Abstract
Epstein-Barr virus (EBV) latent membrane protein 2A (LMP2A) is important for maintenance of latency in infected B lymphocytes. Through its immunoreceptor tyrosine-based activation motif (ITAM) and PY motifs, LMP2A is able to block B-cell receptor (BCR) signaling, bind BCR-associated kinases, and manipulate the turnover of itself and these kinases via a PY-mediated interaction with the Nedd4 family of ubiquitin ligases. In epithelial cells, LMP2A has been shown to activate the phosphatidylinositol 3′-OH kinase/Akt and β-catenin signaling pathways. In the present study, the biological consequences of LMP2A expression in the normal human foreskin keratinocyte (HFK) cell line were investigated and the importance of the ITAM and PY motifs for LMP2A signaling effects in HFK cells was ascertained. The ITAM was essential for the activation of Akt by LMP2A in HFK cells, while both the ITAM and PY motifs contributed to LMP2A-mediated accumulation and nuclear translocation of the oncoprotein β-catenin. LMP2A inhibited induction of differentiation in an assay conducted with semisolid methylcellulose medium, and the PY motifs were critical for this inhibition. LMP2A is expressed in the EBV-associated epithelial malignancies nasopharyngeal carcinoma and gastric carcinoma, and these data indicate that LMP2A affects cellular processes that likely contribute to carcinogenesis.
The Epstein-Barr virus (EBV) is a very successful human herpesvirus that has infected more than 90% of the world's adult population (16). The virus is capable of both lytic and latent forms of infection, and the virus is able to persist throughout the life of the host via establishment of latency. Although most EBV infections are asymptomatic, disease arises in a subset of infected individuals, especially in the immunocompromised population. EBV infection is associated with a variety of malignancies, including Hodgkin lymphoma, endemic Burkitt's lymphoma, gastric carcinoma, and nasopharyngeal carcinoma (27). These cancers are characterized by the proliferation of monoclonal EBV-infected cells, and viral expression in these cells is limited to a subset of latent genes (16, 26, 27). In EBV-associated cancers, EBV nuclear antigen 1 and other EBV nuclear antigen proteins, 2, 3A, 3B, 3C, and LP, as well as latent membrane protein 1 (LMP1), LMP2A, and LMP2B, may be expressed in addition to a series of transcripts from the BamHI-A region of the EBV genome and EBV-encoded RNAs (16).
LMP2A is expressed in most EBV-associated tumors, including Hodgkin lymphoma, nasopharyngeal carcinoma, and gastric carcinoma (4, 5, 24, 32). In infected B lymphocytes, LMP2A functions to maintain viral latency by blocking B-cell receptor (BCR) activation and signaling, which would otherwise reactivate the virus to enter the lytic cycle (19-22). Furthermore, experiments with transgenic mice expressing LMP2A under the control of the Eμ enhancer and promoter targeting expression to the B-cell lineage revealed that LMP2A sends survival signals and allows B cells to bypass developmental checkpoints and escape the bone marrow to colonize peripheral lymphoid organs (6, 7, 18). Critical for these functions is the immunoreceptor tyrosine-based activation motif (ITAM) located in the cytoplasmic amino (N) terminus of LMP2A. Through its ITAM, LMP2A activates phosphatidylinositol 3′-OH kinase (PI3K)/Akt signaling in B lymphocytes and associates with the BCR signaling effector Syk kinase (9, 31). The LMP2A N terminus also contains two proline-rich motifs known as PY motifs that enable it to interact with members of the Nedd4 family of ubiquitin ligases in B cells (13, 14, 35). Through these interactions, LMP2A manipulates the turnover of itself and other binding partners such as Lyn kinase and possibly Syk.
Because of a lack of expression of the BCR and associated kinases within epithelial cells, LMP2A signaling in epithelial cells likely has different effects than in B cells. However, one pathway that is similarly activated by LMP2A in both epithelial and B cells is the PI3K/Akt pathway (23, 28, 31). This pathway has prominent roles in the activation of cell proliferation, migration, and survival; therefore, activation of the PI3K/Akt pathway could confer a growth advantage to cells expressing LMP2A (3, 11, 34). In addition to activation of PI3K/Akt, our laboratory has recently shown that LMP2A is able to activate β-catenin signaling in human foreskin keratinocyte (HFK) cells, a normal epithelial cell line derived from primary HFK cells immortalized via transduction with the catalytic subunit of telomerase (23). LMP2A signaling in HFK cells leads to cytosolic accumulation of the β-catenin oncoprotein and PI3K-dependent nuclear translocation of β-catenin. In the nucleus, β-catenin interacts with members of the T-cell factor/lymphoid enhancer factor family of transcription factors to activate expression of genes such as c-myc and CCND1 (cyclin D1), which could lead to enhancement of cell cycle progression and cell proliferation (10, 12, 25, 29, 33, 36). Therefore, activation of β-catenin signaling provides another mechanism by which LMP2A might stimulate cell growth and contribute to EBV-associated oncogenesis.
Previous studies with the spontaneously immortalized human epithelial cell line HaCaT suggest that LMP2A has oncogenic capacity. HaCaT cells stably expressing LMP2A were able to form colonies in soft agar in a PI3K-dependent manner and formed aggressive, poorly differentiated tumors in nude mice (28). Furthermore, when they were grown in organotypic raft cultures, LMP2A expression in HaCaT cells led to inhibition of epithelial cell differentiation, denoted by a lack of involucrin induction (28).
