Abstract
Importance
Vestibular disorders have been reported following cochlear implant (CI) surgery, but the literature shows a wide discrepancy in the reported clinical impact. The aim of this meta-analysis is to quantify the effect of CI before and after surgery on the outcomes of vestibular tests, postural stability, and subjective perception of dizziness.
Objective
To evaluate the effects of CI surgery on vestibular function in adult patients (≥18 years) with sensorineural hearing loss who underwent unilateral or bilateral implantation.
Data sources
MEDLINE, PubMed, Web of Science and Cochrane Library from January 1, 1995, through July 12, 2016.
Study selection
Published studies of adult patients who received unilateral or bilateral CIs and whose vestibular function or postural stability was assessed before and after surgery.
Data extraction
From each study, test results before and after surgery were compared, for the following five tests: clinical head impulse test (HIT); bi-thermal caloric irrigation of the horizontal semicircular canal; vestibular evoked myogenic potential (VEMP); dizziness handicap inventory (DHI); and computerized dynamic posturography (CDP).
Results
Twenty-seven studies met all inclusion criteria. Most studies performed either bi-thermal caloric irrigation and/or VEMP, with fewer studies investigating changes in HIT, posturography or DHI. CI surgery significantly affected the results of caloric and VEMP testing. However, HIT results, posturography, and DHI, scores were not significantly affected after CI surgery.
Conclusions and relevance
CI surgery has a significant negative effect on the results of caloric as well as VEMP tests. No significant effect of CI surgery was detected in HIT, posturography, or DHI scores. Overall, the clinical effect of CI surgery on the vestibular function was found to be insignificant. Nonetheless, the potential effects of surgery on the vestibular system should be discussed with CI candidates before surgery.
Keywords: Cochlear Implant, Vestibular function, Postural stability, Vestibular disorders
Background
Hearing loss is the most common sensory deficit of all. More than 5% of the world’s population suffer from disabling hearing loss, affecting about one-third of people above 65 years of age [1]. In cases where hearing aids are no longer useful or sufficient, cochlear implant (CI) surgery is the standard procedure for the treatment of hearing loss. CI attempts to replace the function of hair cells that are no longer able to stimulate primary auditory neurons in response to sound. While the effects of CI surgery on residual cochlear function is well studied, less attention has been given to its effects on vestibular function. Such effects occur because CI surgery frequently affects the vestibular apparatus, which is in close anatomical proximity to the auditory system.
Different mechanisms that could lead to vestibular dysfunction during or after CI surgery have been postulated: 1) direct trauma caused by electrode insertion, 2) acute serous labyrinthitis due to cochleostomy, 3) foreign body reaction with labyrinthitis, 4) endolymphatic hydrops, and 5) electrical stimulation from the implant itself [2].
The occurrence of vestibular dysfunction following CI surgery has a very wide range as assessed by bi-thermal caloric testing and vestibular evoked myogenic potential (VEMP) testing [2–6]. However, not all CI recipients suffer from postoperative dizziness [2–5], and CI recipients reported different forms of dizziness after surgery. Postoperative dizziness had different characteristics, onset, and duration [6].
Given the increasing use of bilateral implantation, it would be important to be able to quantify the effects of CI surgery on the vestibular system. This information would be of great benefit both to the CI team and patients. The aim of the current study was to evaluate the effects of CI surgery on vestibular function and postural stability in adult patients having sensorineural hearing loss (SNHL) who underwent unilateral or bilateral implantation. The purpose of the current meta-analysis study was to demonstrate a quantifiable effect of CI surgery on several tests for balance and vestibular function.
Methods
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis) statement was used as our methodology for this systematic review [7].
Study eligibility criteria
The criteria used in the selection were: (1) studies including adult patients (≥18 years old), (2) studies reporting both pre- and postoperative test results, and (3) studies that reported numbers of normal and abnormal patients for the following tests: clinical head impulse test (HIT), caloric, and vestibular evoked myogenic potential (VEMP) testing were included. Studies that reported raw or average data and standard deviations for posturography (Sensory Organization Test (SOT) conditions 5 and 6) or for dizziness handicap inventory (DHI) pre- and postoperatively were also included. Studies involving young patients (<18 years) were excluded.
All studies had CI surgery performed by the same surgical unit, so it was assumed that the techniques between patients were standardized.
Data sources
A thorough search of MEDLINE, PubMed, EMBASE, Web of Science and Cochrane Review was conducted, using the keywords “cochlear implant and vestibular” or “cochlear implant and caloric” or “cochlear implant and VEMP” or “cochlear implant and balance” or “cochlear implant and posturography” or “cochlear implant and dizziness” or “cochlear implant and Dizziness Handicap Inventory”. This meta-analysis included the date range from January 1st, 1995 to July 12th, 2016.
Data extraction
A total of 2006 potential journal articles was identified using the keywords mentioned above. Only articles in English and French were included. Individual studies’ abstracts were screened to select the studies that met the criteria for this meta-analysis. Full texts of selected articles were retrieved and then rescreened for possible inclusion in the current meta-analysis by two different observers independently.
Data presentation
Different tests exist to evaluate different aspects of the state of the vestibular apparatus. The HIT is one test that assesses vestibulo-ocular function. Other tests objectively evaluate parameters associated with different parts of the vestibular apparatus; however, they do not measure the function of the vestibular system. Such tests include the caloric and VEMP tests.
