Abstract
Alterations of cytokine responses are thought to favor the establishment of persistent hepatitis C virus (HCV) infections, enhancing the risk of liver cirrhosis and hepatocellular carcinoma. Here we demonstrate that the expression of the HCV core (C) protein in stably transfected T cells correlates with a selective reduction of interleukin-2 (IL-2) promoter activity and IL-2 production in response to T-cell receptor triggering, whereas the activation of IL-4, IL-10, gamma interferon, and tumor necrosis factor alpha was moderately increased. This altered cytokine expression profile was associated with a perturbation of mitogen-activated protein (MAP) kinase responses. Extracellular regulated kinase and p38 were constitutively phosphorylated in C-expressing cells, while triggering of the costimulatory c-Jun N-terminal kinase (JNK) signaling cascade and activation of the CD28 response element within the IL-2 promoter appeared to be impaired. The perturbations of MAP kinase phosphorylation could be eliminated by cyclosporine A-mediated inhibition of nuclear factor of activated T cells, suggesting that the inactivation of JNK signaling and hyporesponsiveness to IL-2 induction were downstream consequences of C-induced Ca2+ flux in a manner that mimics the induction of clonal anergy.
Hepatitis C virus (HCV), a positive-strand RNA virus belonging to the Flaviviridae family, is estimated to infect approximately 170 million people worldwide. Only a fraction of acute HCV infections resolve within a few months. In the majority of cases, the virus establishes persistent infections, a significant proportion of which progress to fibrosis, cirrhosis, liver failure, or hepatocellular carcinoma (reviewed in reference 35). Although a primary HCV infection often elicits a humoral immune response that modulates the acute infection, antibodies are not detected in many patients or appear late (47). Furthermore, the presence of isolate-specific antibodies against virus glycoproteins does not correlate with virus clearance (4), and their absence in HCV-infected agammaglobulinemic children does not increase the risk of virus persistence (7, 12), suggesting that the humoral response is neither sufficient nor required for the resolution of HCV infection. In contrast, there is compelling evidence for a role of cell-mediated immunity in the outcome of primary HCV infection. HCV clearance is associated with a vigorous and sustained cellular immune response against multiple viral epitopes, whereas individuals with chronic infections often have a relatively weaker and narrower cytotoxic T-lymphocyte (CTL) response (11, 14, 19, 29, 42, 54, 72, 80). Furthermore, HCV-specific CD8+ T cells detected during chronic infections often express a dysfunctional phenotype characterized by a low expression level of gamma interferon (IFN-γ) (2, 33), while CD4+ T cells display a Th2 phenotype characterized by low IFN-γ and interleukin (IL-2) and high IL-4 and IL-10 production (23, 81, 83, 86).
The modulation of T-cell responses is the hallmark of many persistent infections and is often associated with the expression of specific viral products. The HCV genome is translated into a polyprotein of about 3,000 amino acids that is cleaved by cellular and viral proteases into three structural proteins and at least six nonstructural proteins (45). Three HCV gene products have been suggested to have immunomodulatory functions. The structural protein E2 (78) and the nonstructural protein NS5A (27, 28) modulate interferon responses by interacting with the interferon-inducible, double-stranded RNA-dependent protein kinase R (PKR). In addition, the HCV core (C) protein binds to certain members of the tumor necrosis factor (TNF) receptor superfamily and modulates TNF-α responses in some cell types (63). Furthermore, in vitro (8, 9, 15, 55, 71, 76) and some in vivo (43, 57, 58, 76) studies have indicated that HCV may replicate in B and T lymphocytes, suggesting that the activity of immunocompetent cells may be directly influenced by virus infection. In line with this possibility, we have previously shown that the expression of HCV C affects intracellular signaling that is essential for the activation of the IL-2 promoter in T cells (5).
The synthesis of IL-2 is tightly regulated at the transcriptional level and requires simultaneous triggering of the T-cell receptor (TCR) complex and the coreceptor CD28 (59). Triggering of the TCR causes the activation of a complex array of proximal signals, resulting in Ca2+ oscillations and protein kinase C (PKC)/p21ras-mediated activation of two mitogen-activated protein (MAP) kinases, extracellular regulated kinase (ERK) and p38. The costimulatory pathway, induced by simultaneous triggering of the TCR and CD28, includes the activation of NF-κB and a third MAP kinase pathway comprising the c-Jun N-terminal kinase (JNK). We have recently reported that the expression of HCV C in human T cells promotes the leakage of Ca2+ from intracellular stores and causes Ca2+ oscillations that favor the activation of nuclear factor of activated T cell (NFAT)-regulated promoters (6). We have now studied the effects of HCV C expression on cytokine synthesis. We demonstrate here that the stable expression of HCV C in T cells induces an anergic state characterized by a decreased IL-2 response and impaired activation of the JNK signaling pathway.
MATERIALS AND METHODS
Chemicals and immunological reagents.
All chemicals used were of analytical grade. Restriction endonucleases, T4 DNA polymerase, Taq polymerase, and T4 DNA ligase were obtained from MBI Fermentas (Vilnius, Lithuania). Ionomycin, 12-O-tetradecanoylphorbol 13-acetate (TPA), cyclosporine A, and puromycin were purchased from Sigma-Aldrich Corp. (St. Louis, Mo.). The anti-CD3 antibody (Ab) HIT3a and the phycoerythrin-labeled anti-IL-2 Ab MQ1-17H12 were obtained from Pharmingen (San Diego, Calif.). Horseradish peroxidase- and phycoerythrin-linked anti-immunoglobulins were obtained from Dako A/S (Glostrup, Denmark). Beetle luciferin was purchased from Promega Corp. (Madison, Wis.). The monoclonal Ab (MAb) C7-50 (56), which recognizes HCV C, was a gift from Jack Wands (Harvard Medical School, Boston, Mass.). Antibodies to IκB-α and the phosphorylated forms of SEK1, JNK1/2, ERK1/2, p38, and c-Jun were purchased from Cell Signaling (Beverly, Mass.). The MAb 9.3 recognizing CD28 was a gift from Bristol-Myers Squibb Pharmaceutical Research Institute (Seattle, Wash.).
Cells, cell culture, and plasmids.
The human T-lymphoma Jurkat cell line, subclone E6-1, and its HCV C-expressing derivatives JHC.d, JHC.g, and JHC.h (6) were cultured in RPMI 1640 supplemented with 10% fetal calf serum. For production of the pGL2-IL-2-luc plasmid, a PCR-amplified fragment corresponding to positions −393 to +3 of the IL-2 promoter (the primers for amplification were 5′-ACTCTTGCTCTTGTTCAC-3′ and 5′-TGATAGGGAACTCTTGAA-3′) was ligated into the pATg vector by use of a LigATor kit (R&D Systems Europe Ltd). The IL-2 fragment was then transferred to the KpnI and HindIII sites of pGL2-Basic (Promega Corp.) containing the firefly luciferase gene. The following reporter plasmids were used: NFAT-luc, containing the luciferase gene driven by three copies of the NFAT-responsive element (positions −286 to −257 of the human IL-2 gene) linked to the human IL-2 promoter (−72 to +47) (61); IL-4-luc, containing nucleotides −741 to +60 of the murine IL-4 enhancer-promoter cloned upstream of the luciferase gene in pBS-LUC (77); IL-10-luc, containing nucleotides −890 to +120 of the human IL-10 gene cloned into the basic luciferase reporter plasmid pGL3B (49); TNF-α-luc, containing a 1,311-nucleotide intervening sequence between the human TNF-β and TNF-α genes cloned into the luciferase plasmid pXPT2 (64); IFN-γ-lacZ, containing nucleotides −538 to +64 of the human IFN-γ gene cloned upstream of the lacZ gene in pEQ3 (62); and RE/AP-luc, containing four CD28 response element/AP1 sites (positions −168 to −143 of the human IL-2 gene) driving luciferase expression (70). The plasmid p-v-Jun carries the entire human v-jun gene driven by the cytomegalovirus immediate early promoter (3).