The goals of the present study were to further characterize the biological consequences of LMP2A expression in the normal HFK cell line and to ascertain the importance of the ITAM and PY motifs of LMP2A for its signaling effects in HFK cells. The LMP2A ITAM was required for its activation of Akt in these cells. However, both the PY motifs and the ITAM were found to be important for the effects of LMP2A on β-catenin accumulation and nuclear translocation. Furthermore, LMP2A inhibited epithelial cell differentiation in methylcellulose-based differentiation assays, and the PY motifs were essential for this effect. These data reveal that the ability of LMP2A to interact with ubiquitin ligases is critical to its effects on β-catenin and to the inhibition of epithelial cell differentiation.
MATERIALS AND METHODS
Cell lines and materials.
HFK cells (8) were maintained in keratinocyte serum-free medium (K-SFM; Gibco-Invitrogen, Carlsbad, Calif.) supplemented with 0.2 ng of epidermal growth factor (EGF) per ml, 30 μg of bovine pituitary extract (BPE) per ml, and 1% antibiotic-antimycotic solution (Gibco) at 37°C in 5% CO2. For stable cell lines, 0.5 μg of puromycin (Sigma-Aldrich, St. Louis, Mo.) per ml was added to the K-SFM for selection and maintenance of expression. Stable cell lines were generated by transduction with recombinant retroviruses expressing the pBabe vector or the pBabe vector subcloned with hemagglutinin (HA)-tagged LMP2A, LMP2A ΔITAM, or LMP2A ΔPY as previously described (28), and pools were selected in 0.5 μg of puromycin per ml. The LMP2A ITAM is composed of Tyr74 and Tyr85, and the LMP2A ΔITAM mutant protein contains a deletion of residues 80 to 112 of the LMP2A N terminus with mutation of Tyr74 to Ala. The LMP2A N terminus also has two PY (PPPPY) motifs responsible for the interaction of LMP2A with members of the Nedd4 family of ubiquitin ligases in B cells. The LMP2A ΔPY mutant protein has Pro-to-Ala mutations in the central prolines in each PY motif to abolish this interaction, and this construct was a kind gift from M. Ikeda and R. Longnecker (13).
Cells were treated with the PI3K inhibitor LY294002 (LY) (Calbiochem no. 440202; EMD Biosciences, Inc., San Diego, Calif.) at 25 μM or the Akt inhibitor 1L-6-hydroxymethyl-chiro-inositol 2[(R)-2-O-methyl-3-O-octadecylcarbonate] (Calbiochem no. 124005) at 10 μM for approximately 24 h. Inhibitors were solubilized in dimethyl sulfoxide (DMSO; Sigma) as a vehicle control.
Methylcellulose differentiation assays.
Methylcellulose medium (1.6%) was prepared by adding half of the total volume of K-SFM to autoclaved methylcellulose (4,000 cps; Sigma-Aldrich), heating the mixture to 60°C for 20 min, and then adding the remainder of the K-SFM. At this time, supplements such as 30 μg of BPE per ml, 0.2 ng of EGF per ml, 0.5 μg of puromycin per ml, and 1% antibiotic-antimycotic solution were added. Methylcellulose medium was stirred overnight at 4°C. To set up the assay, 30 ml of methylcellulose medium was added to 100-mm-diameter sterile petri dishes. HFK cells stably expressing the pBabe vector, LMP2A, LMP2A ΔITAM, and LMP2A ΔPY were trypsinized and counted, and 2 × 106 to 4 × 106 cells in 1 ml of K-SFM were added dropwise to the methylcellulose medium plated in the petri dishes. With the end of a pipette tip, the cells were stirred throughout the medium to ensure distribution and incubated at 37°C with 5% CO2 for 1 to 2 days. As a control, an equal number of cells were plated in a regular 100-mm-diameter tissue culture plate with a total volume of 10 ml of regular K-SFM (30 μg of BPE per ml, 0.2 ng of EGF per ml, 0.5 μg of puromycin per ml, and 1% antibiotic-antimycotic solution) without methylcellulose.
After the designated time periods, cells were harvested. To harvest cells from the methylcellulose medium, medium with cells was scraped into three 50-ml conicals, diluted in phosphate-buffered saline (PBS), and collected by centrifugation. The remaining methylcellulose was removed by two subsequent PBS washes in 50- and 15-ml conicals with centrifugation. To lyse the cells for analysis, cell pellets were resuspended in Nonidet P-40 (NP-40) lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 2 mM EDTA, 10% glycerol, 1% NP-40, 0.4 mM phenylmethylsulfonyl fluoride [PMSF], 1 mM sodium vanadate [Na3VO4], protease and phosphatase inhibitor cocktails [Sigma] at 1:100). Cell pellets with lysis buffer were incubated on ice for 10 min with occasional vortexing and then clarified by high-speed centrifugation. To harvest the controls in K-SFM without methylcellulose, cells were washed two times in cold PBS on ice and scraped into NP-40 lysis buffer on ice. After a 10-min incubation on ice, cells were clarified by centrifugation as described above.
Western blot analysis.
Protein concentrations of lysates were determined by the Bio-Rad DC protein assay system (Bio-Rad, Hercules, Calif.). Equal amounts of lysates were suspended in 3× sodium dodecyl sulfate sample buffer, boiled for 5 min, and electrophoresed on a sodium dodecyl sulfate-7.5% polyacrylamide gel. Proteins were transferred to an Optitran nitrocellulose membrane (Schleicher & Schuell Bioscience, Keene, N.H.) in a Bio-Rad transfer unit, and Western blot analyses were performed. After blocking in 5% milk in Tris-buffered saline-Tween 20 (TBST), the membranes were incubated overnight in primary antibody on a nutator at 4°C. The antibodies used included anti-involucrin (1:1,000; Sigma), antiactin (1:200), anti-GRP78 (1:200), anti-HA (1:200), and anti-green fluorescent protein (GFP; 1:200) from Santa Cruz Biotechnologies (Santa Cruz, Calif.), anti-phospho Akt (Ser473; 1:500) and anti-Akt (1:500) from Cell Signaling Technology (Beverly, Mass.), and anti-β-catenin (1:500) from BD Transduction Laboratories (San Jose, Calif.). After three 5-min washes in TBST, the membranes were incubated in horseradish peroxidase-tagged secondary antibodies (1:1,000; Amersham Biosciences, Piscataway, N.J., and Dako, Carpinteria, Calif.) at room temperature for 1 h on a nutator. After three 5-min washes in TBST, antibody-bound proteins were detected via autoradiography with the Pierce SuperSignal West Pico System (Pierce, Rockford, Ill.). Densitometry was performed with the Image J software (National Institutes of Health).