Posturography is a set of tests that assess the integrative vestibular performance associated with maintenance of posture, where the vestibular function integrates with other sensory inputs (such as vision & proprioception, in order to maintain posture). When applying the SOT test, posturography assesses the state of compensation, because all the movements are sway-referenced, with no induced movements. The DHI is a subjective test for assessment of the perceived function of the vestibular balance condition.
Data synthesis
Four separate meta-analyses were conducted - one for each test. For HIT, caloric, and VEMP testing, the outcome measure was obtained from the ratio of subjects with normal test results before and after surgery; the effect size was measured using the log relative risk (RR) because outcomes are reported in a dichotomous manner (i.e. either normal or hypo/areflexia). For Posturography and DHI, the outcome measure was the mean difference in scores; the effect size was measured using the mean difference (MD) in scores before and after surgery. The random effects model was used, because of the expected variability in the tests’ conditions and results interpretation in the different test centers, and also because all the heterogeneity analyses were significant. Due to the low number of studies available, a meta-analysis was not performed for the posturography data. To calculate the mean difference in scores, the means and standard deviations for scores were extracted, as well as the number of subjects before and after surgery. All data analyses were performed using R-version-3.1.2. Statistical significance was defined as P < 0.05.
Results
Of the 2006 studies, 1956 articles were excluded at the abstract level because they were either duplicates or because the eligibility criteria did not apply (Fig. 1). Next, the full-text of 50 publications were recovered, and then 23 of these publications were excluded because it was not possible to extract useful data from them. Those reports either did not report numbers of subjects having preoperative normal vestibular function and/or numbers of subjects having postoperative normal vestibular functions, or they applied different forms of tests not evaluated in this study. The remaining 27 reports were included in the meta-analysis (Fig. 1) and the results were described separately (Table 1, Figs. 2, 3, 4 and 5).
Fig. 1.
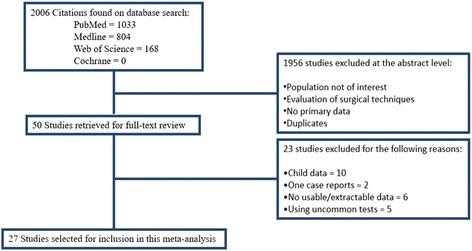
Flow diagram of search and study selection process
Table 1.
Summary of results of all studies included in the meta-analyses
| Source (publication) | Study design | Follow-up (days) | Number of patients | Mean age (range) | HIT + RE |
Caloric + RE |
VEMP + RE | DHI+ RE | CDP + RE |
|---|---|---|---|---|---|---|---|---|---|
| Abramides 2015 [18], Sao Paolo, Brazil | Prospective study | 120 | 24 | 42 (12–65) | Yes P = 0.414 |
||||
| Basta 2008 [12] Berlin, Germany | Prospective study | 42 | 18 | (10–75) | Yes ND (NS) |
Yes ND (NS) |
Yes P < 0.05 |
Yes ND (NS) |
|
| Bateucas 2015 [8] Salamanca, Spain | Prospective descriptive | 2 | 30 | 54 ± 10 | Yes | Yes | |||
| Bonucci 2008 [15] Sao Paolo, Brazil | NI* | NI* | 38 | 30.65 ± 32 4–62 |
Yes ND |
||||
| Brey 1995 [14] Mayo clinic,Rochester, Minnesota | NI* | 45 to 1770 | 52 | 3-87 | Yes P = 0.01 |
Yes ND |
|||
| Buchman 2004 [3] University of North Carolina, USA | Prospectivestudy | 30 | 67 | 2-87 | Yes ND |
Yes ND |
|||
| Coordes 2012 [13] Berlin, Germany | Prospective study | NI* | 17 | 60 (20–73) | Yes ND |
||||
| Ernst 2006 [30] Berlin, Germany | Prospective study | 365 | 18 | 18-62 | Yes ND (NS) |
||||
| Ito 1998 [31] Otsu, Japan | NI* | 30 | 55 | >18 | Yes ND |
||||
| Jutila 2012 [32] Helsinki, Finland | Prospective study | 60 | 44 | 55 (30–76) | Yes P > 0.05 |
||||
| Katsiari 2013 [2] Piraeus, Greece | Prospective study | 30 | 20 | 47.6 ± 20.2 10–77 |
Yes P = 0.01 |
Yes P = 0.002 |
|||
| Kiyomizu 2000 [33] Miyazaki, Japan | NI* | NI* | 23 | 36-75 | Yes ND |
||||
| Kluenter 2009 [6] Fena, Germany | Prospective study | 42 31–368) | 52 | 47(11–74) | Yes ND |
||||
| Kluenter 2010 [25] Fena, Germany | Prospective study | 44 (31–363) | 24 | 51 (20–75) | Yes ND |
||||