Analysis of cytokine gene expression.
Jurkat cells and derivatives thereof were transfected by the DEAE-dextran procedure as previously described (5). The cells were divided into aliquots at 40 h posttransfection and then stimulated with 50 ng of TPA/ml and 1 μM ionomycin or a 1:5,000 dilution of the 9.3 MAb recognizing CD28. Seven hours later, the cells were washed once in phosphate-buffered saline (PBS) and lysed in a buffer containing 25 mM Tris-phosphate (pH 7.8), 2 mM 1,4-dithiothreitol (DTT), 10% glycerol, and 1% Triton X-100. Luciferase and β-galactosidase activities were measured with a Sirius luminometer from Berthold Detection Systems (Pforzheim, Germany) by the use of beetle luciferin from Promega and a Galacto-Star kit from Tropix (Bedford, Mass.), respectively. Statistical significances were determined by Student's two-tailed t test.
Protein expression, degradation, and phosphorylation.
For protein degradation and phosphorylation assays, cell cultures were treated for 20 min at 37°C with TPA (50 ng/ml) and ionomycin (1 μM) or mouse ascites of 9.3 (diluted 1:2,500). Cellular extracts were prepared by solubilization in sodium dodecyl sulfate (SDS) sample buffer (65 mM Tris [pH 6.8], 2% SDS, 10% glycerol, 5% mercaptoethanol, and 1% bromphenol blue). Proteins were resolved by electrophoresis in an SDS-12% polyacrylamide gel and were then transferred to a nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany). HCV C was recognized by MAb C7-50 (mouse ascites diluted 1:5,000) (56). The degradation of IκB-α was determined by quantitation of the remaining IκB-α by the use of specific antibodies in an immunoblot assay. The phosphorylation of ERK1/2, p38, SEK1, and JNK1/2 was analyzed by immunoblotting with phosphospecific antibodies to the respective antigens. Bound secondary Ab was detected by enhanced chemiluminescence (Pierce, Rockford, Ill.).
Flow cytometry.
The expression of CD3 and CD28 was investigated by flow cytometry with a phycoerythrin-conjugated anti-CD3 Ab (BD Biosciences, San Jose, Calif.) and a 1:300 dilution of 9.3 mouse ascites, respectively. A fluorescein isothiocyanate-conjugated anti-mouse Ab was used as a secondary Ab for CD28 staining. As a negative control, an equal mix of Simultest control γ1/γ2a and Simultest control γ2a/γ1 was used. For flow cytometry analyses of IL-2 expression, 0.5 × 106 cells were stimulated with either TPA (10 ng/ml) and ionomycin (1 μM) or monoclonal antibodies to the TCR and CD28 (HIT3a [0.8 μg/ml] and mouse ascites of 9.3 [diluted 1:5,000], respectively) for 6 h in the presence of 2 μM monensin. Washed cells were fixed in the presence of 4% paraformaldehyde and permeabilized in PBS containing 1% fetal calf serum, 0.1% NaN3, and 0.1% saponin. The cells were then stained in the same buffer with 3 μg of R-phycoerythrin-conjugated rat anti-human IL-2 MAb MQ1-17H12/ml. Stained cells were analyzed on a FACScan flow cytometer (BD Biosciences), and data analysis was performed with CellQuest software.
Electrophoretic mobility shift assay.
Cell cultures were stimulated with TPA (50 ng/ml) and ionomycin (1 μM). After 4 h, nuclei were isolated by disruption of the cells with a low-salt buffer followed by centrifugation. The cells were washed in PBS and lysed in a buffer containing 10 mM HEPES (pH 7.4), 10 mM KCl, 1.5 mM MgCl2, 0.5 mM DTT, 0.2 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin/ml, and 0.025% NP-40. The nuclei were pelleted, and nuclear proteins were extracted with a buffer containing 20 mM HEPES (pH 7.4), 420 mM NaCl, 1.5 mM MgCl2, 0.5 mM DTT, 0.2 mM EDTA, 0.2 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin/ml, and 5% glycerol. Nuclear extracts equivalent to 5 μg of bovine serum albumin were incubated together with 0.2 ng of the 32P-labeled, double-stranded monomer of the murine IL-2 distal NFAT binding site (5′-GATCGCCCAAAGAGGAAAATTTGTTTCATACAG-3′) (38) in a buffer containing 10 mM Tris-Cl (pH 7.5), 50 mM NaCl, 1 mM DTT, 0.5 mM EDTA, 5% glycerol, and 1 μg of poly(dI-dC)/ml. After 30 min at 25°C, the reaction mixture was resolved in a 5% nondenaturing polyacrylamide gel, and the protein-DNA complexes were visualized by autoradiography.
ELISA.
Cells were stimulated for 24 h with either TPA (50 ng/ml) and ionomycin (1 μM) or monoclonal antibodies to the TCR and CD28 (HIT3a [100 ng/ml] and mouse ascites of 9.3 [diluted 1:5,000], respectively). The IL-2 concentrations in supernatants from approximately 200,000 cells were determined by use of a human IL-2 Opt EIA ELISA kit (Pharmingen) according to the manufacturer's instructions.
RESULTS
Altered cytokine gene expression and protein production in HCV C-expressing cells.
We have previously shown that the expression of HCV C induces Ca2+ oscillations in Jurkat cells and favors the selective activation of NFAT (5, 6). To investigate this phenomenon further, we investigated cytokine promoter activity and protein production in parental Jurkat cells and in three stable HCV C-expressing sublines, namely, JHC.d, JHC.g, and JHC.h (Fig. 1A). Physiologic induction was mimicked by ligation of the TCR complex and CD28 with specific Abs. The concentration of secreted IL-2 in tissue culture supernatants was determined after 24 h by ELISA. Supernatants from control Jurkat cells contained approximately 1.3 ng of IL-2/ml, while significantly less IL-2 (0.3 to 0.5 ng/ml) was detected in supernatants from HCV C-expressing cell lines (Fig. 1B). This decreased inducibility was not a consequence of a reduced expression of critical surface receptors, since HCV C-expressing cell lines analyzed by flow cytometry were indistinguishable from parental cells with respect to the expression of CD3 and CD28 (data not shown).
FIG. 1.
Decreased IL-2 production in HCV C-expressing cells. (A) The expression of HCV C was investigated in parental Jurkat cells (Jk) and C-transfected sublines (JHC.d, JHC.g, and JHC.h) by Western blotting. The position of the molecular size marker (in kilodaltons) is indicated. (B) IL-2 synthesis was induced by triggering of surface receptors with specific antibodies (anti-CD3 and anti-CD28, left panel) or by treatment with TPA and ionomycin (right panel). The amounts of IL-2 in cell culture supernatants were determined by an ELISA at 24 h postinduction. (C) Numbers of IL-2-secreting cells were determined by flow cytometry. Cell lines were stimulated with TPA and ionomycin in the presence of monensin. After 6 h, the cells were fixed, permeabilized, and stained with a phycoerythrin-labeled Ab recognizing human IL-2. (D) Transcriptional activation of IL-2 gene in cells expressing HCV C. Control and C-expressing cell lines were transfected with a luciferase reporter plasmid driven by the IL-2 promoter. At 40 h posttransfection, the cells were stimulated with TPA and ionomycin for 6 h, and the luciferase activity, presented in relative light units (RLU), was determined. The data presented are representative of three independent experiments.