Transfections and plasmids.
The plasmids used for transient transfection assays included pSG5, pSG5-LMP2A, pSG5-LMP2A ΔPY (gift from M. Ikeda and R. Longnecker) (13), pSG5-LMP2A ΔITAM, XE69 encoding GFP-β-catenin (gift from R. Moon), pCMV6-myrAkt, pBabe-PI3K CAAX, and pBabe-PI3K K227E (gifts from C. Der). Transfections were performed with FuGENE 6 (Roche, Basel, Switzerland) in accordance with the manufacturer's directions.
Cell fractionations.
Cellular fractionations were performed with Optiprep (Sigma), as adapted from the manufacturer's protocol. Briefly, cells were resuspended in cold buffer A (20 mM HEPES, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol, 0.5 mM PMSF, 1 mM Na3VO4, protease and phosphatase inhibitor cocktails at 1:100), incubated on ice for 15 min, and vortexed for 1 min in the presence of 1% NP-40. Crude nuclei were pelleted at low speed, and cytosolic extracts (supernatants) were collected. Nuclei were washed in homogenization medium (0.25 M sucrose, 25 mM KCl, 5 mM MgCl2, 20 mM Tris [pH 7.8]) and purified over an Optiprep gradient with 25, 30, and 35% layers. Banded nuclei were collected and lysed in NE buffer (20 mM Tris-HCl, 1.5 mM MgCl2, 0.2 mM EDTA, 25% glycerol, 0.5 mM PMSF, 1 mM Na3VO4, protease and phosphatase inhibitor cocktails at 1:100) with 400 mM NaCl. After incubation on ice for 10 min with occasional vortexing, nuclear extracts were clarified via high-speed centrifugation.
RESULTS
Epithelial cell differentiation is inhibited in LMP2A-expressing HFK cells.
LMP2A has been shown to have oncogenic capacity when expressed in HaCaT keratinocytes as LMP2A-expressing HaCaT cells were capable of anchorage-independent growth in soft agar and gave rise to aggressive, poorly differentiated tumors when injected into immunodeficient mice (28). Furthermore, differentiation was inhibited when cells were grown in organotypic raft cultures, and expression of the differentiation marker involucrin was decreased (28). To determine if LMP2A expression similarly affects the normal epithelial cell line derived from HFK cells, stable HFK cell lines expressing LMP2A were established. LMP2A expression in HFK cells did not confer anchorage-independent growth or tumor formation in nude mice (data not shown).
To investigate whether LMP2A affects epithelial cell differentiation, HFK cells stably expressing either the vector or LMP2A were induced to differentiate in semisolid methylcellulose medium and assessed for involucrin expression. Involucrin expression was significantly lower in LMP2A-expressing HFK cells than in the vector control cell line when cells were grown in K-SFM (Fig. 1A). During growth in methylcellulose, involucrin expression greatly increased in the vector control cells and this increase was blocked in LMP2A-expressing cells. These findings indicate that LMP2A decreases steady-state levels of involucrin and inhibits differentiation-induced expression of involucrin.
FIG. 1.
The PY motif of LMP2A is important for inhibition of involucrin expression. (A) HFK cells (4 × 106) stably expressing the pBabe vector, LMP2A, LMP2A ΔITAM, and LMP2A ΔPY were resuspended in K-SFM-methylcellulose medium. An equal number of cells were plated in parallel in tissue culture plates with K-SFM. Cells were harvested after 28 and 54 h, and immunoblot analyses were performed. Induction of differentiation was assessed with antibodies against involucrin, and expression of the LMP2A constructs was determined via anti-HA immunoblotting. Antiactin was included as a loading control. (B and C) Densitometry was performed, and involucrin levels at 28 h were normalized to the corresponding actin levels (B) or to expression of LMP2A (C) and graphed.
Two motifs in the LMP2A N-terminal signaling arm are important for various aspects of the signaling capacity of LMP2A. The ITAM of LMP2A is required for its ability to block B-cell receptor signaling and to activate PI3K/Akt signaling in B cells. The PY motifs of LMP2A mediate interaction with members of the Nedd4 family of ubiquitin ligases, and these motifs affect the turnover of LMP2A and associated kinases, such as Lyn, in B cells. To ascertain the importance of the ITAM and PY motifs to LMP2A signaling and effects on epithelial differentiation, stable cell lines expressing mutant LMP2A protein with either the ITAM deleted (LMP2A ΔITAM) or mutated PY motifs (LMP2A ΔPY) were established. Decreased amounts of involucrin were detected in cells expressing LMP2A or the ITAM and PY mutant proteins in K-SFM with a slightly increased amount in the cells expressing the two mutant proteins (Fig. 1A). Involucrin expression significantly increased in the vector control cells when they were grown in methylcellulose but did not increase in cells expressing either LMP2A or the LMP2A ITAM mutant protein at 28 h. In contrast, involucrin expression was induced in cells expressing the PY mutant protein, although the level of induction was reduced compared to that obtained with the vector.