| Krause 2009a [22] Munich, Germany | Prospective study | 28 - 42 | 59 | 54 (15–83) | Yes P < 0.001 |
||||
| Krause 2009b [23] Munich, Germany | Prospectivestudy | 28 | 47 | 54 (16–83) | Yes P < 0.01 |
||||
| Krause 2010 [24] Munich, Germany | Prospectivestudy | 60 | 32 | 55 (15–83) | Yes P < 0.001 |
Yes P < 0.047 |
|||
| Louza 2015 [34] Munich, Germany | Retrospective observational study | 28 - 42 | 41 | >14 56 ± 19 |
Yes ND |
Yes ND |
|||
| Melvin 2009 [5] Johns Hopkins, Maryland, USA | Prospective cohort | 28 - 42 | 16 | 46 (23–69) |
Yes ND |
Yes ND |
Yes ND |
||
| Migliaccio 2005 [10] Johns Hopkins, Maryland, USA | Prospective study | 28 - 42 | 16 | 46 (27–64) | Yes P > 0.05 |
||||
| Nordfalk 2014 [21] Oslo, Norway | Prospective pilot | 28 - 42 | 12 | 32-61 | Yes ND |
||||
| Nordfalk 2015 [19] Oslo, Norway |
Prospective | 42-56 | 39 | 57.5 ± 17.2 (18–83) |
Yes ND |
Yes ND |
|||
| Robard 2015 [11] Caen, France | Prospective study | 150 | 34 | 49 ± 25 (1–86) |
Yes P = 0.0015 |
||||
| Rossi 1998 [35] Turin, Italy | Case series | 180 | 32 | 12-74 | Yes ND |
||||
| Todt 2008 [36] Berlin, Germany | Retrospective cohort | 42 - 56 | 62 | 17-84 | Yes ND |
Yes ND |
|||
| Vankatova 2014 [9] Geneve, Switzerland | Retrospective study | NI* | 50 | 15-72 | Yes ND |
Yes ND |
|||
| Wagner 2010 [17] Berlin, Germany | Retrospective cohort | 42 - 56 | 20 | 41.5 (11–58) | Yes ND |
Yes ND |
HIT* head impulse test, VEMP* vestibular evoked myogenic potential, DHI* dizziness handicap inventory, CDP* computerized dynamic posturography, RE* reported effect, NI* not identified. ND* not detected, NS* non-significant, S* significant. RE* reported effect
Fig. 2.
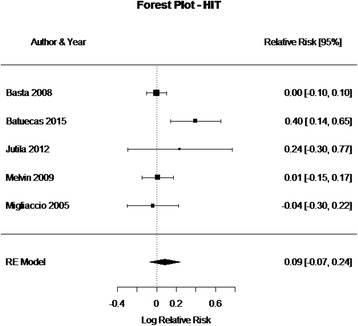
Forest plot (showing relative effect sizes) for the HIT test
Fig. 3.
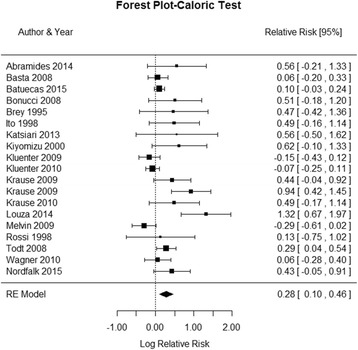
Forest plot (showing relative effect sizes) for the caloric test
Fig. 4.
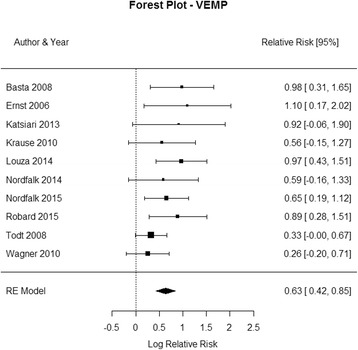
Forest plot (showing relative effect sizes) for the VEMP test
Fig. 5.
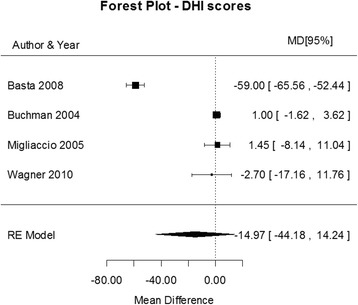
Forest plot (showing relative effect sizes) for the DHI test
HIT results
The number of subjects with normal and abnormal testing results before and after CI surgery who were included in the meta-analysis of the HIT test is shown in Table 2 in Appendix. The statistical analysis revealed a non-significant effect of CI surgery on the HIT test results (RR = 0.0951, 95% CI = −0.0220, 0.2122, P = 0.11). There was substantial variability in the results observed in these studies (I2 = 57.98%, QDF = 5) = 11.2612, P = 0.046). The forest plot indicating the relative strength of each study included in the meta-analysis is illustrated by Fig. 2. Two studies (Batuecas et al., [8] and Vankatova et al. [9]) had a relatively larger number of abnormal postoperative HIT results. However, patients in Batuecas et al. [8] were re-tested after a relatively short postoperative period (2 days). For Vankatova et al. [9], communication with the authors revealed that they had false positive results. Consequently, it was decided to exclude this study from the meta-analysis.
Five out of the six studies that performed HIT, conducted a quantitative HIT, whether a video HIT [8, 9], a search coil HIT [5, 10], or a motorized HIT (mHIT) [11]. Only Basta et al., [12] used a bedside HIT.
Caloric test results
The number of subjects with normal and abnormal testing results before and after CI surgery included in the meta-analysis of the caloric test is shown in Table 3 in Appendix. The statistical analysis revealed a significant effect of CI surgery on the caloric test results (RR = 0.2826, 95% CI = 0.1032, 0.4621, P = 0.0039). There was a considerable heterogeneity observed in the studies (I2 = 74.90%, Q (DF = 18) = 50.8956, P < 0.0001). The forest plot indicating the relative strength of each study included in the meta-analysis is illustrated by Fig. 3. Despite the variability among the reports, the results revealed a tendency for loss of peripheral vestibular function following CI surgery in the majority of the 19 studies involved in this analysis. Several factors could account for the variability among the studies, such as the age range, the test settings and timing of the postoperative retest.