The triggering of CD3 and CD28 initiates a complex signaling cascade including protein tyrosine phosphorylation and inositol phospholipid turnover that can be mimicked by stimulation with TPA and ionomycin, which act as a PKC activator and a Ca2+ ionophore, respectively. Although pharmacological triggering was a more potent inducer of IL-2 in Jurkat cells (30 ng/ml) than the triggering of surface receptors, C-expressing cells were also less (<3 ng/ml) responsive to this treatment (Fig. 1B). To investigate whether this deficiency was due to an impaired secretion of IL-2, we detected intracellular IL-2 in permeabilized cells by flow cytometry. Whereas approximately one-fourth of the parental Jurkat cells or mock-transfected clonal cells that did not contain the C-encoding plasmid expressed IL-2, only a minor fraction (1 to 4%) of the cells expressing HCV C were positive for IL-2 (Fig. 1C).
The synthesis of IL-2 is regulated by both transcriptional and posttranscriptional mechanisms. To investigate whether the hyporesponsiveness of C-expressing cells correlated with a decreased activity of the IL-2 promoter, we transfected an IL-2 promoter-driven luciferase reporter plasmid into C-expressing and control cells. Strong reporter gene activity was observed when control cells were stimulated with TPA and ionomycin, as expected, whereas 5 to 20% of this activity was observed with cell lines expressing HCV C (Fig. 1D).
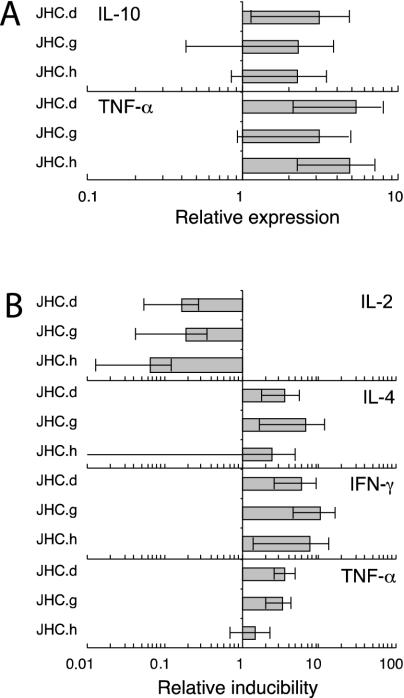
To further characterize the cytokine profile of C-expressing cells, we compared the induction of other cytokine genes associated with either the Th1 (IFN-γ and TNF-α) or Th2 (IL-4 and IL-10) phenotype with that of IL-2 in reporter assays. Whereas the activity of the IL-2, IL-4, and IFN-γ promoters was barely detectable in unstimulated C-expressing cells (data not shown), increased basal activities were observed for the IL-10 (two- to threefold) (P < 0.05; n = 16) and TNF-α (three- to fivefold) (P < 0.05; n = 22) promoters (Fig. 2A). Treatment with TPA and ionomycin induced all cytokine promoters (except IL-10 [data not shown]) in Jurkat and C-expressing cell lines (Fig. 2B). However, whereas the IL-2 gene was less inducible in C-expressing cells, increased expression was observed for IL-4 (2- to 7-fold) (P < 0.05; n = 19), IFN-γ (6- to 11-fold) (P < 0.01; n = 16), and TNF-α (2- to 4-fold) (P < 0.05; n = 22).
FIG. 2.
Altered transcriptional activation of various cytokine promoters in HCV C-expressing cell lines. Cells were transiently transfected with proximal cytokine promoters coupled to either a luciferase (IL-2, IL-4, IL-10, or TNF-α) or a β-galactosidase (IFN-γ) reporter gene. At 40 h posttransfection, the cells were stimulated with TPA and ionomycin for 6 h, and the reporter activities were determined. (A) Relative cytokine induction without stimulation. (B) Relative cytokine induction after stimulation with TPA and ionomycin. For each reporter gene, all activities were normalized to those of control cells. Values are means ± standard errors of the means (SEM) of four to six independent experiments.
Decreased inducibility of the CD28 response element via the costimulatory pathway in HCV C-expressing cell lines.
The induction of IL-2 synthesis is dependent on the triggering of multiple signaling cascades and the activation of transcription factors that bind to specific elements in the IL-2 promoter. Whereas TCR triggering is essentially mediated via the distal, composite NFAT/AP-1 element, the costimulatory activation of IL-2 induction is mediated via the composite CD28 response element (CD28RE), which contains two nonconsensus binding sites, one for NF-κB and one for AP-1 (10, 40, 53, 70). To analyze whether the expression of HCV C affects the integrity of the TCR and costimulatory signaling pathways, we transfected the cell lines with reporter plasmids controlled by either the distal, composite NFAT/AP-1 element or the composite CD28RE. In Jurkat cells, induction by TPA and ionomycin resulted in a potent activation of both promoters, as expected (Fig. 3). In C-expressing cell lines, no significant difference in NFAT activation was observed compared to the parental cell line. In contrast, significantly less (2 to 30%) (P < 0.001; n = 14) activity was observed with the CD28RE, indicating impaired inducibility via the costimulatory pathway.
FIG. 3.
Impaired inducibility of composite CD28 responsive element in cells expressing HCV C. Jurkat (Jk) and C-expressing cell lines (JHC.d, JHC.g, and JHC.h) were transfected with reporter plasmids containing either three distal composite NFAT elements or four composite CD28 responsive elements linked to the luciferase gene. At 40 h posttransfection, the cells were stimulated with TPA and ionomycin for 6 h, and the relative luciferase activities were determined. For each reporter gene, all activities were normalized to those of stimulated control cells. Values are means ± SEM of three independent experiments.
Increased DNA binding of NFAT in HCV C-expressing cells.
Since the NFAT-luc reporter contains a trimer of one of the composite NFAT/AP-1 sites, the difference in inducibility between the entire IL-2 promoter and the NFAT reporter might be a consequence of the number of NFAT elements. To exclude the possibility that a potentially weaker activation of NFAT remained undetected by the more sensitive NFAT-luc reporter, we analyzed the specific DNA binding of NFAT in an electrophoretic mobility shift assay by using a probe corresponding to a monomer of the distal NFAT site. In both cell lines, a mobility shift sensitive to a cold competitor was induced, indicating specific DNA binding (Fig. 4). JHC.d cells displayed increased DNA binding of NFAT compared with parental Jurkat cells.
FIG. 4.
Specific DNA binding of activated NFAT in JHC.d cells. Nuclear extracts were prepared from stimulated Jurkat and JHC.d cells and were incubated with a labeled oligonucleotide corresponding to the IL-2 distal NFAT site in the absence or presence of a 50-fold excess of cold oligonucleotide. The bound complexes were resolved in a 5% native polyacrylamide gel. The positions of DNA complexes containing NFAT/AP1 (upper band) or NFAT (lower band) are indicated with arrows. Results that are representative of three experiments are shown.
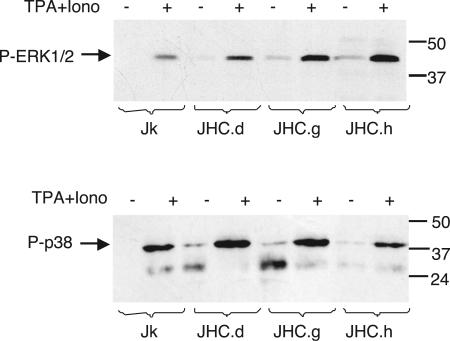
Constitutive phosphorylation of the MAP kinases ERK1/2 and p38 in HCV C-expressing cells.