For comparison, the involucrin levels were normalized to actin (Fig. 1B). After differentiation induction in methylcellulose, involucrin expression in the PY mutant was approximately half the level in the vector control. In the wild-type and ITAM mutant LMP2A-expressing cells, involucrin levels remained low at approximately 1/10 of the level in the vector controls (Fig. 1B). Expression of involucrin in all groups grown in methylcellulose increased further by the 54-h time point. As LMP2A and the mutant proteins were expressed at slightly different levels, the amount of involucrin was normalized to LMP2A expression (Fig. 1C). After 28 h in methylcellulose, the amount of involucrin was unchanged in the LMP2A or the ITAM mutant protein-expressing cells relative to that in their counterparts in K-SFM, and involucrin levels were 10-fold higher in the cells expressing the PY mutant protein compared to those in the wild-type and ITAM mutant protein-expressing cells. These data indicate that the inhibition of involucrin induction by growth in methylcellulose is impaired by loss of the PY motifs while deletion of the ITAM only slightly affected the ability of LMP2A to block differentiation. Therefore, the PY motifs are essential for LMP2A-mediated inhibition of epithelial cell differentiation.
The ITAM of LMP2A is required for LMP2A-mediated Akt activation in HFK cells.
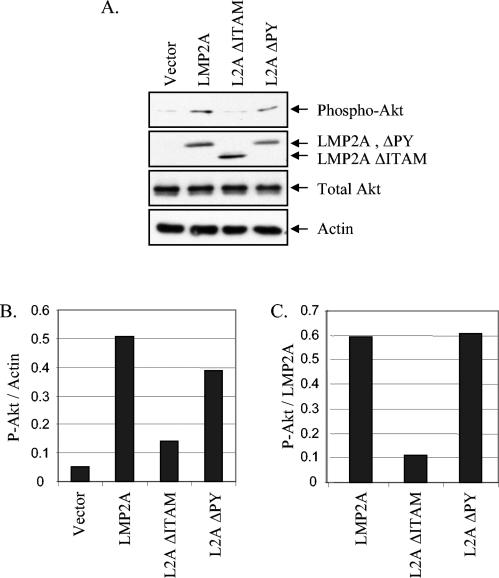
LMP2A has been previously shown to activate Akt in epithelial cells (23, 28). In B cells, LMP2A-mediated activation of Akt occurs via PI3K signaling initiated through the LMP2A ITAM (31). To identify the domains of LMP2A that are responsible for PI3K/Akt activation in epithelial cells, immunoblot analysis of HFK cells stably expressing the vector, LMP2A, LMP2A ΔITAM, and LMP2A ΔPY was performed with an antibody specific for the phosphorylated, activated form of Akt. Phosphorylated Akt was readily detected in HFK cells expressing LMP2A and the PY mutant but was barely detectable in cells expressing the vector control or the ITAM mutant protein (Fig. 2A). The levels of phosphorylated Akt were normalized to the amounts of actin (Fig. 2B) and to the levels of LMP2A and the mutant proteins (Fig. 2C). These data revealed that activation of Akt was equivalent for LMP2A and the PY mutant protein but was lost in the ITAM mutant protein. Thus, the activation of PI3K and its downstream effector Akt by LMP2A in epithelial cells requires the LMP2A ITAM but not the PY motifs.
FIG. 2.
The ITAM is required for LMP2A-mediated Akt activation. (A) HFK cells stably expressing the pBabe vector, LMP2A, LMP2A ΔITAM, and LMP2A ΔPY were harvested at confluency after 48 h of starvation (i.e., with BPE and EGF supplements withheld). Activated Akt was detected by immunoblotting for activated phospho-Akt (P-Akt) in comparison with levels of total Akt. Expression levels of wild-type and mutant LMP2A were determined via anti-HA immunoblotting. Antiactin was included to control for protein loading. (B and C) Densitometry was performed, and phospho-Akt levels were normalized to the corresponding actin levels (B) or to expression of LMP2A (C) and graphed.
The ITAM and PY motifs both contribute to LMP2A-mediated effects on β-catenin in HFK cells.
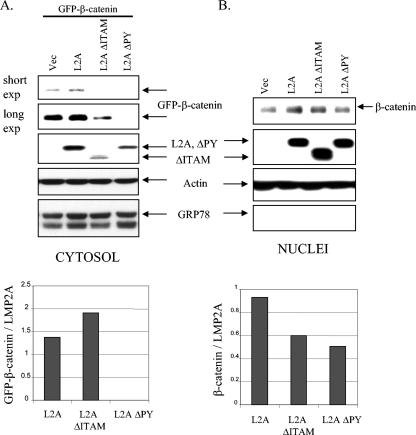
LMP2A has recently been shown to activate the β-catenin pathway in HFK cells, in part via effects on PI3K signaling (23). As the ITAM of LMP2A is required for PI3K/Akt activation in B cells and now in epithelial cells, the contribution of the ITAM and the PY motifs to LMP2A-mediated effects on β-catenin was investigated. HFK cells were cotransfected with GFP-β-catenin in combination with the vector, LMP2A, LMP2A ΔITAM, or LMP2A ΔPY and fractionated into cytosolic and nuclear components, and levels of GFP-β-catenin and endogenous β-catenin were determined by immunoblotting. Increased levels of GFP-β-catenin were detected in the cytosol of LMP2A-tranfected cells relative to those in the vector control (Fig. 3A). Normalizing the amounts of GFP-β-catenin to LMP2A expression revealed that loss of the ITAM domain did not affect the ability of LMP2A to stabilize GFP-β-catenin in the cytosol (Fig. 3A). This finding concurs with previous work that showed that inhibition of PI3K did not affect the LMP2A-mediated increase in cytosolic GFP-β-catenin. Importantly, the PY mutant protein was completely unable to stabilize cotransfected GFP-β-catenin, indicating that this property of LMP2A was dependent on its PY motifs and their putative interactions with ubiquitin ligases.
