Abstract
Background
Since 2005, Ethiopia has aggressively scaled up malaria prevention and case management. As a result, the number of malaria cases and deaths has significantly declined. In order to track progress towards the elimination of malaria in Amhara Region, coverage of malaria control tools and current malaria transmission need to be documented.
Methods
A cross-sectional household survey oversampling children under 5 years of age was conducted during the dry season in 2013. A bivalent rapid diagnostic test (RDT) detecting both Plasmodium falciparum and Plasmodium vivax and serology assays using merozoite antigens from both these species were used to assess the prevalence of malaria infections and exposure to malaria parasites in 16 woredas (districts) in Amhara Region.
Results
7878 participants were included, with a mean age of 16.8 years (range 0.5–102.8 years) and 42.0% being children under 5 years of age. The age-adjusted RDT-positivity for P. falciparum and P. vivax infection was 1.5 and 0.4%, respectively, of which 0.05% presented as co-infections. Overall age-adjusted seroprevalence was 30.0% for P. falciparum, 21.8% for P. vivax, and seroprevalence for any malaria species was 39.4%. The prevalence of RDT-positive infections varied by woreda, ranging from 0.0 to 8.3% and by altitude with rates of 3.2, 0.7, and 0.4% at under 2000, 2000–2500, and >2500 m, respectively. Serological analysis showed heterogeneity in transmission intensity by area and altitude and evidence for a change in the force of infection in the mid-2000s.
Conclusions
Current and historic malaria transmission across Amhara Region show substantial variation by age and altitude with some settings showing very low or near-zero transmission. Plasmodium vivax infections appear to be lower but relatively more stable across geography and altitude, while P. falciparum is the dominant infection in the higher transmission, low-altitude areas. Age-dependent seroprevalence analyses indicates a drop in transmission occurred in the mid-2000s, coinciding with malaria control scale-up efforts. As malaria parasitaemia rates get very low with elimination efforts, serological evaluation may help track progress to elimination.
Electronic supplementary material
The online version of this article (doi:10.1186/s12936-017-1884-y) contains supplementary material, which is available to authorized users.
Keywords: Malaria, Plasmodium falciparum, Plasmodium vivax, Seroconversion, Seroprevalence, Malaria transmission
Background
Malaria is a major public health challenge in Ethiopia, accounting for approximately 12 million cases each year [1]. Malaria epidemiology in Ethiopia is relatively unique in Africa in that both Plasmodium falciparum and Plasmodium vivax are present and malaria transmission is generally unstable, with focal, seasonal outbreaks and occasional epidemics [1].
During the last decade, significant scale-up of interventions—including population-wide distribution of free, long-lasting insecticide-treated nets (LLINs), indoor residual spraying (IRS), nationwide roll-out of rapid diagnostic tests (RDTs) and artemisinin-based combination treatments—to reduce malaria transmission was implemented throughout the country [2]. This resulted in a significant reduction in the prevalence of the malarial infection and illness and its consequences [3–6]. It is estimated that, if these activities are continued and sustained in the coming 5 years, 50 million malaria cases will be averted and $39 million in costs associated with patient care and treatment will be saved [7]. It is in this context that there is a renewed commitment to advance toward malaria elimination in Ethiopia.
To facilitate malaria elimination in geographical areas with historically low transmission [8], it is imperative to first document the current levels of malaria transmission and to provide the necessary evidence to extend effective malaria elimination activities. Measuring malaria transmission in low transmission areas is challenging, as microscopy and/or RDT results both underestimate infections and typically present a narrow dynamic range of malaria prevalence estimates, which require large sample sizes [9–11]. While molecular diagnostic tests such as PCR are more sensitive and may capture a more accurate representation of infection prevalence, they are currently impractical in terms of complexity and cost, and in the case of P. vivax, require a larger specimen volume [12, 13]. Serological markers measuring cumulative exposure can be a more practical and cost effective alternative, and may provide improved discrimination of transmission rates in the low to medium transmission areas by comparing seroprevalence and seroconversion rates. A recent study based on school surveys from the Oromia Regional State of Ethiopia, demonstrate the value of serological marker-based indicators in low transmission areas (seroprevalence of 0–12.7% for P. falciparum and 0–4.5% for P. vivax) [14].
A collaborative effort between the Federal Ministry of Health and Amhara National Regional State Health Bureau (ANRSHB); the Malaria Control and Elimination Partnership in Africa (MACEPA), a programme at PATH; and The Carter Center (TCC) was established to develop, implement, and evaluate a comprehensive malaria elimination strategy in selected areas of Amhara Region, Ethiopia. Before the implementation of activities, this study was conducted to estimate 2013 baseline rates of: (1) core malaria interventions including LLIN ownership and use, and IRS coverage; and (2) current and cumulative malaria infections due to P. falciparum and/or P. vivax using a population-based survey with malaria RDTs and malaria serology.
Methods
Study area
This study was conducted in Ethiopia’s Amhara Region, which has a population of approximately 20 million people. In 2012, the reported annual incidence of malaria in the Amhara Region was 60 cases per 1000 population, accounting for 19% of the national malaria burden [15, 16]. The Amhara Region has significant variations in altitude, temperature, and annual rainfall resulting in areas of differing malaria risk and transmission for both P. falciparum and P. vivax. Malaria transmission is seasonal, with the major malaria transmission season being between September and December. For the purpose of this study, woredas (districts) of Amhara were divided into four eco-epidemiological zones (very low, very low to low, low, and low to moderate) based on weekly malaria incidence data from 2012. A total of 16 woredas from these four unique eco-epidemiological zones were selected (Fig. 1a, b).
Fig. 1.