VEMP test results
The studies included in the meta-analysis of VEMP test are shown in Table 4 in Appendix. All included studies used cVEMP. The statistical analysis revealed a significant detrimental effect of CI surgery on VEMP test results (RR = 0.5099, 95% CI = 0.2941, 0.7256, P < 0.0001). There was a substantial heterogeneity in the studies (I2 = 51.68%, Q (DF = 11) = 20.7693, P = 0.0293). The forest plot indicating the relative strength of each study included in the meta-analysis is illustrated by Fig. 4. Two studies (Coordes et al. [13], and Melvin et al. [5]) had a relatively higher number of patients who retained normal VEMP test results postoperatively. This could be due to the use of bone-conduction VEMP, which is more sensitive compared to air-conduction VEMP [13].
Posturography results
The results from the studies that investigated posturography, particularly the conditions 5 and 6 are shown in Table 5 in Appendix. Meta-analysis could not be conducted because only two studies were retrieved [3, 14]. Brey et al. [14]. found a non-significant difference between pre- and post- implantation, where the difference in conditions 5 and 6 scores was very subtle: These results did not differ much from the results reported by Buchman et al. [3]. Overall, postural stability performance did not seem to be affected by the CI surgery.
DHI results
Results from the studies that were included in the meta-analysis of the DHI test are shown in Table 6 in Appendix. The statistical analysis revealed a non-significant effect of CI surgery on the DHI scores (MD = −14.9718, 95% CI = −44.1804, 14.23, P = 0.3151). There was a considerable heterogeneity in the studies (I2 = 98.65%, Q (df = 3) = 280.0102, P.0001). The forest plot showing the relative strength of each study included in the meta-analysis is illustrated by Fig. 5. Basta et al., [12] reported an unusually high postoperative mean score. However, these authors analyzed only five patients with a significant increase in their DHI scores after the surgery. All of them were significantly older (68.8 ± 6.5 years), as compared to the other studies (mean 46.7 ± 18.2 years). Results from DHI scores agree with posturography results, where in most studies, even those reporting increased DHI scores did not result in a change that required further investigation and/or intervention.
Discussion
Vestibular disorders have been reported following CI surgery. This systematic review and meta-analysis showed great variability in the tests’ results. This variability might be due to the different testing measures employed. Both HIT and caloric tests are strongly affected by the lateral semicircular canal function. VEMP testing is strongly influenced by the saccular function. Posturography testing is closely related to the compensatory mechanisms of postural performance. DHI assessments characterize a patient’s subjective impression about their balance perception. Thus it appears that CI may affect some aspects of vestibular function [5]. The variability may also be partly explained by the differences in the criteria and/or test techniques such as the cut-off to determine the normal versus abnormal test results [2]. However, not all studies reported their criteria.
Two studies [8, 9] had a relatively larger number of abnormal postoperative HIT results. Maybe this can be explained by the short postoperative re-test period (2 days) [8]. Unfortunally, was not possible to pool and analyze studies based on follow-up periods because several papers were not specific, either they did not specify the period [15], or provided a very wide range for it [14].
For VEMP results, two studies [5, 13] showed better postoperative results. This could be due to the use of bone-conduction VEMP, which is more sensitive compared to air-conduction VEMP [13], and hence were not included in the meta-analysis. For DHI results, Basta et al., [12] reported an unusually high postoperative mean score. However, these authors analyzed only five patients with a significant increase in their DHI scores after the surgery. All of them were significantly older (68.8 ± 6.5 years), as compared to the other studies (mean 46.7 ± 18.2 years). Results from DHI scores agree with posturography results, where in most studies, even those reporting increased DHI scores did not result in a change that required further investigation and/or intervention.
Another factor that contributes to variability of the results is the fact that CI users are not a homogenous population. They come from different age groups involving newborns to older adults suffering from severe-to-profound SNHL. Thus, age and etiology of SNHL can affect the vestibular function either before, after, or both before and after CI surgery. For example, meningitis often results in disturbed vestibular function due to ossification of the labyrinth (Cushing et al., [16]). From the pooled results in the current meta-analysis, it was found that before surgery, 39.5% had abnormal caloric test results, 31.7% had abnormal VEMP test results, and 11.5% had abnormal HIT results [see Table 1 and Appendix]. Two studies [10, 17] showed a preoperative average DHI scores higher than ten indicating a previous vestibular problem. Few studies reported the number of patients with preoperative caloric or VEMP hyporeflexia who had a deterioration (areflexia) postoperatively [2, 15, 18]. For example, Bonucci et al. [15] found that 10% of the patients who had preoperative hyporeflexia in the caloric test had postoperative areflexia, however, it was not clear whether it was the implanted ear or the contralateral ear. Abramides et al. [18] and Katsiari et al. [2] reported that a deterioration in the non-implanted ear might occur either because the insertion of the electrode in the scala tympani in one ear alters the vestibular input to the brain, and hence modifies the contralateral ear response, or because the reproducibility of the response in these individuals over time is not perfect.
Surgical technique can also affect the outcome. Factors such as electrode insertion site (whether through a cochleostomy, anteroinferior to the round window, or directly through the round window), the electrode length (short or long electrode), the electrode insertion speed, and the electrode insertion depth [19]. The current literature does not provide details about the surgical procedure and only mention the technique used (cochleostomy versus round window approach). The majority of the articles reported the cochleostomy (anteroinferior to the round window) as the standard approach.1 Unfortunally, it was not mentioned whether soft surgical techniques were used to minimize trauma to the labyrinth [20].