FIG. 3.
The ITAM and PY motifs contribute to the effects of LMP2A on β-catenin stabilization and nuclear translocation. HFK cells were cotransfected with GFP-β-catenin in combination with pSG5, pSG5-LMP2A, pSG5-LMP2A ΔITAM, or pSG5-LMP2A ΔPY. Supplements were withheld for 24 h prior to harvesting. Upon harvesting, cells were fractionated into their cytosolic (A) and nuclear (B) compartments and analyzed by immunoblotting. (A, top) Anti-GFP immunoblotting determined levels of GFP-β-catenin in the cytosols of transfected cells, and anti-HA detected expression of the LMP2A constructs. Antiactin and anti-GRP78 blot assays were included to assess protein loading and the integrity of the fractionation procedure, respectively. (A, bottom) Densitometry was performed, and GFP-β-catenin levels were normalized to the expression of LMP2A and graphed. (B, top) Nuclear β-catenin was detected with antibodies against β-catenin, and LMP2A expression was determined with anti-HA antibodies. GRP78 is an endoplasmic reticulum marker, and anti-GRP78 immunoblotting indicated the purity of the nuclear extracts. An antiactin immunoblot served as a protein loading control. (B, bottom) Densitometry was performed, and β-catenin levels were normalized to the expression of LMP2A and graphed. Vec, vector; exp, exposure.
The transfected GFP-β-catenin was not detectable in the nucleus; therefore, the effects of LMP2A and domain mutant proteins were determined by assessing the levels of endogenous β-catenin. Consistent with our previously published data, LMP2A expression led to an increase in nuclear levels of β-catenin compared to the vector control (Fig. 3B) (23). This accumulation was less dramatic with the ITAM mutant protein, consistent with our previously identified role for PI3K signaling in LMP2A-mediated nuclear β-catenin accumulation. Nuclear β-catenin levels in cells expressing LMP2A ΔPY were comparable to the levels detected in vector control-transfected cells (Fig. 4B). This is consistent with the lower levels of cytosolic β-catenin observed in cells expressing the PY mutant protein and suggests that the low levels of cytoplasmic β-catenin result in decreased nuclear β-catenin (Fig. 3A and 4A). Normalization of nuclear β-catenin to LMP2A expression confirmed that both domains contributed to the higher levels of nuclear β-catenin (Fig. 3B).
FIG. 4.
PI3K and Akt activation regulates basal levels of β-catenin in LMP2A-expressing cells. (A) HFK cells were transfected with pSG5, pSG5-LMP2A, and pSG5-LMP2A ΔPY. EGF and BPE supplements were withheld for 48 h, and the cells were treated with 25 μM LY, 10 μM Akt inhibitor, or a DMSO control for 24 h prior to being harvested. At 48 h after transfection, cells were harvested, fractionated to isolate the cytosol, and subjected to immunoblot analysis to determine cytosolic β-catenin levels (top). Anti-HA immunoblotting revealed levels of the transfected LMP2A constructs (middle), and antiactin and anti-GRP78 (an endoplasmic reticulum chaperone) were included as loading controls (bottom). (B) Densitometry was performed, and GFP-β-catenin levels were normalized to the expression of LMP2A and graphed. Vec, vector; Inhib, inhibitor.
PI3K and Akt activation contributes to stabilization of cytoplasmic GFP-β-catenin.
To further investigate the role of the ITAM and PY motifs and activation of PI3K and Akt on the effects of LMP2A on β-catenin, cells were transfected with the vector, LMP2A, or the PY mutant protein and treated with the PI3K-specific inhibitor LY, an Akt inhibitor, or the vehicle control (DMSO) and fractionated to assess β-catenin levels in the cytosol. The amount of GFP-β-catenin was increased in the cytosol of LMP2A-expressing cells compared to that in the vector control and was not increased in cells expressing the PY mutant protein (Fig. 4A). Normalization of GFP-β-catenin expression to LMP2A levels confirmed the requirement for the PY motif in the stabilization of β-catenin (Fig. 3A and 4B). Inhibition of PI3K or Akt did not affect the levels of β-catenin in the vector control cells; however, this inhibition further decreased the β-catenin level in the LMP2A ΔPY mutant cells to less than that in the vector control cells (Fig. 4A). These data indicate that activation of PI3K and Akt by LMP2A governs the steady-state levels of β-catenin in LMP2A-expressing cells and that the PY motifs of LMP2A are required for the accumulation of elevated levels of β-catenin.
PI3K induces β-catenin stabilization.