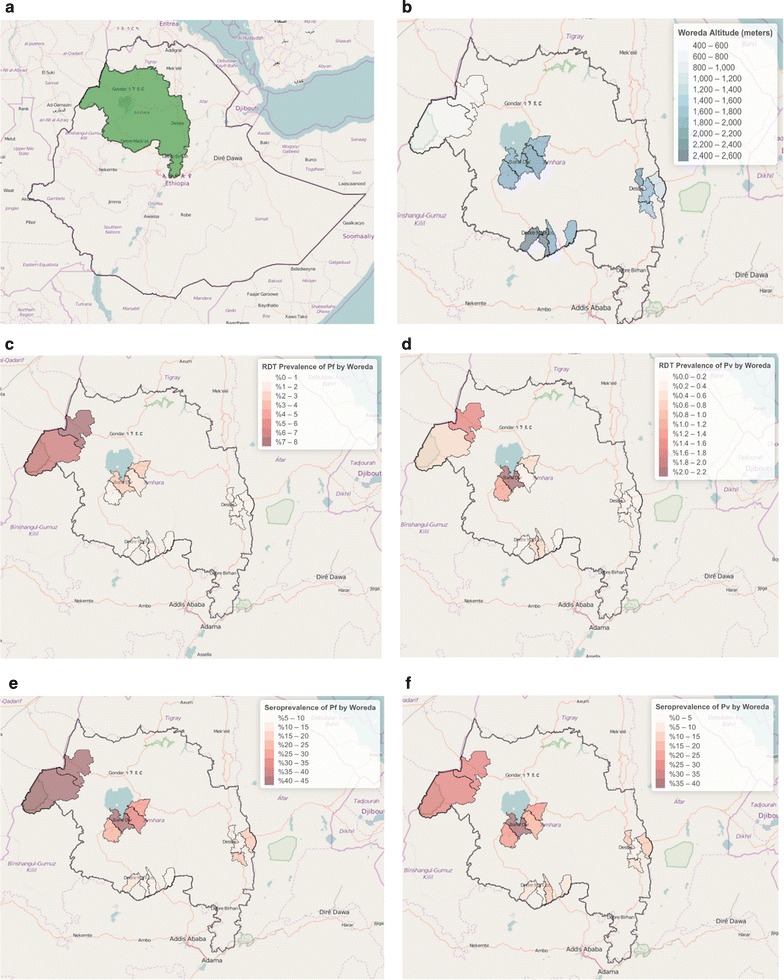
a Amhara Region, Ethiopia, b survey areas (woredas) selected for the Malaria Elimination, colour-coded light to dark from lowest to highest for altitude, c Plasmodium falciparum RDT prevalence, d Plasmodium vivax RDT prevalence, e Plasmodium falciparum seroprevalence, and f Plasmodium vivax seroprevalence
Ethical considerations
This research protocol was reviewed and approved by ethical review committees at Amhara National Regional State (Amhara Research Ethics Review Committee), PATH, and Emory University.
Study design and data collection
A cross-sectional household survey was conducted in March–April 2013 during the dry, low-transmission season to provide estimates of malaria burden and coverage of malaria control tools at woreda level in 16 target woredas. Woreda-stratified sampling was used to draw a sample from each woreda that was proportional to the woreda population. Within each woreda, enumeration areas (EAs) were selected with probability proportional to size. A total of 278 EAs were selected from woreda listings of all EAs developed by the Central Statistical Agency for the 2007 census. In each selected EA, 25 households were selected using simple random sampling from a sampling frame of all households created by EA mapping. EAs were mapped using personal digital assistants (PDAs). Within each sampled household, all children aged 6 months to 4 years old were eligible for malaria testing. Household members over 5 years of age were eligible in one out of every four households. Individuals were invited to participate and were included after verbal informed consent was obtained.
A standardized questionnaire to collect information on socio-demographic characteristics, knowledge and attitudes about malaria, and use of malaria control tools was administered to each participant. Data were collected using PDAs. Finger-prick blood samples were collected from consenting participants to perform an RDT (bivalent SD Bioline Malaria Ag P.f./P.v. HRP-2/pLDH POCT) and prepare dried blood spots (DBS) on filter paper. Participants with a positive RDT result received antimalarial treatment per national guidelines. Those seriously ill were referred to seek immediate care at the nearest health facility.
Laboratory methods
Samples were assayed for anti-malarial IgG antibody responses to P. falciparum and P. vivax merozoite surface protein (MSP-119) and apical membrane antigen 1 (AMA-1) recombinant protein [11] by enzyme-linked immunosorbent assay (ELISA) at the Amhara Region Health Research Laboratory Centre. Three-millimetre diameter circles were punched from DBS and reconstituted in PBS buffer as previously described [10, 17]. Plates were coated with antigens at a concentration of 0.5 μg/mL overnight. Eluates from test samples were added at a dilution of 1:1000 and species-specific positive controls sera were added at serial dilutions on each plate to generate standard curves. After overnight incubation, horseradish-peroxidase-conjugated rabbit-anti-human IgG (DAKO, USA) secondary antibody was added followed by substrate Tetramethylbenzidine (TMB) (SurModics, USA) as color detection. Optical density (OD) values were read at 450 nm after 15 min incubation with the substrate.
Data analysis
Data analysis was done using Stata (College Station, USA). Estimates of seroprevalence and prevalence of malaria infection by RDT were weighted to account for the sampling design.
Serology samples with duplicate OD values that varied by more than 50% were excluded from the analysis. Duplicate OD results were averaged, the raw ODs were transformed into titres using the standard curve generated by the positive control on each assay plate. Seropositivity was calculated by fitting a Gaussian distribution to normalized OD values using maximum-likelihood methods assuming two distributions, a narrow seronegative, and broad seropositive [18]. For each antigen, the cutoff value was determined by the mean OD of the seronegative population plus three standard deviations [11]. Plasmodium falciparum and P. vivax seropositivity was defined as an individual being positive for either or both respective antigens, and was analysed by age, altitude, and woreda. Simple reverse catalytic models were fitted to seroprevalence and age data using maximum likelihood methods and seroconversion rates (SCR) were estimated [11]. Evidence for models with two forces of infection were investigated using profile likelihood plots and compared with a model with a single force of infection using log-likelihood tests [10].