Besides the requirement of NFAT, the induction of IL-2 requires the activation of different MAP kinase signaling pathways. Two of these, the ERK and p38 MAP kinase pathways, are activated by triggering of the TCR without concomitant costimulation (18). To elucidate whether the expression of C would compromise the activation of the TCR-triggered MAP kinase pathways, we analyzed the phosphorylation status of ERK1/2 and p38 in unstimulated and stimulated cells. Whereas no basal phosphorylation of ERK1/2 and p38 was detected in Jurkat cells, the addition of TPA and ionomycin resulted in phosphorylation, as expected (Fig. 5). In C-expressing cells, ERK1/2 and p38 were constitutively phosphorylated under basal conditions and could be further phosphorylated by stimulation.
FIG. 5.
Activation of MAP kinases ERK1/2 and p38 in HCV C-expressing cells. Phosphorylated ERK1/2 and p38 in control cells (Jk) and HCV C-expressing cells (JHC.d, JHC.g, and JHC.h) were detected in Western blots by the use of phosphospecific antibodies to ERK1/2 and p38. Where indicated, the cells were stimulated with TPA and ionomycin (Iono) for 20 min. The expected positions of the two isoforms of ERK1/2 (42 and 44 kDa) and p38 are indicated to the left. The positions of molecular size markers (in kilodaltons) run in parallel are indicated to the right. The data presented are representative of three independent experiments.
No effect of HCV C on inducible degradation of IκB-α.
Although TCR triggering is sufficient to activate NFAT and ERK, a simultaneous costimulation of CD28 is required for the activation of NF-κB and JNK (65, 75). The activation of NF-κB requires phosphorylation-dependent degradation of the NF-κB-associated inhibitor IκB-α, which acts by sequestering NF-κB in the cytosol. To examine whether HCV C affected IκB-α degradation, we stimulated the cell lines with TPA and ionomycin and analyzed IκB-α expression by Western blotting. A time course analysis revealed that IκB-α levels reached a minimum at 20 min postinduction in Jurkat cells (data not shown). The degradation of IκB-α in C-expressing clones was not significantly different from that in parental cells (Fig. 6).
FIG. 6.
Degradation of IκB-α in cells expressing the C protein. The degradation of IκB-α in control cells (Jk) and cells expressing HCV C (JHC.d, JHC.g, and JHC.h) was detected by immunoblotting. Where indicated, the cells were stimulated with TPA and ionomycin (Iono) for 20 min. The expected position of IκB-α (37 kDa) is indicated to the left, and the positions of molecular size markers (in kilodaltons) are indicated to the right. The data presented are representative of three independent experiments.
HCV C confers hyporesponsiveness to costimulation via the JNK signaling pathway.
Besides NK-κB activation, costimulatory signaling triggers the JNK pathway, which ultimately causes the phosphorylation-dependent activation of c-Jun. To investigate whether the expression of HCV affected JNK-mediated signaling, we analyzed the phosphorylation status of c-Jun and the upstream kinases SEK1 and JNK. Treatment with TPA and ionomycin resulted in the phosphorylation of SEK1, JNK, and c-Jun in Jurkat cells, as expected (Fig. 7). In contrast, no or only minimal phosphorylation of SEK1, JNK, and c-Jun was detected in C-expressing clones, indicating that these cells were relatively insensitive to costimulation via the JNK signaling pathway.
FIG. 7.
Decreased inducibility of JNK signaling pathway in cells expressing HCV C. The activation of the JNK signaling pathway in control cells (Jk) and HCV C-expressing cells (JHC.d, JHC.g, and JHC.h) was analyzed in immunoblots by the use of phosphospecific antibodies to SEK1, JNK1/2, and c-Jun. Where indicated, the cells were stimulated with TPA and ionomycin (Iono) for 20 min. The expected positions of SEK1 (46 kDa), the two isoforms of JNK1/2 (46 and 54 kDa), and c-Jun (48 kDa) are indicated to the left. The positions of molecular size markers (in kilodaltons) are indicated to the right. The data presented are representative of four independent experiments.
Altered MAP kinase activation can be restored by calcium depletion or by cyclosporine A treatment.
Since NFAT is activated by the Ca2+-dependent phosphatase calcineurin (26), we hypothesized that hyporeponsiveness in C-expressing cells might be a downstream consequence of HCV C-induced Ca2+ oscillations. To address this question, we analyzed whether constitutive ERK1/2 and p38 phosphorylation and the defect in JNK signaling could be abolished by Ca2+ depletion or by a treatment with cyclosporine A, an inhibitor of calcineurin. After C-expressing cells were cultured for 24 h in the presence of cyclosporine A, the phosphorylation of ERK1/2 or p38 was no longer observed (Fig. 8A and B).
FIG. 8.
Altered MAP kinase activation is restored by treatment with cyclosporine A. Control cells (Jk) and cells expressing HCV C (JHC.d, JHC.g, and JHC.h) were stimulated with TPA and an anti-CD28 antibody (α-CD28) for 20 min. Where indicated, the cells had been pretreated with cyclosporine A (CsA; 50 ng/ml) or cultured in calcium-free medium (−Ca2+) for 24 h. Phosphorylated forms of ERK1/2 (A), p38 (B), and JNK1/2 (C) in cellular extracts were detected by Western blotting as described in the legend to Fig. 6. The positions of molecular size markers (in kilodaltons) are shown to the right. The data presented are representative of three independent experiments.
Besides its role in the activation of NFAT, calcineurin also cooperates with PKC-θ in the activation of JNK (85). Since the inhibition of calcineurin would interfere with the ionomycin-induced activation of JNK, a combination of TPA and a MAb recognizing CD28 was used for the induction of JNK phosphorylation in this experiment. As with TPA and ionomycin, the phosphorylation of JNK was induced in Jurkat cells by this treatment but was not induced in HCV C-expressing clones (Fig. 8C). However, after overnight culturing in the presence of cyclosporine A, the phosphorylation of JNK could be induced in two (JHC.d and JHC.g) of the three C-expressing clones, indicating that defective JNK activation can be abolished by an inhibition of the Ca2+-calcineurin pathway.
Restored inducibility of the CD28 responsive element by ectopic expression of v-Jun.
To investigate whether the impaired activation of c-Jun in C-expressing cells was a direct cause of the decreased inducibility of the CD28RE, we bypassed the JNK signaling cascade by cotransfection of a plasmid expressing v-Jun, which is active independent of JNK. The expression of v-Jun caused increased reporter activity in both Jurkat and JHC.d cells (Fig. 9). However, the effect of v-Jun was much more potent in JHC.d cells than in Jurkat cells, and the significantly decreased inducibility of the CD28RE observed in C-expressing cells was eliminated.
FIG. 9.
Restored inducibility of CD28 responsive element by ectopic expression of v-Jun. Jurkat (Jk) and C-expressing (JHC.d) cells were cotransfected with a luciferase reporter plasmid driven by four composite CD28REs and either an empty vector control (−) or a plasmid encoding v-Jun. At 40 h posttransfection, the cells were stimulated with TPA and an Ab recognizing CD28 (α-CD28) for 6 h, and luciferase activities was determined. The data presented are means ± SEM of four independent experiments, which were normalized to stimulated Jurkat cells.
