Abstract
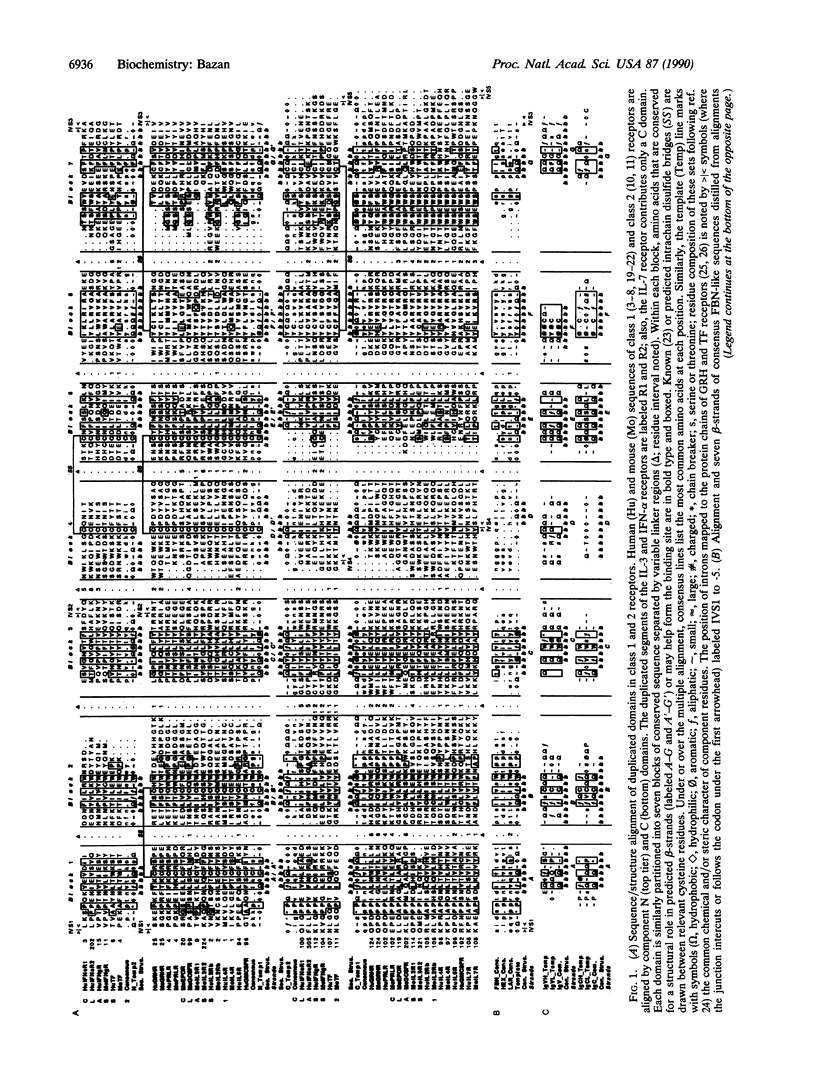
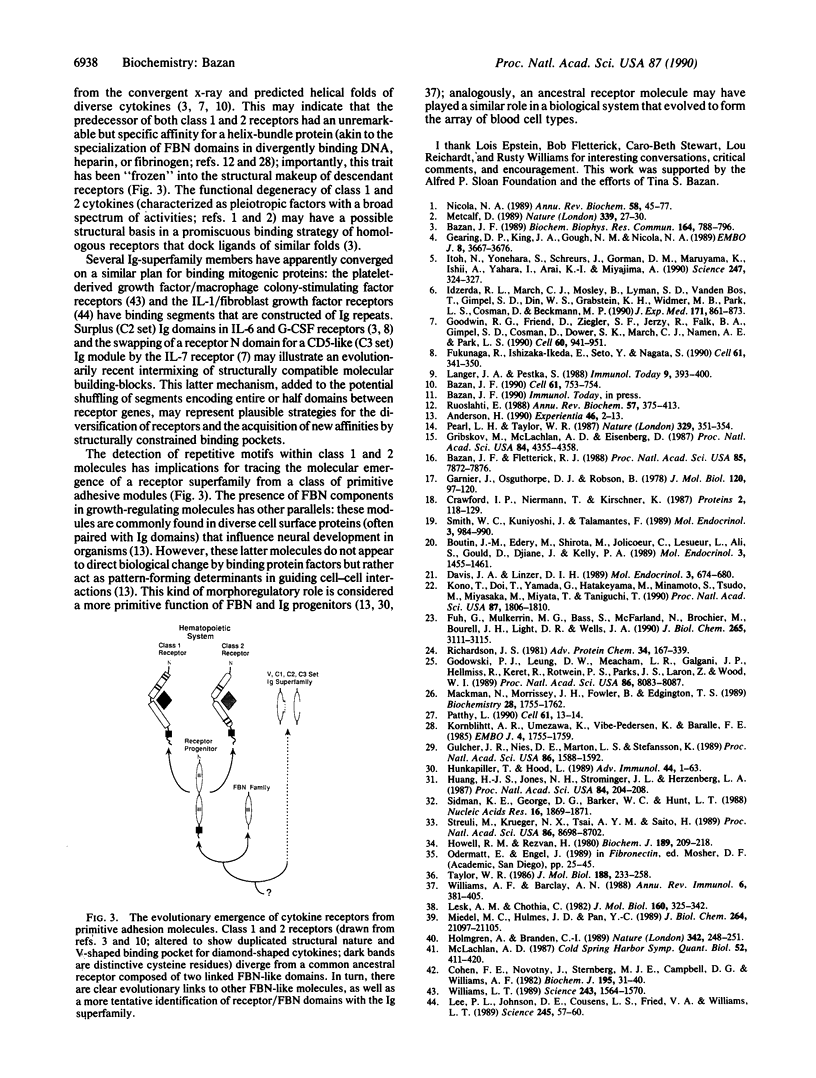
A family of cytokine receptors comprising molecules specific for a diverse group of hematopoietic factors and growth hormones has been principally defined by a striking homology of binding domains. This work proposes that the approximately 200-residue binding segment of the canonical cytokine receptor is composed of two discrete folding domains that share a significant sequence and structural resemblance. Analogous motifs are found in tandem approximately 100-amino acid domains in the extracellular segments of a receptor family formed by the interferon-alpha/beta and -gamma receptors and tissue factor, a membrane tether for a coagulation protease. Domains from the receptor supergroup reveal clear evolutionary links to fibronectin type III structures, approximately 90-amino acid modules that are typically found in cell surface molecules with adhesive functions. Predictive structural analysis of the shared receptor and fibronectin domains locates seven beta-strands in conserved regions of the chain; these strands are modeled to fold into antiparallel beta-sandwiches with a topology that is similar to immunoglobulin constant domains. These findings have strong implications for understanding the evolutionary emergence of an important class of regulatory molecules from primitive adhesive modules. In addition, the resulting double-barrel design of the receptors and the spatial clustering of conserved residues suggest a likely binding site for cytokine ligands.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson H. Adhesion molecules and animal development. Experientia. 1990 Jan 15;46(1):2–13. doi: 10.1007/BF01955407. [DOI] [PubMed] [Google Scholar]
- Bazan J. F. A novel family of growth factor receptors: a common binding domain in the growth hormone, prolactin, erythropoietin and IL-6 receptors, and the p75 IL-2 receptor beta-chain. Biochem Biophys Res Commun. 1989 Oct 31;164(2):788–795. doi: 10.1016/0006-291x(89)91528-3. [DOI] [PubMed] [Google Scholar]
- Bazan J. F., Fletterick R. J. Viral cysteine proteases are homologous to the trypsin-like family of serine proteases: structural and functional implications. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7872–7876. doi: 10.1073/pnas.85.21.7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan J. F. Shared architecture of hormone binding domains in type I and II interferon receptors. Cell. 1990 Jun 1;61(5):753–754. doi: 10.1016/0092-8674(90)90182-e. [DOI] [PubMed] [Google Scholar]
- Boutin J. M., Edery M., Shirota M., Jolicoeur C., Lesueur L., Ali S., Gould D., Djiane J., Kelly P. A. Identification of a cDNA encoding a long form of prolactin receptor in human hepatoma and breast cancer cells. Mol Endocrinol. 1989 Sep;3(9):1455–1461. doi: 10.1210/mend-3-9-1455. [DOI] [PubMed] [Google Scholar]