Logistic regression was employed to determine the likelihood of being RDT-positive for P. vivax and/or P. falciparum (odds ratios [OR]; 95% confidence intervals [CI]) as compared to having a negative RDT test result, for sex, age, altitude, fever, malaria history, bed net ownership, bed net use, and IRS spraying in the last 12 months. Univariate and multivariate models including all variables to estimate adjusted ORs were run. Two independent models were run for altitude (<2000 and ≥2000 m) because there was a significant effect modification between altitude and bed net use/ownership and altitude and IRS. Moreover, the national malaria programme uses the 2000 m altitude cut-off for targeted deployment of malaria control tools, hence supporting the a priori plan to run the two models separately. Also, it was not possible to add a third altitude band above 2500 m for the regression as was done for the descriptive analysis because the number of RDT positives was too small. Similar regression models were employed for P. falciparum and P. vivax seropositivity.
Results
Study population
A total of 7878 individuals from 16 woredas (Fig. 1a, b) participated in the cross-sectional survey and had questionnaire data, RDT malaria blood testing results, and serology results available for analysis. 52.1% of participants were female, 42.0% were less than 4 years of age, and 55.1% lived below 2000 m (Table 1). At the household level, 59.2% owned a bed net and 31.4% had received IRS in the previous 12 months. Only 21.7% of participants reported having used a bed net the previous night, and 11.6% reported having had a fever in the previous 2 weeks. A total of 151 (1.9%) were RDT-positive (age-adjusted prevalence) (Table 2); 120 (1.5%) were RDT-positive for P. falciparum, 35 (0.4%) for P. vivax, and 4 (0.05%) for both P. falciparum and P. vivax. Among RDT-positive individuals, 41.0% were asymptomatic (no reported fever in the last 2 weeks), with no significant differences by age group. The overall seroprevalence was 39.4% (n = 3102), with 30.0% (2367) being P. falciparum seropositive and 21.8% (1720) P. vivax seropositive.
Table 1.
Characteristics of survey participants (n = 7878)
| Number | Percent (%) | |
|---|---|---|
| Sex | ||
| Female | 4101 | 52.1 |
| Male | 3777 | 47.9 |
| Age (years) | ||
| 6 months–4 | 3307 | 42.0 |
| 5–9 | 924 | 11.7 |
| 10–19 | 1094 | 13.9 |
| 20–39 | 1486 | 18.9 |
| 40–59 | 696 | 8.8 |
| 60+ | 371 | 4.7 |
| Woreda | ||
| Very low transmission | ||
| Aneded | 250 | 3.2 |
| Awabel | 391 | 5.0 |
| Gozamin | 393 | 5.0 |
| Shebel Berenta | 315 | 4.0 |
| Very low to low transmission | ||
| Bati | 396 | 5.0 |
| Dawa Chef | 589 | 7.5 |
| Kalu | 631 | 8.0 |
| Tehuledere | 275 | 3.5 |
| Low transmission | ||
| Bahir Dar Zuriya | 771 | 9.8 |
| Dera | 830 | 10.5 |
| Fogera | 900 | 11.4 |
| Mecha | 1249 | 15.9 |
| Low to moderate transmission | ||
| Genda Wuha Town | 53 | 0.7 |
| Gulegu | 78 | 1.0 |
| Metema | 424 | 5.4 |
| Quara | 333 | 4.2 |
| Altitude (m) | ||
| <2000 | 4388 | 55.1 |
| 2000–2500 | 3175 | 40.3 |
| >2500 | 365 | 4.6 |
| Bed net use the previous night | ||
| No | 6165 | 78.3 |
| Yes | 1713 | 21.7 |
| Fever in last 2 weeks | ||
| No | 6951 | 88.4 |
| Yes | 909 | 11.6 |
Unweighted estimates
Table 2.
Prevalence of malaria infection by RDT-positivity and seroprevalence (n = 7878)
| Number | Percent (%) | |
|---|---|---|
| RDT-positivity | ||
| P. falciparum | 120 | 1.5 |
| P. vivax | 35 | 0.4 |
| P. falciparum and P. vivax co-infection | 4 | 0.05 |
| Total P. falciparum and/or P. vivax | 151 | 1.9 |
| Seroprevalence | ||
| P. falciparum | 2367 | 30.0 |
| P. vivax | 1720 | 21.8 |
| P. falciparum and P. vivax co-infection | 985 | 12.5 |
| Total P. falciparum and/or P. vivax | 3102 | 39.4 |
Weighted estimates based on sampling strategy
Geospatial distribution of RDT-positivity and seroprevalence
There was substantial variation in RDT-positivity for P. falciparum (from <1.0% to roughly 8.0%) (Fig. 1c) and less variation for P. vivax (from 0% to roughly 1.5%) (Fig. 1d) over the 16 woredas. Plasmodium falciparum seroprevalence by woreda ranged from 61.5% in Metema to 4.8% in Tehuledere (Fig. 1e); and P. vivax seroprevalence by woreda ranged from 43.7% in Bahir Dar Zuriya to 3.7% in Aneded (Fig. 1f). Overall, the RDT-positivity rates and the seroprevalence for both P. falciparum and P. vivax decreased with increasing altitude (Fig. 2). While higher RDT and serology prevalence was observed in woredas located at lower altitudes, there was heterogeneity within the different elevation strata (Fig. 2). The RDT-positivity by altitude was 3.2, 0.7, and 0.4% for <2000, 2000–2500, and >2500 m, respectively. Respective RDT-positivity for P. falciparum and P. vivax at these altitudes were 2.7 and 0.5% (<2000 m), 0.3 and 0.4% (2000–2500 m), and 0 and 0.4% (≥2500 m) (Fig. 3a). Additionally, the respective seroprevalence for P. falciparum and P. vivax at these altitudes were 41.6 and 28.8% (<2000 m), 19.8 and 18.9% (2000–2500 m), and 10.7 and 10.6% (≥2500 m) (Fig. 3a). There were no significant differences in seroprevalence by gender across the different elevation strata.