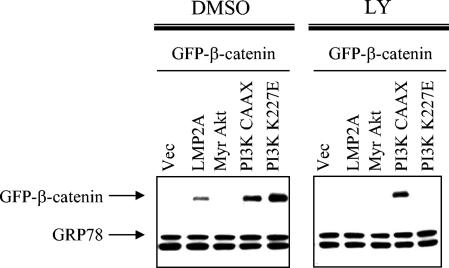
These data implicate both the PY motifs and the ITAM of LMP2A in its effects on β-catenin. The ITAM is responsible for activation of PI3K/Akt signaling by LMP2A, and LY-mediated inhibition of effects on β-catenin indicates that the PI3K pathway also contributes to β-catenin accumulation and nuclear translocation in LMP2A-expressing cells. To further ascertain the role of PI3K and Akt in this process, HFK cells were transiently transfected with two forms of constitutively active PI3K and a constitutively activated form of Akt. PI3K-CAAX is targeted to the membrane via farnesylation, where it leads to activation of downstream kinases that can activate Akt. PI3K K227E has a mutation in its Ras interaction domain that mimics Ras-mediated activation of PI3K. Myristylated Akt (myr-Akt) is also targeted to the membrane, resulting in constitutively activated, membrane-bound Akt. These constructs were cotransfected with GFP-β-catenin, the cells were fractionated, and effects on GFP-β-catenin levels were determined by immunoblot analysis and compared with vector and LMP2A cotransfections. Expression of both constitutively active PI3K constructs dramatically increased the cytosolic levels of GFP-β-catenin and exceeded the levels in LMP2A-expressing cells (Fig. 5). These data indicate that PI3K activation affects β-catenin stabilization in these cells. Interestingly, myr-Akt did not affect GFP-β-catenin levels (Fig. 5). These data may indicate that some other PI3K effector is responsible for β-catenin signaling effects. However, the inhibition of GFP-β-catenin accumulation by the Akt inhibitor (Fig. 4) suggests that Akt activity is required. These findings suggest that the localization of Akt is a critical factor and that membrane-bound Akt cannot affect β-catenin levels. Treatment with LY, which inhibits the kinase domain of PI3K, blocked cytosolic GFP-β-catenin accumulation in cells cotransfected with either LMP2A or PI3K K227E but not with PI3K CAAX (Fig. 5, right panel). These data indicate that PI3K activation and signaling via the LMP2A ITAM contribute to the accumulation of β-catenin.
FIG. 5.
Constitutively active PI3K, but not myr-Akt, stabilizes cytoplasmic β-catenin. HFK cells were cotransfected with GFP-β-catenin in combination with vector (Vec), LMP2A, myr-Akt, PI3K CAAX, and PI3K K227E. Supplements were withheld, and cells were treated with DMSO or 25 μM LY for 24 h prior to being harvested. Cells were analyzed by immunoblotting with anti-GFP antibodies to detect levels of GFP-β-catenin in the cytosol. Anti-GRP78 immunoblotting revealed equal loading of cytosolic proteins.
DISCUSSION
This study reveals that LMP2A expression in telomerase-immortalized, normal HFK cells inhibited epithelial cell differentiation in semisolid methylcellulose-based differentiation assays. The ITAM and PY motifs in the cytoplasmic, N-terminal signaling arm of LMP2A contributed to the observed inhibition of differentiation, with a more prominent role for the PY motifs, as their deletion led to an inefficient block of involucrin expression (Fig. 1). The PY motifs were also found to significantly contribute to another pathway activated by LMP2A in epithelial cells, the β-catenin pathway. In cells expressing the LMP2A ΔPY mutant protein, the levels of both cytosolic and nuclear β-catenin were decreased relative to those in wild-type LMP2A-expressing cells (Fig. 3 and 4). The ITAM of LMP2A was responsible for activation of PI3K and Akt (Fig. 2) and also contributed to LMP2A-mediated effects on β-catenin, as its deletion was associated with decreased levels of nuclear β-catenin relative to wild-type LMP2A (Fig. 3).
In contrast to previous data detailing the oncogenic capacity of LMP2A in the keratinocyte cell line HaCaT (28), LMP2A was unable to transform the normal HFK cell line. LMP2A-expressing HFK cells were incapable of colony formation in soft agar and did not induce tumor formation in nude mice (data not shown). However, the data presented in this report imply that although LMP2A is not oncogenic outright, it does alter properties of the cell such that in certain contexts, it may confer a selective growth advantage. As shown here and in previous studies, LMP2A blocks epithelial cell differentiation, activates PI3K/Akt signaling, and activates the β-catenin pathway (23, 28). These properties could poise the cells at the threshold of transformation such that in combination with other viral proteins or genetic mutations, LMP2A might encourage or enable oncogenesis to proceed. This may, in part, explain why LMP2A conferred oncogenic properties on the HaCaT cell line (28). HaCaT cells were spontaneously immortalized, are aneuploid, and have collected a variety of genetic aberrations over time such as mutation and inactivation of the tumor suppressor p53 (1, 2, 17). Expression of LMP2A might have served as the catalyst needed to transform the HaCaT cells. In relatively normal HFK cells, the threshold for transformation is likely higher than that in HaCaT cells, and additional genetic alterations or expression of other viral proteins are required for oncogenesis. These data support a contributory role for LMP2A in the genesis of EBV-associated malignancies, with coexpression of other viral proteins and perhaps in the context of altered cellular milieus.
The present study reveals an important role for the PY motifs of LMP2A in its effects on epithelial cell differentiation and β-catenin signaling. Mutation of the PY motifs blocked the ability of LMP2A to increase cytosolic β-catenin levels with subsequent decreased nuclear translocation (Fig. 3 and 4). Furthermore, the PY motifs contributed to the inhibition by LMP2A on induction of involucrin expression in a methylcellulose-based differentiation assay (Fig. 1). The PY motifs mediate interaction with the WW domain-containing ubiquitin ligases that make up the Nedd4 family (13). In B cells, interaction of LMP2A with members of the Nedd4 ubiquitin ligase family enables it to increase turnover of proteins such as Lyn kinase associated with BCR signaling via ubiquitination and degradation (13, 14, 35). This contributes to LMP2A-mediated silencing of BCR activation and signaling to enable maintenance of viral latency. Members of the Nedd4 family are also expressed in epithelial cells, and it is likely that a similar interaction occurs in epithelial cells (15, 30). As some BCR-associated kinases such as Lyn are not ubiquitously expressed in epithelial cells, the substrates of this interaction are probably different, resulting in distinct consequences. The fact that LMP2A-mediated effects on epithelial differentiation and β-catenin signaling were affected by mutation of the PY motifs indicates that interactions between ubiquitin ligases and LMP2A are a major contributing factor to the effects of LMP2A on epithelial cell growth and signaling. It will be important to further characterize these putative interactions and target substrates in LMP2A-expressing epithelial cells.