- Cohen F. E., Novotný J., Sternberg M. J., Campbell D. G., Williams A. F. Analysis of structural similarities between brain Thy-1 antigen and immunoglobulin domains. Evidence for an evolutionary relationship and a hypothesis for its functional significance. Biochem J. 1981 Apr 1;195(1):31–40. doi: 10.1042/bj1950031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P., Niermann T., Kirschner K. Prediction of secondary structure by evolutionary comparison: application to the alpha subunit of tryptophan synthase. Proteins. 1987;2(2):118–129. doi: 10.1002/prot.340020206. [DOI] [PubMed] [Google Scholar]
- Davis J. A., Linzer D. I. Expression of multiple forms of the prolactin receptor in mouse liver. Mol Endocrinol. 1989 Apr;3(4):674–680. doi: 10.1210/mend-3-4-674. [DOI] [PubMed] [Google Scholar]
- Fuh G., Mulkerrin M. G., Bass S., McFarland N., Brochier M., Bourell J. H., Light D. R., Wells J. A. The human growth hormone receptor. Secretion from Escherichia coli and disulfide bonding pattern of the extracellular binding domain. J Biol Chem. 1990 Feb 25;265(6):3111–3115. [PubMed] [Google Scholar]
- Fukunaga R., Ishizaka-Ikeda E., Seto Y., Nagata S. Expression cloning of a receptor for murine granulocyte colony-stimulating factor. Cell. 1990 Apr 20;61(2):341–350. doi: 10.1016/0092-8674(90)90814-u. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gearing D. P., King J. A., Gough N. M., Nicola N. A. Expression cloning of a receptor for human granulocyte-macrophage colony-stimulating factor. EMBO J. 1989 Dec 1;8(12):3667–3676. doi: 10.1002/j.1460-2075.1989.tb08541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godowski P. J., Leung D. W., Meacham L. R., Galgani J. P., Hellmiss R., Keret R., Rotwein P. S., Parks J. S., Laron Z., Wood W. I. Characterization of the human growth hormone receptor gene and demonstration of a partial gene deletion in two patients with Laron-type dwarfism. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8083–8087. doi: 10.1073/pnas.86.20.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin R. G., Friend D., Ziegler S. F., Jerzy R., Falk B. A., Gimpel S., Cosman D., Dower S. K., March C. J., Namen A. E. Cloning of the human and murine interleukin-7 receptors: demonstration of a soluble form and homology to a new receptor superfamily. Cell. 1990 Mar 23;60(6):941–951. doi: 10.1016/0092-8674(90)90342-c. [DOI] [PubMed] [Google Scholar]
- Gribskov M., McLachlan A. D., Eisenberg D. Profile analysis: detection of distantly related proteins. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4355–4358. doi: 10.1073/pnas.84.13.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulcher J. R., Nies D. E., Marton L. S., Stefansson K. An alternatively spliced region of the human hexabrachion contains a repeat of potential N-glycosylation sites. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1588–1592. doi: 10.1073/pnas.86.5.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A., Bränden C. I. Crystal structure of chaperone protein PapD reveals an immunoglobulin fold. Nature. 1989 Nov 16;342(6247):248–251. doi: 10.1038/342248a0. [DOI] [PubMed] [Google Scholar]