DISCUSSION
The mechanisms by which HCV evades immune responses to establish and maintain persistent infections remain poorly understood. Whereas a cell-mediated immune response may result in the resolution of an acute HCV infection, it is most often inadequate and may display several abnormalities. The induction of adaptive immunity to HCV is remarkably slow and weak, as it often takes between 4 and 8 weeks before HCV-specific CD8+ T cells can be detected (79). Furthermore, in patients who develop chronic infections, the frequencies of virus-specific CTL precursors are much lower than those typically observed for other viral infections (84). The effector T cells that recognize HCV peptides appear to be incompletely differentiated and functionally impaired (2, 33, 84), and immature CTLs directed to unrelated viral peptides have recently been discovered in HCV-infected individuals (48). Functionally impaired T cells are found in other persistent viral infections (2, 69, 87), and it has been suggested that these cells are arrested due to a lack of CD4+-T-cell help (39). During chronic HCV infections, CD4+ T cells are poorly activated (11, 54, 83), and since up to 40% of intrahepatic CD4+ T cells have been shown to express viral antigens (8), we reasoned that the altered T-cell responsiveness may be due to the activity of HCV gene products. In line with such a scenario, we have previously shown that the expression of HCV C in the CD4+ Jurkat T-cell line induces Ca2+ oscillations that favor the selective activation of NFAT during transient expression (5, 6).
In the present study, we found that the stable expression of HCV C in transfected Jurkat cells altered the basal cytokine profile and induced a hyporesponsive state characterized by decreased IL-2 inducibility. The overall alterations in the cytokine profile suggest that C expression drives Jurkat cells to a Th2-like phenotype, which is in line with the observation that chronic HCV infections are associated with a skewed cytokine profile, including low IL-2 and high IL-10 production (23, 81, 83, 86). Whereas IL-2 promotes Th1 cell proliferation and is required for sustained CD8+-T-cell responses (22), IL-10 acts as a general suppressor of inflammatory responses and induces an anergic state in Th1 and Th2 cells (32). The hyporesponsiveness of the C-expressing Jurkat cells shared many features with clonal anergy, which is induced by the triggering of Ca2+ signaling without concomitant costimulation (reviewed in reference 67). Besides compromising IL-2 induction, the anergization of primary T cells is also accompanied by defective proliferation, and although this effect cannot be addressed in the IL-2-independent Jurkat lines, it is noteworthy that a decreased proliferative capacity of CD4+ T cells has been observed during chronic HCV infections (11, 54, 83). Notably, our C-expressing Jurkat clones were hyporesponsive not only to triggering of the TCR but also to treatment with TPA and ionomycin. Whereas potent pharmacological agents often overcome T-cell anergy (44), exceptions have been reported when short incubation times between anergy induction and restimulation are used (18). The fact that this did not occur in C-expressing cells supports the significance of our study.
Luciferase reporter assays indicated a decreased inducibility of the IL-2 promoter in C-expressing cells. The IL-2 promoter contains binding sites for a variety of transcription factors, including NFAT, AP-1, NF-κB, and Oct (reviewed in reference 68) (Fig. 10). The binding and transcriptional activation of these factors are regulated by a complex array of signals delivered by the TCR and CD28, including protein tyrosine kinases, inositol phospholipid turnover, intracellular Ca2+ flux, calcineurin, protein kinase C, and MAP kinases. Whereas anergic T cells induced by TCR triggering display normal Ca2+/NFAT signaling, defects have been described in signaling that is essential for the activation of AP-1, e.g., p21ras and the MAP kinases ERK, p38, and JNK (16, 25, 44). It is not clear whether an ERK deficiency contributes to anergization or is a consequence of anergy, since the inhibition of ERK1/2 activity by the pharmacological agent PD90859 has no effect on the induction of anergy (17). In C-expressing cells, the TCR-dependent signaling pathways remained inducible. As reported previously, triggering of the TCR evokes a sustained Ca2+ flux in cells constitutively expressing HCV C (6). We have now demonstrated that Ca2+- and calcineurin-dependent DNA binding and activation of NFAT can be induced in C-expressing cells. NFAT binds in cooperation with AP-1, and the activation of the composite distal NFAT element is thus dependent on intact ERK signaling, since ERK-dependent phosphorylation of Elk is required for synthesis of the AP-1 component c-Fos. The phosphorylation of ERK1/2 and p38 was consistently induced by TPA and ionomycin. In addition, ERK1/2 and p38 were slightly phosphorylated even in the absence of stimulation.
FIG. 10.
Simplified scheme of IL-2 promoter and the signaling pathways involved in its regulation. The targets for artificial inducers and inhibitors used in this study are indicated along with the targets affected by HCV C (arrows pointing up- or downwards). The composite distal NFAT/AP-1 and CD28RE/AP-1 elements are indicated by gray boxes. Notably, the effects on MAP kinase phosphorylation were likely downstream consequences of C-induced Ca2+ flux, since these perturbations could be eliminated by a cyclosporine A treatment. For further details, see the text. DAG, diacylglycerol; PLC-γ, phospholipase C-γ1; PTKs, protein tyrosine kinases.
The costimulatory pathway integrates signals from the TCR and CD28, resulting in the activation of NF-κB and the JNK signaling cascade. Together with c-Rel, phosphorylated c-Jun mediates the costimulatory signal to the composite CD28RE in the IL-2 promoter. We did not observe significant differences in the degradation of the NF-κB inhibitor IκB-α in control and HCV-expressing cells, suggesting that this signaling pathway remained intact. In contrast, the induction of the costimulatory MAP kinase cascade involving SEK1, JNK1/2, and c-Jun was blocked and the inducibility of the composite CD28RE was decreased, suggesting that the hyporesponsiveness in C-expressing cells was a consequence of selective inactivation of the JNK signaling cascade. Consistently, the inducibility of the CD28RE could be restored by the ectopic expression of v-Jun, which is active independent of JNK.
The effects of HCV C on signaling via the MAP kinases ERK, p38, and JNK were observed earlier in cells of nonhematopoietic origin, e.g., HepG2, BALB/3T3, and HEK293 cells (24, 30, 34, 73, 82). The MAP kinases are activated indirectly in a cell-dependent manner by members of the conventional (α, β1, β2, and γ) and novel (∂, ɛ, θ, and η) PKCs, with the latter being insensitive to Ca2+ (reviewed in reference 37). Whereas in most cells both ERK and JNK kinases can be activated by PKC-α, only ERK is activated by conventional PKCs in T cells. Since conventional PKCs are activated by Ca2+, a conceivable explanation is thus that the stimulatory effects that we and others have observed for HCV C on ERK are a consequence of the Ca2+-dependent activation of conventional PKCs. Furthermore, the different effects of HCV C on JNK phosphorylation, by which JNK is activated in nonhematopoietic cells but not in T cells, may reflect the T-cell-specific inability of conventional PKCs to activate JNK.
The different effects of HCV C on cytokine expression likely reflect structural differences in respective promoter regions and specific requirements for induction. Except for IL-10, all analyzed cytokine promoters contain binding sites for NFAT (52) and were hence responsive to TPA and ionomycin. However, whereas most NFAT elements in the IL-2, IL-4, and IFN-γ genes in fact are composite sites that require the cooperation of NFAT with AP-1, the predominant NFAT site in the TNF-α promoter is a quasi-palindromic κ3 site that recruits NFAT dimers and is hence inducible by triggering of the Ca2+/NFAT pathway alone (51). Consistent with increased Ca2+ signaling, the basal expression of TNF-α was indeed increased in C-expressing cells. Finally, the IL-10 promoter is mainly regulated via the p38 pathway (49), and the increase in constitutive IL-10 production observed in C-expressing cells was conceivably a consequence of increased basal p38 activity.