The data in the current meta-analysis showed no significant increase in DHI in the majority of patients (84.4%), suggesting that CI did not affect balance. Seventy-two percent of the patients retained a normal caloric function after surgery, 60% retained normal HIT results, and 56% retained normal VEMP test results, thus it can be concluded that the impact of CI surgery on the vestibular apparatus was not clinically significant. It is worth noting that some conditions such as the use of ototoxic drugs or Meniere’s disease might be present in CI users, and could limit the interpretation of abnormal balance tests in case testing was done only postoperatively. However, the studies did not report detailed patients’ medical history to be conclusive.
It is important to note that some studies were performed by the same group (Nordfalk et al. [19, 21], Krause et al. [22–24], and Kluenter et al. [6, 25]). The authors were contacted to verify whether these studies have an overlap. Nordfalk et al. have different sets of patient populations, so they do not overlap. Kluenter et al. had 12 patients who participated in both studies. No response was received from Krause et al.
We found that CI surgery can significantly affect the results of both the caloric test and VEMP test. This finding is in accord with the systematic review of Kuang et al. [26], where they found that 37% of patients had reduced reflex, and 34% had caloric asymmetry after CI surgery. Other authors [27, 28] reported that one-third of CI recipients complain of dizziness after surgery. A recent review aimed at determining the best test to evaluate vestibular function before and after CI surgery was published by Abouzayd et al., [29]. They found that the caloric test was least sensitive, VEMP results were most often impaired, and HIT results were generally conserved. Our study provides a quantified evidence that CI surgery can significantly affect some vestibular test results (although it might not be clinically significant, as evident from the pre- and postoperative DHI scores). It also provides estimates of vestibular dysfunction in CI candidates. The current study confirms that it is important to pursue a case-by-case approach with CI surgery candidates, based on each patient’s history and symptoms.
To summarize, several factors can contribute to the variability of the results within and between the vestibular function tests, both before and after CI surgery, that are difficult to control for. Those factors include age and etiology of hearing loss, the surgical technique used, and the incidence of trauma to the inner ear. Because congenital, genetic, and post-meningitis hearing loss is more common in children, a separate analysis of pediatric vestibular function before and after CI surgery, and comparing the results to adults, would be a useful area of future research.
Conclusion
According to the results of the current meta-analysis, CI surgery can significantly affect the results of caloric as well as VEMP tests. No significant effect was detected in HIT results, posturography, or DHI scores. Drawing a definitive conclusion is rather difficult for a number of reasons, such as heterogeneity in study design, variability among patient populations, pre-existing condition, and measurement and reporting differences. Whilst studies showed that some postoperative scores were worse after CI, the proportion of patients affected appears low. Age and etiology of hearing loss appear to affect the vestibular function after CI surgery. Nonetheless, the possible effects of CI surgery on the vestibular system should be communicated to CI recipients before surgery.
Acknowledgements
The authors would like to thank Dr. Xun Zhang, PhD - McGill University Health Center. For revising R codes and results—as well as the McGill Medical librarians at MUHC, for their valuable assistance in building the search strategy and retrieving the articles from the various databases.
Funding
Canada Graduate Scholarship Master’s (CGS-M) Award - Canadian Institutes of Health Research (CIHR).
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analysed during the current study
Authors’ contributions
II: data collection, data analysis, manuscript drafting. SDS: data analysis, manuscript editing and revision. BS: data analysis, manuscript editing and revision. AZ: Concept & design, data analysis, manuscript editing and revision. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- CI
Cochlear Implant
- DHI
Dizziness Handicap Inventory
- HIT
Head Impulse Test
- MD
Mean Difference
- RR
Relative Risk
- SOT
Sensory Organization Test
- VEMP
Vestibular Evoked Myogenic Potential
Appendix
Table 2.
Number of subjects with normal and abnormal testing results before and after surgery in studies included in the meta-analysis for the HIT test
| Study | Year | Normal pre | Abnormal pre | Normal post | Abnormal post | Number pre | Number post |
|---|---|---|---|---|---|---|---|
| Basta [12] | 2008 | 18 | 0 | 18 | 0 | 18 | 18 |
| Batuecas [8] | 2015 | 30 | 0 | 20 | 10 | 30 | 30 |
| Jutila [32] | 2012 | 19 | 25 | 15 | 29 | 44 | 44 |
| Melvin [5] | 2009 | 14 | 0 | 10 | 0 | 14 | 10 |
| Migliaccio [10] | 2005 | 14 | 2 | 10 | 1 | 16 | 11 |
| Vankatova [9] | 2014 | 50 | 0 | 43 | 7 | 50 | 50 |
Normal pre = number of individuals with normal test results before surgery. Abnormal Pre = number of individuals with abnormal test results before surgery. Normal post = number of individuals with normal test results after surgery. Abnormal post = number of individuals with abnormal test results after surgery. Number pre = number of individuals tested before surgery. Number post = number of individuals tested after surgery
Table 3.
