [PubMed] [Google Scholar]
- 11.Forsdahl SH, Singh K, Solberg S, Jacobsen BK. Risk factors for abdominal aortic aneurysms: a 7-year prospective study: the Tromso Study, 1994–2001. Circulation. 2009;119:2202–2208. doi: 10.1161/CIRCULATIONAHA.108.817619. [DOI] [PubMed] [Google Scholar]
- 12.Chun KC, Teng KY, Chavez LA, et al. Risk factors associated with the diagnosis of abdominal aortic aneurysm in patients screened at a regional Veterans Affairs health care system. Ann Vasc Surg. 2014;28:87–92. doi: 10.1016/j.avsg.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Stanley JC, Fry WJ. Pathogenesis and clinical significance of splenic artery aneurysms. Surgery. 1974;76:898–909. [PubMed] [Google Scholar]
- 14.Puttini M, Aseni P, Brambilla G, Belli L. Splenic artery aneurysms in portal hypertension. J Cardiovasc Surg (Torino) 1982;23:490–493. [PubMed] [Google Scholar]
- 15.Tétreau R, Beji H, Henry L, Valette P-J, Pilleul F. Arterial splanchnic aneurysms: presentation, treatment and outcome in 112 patients. Diagn Interv Imaging. 2016;97:81–90. doi: 10.1016/j.diii.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Bolen MA, Brinza E, Renapurkar RD, Kim ESH, Gornik HL. Screening CT angiography of the aorta, visceral branch vessels, and pelvic arteries in fibromuscular dysplasia. JACC Cardiovasc Imaging. 2016 doi: 10.1016/j.jcmg.2016.04.010. DOI: 10.1016/j.jcmg.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Stanley JC, Gewertz BL, Bove EL, Sottiurai V, Fry WJ. Arterial fibrodysplasia. Histopathologic character and current etiologic concepts. Arch Surg. 1975;110:561–566. doi: 10.1001/archsurg.1975.01360110107018. [DOI] [PubMed] [Google Scholar]
- 18.Ueda J, Takahashi S, Furukawa T, Araki Y. Portal hypertension with intra- and extrasplenic arterial aneurysms and large venous varices. Cardiovasc Intervent Radiol. 1995;18:243–246. doi: 10.1007/BF00239420. [DOI] [PubMed] [Google Scholar]
- 19.Onuchic LF, Furu L, Nagasawa Y, et al. PKHD1, the polycystic kidney and hepatic disease 1 gene, encodes a novel large protein containing multiple immunoglobulin-like plexin-transcription-factor domains and parallel beta-helix 1 repeats. Am J Hum Genet. 2002;70:1305–1317. doi: 10.1086/340448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ward CJ, Hogan MC, Rossetti S, et al. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet. 2002;30:259–269. doi: 10.1038/ng833. [DOI] [PubMed] [Google Scholar]
- 21.Menezes LFC, Cai Y, Nagasawa Y, et al. Polyductin, the PKHD1 gene product, comprises isoforms expressed in plasma membrane, primary cilium, and cytoplasm. Kidney Int. 2004;66:1345–1355. doi: 10.1111/j.1523-1755.2004.00844.x. [DOI] [PubMed] [Google Scholar]
- 22.Nagasawa Y, Matthiesen S, Onuchic LF, et al. Identification and characterization of Pkhd1, the mouse orthologue of the human ARPKD gene. J Am Soc Nephrol. 2002;13:2246–2258. doi: 10.1097/01.asn.0000030392.19694.9d. [DOI] [PubMed] [Google Scholar]
- 23.Rossetti S, Consugar MB, Chapman AB, et al. Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2007;18:2143–2160. doi: 10.1681/ASN.2006121387. [DOI] [PubMed] [Google Scholar]
- 24.Harris PC, Torres VE. Polycystic kidney disease. Annu Rev Med. 2009;60:321–337. doi: 10.1146/annurev.med.60.101707.125712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ward CJ, Turley H, Ong AC, et al. Polycystin, the polycystic kidney disease 1 protein, is expressed by epithelial cells in fetal, adult, and polycystic kidney. Proc Natl Acad Sci USA. 1996;93:1524–1528. doi: 10.1073/pnas.93.4.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian F, Germino FJ, Cai Y, Zhang X, Somlo S, Germino GG. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat Genet. 1997;16:179–183. doi: 10.1038/ng0697-179. [DOI] [PubMed] [Google Scholar]
- 27.Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol. 2002;13:2508–2516. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- 28.Yoder BK. Role of primary cilia in the pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2007;18:1381–1388. doi: 10.1681/ASN.2006111215. [DOI] [PubMed] [Google Scholar]
- 29.Kim I, Li C, Liang D, et al. Polycystin-2 expression is regulated by a PC2-binding domain in the intracellular portion of fibrocystin. J Biol Chem. 2008;283:31559–31566. doi: 10.1074/jbc.M805452200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim I, Fu Y, Hui K, et al. Fibrocystin/polyductin modulates renal tubular formation by regulating polycystin-2 expression and function. J Am Soc Nephrol. 2008;19:455–468. doi: 10.1681/ASN.2007070770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S, Zhang J, Nauli SM, et al. Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia. Mol Cell Biol. 2007;27:3241–3252. doi: 10.1128/MCB.00072-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graf S, Schischma A, Eberhardt KE, Istel R, Stiasny B, Schulze BD. Intracranial aneurysms and dolichoectasia in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2002;17:819–823. doi: 10.1093/ndt/17.5.819. [DOI] [PubMed] [Google Scholar]