The facts that TCR-triggered anergy induction can be prevented by cyclosporine A (66) and that it is associated with the expression of a specific set of NFAT-regulated genes (50) underscore the critical involvement of the Ca2+/calcineurin pathway for the induction of clonal anergy. In C-expressing cells, inactivation of the Ca2+/calcineurin pathway by calcium deprivation or cyclosporine A treatment eliminated the constitutive phosphorylation of ERK1/2 and p38 and restored the inducibility of JNK1/2 activation, indicating that the altered signaling of these pathways was a downstream consequence of the increased Ca2+ flux. Conceivable targets of the Ca2+/calcineurin pathway include the NFAT-driven, anergy-associated genes (50) and the MAP kinase phosphatases, which inhibit JNK activity by dephosphorylation (31, 46). Notably, treatment with cyclosporine A has been demonstrated to reduce the viral load during anti-HCV therapy (1, 36). Whereas cyclosporine A is normally used for the suppression of allograft reactions, its inhibitory effects on NFAT can also prevent the acquisition of an anergic phenotype. Although direct antiviral effects cannot be excluded, it is therefore conceivable that cyclosporine A treatment facilitates the reversal of an anergic state, which would then allow a restart of the immune response. Furthermore, it should be stressed that the effects of C expression appear to be different in transient transfections than in stable cell lines, since the transient expression of C is not associated with an anergic phenotype in spite of its potent activation of NFAT (5). These contradictory results may reflect different exposure times to C in respective settings, since the acquisition of the anergic phenotype requires sufficient time to allow the expression of NFAT-regulated genes.
The ability of the C protein to activate NFAT can be abolished by the deletion of its carboxy-terminal domain, which targets C to the endoplasmic reticulum (ER) (5). Infection by viruses that assemble on the ER membrane often results in the overexpression and accumulation of structural proteins, which may cause nonspecific perturbations of the ER membrane that lead to activation of the Ca2+/NFAT pathway and subsequent conversion to an anergic state. The overexpression of transfected C may have a similar nonspecific effect. However, it seems unlikely that the ectopic overexpression of C is the sole cause of the phenotype observed in our experiments since we were able to isolate a C deletion mutant that failed to activate NFAT despite high-level accumulation in the ER (A. Bergqvist, unpublished observation). It should be stressed that although the high replication rate (up to 1012 infectious particles per day [60]) suggests that a substantial amount of the main virion component C is produced during a natural HCV infection, the levels of C in infected lymphocytes have not been determined and further studies are warranted to determine whether the expression levels in the Jurkat lines reflect what occurs in vivo.
Our findings are in line with studies of the in vivo effects of HCV C expression and JNK deficiency on T-cell responses. In heterologous expression systems based on either infection with recombinant vaccinia virus or T-cell-specific transgenic expression, HCV C was demonstrated to be associated with immune suppression characterized by a decreased activation of CTLs and a decreased expression of IL-2 and IFN-γ (41, 74). Experiments with knockout mice suggest that JNKs are critical for Th1 differentiation, CTL function, and the resolution of infections with intracellular parasites (13, 20, 21). In summary, our results indicate that HCV C expression in vitro leads to an inactivation of JNK signaling and hyporesponsiveness to IL-2 induction as downstream consequences of the C-induced Ca2+ flux in a manner that mimics the induction of clonal anergy. Given the requirement for IL-2 for sustained CD8+-T-cell proliferation in nonlymphoid tissues, an anergic state would reduce the prospects for a strong intrahepatic T-cell response, which is necessary to control the infection. Although the relevance of our findings in the context of HCV infection remains to be established and although other factors conceivably contribute to chronicity, our data provide a plausible model for the insufficient CTL response that is typically associated with HCV persistence.
Acknowledgments
We thank J. Economou, A. Kumar, K. M. Murphy, J. R. Wands, A. Weiss, and C. B. Wilson for providing antibodies and plasmids. We are also grateful to Victor Levitsky and Göran Magnusson for critically reading the manuscript.
This work was supported by grants from the Swedish Cancer Society and the Swedish Foundation for Strategic Research (to A.B. and M.G.M.) and by the Swedish Research Council, the Swedish Society for Medical Research, Åke Wibergs Stiftelse, Claes Groschinskys Stiftelse, and Magnus Bergvalls Stiftelse (to A.B.).
REFERENCES
- 1.Akiyama, H., H. Yoshinaga, T. Tanaka, K. Hiruma, S. Tanikawa, H. Sakamaki, Y. Onozawa, T. Wakita, and M. Kohara. 1997. Effects of cyclosporin A on hepatitis C virus infection in bone marrow transplant patients. Bone Marrow Transplantation Team. Bone Marrow Transplant. 20:993-995. [DOI] [PubMed] [Google Scholar]
- 2.Appay, V., P. R. Dunbar, M. Callan, P. Klenerman, G. M. Gillespie, L. Papagno, G. S. Ogg, A. King, F. Lechner, C. A. Spina, S. Little, D. V. Havlir, D. D. Richman, N. Gruener, G. Pape, A. Waters, P. Easterbrook, M. Salio, V. Cerundolo, A. J. McMichael, and S. L. Rowland-Jones. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8:379-385. [DOI] [PubMed] [Google Scholar]
- 3.Baichwal, V. R., and R. Tjian. 1990. Control of c-Jun activity by interaction of a cell-specific inhibitor with regulatory domain delta: differences between v- and c-Jun. Cell 63:815-825. [DOI] [PubMed] [Google Scholar]
- 4.Bassett, S. E., K. M. Brasky, and R. E. Lanford. 1998. Analysis of hepatitis C virus-inoculated chimpanzees reveals unexpected clinical profiles. J. Virol. 72:2589-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergqvist, A., and C. M. Rice. 2001. Transcriptional activation of the interleukin-2 promoter by hepatitis C virus core protein. J. Virol. 75:772-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergqvist, A., S. Sundström, L. Dimberg, E. Gylfe, and M. G. Masucci. 2003. The hepatitis C virus core protein modulates T cell responses by inducing spontaneous and altering TCR triggered Ca2+ oscillations. J. Biol. Chem. 278:18877-18883. [DOI] [PubMed] [Google Scholar]
- 7.Bjoro, K., S. S. Froland, Z. Yun, H. H. Samdal, and T. Haaland. 1994. Hepatitis C infection in patients with primary hypogammaglobulinemia after treatment with contaminated immune globulin. N. Engl. J. Med. 331:1607-1611. [DOI] [PubMed] [Google Scholar]
- 8.Blight, K. J., R. R. Lesniewski, J. T. LaBrooy, and E. J. Gowans. 1994. Detection and distribution of hepatitis C-specific antigens in naturally infected liver. Hepatology 20:553-557. [PubMed] [Google Scholar]