Number of subjects with normal and abnormal testing results before and after surgery in studies included in the meta-analysis for the caloric test
| Study | Year | Normal Pre | Abnormal pre |
Normal post |
Abnormal post | Number pre | Number post |
|---|---|---|---|---|---|---|---|
| Abramides [18] | 2014 | 14 | 34 | 8 | 40 | 24 | 24 |
| Basta [12] | 2008 | 16 | 2 | 15 | 3 | 18 | 18 |
| Batuecas [8] | 2015 | 30 | 0 | 27 | 3 | 30 | 30 |
| Bonucci [15] | 2008 | 15 | 23 | 9 | 29 | 38 | 38 |
| Brey [14] | 1995 | 8 | 9 | 5 | 12 | 17 | 17 |
| Ito [31] | 1998 | 18 | 37 | 11 | 44 | 55 | 55 |
| Katsiari [2] | 2013 | 7 | 13 | 4 | 16 | 20 | 20 |
| Kiyomizu [33] | 2000 | 13 | 10 | 7 | 16 | 23 | 23 |
| Kluenter [6] | 2009 | 18 | 6 | 21 | 3 | 24 | 24 |
| Kluenter [25] | 2010 | 41 | 11 | 44 | 8 | 52 | 52 |
| Krausea [22] | 2009 | 25 | 20 | 15 | 27 | 45 | 42 |
| Krauseb [23] | 2009 | 35 | 21 | 13 | 40 | 56 | 53 |
| Krause [24] | 2010 | 13 | 9 | 8 | 14 | 32 | 32 |
| Louza [34] | 2014 | 30 | 11 | 8 | 33 | 41 | 41 |
| Melvin [5] | 2009 | 14 | 6 | 15 | 1 | 20 | 16 |
| Nordfalk [19] | 2015 | 20 | 10 | 13 | 17 | 30 | 30 |
| Rossi [35] | 1998 | 8 | 24 | 7 | 25 | 32 | 32 |
| Todt [36] | 2008 | 48 | 14 | 36 | 26 | 62 | 62 |
| Wagner [17] | 2010 | 17 | 5 | 16 | 6 | 22 | 22 |
Normal pre = number of individuals with normal test results before surgery. Abnormal Pre = number of individuals with abnormal test results before surgery. Normal post = number of individuals with normal test results after surgery. Abnormal post = number of individuals with abnormal test results after surgery. Number pre = number of individuals tested before surgery. Number post = number of individuals tested after surgery
Table 4.
Number of subjects with normal and abnormal testing results before and after surgery in studies included in the meta-analysis for the VEMP test
| Study | Years | Normal. pre |
Abnormal. pre | Normal. post | Abnormal. post | Number. pre | Number. post |
|---|---|---|---|---|---|---|---|
| Basta [12] | 2008 | 16 | 2 | 6 | 12 | 18 | 18 |
| Coordes [13] | 2012 | 17 | 0 | 14 | 3 | 17 | 17 |
| Ernst [30] | 2006 | 12 | 6 | 4 | 14 | 18 | 18 |
| Katsiari [2] | 2013 | 10 | 10 | 4 | 16 | 20 | 20 |
| Krause [24] | 2010 | 14 | 16 | 8 | 22 | 30 | 30 |
| Louza [34] | 2014 | 29 | 12 | 11 | 30 | 41 | 41 |
| Melvin [5] | 2009 | 12 | 7 | 11 | 5 | 19 | 16 |
| Nordfalk [21] | 2014 | 9 | 3 | 5 | 7 | 12 | 12 |
| Nordfalk [19] | 2015 | 25 | 8 | 13 | 20 | 33 | 33 |
| Robard [11] | 2015 | 22 | 12 | 9 | 25 | 34 | 34 |
| Todt [36] | 2008 | 39 | 23 | 28 | 34 | 62 | 62 |
| Wagner [17] | 2010 | 22 | 18 | 17 | 23 | 20 | 20 |
Normal pre = number of individuals with normal test results before surgery. Abnormal Pre = number of individuals with abnormal test results before surgery. Normal post = number of individuals with normal test results after surgery. Abnormal post = number of individuals with abnormal test results after surgery. Number pre = number of individuals tested before surgery. Number post = number of individuals tested after surgery
Table 5.
Test results (Sensory Organization test scores) before and after surgery in studies included in the meta-analysis for the posturography test
| Study | Year | Mean1 | SD1 | Number1 | Mean2 | SD2 | Number2 |
|---|---|---|---|---|---|---|---|
| Brey [14] | 1995 | 46.99 | 25.68 | 22 | 45 | 31.04 | 22 |
| Brey [14] | 1995 | 43.5 | 22.1 | 22 | 42.17 | 28.76 | 22 |
| Buchman [3] | 2004 | 39 | 26 | 82 | 40 | 27 | 67 |
| Buchman [3] | 2004 | 33 | 26 | 82 | 31 | 26 | 67 |
Mean1 = mean scores before surgery. SD1 = scores standard deviations before surgery. Number1 = number of patients tested before surgery. Mean2 = mean scores after surgery. SD2 = scores standard deviations after surgery. Number2 = number of patients tested after surgery
Table 6.
Test results (DHI scores) before and after surgery in studies included in the meta-analysis for the DHI test
| Study | Year | Mean1 | SD1 | Number1 | Mean2 | SD2 | Number2 |
|---|---|---|---|---|---|---|---|
| Basta [12] | 2008 | 5 | 1.41 | 18 | 64 | 14.14 | 18 |
| Buchman [3] | 2004 | 5 | 8 | 78 | 4 | 8 | 66 |
| Migliaccio [10] | 2005 | 10.54 | 11.76 | 11 | 9.09 | 11.18 | 11 |
| Wagner [17] | 2010 | 14.9 | 24.4 | 20 | 17.6 | 22.2 | 20 |
Mean1 = mean scores before surgery. SD1 = scores standard deviations before surgery. Number1 = number of patients tested before surgery. Mean2 = mean scores after surgery. SD2 = scores standard deviations after surgery. Number2 = number of patients tested after surgery
Footnotes
Todt et al., 2008 [36] claimed that the use of round window approach for electrode insertion would decrease the probability of loss of vestibular function postoperatively, compared to the standard cochleostomy approach.