- Howell R. M., Rezvan H. Circular dichroic, infrared and other studies on the protein component of pig brain thromboplastin. Biochem J. 1980 Aug 1;189(2):209–218. doi: 10.1042/bj1890209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. J., Jones N. H., Strominger J. L., Herzenberg L. A. Molecular cloning of Ly-1, a membrane glycoprotein of mouse T lymphocytes and a subset of B cells: molecular homology to its human counterpart Leu-1/T1 (CD5). Proc Natl Acad Sci U S A. 1987 Jan;84(1):204–208. doi: 10.1073/pnas.84.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller T., Hood L. Diversity of the immunoglobulin gene superfamily. Adv Immunol. 1989;44:1–63. doi: 10.1016/s0065-2776(08)60639-2. [DOI] [PubMed] [Google Scholar]
- Idzerda R. L., March C. J., Mosley B., Lyman S. D., Vanden Bos T., Gimpel S. D., Din W. S., Grabstein K. H., Widmer M. B., Park L. S. Human interleukin 4 receptor confers biological responsiveness and defines a novel receptor superfamily. J Exp Med. 1990 Mar 1;171(3):861–873. doi: 10.1084/jem.171.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Schreurs J., Gorman D. M., Maruyama K., Ishii A., Yahara I., Arai K., Miyajima A. Cloning of an interleukin-3 receptor gene: a member of a distinct receptor gene family. Science. 1990 Jan 19;247(4940):324–327. doi: 10.1126/science.2404337. [DOI] [PubMed] [Google Scholar]
- Kono T., Doi T., Yamada G., Hatakeyama M., Minamoto S., Tsudo M., Miyasaka M., Miyata T., Taniguchi T. Murine interleukin 2 receptor beta chain: dysregulated gene expression in lymphoma line EL-4 caused by a promoter insertion. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1806–1810. doi: 10.1073/pnas.87.5.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblihtt A. R., Umezawa K., Vibe-Pedersen K., Baralle F. E. Primary structure of human fibronectin: differential splicing may generate at least 10 polypeptides from a single gene. EMBO J. 1985 Jul;4(7):1755–1759. doi: 10.1002/j.1460-2075.1985.tb03847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer J. A., Pestka S. Interferon receptors. Immunol Today. 1988 Dec;9(12):393–400. doi: 10.1016/0167-5699(88)91241-8. [DOI] [PubMed] [Google Scholar]
- Lee P. L., Johnson D. E., Cousens L. S., Fried V. A., Williams L. T. Purification and complementary DNA cloning of a receptor for basic fibroblast growth factor. Science. 1989 Jul 7;245(4913):57–60. doi: 10.1126/science.2544996. [DOI] [PubMed] [Google Scholar]
- Lesk A. M., Chothia C. Evolution of proteins formed by beta-sheets. II. The core of the immunoglobulin domains. J Mol Biol. 1982 Sep 15;160(2):325–342. doi: 10.1016/0022-2836(82)90179-6. [DOI] [PubMed] [Google Scholar]