- 9.Bronowicki, J. P., M. A. Loriot, V. Thiers, Y. Grignon, A. L. Zignego, and C. Brechot. 1998. Hepatitis C virus persistence in human hematopoietic cells injected into SCID mice. Hepatology 28:211-218. [DOI] [PubMed] [Google Scholar]
- 10.Butscher, W. G., C. Powers, M. Olive, C. Vinson, and K. Gardner. 1998. Coordinate transactivation of the interleukin-2 CD28 response element by c-Rel and ATF-1/CREB2. J. Biol. Chem. 273:552-560. [DOI] [PubMed] [Google Scholar]
- 11.Chang, K. M., R. Thimme, J. J. Melpolder, D. Oldach, J. Pemberton, J. Moorhead-Loudis, J. G. McHutchison, H. J. Alter, and F. V. Chisari. 2001. Differential CD4(+) and CD8(+) T-cell responsiveness in hepatitis C virus infection. Hepatology 33:267-276. [DOI] [PubMed] [Google Scholar]
- 12.Christie, J. M., C. J. Healey, J. Watson, V. S. Wong, M. Duddridge, N. Snowden, W. M. Rosenberg, K. A. Fleming, H. Chapel, and R. W. Chapman. 1997. Clinical outcome of hypogammaglobulinaemic patients following outbreak of acute hepatitis C: 2 year follow up. Clin. Exp. Immunol. 110:4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Constant, S. L., C. Dong, D. D. Yang, M. Wysk, R. J. Davis, and R. A. Flavell. 2000. JNK1 is required for T cell-mediated immunity against Leishmania major infection. J. Immunol. 165:2671-2676. [DOI] [PubMed] [Google Scholar]
- 14.Cooper, S., A. L. Erickson, E. J. Adams, J. Kansopon, A. J. Weiner, D. Y. Chien, M. Houghton, P. Parham, and C. M. Walker. 1999. Analysis of a successful immune response against hepatitis C virus. Immunity 10:439-449. [DOI] [PubMed] [Google Scholar]
- 15.Cribier, B., C. Schmitt, A. Bingen, A. Kirn, and F. Keller. 1995. In vitro infection of peripheral blood mononuclear cells by hepatitis C virus. J. Gen. Virol. 76:2485-2491. [DOI] [PubMed] [Google Scholar]
- 16.DeSilva, D. R., W. S. Feeser, E. J. Tancula, and P. A. Scherle. 1996. Anergic T cells are defective in both Jun NH2-terminal kinase and mitogen-activated protein kinase signaling pathways. J. Exp. Med. 183:2017-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeSilva, D. R., E. A. Jones, M. F. Favata, B. D. Jaffee, R. L. Magolda, J. M. Trzaskos, and P. A. Scherle. 1998. Inhibition of mitogen-activated protein kinase kinase blocks T cell proliferation but does not induce or prevent anergy. J. Immunol. 160:4175-4181. [PubMed] [Google Scholar]
- 18.DeSilva, D. R., E. A. Jones, W. S. Feeser, E. J. Manos, and P. A. Scherle. 1997. The p38 mitogen-activated protein kinase pathway in activated and anergic Th1 cells. Cell. Immunol. 180:116-123. [DOI] [PubMed] [Google Scholar]
- 19.Diepolder, H. M., R. Zachoval, R. M. Hoffmann, E. A. Wierenga, T. Santantonio, M. C. Jung, D. Eichenlaub, and G. R. Pape. 1995. Possible mechanism involving T-lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet 346:1006-1007. [DOI] [PubMed] [Google Scholar]
- 20.Dong, C., D. D. Yang, C. Tournier, A. J. Whitmarsh, J. Xu, R. J. Davis, and R. A. Flavell. 2000. JNK is required for effector T-cell function but not for T-cell activation. Nature 405:91-94. [DOI] [PubMed] [Google Scholar]
- 21.Dong, C., D. D. Yang, M. Wysk, A. J. Whitmarsh, R. J. Davis, and R. A. Flavell. 1998. Defective T cell differentiation in the absence of Jnk1. Science 282:2092-2095. [DOI] [PubMed] [Google Scholar]
- 22.D'Souza, W. N., and L. Lefrancois. 2003. IL-2 is not required for the initiation of CD8 T cell cycling but sustains expansion. J. Immunol. 171:5727-5735. [DOI] [PubMed] [Google Scholar]
- 23.Eckels, D. D., N. Tabatabail, T.-H. Bian, H. Wang, S. S. Muheisen, C. M. Rice, K. Yoshizawa, and J. U. Gill. 1999. In vitro human Th-cell responses to a recombinant hepatitis C virus antigen: failure in IL-2 production despite proliferation. Hum. Immunol. 60:187-199. [DOI] [PubMed] [Google Scholar]
- 24.Erhardt, A., M. Hassan, T. Heintges, and D. Haussinger. 2002. Hepatitis C virus core protein induces cell proliferation and activates ERK, JNK, and p38 MAP kinases together with the MAP kinase phosphatase MKP-1 in a HepG2 Tet-Off cell line. Virology 292:272-284. [DOI] [PubMed] [Google Scholar]
- 25.Fields, P. E., T. F. Gajewski, and F. W. Fitch. 1996. Blocked Ras activation in anergic CD4+ T cells. Science 271:1276-1278. [DOI] [PubMed] [Google Scholar]
- 26.Fraser, J. D., B. A. Irving, G. R. Crabtree, and A. Weiss. 1991. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science 251:313-316. [DOI] [PubMed] [Google Scholar]
- 27.Gale, M., Jr., C. M. Blakely, B. Kwieciszewski, S. L. Tan, M. Dossett, N. M. Tang, M. J. Korth, S. J. Polyak, D. R. Gretch, and M. G. Katze. 1998. Control of PKR protein kinase by hepatitis C virus nonstructural 5A protein: molecular mechanisms of kinase regulation. Mol. Cell. Biol. 18:5208-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gale, M. J., Jr., M. J. Korth, N. M. Tang, S. L. Tan, D. A. Hopkins, T. E. Dever, S. J. Polyak, D. R. Gretch, and M. G. Katze. 1997. Evidence that hepatitis C virus resistance to interferon is mediated through repression of the PKR protein kinase by the nonstructural 5A protein. Virology 230:217-227. [DOI] [PubMed] [Google Scholar]
- 29.Gerlach, J. T., H. M. Diepolder, M. C. Jung, N. H. Gruener, W. W. Schraut, R. Zachoval, R. Hoffmann, C. A. Schirren, T. Santantonio, and G. R. Pape. 1999. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C. Gastroenterology 117:933-941. [DOI] [PubMed] [Google Scholar]
- 30.Giambartolomei, S., F. Covone, M. Levrero, and C. Balsano. 2001. Sustained activation of the Raf/MEK/Erk pathway in response to EGF in stable cell lines expressing the hepatitis C virus (HCV) core protein. Oncogene 20:2606-2610. [DOI] [PubMed] [Google Scholar]
- 31.Groom, L. A., A. A. Sneddon, D. R. Alessi, S. Dowd, and S. M. Keyse. 1996. Differential regulation of the MAP, SAP and RK/p38 kinases by Pyst1, a novel cytosolic dual-specificity phosphatase. EMBO J. 15:3621-3632. [PMC free article] [PubMed] [Google Scholar]
- 32.Groux, H., M. Bigler, J. E. de Vries, and M. G. Roncarolo. 1996. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J. Exp. Med. 184:19-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gruener, N. H., F. Lechner, M. C. Jung, H. Diepolder, T. Gerlach, G. Lauer, B. Walker, J. Sullivan, R. Phillips, G. R. Pape, and P. Klenerman. 2001. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J. Virol. 75:5550-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayashi, J., H. Aoki, K. Kajino, M. Moriyama, Y. Arakawa, and O. Hino. 2000. Hepatitis C virus core protein activates the MAPK/ERK cascade synergistically with tumor promoter TPA, but not with epidermal growth factor or transforming growth factor alpha. Hepatology 32:958-961. [DOI] [PubMed] [Google Scholar]
- 35.Houghton, M. 1996. Hepatitis C viruses, p. 1035-1058. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed., vol. 1. Lippincott-Raven Publishers, Philadelphia, Pa.