Fig. 2.
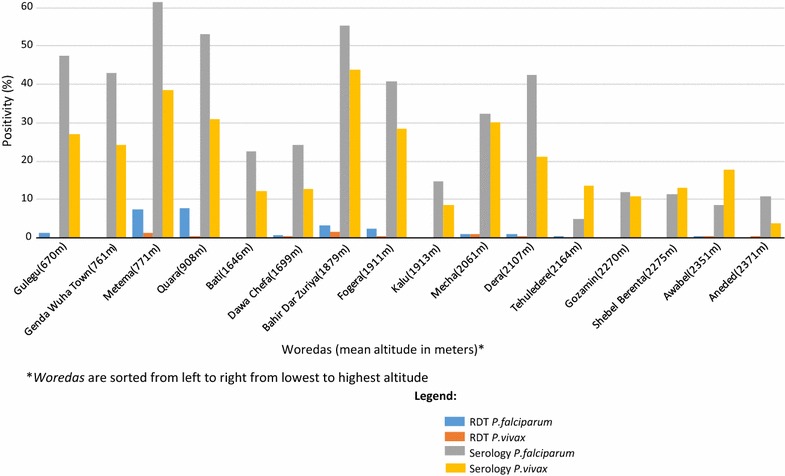
Plasmodium falciparum and P. vivax RDT-positivity and seroprevalence by woreda. The woredas are arranged from highest to lowest altitude
Fig. 3.
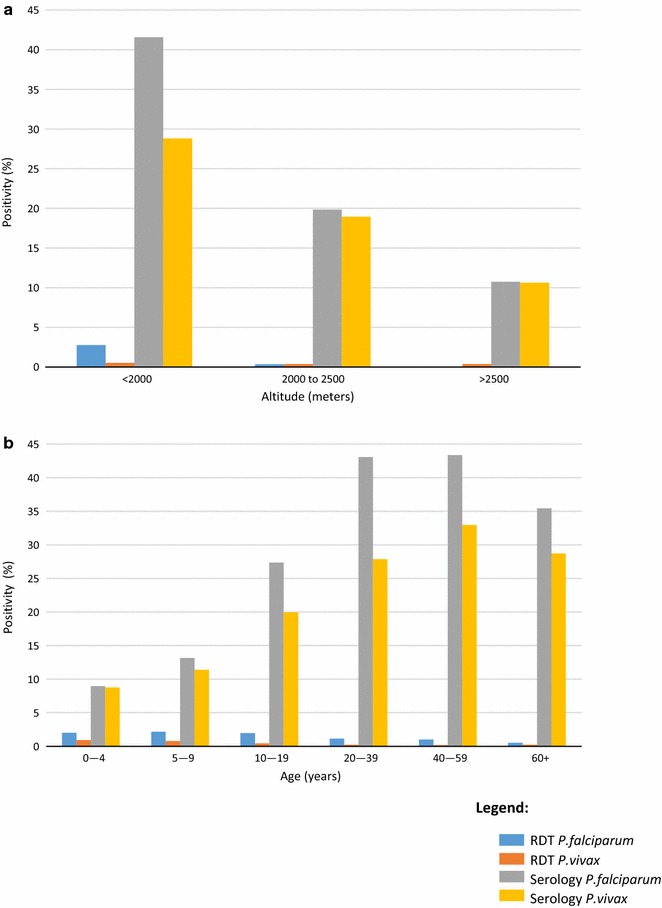
Plasmodium falciparum and P. vivax RDT-positivity and seroprevalence by a altitude and b age group
RDT-positivity and seropositivity by age
During this low transmission season, RDT-positivity for both P. falciparum and P. vivax was highest amongst 0–9-year-olds, after which RDT-positivity decreased with increasing age (Fig. 3b). Across all age groups, P. falciparum RDT-positivity was higher than P. vivax RDT-positivity.
Plasmodium falciparum and P. vivax seroprevalence increased by age group, although a slight decrease among those aged 60 years and older was observed (Fig. 3b). This general pattern of increasing P. falciparum and P. vivax seroprevalence by age was seen in each of the altitude strata (Fig. 4). Overall, P. falciparum seroprevalence was higher than P. vivax seroprevalence across all age groups. However, when stratified by altitude, this pattern was only observed for those living at <2000 m. Plasmodium falciparum and P. vivax seroprevalences were more similar at higher altitudes, although there was variability observed in the >2500 m stratum, which is likely due to small number of positives per age group.
Fig. 4.
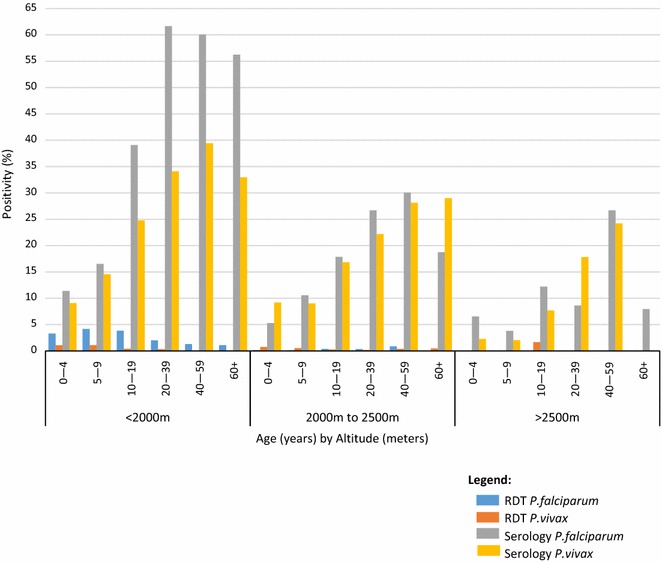
Plasmodium falciparum and P. vivax RDT-positivity and seroprevalence by altitude and age group
Seroconversion rates
The age seroconversion plots for antibody responses for P. falciparum and P. vivax are shown in Fig. 5. The model with two forces of infection (a higher force of infection prior to approximately 15 years ago and a lower force of infection in the last 15 years) provided a better fit to the data than a model with a single force of infection. These models suggest that a substantial reduction in the exposure to both P. falciparum and P. vivax infections has occurred within the last 10–15 years (P. falciparum: current SCR 0.032 year−1 [0.029–0.035], previous SCR 0.26 year−1 [0.11–0.57]; P. vivax: current SCR 0.034 year−1 [0.03–0.038], previous SCR 0.22 year−1 [0.053–0.91]). The age seroconversion plots for antibody responses for P. falciparum and P. vivax by altitude strata are shown in Additional file 1, showing similar trends in all altitudes.