- Mackman N., Morrissey J. H., Fowler B., Edgington T. S. Complete sequence of the human tissue factor gene, a highly regulated cellular receptor that initiates the coagulation protease cascade. Biochemistry. 1989 Feb 21;28(4):1755–1762. doi: 10.1021/bi00430a050. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D. Gene duplication and the origin of repetitive protein structures. Cold Spring Harb Symp Quant Biol. 1987;52:411–420. doi: 10.1101/sqb.1987.052.01.048. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989 May 4;339(6219):27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
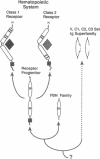
- Miedel M. C., Hulmes J. D., Pan Y. C. Limited proteolysis of recombinant human soluble interleukin-2 receptor. Identification of an interleukin-2 binding core. J Biol Chem. 1989 Dec 15;264(35):21097–21105. [PubMed] [Google Scholar]
- Nicola N. A. Hemopoietic cell growth factors and their receptors. Annu Rev Biochem. 1989;58:45–77. doi: 10.1146/annurev.bi.58.070189.000401. [DOI] [PubMed] [Google Scholar]
- Patthy L. Homology of a domain of the growth hormone/prolactin receptor family with type III modules of fibronectin. Cell. 1990 Apr 6;61(1):13–14. doi: 10.1016/0092-8674(90)90208-v. [DOI] [PubMed] [Google Scholar]
- Pearl L. H., Taylor W. R. A structural model for the retroviral proteases. Nature. 1987 Sep 24;329(6137):351–354. doi: 10.1038/329351a0. [DOI] [PubMed] [Google Scholar]
- Richardson J. S. The anatomy and taxonomy of protein structure. Adv Protein Chem. 1981;34:167–339. doi: 10.1016/s0065-3233(08)60520-3. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- Sidman K. E., George D. G., Barker W. C., Hunt L. T. The protein identification resource (PIR). Nucleic Acids Res. 1988 Mar 11;16(5):1869–1871. doi: 10.1093/nar/16.5.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. C., Kuniyoshi J., Talamantes F. Mouse serum growth hormone (GH) binding protein has GH receptor extracellular and substituted transmembrane domains. Mol Endocrinol. 1989 Jun;3(6):984–990. doi: 10.1210/mend-3-6-984. [DOI] [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Tsai A. Y., Saito H. A family of receptor-linked protein tyrosine phosphatases in humans and Drosophila. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8698–8702. doi: 10.1073/pnas.86.22.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W. R. Identification of protein sequence homology by consensus template alignment. J Mol Biol. 1986 Mar 20;188(2):233–258. doi: 10.1016/0022-2836(86)90308-6. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- Williams L. T. Signal transduction by the platelet-derived growth factor receptor. Science. 1989 Mar 24;243(4898):1564–1570. doi: 10.1126/science.2538922. [DOI] [PubMed] [Google Scholar]