- 33.Schievink WI, Torres VE, Wiebers DO, Huston J. Intracranial arterial dolichoectasia in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1997;8:1298–1303. doi: 10.1681/ASN.V881298. [DOI] [PubMed] [Google Scholar]
- 34.Kim J, Kim SM, Lee SY, et al. A case of severe aortic valve regurgitation caused by an ascending aortic aneurysm in a young patient with autosomal dominant polycystic kidney disease and normal renal function. Korean Circ J. 2012;42:136–139. doi: 10.4070/kcj.2012.42.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Afari ME, Quddus A, Bhattarai M, John AR, Broderick RJ. Spontaneous coronary dissection in polycystic kidney disease. R I Med J. 2013;96:44–45. [PubMed] [Google Scholar]
- 36.Lee C-C, Fang C-Y, Huang C-C, Ng S-H, Yip H-K, Ko S-F. Computed tomography angiographic demonstration of an unexpected left main coronary artery dissection in a patient with polycystic kidney disease. J Thorac Imaging. 2011;26:W4–W6. doi: 10.1097/RTI.0b013e3181dc2a53. [DOI] [PubMed] [Google Scholar]
- 37.Larranaga J, Rutecki GW, Whittier FC. Spontaneous vertebral artery dissection as a complication of autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1995;25:70–74. doi: 10.1016/0272-6386(95)90629-0. [DOI] [PubMed] [Google Scholar]
- 38.Seshadri A, Byrne C, Kramer A, Bartlett ST, Sarkar R. Revascularization and rescue of a failed kidney transplant in a case of autosomal dominant polycystic kidney disease. J Vasc Surg. 2012;55:1766–1768. doi: 10.1016/j.jvs.2011.12.061. [DOI] [PubMed] [Google Scholar]
- 39.Inoguchi H, Komori K, Maehara Y. Surgical management of abdominal aortic aneurysm associated with polycystic kidney disease: report of two cases. Surg Today. 2008;38:253–257. doi: 10.1007/s00595-007-3615-4. [DOI] [PubMed] [Google Scholar]
- 40.Kato A, Takita T, Furuhashi M, Maruyama Y, Hishida A. Abdominal aortic aneurysms in hemodialysis patients with autosomal dominant polycystic kidney disease. Nephron. 2001;88:185–186. doi: 10.1159/000045984. [DOI] [PubMed] [Google Scholar]
- 41.Takagi H, Umemoto T. Abdominal aortic aneurysm and autosomal-dominant polycystic kidney disease. Kidney Int. 2005;67:376. doi: 10.1111/j.1523-1755.2005.091_2.x. [DOI] [PubMed] [Google Scholar]
- 42.Torra R, Nicolau C, Badenas C, et al. Abdominal aortic aneurysms and autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1996;7:2483–2486. doi: 10.1681/ASN.V7112483. [DOI] [PubMed] [Google Scholar]
- 43.Perrone RD, Malek AM, Watnick T. Vascular complications in autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2015 doi: 10.1038/nrneph.2015.128. DOI: 10.1038/nrneph.2015.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim K, Drummond I, Ibraghimov-Beskrovnaya O, Klinger K, Arnaout MA. Polycystin 1 is required for the structural integrity of blood vessels. Proc Natl Acad Sci USA. 2000;97:1731–1736. doi: 10.1073/pnas.040550097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fick-Brosnahan GM. Endothelial dysfunction and angiogenesis in autosomal dominant polycystic kidney disease. Curr Hypertens Rev. 2013;9:32–36. doi: 10.2174/1573402111309010006. [DOI] [PubMed] [Google Scholar]
- 46.Mohieldin AM, Zubayer HSM, Al Omran AJ, et al. Vascular endothelial primary cilia: mechanosensation and hypertension. Curr Hypertens Rev. 2016;12:57–67. doi: 10.2174/1573402111666150630140615. [DOI] [PubMed] [Google Scholar]
- 47.Outeda P, Huso DL, Fisher SA, et al. Polycystin signaling is required for directed endothelial cell migration and lymphatic development. Cell Rep. 2014;7:634–644. doi: 10.1016/j.celrep.2014.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang JL, Woolf AS, Long DA. Angiogenesis and autosomal dominant polycystic kidney disease. Pediatr Nephrol. 2013;28:1749–1755. doi: 10.1007/s00467-012-2305-7. [DOI] [PubMed] [Google Scholar]
- 49.Liu D, Wang CJ, Judge DP, et al. A Pkd1-Fbn1 genetic interaction implicates TGF-β signaling in the pathogenesis of vascular complications in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2014;25:81–91. doi: 10.1681/ASN.2012050486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu R, Franchi F, Miller B, et al. Polycystic kidneys have decreased vascular density: a micro-CT study. Microcirculation. 2013;20:183–189. doi: 10.1111/micc.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peterson KM, Franchi F, Loeffler DL, et al. Endothelial dysfunction occurs prior to clinical evidence of polycystic kidney disease. Am J Nephrol. 2013;38:233–240. doi: 10.1159/000354236. [DOI] [PMC free article] [PubMed] [Google Scholar]