Fig. 5.
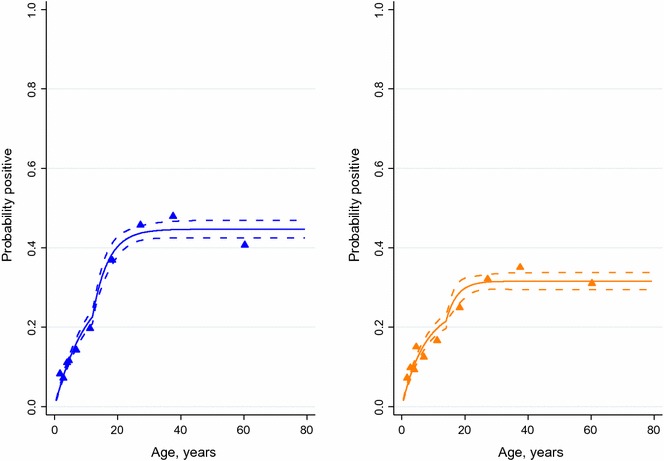
Age-seroconversion plots for antibody responses to Plasmodium falciparum antigens (left) and P. vivax antigens (right). Triangles represent deciles of observed data, solid lines represent the fit to the data of a reverse catalytic conversion model and broken blue lines provide the 95% confidence interval for this fit
The prevalence of RDT-positivity, reported fever and seroprevalence decreased with increasing altitude (Additional file 2). Malaria risk factors associated with RDT-positivity and seroprevalence by altitude (<2000 and ≥2000 m) are shown in Additional files 3 and 4. In both models, individuals with a reported fever in the past 2 weeks were significantly more likely to be RDT-positive, and there was a decreasing risk of RDT-positivity with increasing age. In altitudes below 2000 m, household ownership of a bed net was significantly protective against RDT-positivity; in altitudes ≥2000 m, those with a recent history of malaria were significantly more likely to be RDT-positive, and households that received IRS in the last 12 months were significantly protected against RDT-positivity. For seroprevalence, in altitudes under 2000 m, individuals with reported fever in the previous 2 weeks were significantly more likely to be seropositive, and household ownership of a bed net was significantly protective of seropositivity. In altitudes ≥2000 m, individuals with a history of malaria and households that had received IRS in the previous 12 months were significantly more likely to be seropositive.
Additional file 5 shows that seropositivity for P. falciparum is present in younger children across the different elevation strata, and persists even at the highest altitudes (>2500 m). However, all seropositive cases seen above 2500 m are borderline positive, as they are near the limit of detection of 0.02 OD units.
Discussion
The overall RDT-positivity in the study area during the dry season (March–April) was 1.9%, when the prevalence of infection is expected to be significantly lower than during the major malaria transmission season, which is between September and December in Amhara region. This low prevalence of parasitaemia is probably an underestimate due to the imperfect RDT sensitivity, however it confirms that ongoing transmission persists in this region at a low level into the dry/low transmission season. Another study conducted in July 2013 at the end of the dry season found a high prevalence (12.0%) of RDT-diagnosed Plasmodium infection in nearby low-land farming areas of Amhara [19]. The use of small-scale irrigation schemes employed to ensure food security in the area during the dry season might contribute to maintaining the transmission. A recent study found that malaria transmission was significantly higher in irrigated areas and that was primarily due to the higher number of mosquito breeding sites such as waterlogged agricultural field puddles, leakage pools from irrigation canals and poorly functioning irrigation canals [20]. The relatively low use of malaria prevention tools (e.g., 59.2% household bed net ownership and 22% of individuals sleeping under a bed net the previous night) might represent the specific targeting of LLINs to areas <2000 m and the study sample included many living at higher elevations (areas not targeted for LLINs). However, the relatively low use despite ownership highlights the continuous need to educate and increase the awareness that malaria persists year round, and therefore the use of bed nets and other malaria prevention strategies should be reinforced throughout the year [21].
RDT-positivity was highest among children 0–9 years of age, although this pattern was only seen in the lowest altitude areas. The higher prevalence of infection in this young age group is likely to be driven by lower levels of naturally acquired immunity, with higher parasitaemia densities and potentially longer persistence of parasitaemia at a level that could be detected by RDTs [22]. This shows that, even in low transmission areas like this one in Ethiopia, an age pattern reflecting build-up of immunity is observed [23].
Seroprevalence reflects cumulative exposure to malaria, and, with the antigenic targets used in this study, is expected to fluctuate less between the different transmission seasons. As expected, seroprevalence increased with age for both P. falciparum and P. vivax antigens, reflecting the cumulative exposure to these infections over time, given that, once acquired, antibody responses to MSP-119 and AMA-1 can persist for several years [24]. Thus, seroprevalence in the youngest age group, which ranged from 2.3 to 11.4%, was associated with altitude, and supports the observation of ongoing transmission showed by the RDT results in the same age range. The slight decline in seroprevalence among individuals aged 60 years and older may be due to waning of antibody titers with age, or perhaps reflect decreased exposure to malaria transmission associated with a reduction of farming activities and/or less travel.