In addition to the PY motifs, the data presented here also implicate the ITAM in LMP2A signaling effects in epithelial cells. It has been established previously that the ITAM is required for the ability of LMP2A to activate PI3K and Akt signaling in B cells (31). The present study establishes that the ITAM is also required for LMP2A-mediated Akt activation in epithelial cells (Fig. 3). Furthermore, the ITAM of LMP2A contributes to its effects on the nuclear accumulation of β-catenin (Fig. 4). This is consistent with our previously published finding that activation of the PI3K pathway is required for LMP2A-mediated nuclear accumulation of β-catenin, the hallmark of β-catenin pathway activation. In further support of a prominent role for LMP2A-mediated activation of PI3K in β-catenin signaling, constitutively active PI3K constructs were able to recapitulate the β-catenin accumulation previously observed in LMP2A-expressing HFK cells. Interestingly, however, the constitutively active myr-Akt construct was unable to affect cytosolic or nuclear levels of β-catenin. This may indicate that a PI3K effector other than Akt is involved in β-catenin accumulation and nuclear translocation. Alternatively, Akt is involved, but myr-Akt, although constitutively active, is unable to translocate to parts of the cell required to execute its effects on β-catenin signaling because it is trapped at the plasma membrane via its myristylation moiety.
In summary, these data reveal important effects of LMP2A on differentiation and signaling pathways in epithelial cells. The ITAM of LMP2A is important for β-catenin nuclear translocation and accumulation, and these findings implicate the PI3K signaling pathway in the mechanism of these effects. Importantly, the PY motifs are required for the ability of LMP2A to inhibit HFK cell differentiation and are essential for its effects on β-catenin. These data suggest that differentiation is inversely correlated with β-catenin activation and that LMP2A effects on ubiquitin ligases are a major factor in LMP2A signaling and its effects on epithelial growth regulation.
Acknowledgments
This work was supported by grants CA32979 and CA103634 from the National Institutes of Health.
We thank Masato Ikeda and Richard Longnecker for generously providing the pSG5-LMP2A ΔPY mutant and Frauke Fehrmann and Laimonis Laimins for advice and assistance with the methylcellulose differentiation assay protocol. We are further indebted to Thomas Morrison and Natalie Thornburg for critical reviews of the manuscript.
REFERENCES
- 1.Boukamp, P., R. T. Petrussevska, D. Breitkreutz, J. Hornung, A. Markham, and N. E. Fusenig. 1988. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 106:761-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boukamp, P., S. Popp, S. Altmeyer, A. Hulsen, C. Fasching, T. Cremer, and N. E. Fusenig. 1997. Sustained nontumorigenic phenotype correlates with a largely stable chromosome content during long-term culture of the human keratinocyte line HaCaT. Genes Chromosomes Cancer 19:201-214. [DOI] [PubMed] [Google Scholar]
- 3.Brazil, D. P., and B. A. Hemmings. 2001. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem. Sci. 26:657-664. [DOI] [PubMed] [Google Scholar]
- 4.Brooks, L., Q. Y. Yao, A. B. Rickinson, and L. S. Young. 1992. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: coexpression of EBNA1, LMP1, and LMP2 transcripts. J. Virol. 66:2689-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busson, P., R. McCoy, R. Sadler, K. Gilligan, T. Tursz, and N. Raab-Traub. 1992. Consistent transcription of the Epstein-Barr virus LMP2 gene in nasopharyngeal carcinoma. J. Virol. 66:3257-3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caldwell, R. G., R. C. Brown, and R. Longnecker. 2000. Epstein-Barr virus LMP2A-induced B-cell survival in two unique classes of EmuLMP2A transgenic mice. J. Virol. 74:1101-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caldwell, R. G., J. B. Wilson, S. J. Anderson, and R. Longnecker. 1998. Epstein-Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity 9:405-411. [DOI] [PubMed] [Google Scholar]
- 8.Farwell, D. G., K. A. Shera, J. I. Koop, G. A. Bonnet, C. P. Matthews, G. W. Reuther, M. D. Coltrera, J. K. McDougall, and A. J. Klingelhutz. 2000. Genetic and epigenetic changes in human epithelial cells immortalized by telomerase. Am. J. Pathol. 156:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fruehling, S., and R. Longnecker. 1997. The immunoreceptor tyrosine-based activation motif of Epstein-Barr virus LMP2A is essential for blocking BCR-mediated signal transduction. Virology 235:241-251. [DOI] [PubMed] [Google Scholar]
- 10.Giles, R. H., J. H. van Es, and H. Clevers. 2003. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim. Biophys. Acta 1653:1-24. [DOI] [PubMed] [Google Scholar]
- 11.Hanada, M., J. Feng, and B. A. Hemmings. 2004. Structure, regulation and function of PKB/AKT—a major therapeutic target. Biochim. Biophys. Acta 1697:3-16. [DOI] [PubMed] [Google Scholar]
- 12.He, T. C., A. B. Sparks, C. Rago, H. Hermeking, L. Zawel, L. T. da Costa, P. J. Morin, B. Vogelstein, and K. W. Kinzler. 1998. Identification of c-MYC as a target of the APC pathway. Science 281:1509-1512. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda, M., A. Ikeda, L. C. Longan, and R. Longnecker. 2000. The Epstein-Barr virus latent membrane protein 2A PY motif recruits WW domain-containing ubiquitin-protein ligases. Virology 268:178-191. [DOI] [PubMed] [Google Scholar]
- 14.Ikeda, M., A. Ikeda, and R. Longnecker. 2001. PY motifs of Epstein-Barr virus LMP2A regulate protein stability and phosphorylation of LMP2A-associated proteins. J. Virol. 75:5711-5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ingham, R. J., G. Gish, and T. Pawson. 2004. The Nedd4 family of E3 ubiquitin ligases: functional diversity within a common modular architecture. Oncogene 23:1972-1984. [DOI] [PubMed] [Google Scholar]
- 16.Kieff, E., and A. B. Rickinson. 2001. Epstein-Barr virus and its replication, p. 2511-2573. In B. N. Fields, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, S. E. Straus, and D. M. Knipe (ed.), Fields virology, fourth ed., vol. 2. Lippincott Williams & Wilkins Publishers, Philadelphia, Pa.