Contributor Information
Iman Ibrahim, Email: iman.ibrahim@mail.mcgill.ca.
Sabrina Daniela da Silva, Email: sabrina.wurzba@mcgill.ca.
Bernard Segal, Email: bernard.segal@mcgill.ca.
Anthony Zeitouni, Email: anthony.zeitouni@mcgill.ca.
References
- 1.WHO. http://www.who.int/mediacentre/factsheets/fs300/en/. Accessed 23 June 2015.
- 2.Katsiari E, Balatsouras DG, Sengas J, Riga M, Korres GS, Xenelis J. Influence of cochlear implantation on the vestibular function. Eur Arch Otorhinolaryngol. 2013;270(2):489–495. doi: 10.1007/s00405-012-1950-6. [DOI] [PubMed] [Google Scholar]
- 3.Buchman CA, Joy J, Hodges A, Telischi FF, Balkany TJ. Vestibular effects of cochlear implantation. Laryngoscope. 2004;114(10 Pt 2 Suppl 103):1–22. doi: 10.1097/00005537-200410001-00001. [DOI] [PubMed] [Google Scholar]
- 4.Fina M, Skinner M, Goebel JA, Piccirillo JF, Neely JG, Black O. Vestibular dysfunction after cochlear implantation. Otol Neurotol. 2003;24(2):234–242. doi: 10.1097/00129492-200303000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Melvin T-AN, Della Santina CC, Carey JP, Migliaccio AA. The effects of cochlear implantation on vestibular function. Otol Neurotol. 2009;30(1):87–94. doi: 10.1097/MAO.0b013e31818d1cba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kluenter H-D, Lang-Roth R, Guntinas-Lichius O. Static and dynamic postural control before and after cochlear implantation in adult patients. Eur Arch Otorhinolaryngol. 2009;266(10):1521–1525. doi: 10.1007/s00405-009-0936-5. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Batuecas-Caletrio A, Klumpp M, Santacruz-Ruiz S, Gonzalez FB, Sánchez EG, Arriaga M. Vestibular function in cochlear implantation: Correlating objectiveness and subjectiveness. Laryngoscope. 2015 doi: 10.1002/lary.25299. [DOI] [PubMed] [Google Scholar]
- 9.Vankatova L, Cao Van H, Perez Fornos A, Guinarnd N. Cochlear implantation - better safe than sorry. Rev Med Suisse. 2014;10(444):1820. [PubMed] [Google Scholar]
- 10.Migliaccio AA, Della Santina CC, Carey JP, Niparko JK, Minor LB. The vestibulo-ocular reflex response to head impulses rarely decreases after cochlear implantation. Otol Neurotol. 2005;26(4):655–660. doi: 10.1097/01.mao.0000178125.20741.27. [DOI] [PubMed] [Google Scholar]
- 11.Robard L, Hitier M, Lebas C, Moreau S. Vestibular function and cochlear implant. Eur Arch Otorhinolaryngol. 2015;272(3):523–530. doi: 10.1007/s00405-014-3040-4. [DOI] [PubMed] [Google Scholar]
- 12.Basta D, Todt I, Goepel F, Ernst A. Loss of saccular function after cochlear implantation: the diagnostic impact of intracochlear electrically elicited vestibular evoked myogenic potentials. Audiol Neurootol. 2008;13(3):187–192. doi: 10.1159/000113509. [DOI] [PubMed] [Google Scholar]
- 13.Coordes A, Basta D, Götze R, et al. Sound-induced vertigo after cochlear implantation. Otol Neurotol. 2012;33(3):335–342. doi: 10.1097/MAO.0b013e318245cee3. [DOI] [PubMed] [Google Scholar]
- 14.Brey RH, Facer GW, Trine MB, Lynn SG, Peterson AM, Suman VJ. Vestibular effects associated with implantation of a multiple channel cochlear prosthesis. The American journal of otology. 1995;16(4):424–430. [PubMed] [Google Scholar]
- 15.Bonucci AS, Costa Filho OA, Mariotto LDF, Amantini RCB, Alvarenga K. de F. Vestibular function in cochlear implant users. Brazilian journal of otorhinolaryngology. 2008;74(2):273–278. doi: 10.1016/S1808-8694(15)31100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cushing SL, Papsin BC, Rutka JA, James AL, Blaser SL, Gordon KA. Vestibular End-Organ and Balance Deficits After Meningitis and Cochlear Implantation in Children Correlate Poorly With Functional Outcome. Otology and Neurotology. 2009;30(4):488–495. doi: 10.1097/MAO.0b013e31819bd7c8. [DOI] [PubMed] [Google Scholar]
- 17.Wagner JH, Basta D, Wagner F, Seidl RO, Ernst A, Todt I. Vestibular and taste disorders after bilateral cochlear implantation. Eur Arch Otorhinolaryngol. 2010;267(12):1849–1854. doi: 10.1007/s00405-010-1320-1. [DOI] [PubMed] [Google Scholar]
- 18.Abramides PA, Bittar RSM, Tsuji RK, Bento RF. Caloric test as a predictor tool of postural control in CI users. Acta Otolaryngol. 2015;135(7):685–691. doi: 10.3109/00016489.2015.1020395. [DOI] [PubMed] [Google Scholar]