The RDT-positivity and the seroprevalence for both P. falciparum and P. vivax decreased with increasing altitude, which reflects the well-known phenomenon that transmission is generally higher at lower altitudes. In areas below 2000 m, RDT positivity for P. falciparum was higher than for P. vivax (2.7 and 0.5%, respectively). However, at altitudes over 2500 m the opposite was found: the RDT prevalence for P. vivax was 0.4 and 0% for P. falciparum, which may be related to the fact that P. vivax can have persistent liver stages and infection relapses and is also more tolerant of lower ambient temperatures [25]. Moreover, a recent meta-analysis suggests that the proportion of submicroscopic P. vivax infections is higher than for P. falciparum particularly in low transmission intensity areas [26].
The 2011 Malaria Indicator Survey (MIS) found the same altitude pattern, with a 13-fold higher prevalence of malaria infection in areas under 2000 m, as compared to higher altitudes [1]. Of note, in the 2011 MIS, at altitudes above 2000 m, P. falciparum was not detected by microscopy and P. falciparum positivity by RDT was of 0.3%, showing the variation due to the different sensitivities of the tests used and highlighting the challenges of monitoring transmission in very low transmission areas.
The dominance of P. falciparum RDT-positivity and seroprevalence in areas under 2000 m suggests that these areas are driving the transmission in the Amhara Region by exporting infections to the higher altitude areas. At elevations over 2000 m, RDT prevalence was almost zero, and P. falciparum and P. vivax seroprevalences, which were nearly equivalent, were much lower. All age groups showed seropositivity, although seropositive children in areas above 2500 m had very low antibody titers. This suggests that local transmission in higher altitude areas is very low and that exposure to infection is likely to be acquired elsewhere and imported as a result of the population’s mobility and/or migrant labour workforce [27, 28].
Finally, seroconversion rates showed a statistically significant change in the force of malaria infection, suggesting that malaria transmission intensity was higher and was reduced nearly tenfold (from approximately 25% of individuals seroconverting per year previously to 3% currently) in the early-to-mid-2000s in Amhara. This finding probably reflects the success of the recent aggressive scale-up of malaria control activities in the area [7].
In a low transmission setting such as Amhara, the typical malaria age pattern, where younger individuals, especially children, have the highest risk of infections, is also observed. Although reported fever in the past 2 weeks was a significant predictor of RDT-positivity, 63.1 and 46.4% of RDT-positive individuals in altitudes <2000 and ≥2000 m, respectively, did not report a history of fever. This suggests that naturally acquired immunity is attained early in life [22]. Similar results were observed during a population-wide malaria testing and treatment intervention implemented in six villages in Amhara Region, Ethiopia [29].
Household bed net ownership was significantly protective against both RDT positivity and seropositivity at altitudes under 2000 m, but not at 2000 m and above, primarily because bed net distribution programmes were focused specifically in lower altitudes where malaria transmission is known to be higher. Above 2000 m, IRS in the past 12 months was protective against RDT positivity, but was a significant risk factor for seropositivity. This may be due to the fact that certain IRS targeting programmes were implemented in areas ≥2000 m based on previous malaria outbreaks, and therefore may have masked the true effect of IRS spraying.
Conclusions
As observed in other malarious areas [30–34], there was both RDT-positivity and seroprevalence heterogeneity across and within the different malaria transmission eco-epidemiological zones in Amhara, with overall higher rates for P. falciparum compared to P. vivax and substantially higher transmission in lower altitude areas. Progress made in Ethiopia during the last decade in decreasing malaria transmission should continue with effective interventions tailored to the socio-epidemiological characteristics of the area. Given the apparent importance of population mobility in the parasite transmission patterns, specific interventions targeting the source areas at lower elevations and the mobile population would be warranted to eliminate transmission in the highest altitude areas. Serology offers an opportunity to monitor transmission in low and seasonal transmission areas. Measuring changes in the transmission of malaria will be crucial to monitor progress toward malaria elimination, and serological surveys may be a critical tool in addition to infection detection to describe changes in malaria transmission.
Additional files
Additional file 1. Age-seroconversion plots for antibody responses to Plasmodium falciparum antigens (left) and P. vivax antigens (right) for altitudes below 2000 metres (A), 2000 to 2500 metres (B), and greater than 2500 metres (C). Triangles represent deciles of observed data, solid lines represent the fit to the data of a reverse catalytic conversion model and broken blue lines provide the 95% confidence interval for this fit.
Additional file 2. Percent of RDT-positivity (Plasmodium falciparum and/or P. vivax), serology positivity and presence of fever by bed net ownership, bed net use and IRS use, in different altitude strata.
Additional file 3. Percent and odds ratios for RDT-positivity (Plasmodium falciparum and/or P. vivax) by sociodemographic characteristics and malaria risk factors, for altitudes <2000 metres and ≥2000 metres.
Additional file 4. Percent and odds ratios for seropositivity (Plasmodium falciparum and/or P. vivax) by sociodemographic characteristics and malaria risk factors, for altitudes <2000 metres and ≥2000 metres.
Additional file 5. Dot plots of seropositivity to Plasmodium falciparum antigens among children aged 1 to 5 years, by altitude.
Authors’ contributions
CS, ML, GSN, GD, CD and RS designed the study. ML, CS, BAS and AG designed the study procedures and data collection materials. BAS, BHT, BBB and AG supervised data collection. WY, SP, RD, KT and LY designed serology lab procedures, trained and supervised serology lab analysis. WY conducted serology lab analysis. PB and MK analysed the survey and serology data. WP, SP, PB, CG, GD AG, CD and RS assisted with interpretation of results, drafted and edited the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Informed verbal consent was obtained from eligible participants or their guardians prior to enrolment in the study. The Amhara National Regional State, PATH, and Emory University ethical review committees reviewed and approved the entire protocol. Participation in the study was voluntary.