- 17.Lehman, T. A., R. Modali, P. Boukamp, J. Stanek, W. P. Bennett, J. A. Welsh, R. A. Metcalf, M. R. Stampfer, N. Fusenig, E. M. Rogan, et al. 1993. p53 mutations in human immortalized epithelial cell lines. Carcinogenesis 14:833-839. [DOI] [PubMed] [Google Scholar]
- 18.Merchant, M., R. G. Caldwell, and R. Longnecker. 2000. The LMP2A ITAM is essential for providing B cells with development and survival signals in vivo. J. Virol. 74:9115-9124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller, C. L., A. L. Burkhardt, J. H. Lee, B. Stealey, R. Longnecker, J. B. Bolen, and E. Kieff. 1995. Integral membrane protein 2 of Epstein-Barr virus regulates reactivation from latency through dominant negative effects on protein-tyrosine kinases. Immunity 2:155-166. [DOI] [PubMed] [Google Scholar]
- 20.Miller, C. L., J. H. Lee, E. Kieff, A. L. Burkhardt, J. B. Bolen, and R. Longnecker. 1994. Epstein-Barr virus protein LMP2A regulates reactivation from latency by negatively regulating tyrosine kinases involved in sIg-mediated signal transduction. Infect. Agents Dis. 3:128-136. [PubMed] [Google Scholar]
- 21.Miller, C. L., J. H. Lee, E. Kieff, and R. Longnecker. 1994. An integral membrane protein (LMP2) blocks reactivation of Epstein-Barr virus from latency following surface immunoglobulin crosslinking. Proc. Natl. Acad. Sci. USA 91:772-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller, C. L., R. Longnecker, and E. Kieff. 1993. Epstein-Barr virus latent membrane protein 2A blocks calcium mobilization in B lymphocytes. J. Virol. 67:3087-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrison, J. A., A. J. Klingelhutz, and N. Raab-Traub. 2003. Epstein-Barr virus latent membrane protein 2A activates beta-catenin signaling in epithelial cells. J. Virol. 77:12276-12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niedobitek, G., E. Kremmer, H. Herbst, L. Whitehead, C. W. Dawson, E. Niedobitek, C. von Ostau, N. Rooney, F. A. Grasser, and L. S. Young. 1997. Immunohistochemical detection of the Epstein-Barr virus-encoded latent membrane protein 2A in Hodgkin's disease and infectious mononucleosis. Blood 90:1664-1672. [PubMed] [Google Scholar]
- 25.Polakis, P. 2000. Wnt signaling and cancer. Genes Dev. 14:1837-1851. [PubMed] [Google Scholar]
- 26.Raab-Traub, N., and K. Flynn. 1986. The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell 47:883-889. [DOI] [PubMed] [Google Scholar]
- 27.Rickinson, A. B., and E. Kieff. 2001. Epstein-Barr virus, p. 2575-2627. In B. N. Fields, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, S. E. Straus, and D. M. Knipe (ed.), Fields virology, fourth ed., vol. 2. Lippincott Williams & Wilkins Publishers, Philadelphia, Pa.
- 28.Scholle, F., K. M. Bendt, and N. Raab-Traub. 2000. Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J. Virol. 74:10681-10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shtutman, M., J. Zhurinsky, I. Simcha, C. Albanese, M. D'Amico, R. Pestell, and A. Ben-Ze'ev. 1999. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. USA 96:5522-5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staub, O., I. Gautschi, T. Ishikawa, K. Breitschopf, A. Ciechanover, L. Schild, and D. Rotin. 1997. Regulation of stability and function of the epithelial Na+ channel (ENaC) by ubiquitination. EMBO J. 16:6325-6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swart, R., I. K. Ruf, J. Sample, and R. Longnecker. 2000. Latent membrane protein 2A-mediated effects on the phosphatidylinositol 3-kinase/Akt pathway. J. Virol. 74:10838-10845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takada, K. 2000. Epstein-Barr virus and gastric carcinoma. Mol. Pathol. 53:255-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tetsu, O., and F. McCormick. 1999. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398:422-426. [DOI] [PubMed] [Google Scholar]
- 34.Vanhaesebroeck, B., and D. R. Alessi. 2000. The PI3K-PDK1 connection: more than just a road to PKB. Biochem. J. 346(Pt. 3):561-576. [PMC free article] [PubMed] [Google Scholar]
- 35.Winberg, G., L. Matskova, F. Chen, P. Plant, D. Rotin, G. Gish, R. Ingham, I. Ernberg, and T. Pawson. 2000. Latent membrane protein 2A of Epstein-Barr virus binds WW domain E3 protein-ubiquitin ligases that ubiquitinate B-cell tyrosine kinases. Mol. Cell. Biol. 20:8526-8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong, N. A., and M. Pignatelli. 2002. Beta-catenin—a linchpin in colorectal carcinogenesis? Am. J. Pathol. 160:389-401. [DOI] [PMC free article] [PubMed] [Google Scholar]