- 19.Nordfalk KF, Rasmussen K, Hopp E, Bunne M, Silvola JT, Jablonski GE. Insertion Depth in Cochlear Implantation and Outcome in Residual Hearing and Vestibular Function. Ear Hear. 2015 doi: 10.1097/AUD.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 20.Cohen NL. Cochlear implant soft surgery: fact or fantasy? Otolaryngol Head Neck Surg. 1997;117(3 Pt 1):214–216. doi: 10.1016/S0194-5998(97)70176-1. [DOI] [PubMed] [Google Scholar]
- 21.Nordfalk KF, Rasmussen K, Hopp E, Greisiger R, Jablonski GE. Scalar position in cochlear implant surgery and outcome in residual hearing and the vestibular system. Int J Audiol. 2014;53(2):121–127. doi: 10.3109/14992027.2013.854413. [DOI] [PubMed] [Google Scholar]
- 22.Krause E, Louza JPR, Hempel J-M, Wechtenbruch J, Rader T, Gürkov R. Effect of cochlear implantation on horizontal semicircular canal function. Eur Arch Otorhinolaryngol. 2009;266(6):811–817. doi: 10.1007/s00405-008-0815-5. [DOI] [PubMed] [Google Scholar]
- 23.Krause E, Wechtenbruch J, Rader T, Berghaus A, Gürkov R. Impaired fixation suppression is a risk factor for vertigo after cochlear implantation. J Laryngol Otol. 2009;123(8):845–850. doi: 10.1017/S0022215109004812. [DOI] [PubMed] [Google Scholar]
- 24.Krause E, Louza JPR, Wechtenbruch J, Gürkov R. Influence of cochlear implantation on peripheral vestibular receptor function. Otolaryngol Head Neck Surg. 2010;142(6):809–813. doi: 10.1016/j.otohns.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Kluenter H-D, Lang-Roth R, Beutner D, Hüttenbrink K-B, Guntinas-Lichius O. Postural control before and after cochlear implantation: standard cochleostomy versus round window approach. Acta Otolaryngol. 2010;130(6):696–701. doi: 10.3109/00016480903373732. [DOI] [PubMed] [Google Scholar]
- 26.Kuang H, Haversat HH, Michaelides EM. Impairment of caloric function after cochlear implantation. J Speech Lang Hear Res. 2015 doi: 10.1044/2015_JSLHR-H-15-0010. [DOI] [PubMed] [Google Scholar]
- 27.Zawawi F, Faisal A, Tony L, Anthony GZ. Patients Reported Outcome Post-Cochlear Implantation: How Severe Is Their Dizziness? Journal of Otolaryngology - Head and Neck Surgery = Le Journal D’oto-Rhino-Laryngologie Et De Chirurgie Cervico-Faciale 43, no. 1 (December 10, 2014): 49. doi:10.1186/s40463-014-0049-z. [DOI] [PMC free article] [PubMed]
- 28.Shoman N, Ngo R, Archibald J, Pijl S, Chan S, Westerberg BD. Prevalence of new-onset vestibular symptoms following cochlear implantation. J Otolaryngol. 2008;37:388–394. [PubMed] [Google Scholar]
- 29.Abouzayd M, Smith PF, Moreau S, Hitier M. What vestibular tests to choose in symptomatic patients after a cochlear implant? A systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2016 doi: 10.1007/s00405-016-4007-4. [DOI] [PubMed] [Google Scholar]
- 30.Ernst A, Todt I, Seidl RO, Eisenschenk A, Blödow A, Basta D. The application of vestibular-evoked myogenic potentials in otoneurosurgery. Otolaryngol Head Neck Surg. 2006;135(2):286–290. doi: 10.1016/j.otohns.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Ito J. Influence of the multichannel cochlear implant on vestibular function. Otolaryngology - Head and Neck Surgery. 1998;118(6):900–902. doi: 10.1016/S0194-5998(98)70295-5. [DOI] [PubMed] [Google Scholar]
- 32.Jutila T, Aalto H, Hirvonen TP. Cochlear implantation rarely alters horizontal vestibulo-ocular reflex in motorized head impulse test. Otol Neurotol. 2013;34(1):48–52. doi: 10.1097/MAO.0b013e318277a430. [DOI] [PubMed] [Google Scholar]
- 33.Kiyomizu K, Tono T, Komune S, Ushisako Y, Morimitsu T. Dizziness and vertigo after cochlear implantation. Adv Otorhinolaryngol. 2000;57:173–175. doi: 10.1159/000059231. [DOI] [PubMed] [Google Scholar]
- 34.Louza J, Mertes L, Braun T, Gürkov R, Krause E. Influence of insertion depth in cochlear implantation on vertigo symptoms and vestibular function. Am J Otolaryngol. 2015;36(2):254–258. doi: 10.1016/j.amjoto.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Rossi G, Solero P, Rolando M, Spadola BM. Vestibular function and cochlear implant. ORL. 1998;60(2):85–87. doi: 10.1159/000027570. [DOI] [PubMed] [Google Scholar]
- 36.Todt I, Basta D, Ernst A. Does the surgical approach in cochlear implantation influence the occurrence of postoperative vertigo? Otolaryngol Head Neck Surg. 2008;138(1):8–12. doi: 10.1016/j.otohns.2007.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study