Funding
This publication is based on research funded by the Bill & Melinda Gates Foundation (OPP1089412). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The findings and conclusions contained within are those of the authors and do not necessarily reflect positions or policies of the Bill & Melinda Gates Foundation.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- AMA
apical membrane antigen
- ANRSHB
Amhara National Regional State Health Bureau
- DBS
dried blood spots
- EA
enumeration area
- IRS
indoor residual spraying
- LLIN
long-lasting insecticide-treated nets
- MSP
merozoite surface protein
- TCC
The Carter Center
- OD
optical density
- OR
odds ratio
- PDA
personal digital assistants
- RDT
rapid diagnostic test
- SCR
seroconversion rates
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s12936-017-1884-y) contains supplementary material, which is available to authorized users.
Contributor Information
Woyneshet G. Yalew, Email: woyn1978mam@yahoo.com
Sampa Pal, Email: spal@path.org.
Pooja Bansil, Email: pbansil@path.org.
Rebecca Dabbs, Email: Rebecca.Dabbs@lshtm.ac.uk.
Kevin Tetteh, Email: Kevin.Tetteh@lshtm.ac.uk.
Caterina Guinovart, Email: cguinovart@path.org.
Michael Kalnoky, Email: mkalnoky@path.org.
Belendia A. Serda, Email: bserda@path.org
Berhane H. Tesfay, Email: btesfay@path.org
Belay B. Beyene, Email: fiametaye@gmail.com
Catherine Seneviratne, Email: cseneviratne@path.org.
Megan Littrell, Email: mlittrell@path.org.
Lindsay Yokobe, Email: lyokobe@path.org.
Gregory S. Noland, Email: gregory.noland@cartercenter.org
Gonzalo J. Domingo, Email: gdomingo@path.org
Asefaw Getachew, Email: agetachew@path.org.
Chris Drakeley, Email: Chris.Drakeley@lshtm.ac.uk.
Richard W. Steketee, Email: rsteketee@path.org
References
- 1.President’s Malaria Initiative: Ethiopia, malaria operational plan FY 2014. http://www.pmi.gov/docs/default-source/default-document-library/malaria-operational-plans/fy14/ethiopia_mop_fy14.pdf?sfvrsn=14. Accessed 23 Feb 2015.
- 2.Jima D, Getachew A, Bilak H, Steketee RW, Emerson PM, Graves PM, et al. Malaria indicator survey 2007, Ethiopia: coverage and use of major malaria prevention and control interventions. Malar J. 2010;9:58. doi: 10.1186/1475-2875-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes KI, Chanda P, AbBarnabas G. Impact of the large-scale deployment of artemether/lumefantrine on the malaria disease burden in Africa: case studies of South Africa, Zambia and Ethiopia. Malar J. 2009;8(Suppl 1):S8. doi: 10.1186/1475-2875-8-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jima D, Wondabeku M, Alemu A, Teferra A, Awel N, Deressa W, et al. Analysis of malaria surveillance data in Ethiopia: what can be learned from the integrated disease surveillance and response system? Malar J. 2012;11:330. doi: 10.1186/1475-2875-11-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shargie EB, Gebre T, Ngondi J, Graves PM, Mosher AW, Emerson PM, et al. Malaria prevalence and mosquito net coverage in Oromia and SNNPR regions of Ethiopia. BMC Public Health. 2008;8:321. doi: 10.1186/1471-2458-8-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guthmann JP, Bonnet M, Ahoua L, Dantoine F, Balkan S, van Herp M, et al. Death rates from malaria epidemics, Burundi and Ethiopia. Emerg Infect Dis. 2007;1:140–143. doi: 10.3201/eid1301.060546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The African Leaders Malaria Alliance (ALMA), the Evidence to Policy Initiative (E2Pi) in UCSF’s Global Health Group, and the Clinton Health Access Initiative (CHAI). Maintaining the gains in malaria control. http://globalhealthsciences.ucsf.edu/sites/default/files/content/ghg/e2pi-maintaining-the-gains-country-briefs.pdf. Accessed 23 Feb 2016.
- 8.Roll back malaria—Ethiopia national strategic plan. http://www.rollbackmalaria.org/files/files/countries/Ethiopia-The-malaria-program-performance-review-2011.pdf. Accessed 23 Feb 2016.
- 9.Tusting LS, Bousema T, Smith DL, Drakeley C. Measuring changes in Plasmodium falciparum transmission: precision, accuracy and costs of metrics. Adv Parasitol. 2014;84:151–208. doi: 10.1016/B978-0-12-800099-1.00003-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart L, Gosling R, Griffin J, Gesase S, Campo J, Hashim R, et al. Rapid assessment of malaria transmission using age-specific sero-conversion rates. PLoS ONE. 2009;4:e6083. doi: 10.1371/journal.pone.0006083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drakeley C, Corran P, Coleman P, Tongren JE, McDonald SL, Carneiro I, Malima R, et al. Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc Natl Acad Sci USA. 2005;102:5108–5113. doi: 10.1073/pnas.0408725102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thongdee P, Chaijaroenkul W, Kuesap J, Na-Bangchang K. Nested-PCR and a new ELISA-based NovaLisa test kit for malaria diagnosis in an endemic area of Thailand. Korean J Parasitol. 2014;52:377–381. doi: 10.3347/kjp.2014.52.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noedl H, Yingyuen K, Laoboonchai A, Fukuda M, Sirichaisinthop J, Miller RS. Sensitivity and specificity of an antigen detection ELISA for malaria diagnosis. Am J Trop Med Hyg. 2006;75:1205–1208. [PubMed] [Google Scholar]
- 14.Ashton RA, Kefyalew T, Rand A, Sime H, Assefa A, Mekasha A, et al. Geostatistical modeling of malaria endemicity using serological indicators of exposure collected through school surveys. Am J Trop Med Hyg. 2015;93:168–177. doi: 10.4269/ajtmh.14-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO. Global malaria programme: updated WHO policy recommendation: single dose primaquine as a gametocytocide in Plasmodium falciparum malaria. Geneva: World Health Organization; 2012. http://www.who.int/malaria/publications/atoz/who_pq_policy_recommendation/en/. Accessed 23 Feb 2016.
- 16.Amhara National Regional State Health Bureau Public Health Emergency Management (PHEM) Annual Report; 2012.
- 17.Corran PH, Cook J, Lynch C, Leendertse H, Manjurano A, Griffin J, et al. Dried blood spots as a source of anti-malarial antibodies for epidemiological studies. Malar J. 2008;7:195. doi: 10.1186/1475-2875-7-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corran P, Coleman P, Riley E, Drakeley C. Serology: a robust indicator of malaria transmission intensity? Trends Parasitol. 2007;23:575–582. doi: 10.1016/j.pt.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Schicker RS, Hiruy N, Melaj B, Gelaye W, Bezabih B, Stephenson R, et al. A venue-based survey of malaria, anemia and mobility patterns among migrant farm workers in Amhara Region, Ethiopia. PLoS ONE. 2015;10:e0143829. doi: 10.1371/journal.pone.0143829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kibret S, Wilson GG, Tekie H, Petros B. Increased malaria transmission around irrigation schemes in Ethiopia and the potential of canal water management for malaria vector control. Malar J. 2014;13:360. doi: 10.1186/1475-2875-13-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deressa W, Fentie G, Girma S, Reithinger R. Ownership and use of insecticide-treated nets in Oromia and Amhara regional states of Ethiopia 2 years after a nationwide campaign. Trop Med Int Health. 2011;16:1552–1561. doi: 10.1111/j.1365-3156.2011.02875.x. [DOI] [PubMed] [Google Scholar]
- 22.Doolan DL, Dobaño C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev. 2009;22:13–36. doi: 10.1128/CMR.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bousema T, Okell L, Felger I, Drakeley C. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol. 2014;12:833–840. doi: 10.1038/nrmicro3364. [DOI] [PubMed] [Google Scholar]
- 24.Okell LC, Bousema T, Griffin JT, Ouedraogo AL, Ghani AC, Drakeley CJ. Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nat Commun. 2012;3:1237. doi: 10.1038/ncomms2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shanks GD. Control and elimination of Plasmodium vivax. Adv Parasitol. 2012;80:301–341. doi: 10.1016/B978-0-12-397900-1.00006-2. [DOI] [PubMed] [Google Scholar]
- 26.Cheng Q, Cunningham J, Gatton ML. Systematic review of sub-microscopic P. vivax infections: prevalence and determining factors. PLoS Negl Trop Dis. 2015;9:e3413. doi: 10.1371/journal.pntd.0003413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alemu K, Worku A, Berhane Y, Kumie A. Men traveling away from home are more likely to bring malaria into high altitude villages, northwest Ethiopia. PLoS ONE. 2014;9:e95341. doi: 10.1371/journal.pone.0095341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yukich JO, Taylor C, Eisele TP, Reithinger R, Nauhassenay H, Berhane Y, et al. Travel history and malaria infection risk in a low-transmission setting in Ethiopia: a case control study. Malar J. 2013;12:33. doi: 10.1186/1475-2875-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott CA, Yeshiwondim AK, Serda B, Guinovart C, Tesfay BH, Agmas A, et al. Mass testing and treatment for malaria in low transmission areas in Amhara Region, Ethiopia. Malar J. 2016;15:305. doi: 10.1186/s12936-016-1333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lynch C, Cook J, Nanyunja S, Bruce J, Bhasin A, Drakeley C, et al. Application of serological tools and spatial analysis to investigate malaria transmission dynamics in highland areas of Southwest Uganda. Am J Trop Med Hyg. 2016;94:1251–1258. doi: 10.4269/ajtmh.15-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bousema T, Griffin JT, Sauerwein RW, Smith DL, Churcher TS, Takken W, et al. Hitting hotspots: spatial targeting of malaria for control and elimination. PLoS Med. 2012;9:e1001165. doi: 10.1371/journal.pmed.1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burkot TR, Graves PM, Paru R, Wirtz RA, Heywood PF. Human malaria transmission studies in the Anopheles punctulatus complex in Papua New Guinea: sporozoite rates, inoculation rates, and sporozoite densities. Am J Trop Med Hyg. 1988;39:135–144. doi: 10.4269/ajtmh.1988.39.135. [DOI] [PubMed] [Google Scholar]
- 33.Mbogo CM, Mwangangi JM, Nzovu J, Gu W, Yan G, Gunter JT, et al. Spatial and temporal heterogeneity of Anopheles mosquitoes and Plasmodium falciparum transmission along the Kenyan coast. Am J Trop Med Hyg. 2003;68:734–742. [PubMed] [Google Scholar]
- 34.Trape JF, Lefebvre-Zante E, Legros F, Ndiaye G, Bouganali H, Druilhe P, et al. Vector density gradients and the epidemiology of urban malaria in Dakar, Senegal. Am J Trop Med Hyg. 1992;47:181–189. doi: 10.4269/ajtmh.1992.47.181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Age-seroconversion plots for antibody responses to Plasmodium falciparum antigens (left) and P. vivax antigens (right) for altitudes below 2000 metres (A), 2000 to 2500 metres (B), and greater than 2500 metres (C). Triangles represent deciles of observed data, solid lines represent the fit to the data of a reverse catalytic conversion model and broken blue lines provide the 95% confidence interval for this fit.
Additional file 2. Percent of RDT-positivity (Plasmodium falciparum and/or P. vivax), serology positivity and presence of fever by bed net ownership, bed net use and IRS use, in different altitude strata.
Additional file 3. Percent and odds ratios for RDT-positivity (Plasmodium falciparum and/or P. vivax) by sociodemographic characteristics and malaria risk factors, for altitudes <2000 metres and ≥2000 metres.
Additional file 4. Percent and odds ratios for seropositivity (Plasmodium falciparum and/or P. vivax) by sociodemographic characteristics and malaria risk factors, for altitudes <2000 metres and ≥2000 metres.
Additional file 5. Dot plots of seropositivity to Plasmodium falciparum antigens among children aged 1 to 5 years, by altitude.
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.