- 36.Inoue, K., K. Sekiyama, M. Yamada, T. Watanabe, H. Yasuda, and M. Yoshiba. 2003. Combined interferon alpha2b and cyclosporin A in the treatment of chronic hepatitis C: controlled trial. J. Gastroenterol. 38:567-572. [DOI] [PubMed] [Google Scholar]
- 37.Isakov, N., and A. Altman. 2002. Protein kinase C(theta) in T cell activation. Annu. Rev. Immunol. 20:761-794. [DOI] [PubMed] [Google Scholar]
- 38.Jain, J., P. G. McCaffrey, V. E. Valge-Archer, and A. Rao. 1992. Nuclear factor of activated T cells contains Fos and Jun. Nature 356:801-804. [DOI] [PubMed] [Google Scholar]
- 39.Kalams, S. A., and B. D. Walker. 1998. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J. Exp. Med. 188:2199-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kempiak, S. J., T. S. Hiura, and A. E. Nel. 1999. The Jun kinase cascade is responsible for activating the CD28 response element of the IL-2 promoter: proof of cross-talk with the I kappa B kinase cascade. J. Immunol. 162:3176-3187. [PubMed] [Google Scholar]
- 41.Large, M. K., D. J. Kittlesen, and Y. S. Hahn. 1999. Suppression of host immune response by the core protein of hepatitis C virus: possible implications for hepatitis C virus persistence. J. Immunol. 162:931-938. [PubMed] [Google Scholar]
- 42.Lechner, F., D. K. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191:1499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lerat, H., F. Berby, M.-A. Trabaud, O. Vidalin, M. Major, C. Trepo, and G. Inchauspe. 1996. Specific detection of hepatitis C virus minus strand RNA in hematopoietic cells. J. Clin. Investig. 97:845-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li, W., C. D. Whaley, A. Mondino, and D. L. Mueller. 1996. Blocked signal transduction to the ERK and JNK protein kinases in anergic CD4+ T cells. Science 271:1272-1276. [DOI] [PubMed] [Google Scholar]
- 45.Lindenbach, B., and C. Rice. 2001. Flaviviridae: the viruses and their replication, p. 991-1041. In D. M. Knipe et al. (ed.), Fields virology, 4th ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 46.Liu, Y., M. Gorospe, C. Yang, and N. J. Holbrook. 1995. Role of mitogen-activated protein kinase phosphatase during the cellular response to genotoxic stress. Inhibition of c-Jun N-terminal kinase activity and AP-1-dependent gene activation. J. Biol. Chem. 270:8377-8380. [DOI] [PubMed] [Google Scholar]
- 47.Logvinoff, C., M. E. Major, D. Oldach, S. Heyward, A. Talal, P. Balfe, S. M. Feinstone, H. Alter, C. M. Rice, and J. A. McKeating. 2004. Neutralizing antibody response during acute and chronic hepatitis C virus infection. Proc. Natl. Acad. Sci. USA 101:10149-10154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lucas, M., A. L. Vargas-Cuero, G. M. Lauer, E. Barnes, C. B. Willberg, N. Semmo, B. D. Walker, R. Phillips, and P. Klenerman. 2004. Pervasive influence of hepatitis C virus on the phenotype of antiviral CD8+ T cells. J. Immunol. 172:1744-1753. [DOI] [PubMed] [Google Scholar]
- 49.Ma, W., W. Lim, K. Gee, S. Aucoin, D. Nandan, M. Kozlowski, F. Diaz-Mitoma, and A. Kumar. 2001. The p38 mitogen-activated kinase pathway regulates the human interleukin-10 promoter via the activation of Sp1 transcription factor in lipopolysaccharide-stimulated human macrophages. J. Biol. Chem. 276:13664-13674. [DOI] [PubMed] [Google Scholar]
- 50.Macian, F., F. Garcia-Cozar, S. H. Im, H. F. Horton, M. C. Byrne, and A. Rao. 2002. Transcriptional mechanisms underlying lymphocyte tolerance. Cell 109:719-731. [DOI] [PubMed] [Google Scholar]
- 51.Macian, F., C. Garcia-Rodriguez, and A. Rao. 2000. Gene expression elicited by NFAT in the presence or absence of cooperative recruitment of Fos and Jun. EMBO J. 19:4783-4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Macian, F., C. Lopez-Rodriguez, and A. Rao. 2001. Partners in transcription: NFAT and AP-1. Oncogene 20:2476-2489. [DOI] [PubMed] [Google Scholar]
- 53.McGuire, K. L., and M. Iacobelli. 1997. Involvement of Rel, Fos, and Jun proteins in binding activity to the IL-2 promoter CD28 response element/AP-1 sequence in human T cells. J. Immunol. 159:1319-1327. [PubMed] [Google Scholar]
- 54.Missale, G., R. Bertoni, V. Lamonaca, A. Valli, M. Massari, C. Mori, M. G. Rumi, M. Houghton, F. Fiaccadori, and C. Ferrari. 1996. Different clinical behaviors of acute hepatitis C virus infection are associated with different vigor of the anti-viral cell-mediated immune response. J. Clin. Investig. 98:706-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mizutani, T., N. Kato, M. Ikeda, K. Sugiyama, and K. Shimotohno. 1996. Long-term human T-cell culture system supporting hepatitis C virus replication. Biochem. Biophys. Res. Commun. 227:822-826. [DOI] [PubMed] [Google Scholar]
- 56.Moradpour, D., T. Wakita, K. Tokushige, R. I. Carlson, K. Krawczynski, and J. R. Wands. 1996. Characterization of three novel monoclonal antibodies against hepatitis C virus core protein. J. Med. Virol. 48:234-241. [DOI] [PubMed] [Google Scholar]
- 57.Morsica, G., G. Tambussi, G. Sitia, R. Novati, A. Lazzarin, L. Lopalco, and S. Mukenge. 1999. Replication of hepatitis C virus in B lymphocytes (CD19+). Blood 94:1138-1139. [PubMed] [Google Scholar]
- 58.Muller, H. M., E. Pfaff, T. Goeser, B. Kallinowski, C. Solbach, and L. Theilmann. 1993. Peripheral blood leukocytes serve as a possible extrahepatic site for hepatitis C virus replication. J. Gen. Virol. 74:669-676. [DOI] [PubMed] [Google Scholar]
- 59.Nel, A. E. 2002. T-cell activation through the antigen receptor. Part 1: signaling components, signaling pathways, and signal integration at the T-cell antigen receptor synapse. J. Allergy Clin. Immunol. 109:758-770. [DOI] [PubMed] [Google Scholar]
- 60.Neumann, A. U., N. P. Lam, H. Dahari, D. R. Gretch, T. E. Wiley, T. J. Layden, and A. S. Perelson. 1998. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science 282:103-107. [DOI] [PubMed] [Google Scholar]
- 61.Northrop, J. P., K. S. Ullman, and G. R. Crabtree. 1993. Characterization of the nuclear and cytoplasmic components of lymphoid specific nuclear factor of activated T cells (NF-AT) complex. J. Biol. Chem. 268:2917-2923. [PubMed] [Google Scholar]
- 62.Penix, L., W. M. Weaver, Y. Pang, H. A. Young, and C. B. Wilson. 1993. Two essential regulatory elements in the human interferon gamma promoter confer activation specific expression in T cells. J. Exp. Med. 178:1483-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ray, R. B., and R. Ray. 2001. Hepatitis C virus core protein: intriguing properties and functional relevance. FEMS Microbiol. Lett. 202:149-156. [DOI] [PubMed] [Google Scholar]
- 64.Rhoades, K., S. Golub, and J. Economou. 1992. The regulation of the human tumor necrosis factor alpha promoter region in macrophage, T cell, and B cell lines. J. Biol. Chem. 267:22102-22107. [PubMed] [Google Scholar]
- 65.Rincon, M., and R. A. Flavell. 1994. AP-1 transcriptional activity requires both T-cell receptor-mediated and co-stimulatory signals in primary T lymphocytes. EMBO J. 13:4370-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schwartz, R. H. 1996. Models of T cell anergy: is there a common molecular mechanism? J. Exp. Med. 184:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwartz, R. H. 2003. T cell anergy. Annu. Rev. Immunol. 21:305-334. [DOI] [PubMed] [Google Scholar]
- 68.Serfling, E., A. Avots, and M. Neumann. 1995. The architecture of the interleukin-2 promoter: a reflection of T lymphocyte activation. Biochim. Biophys. Acta 1263:181-200. [DOI] [PubMed] [Google Scholar]
- 69.Shankar, P., M. Russo, B. Harnisch, M. Patterson, P. Skolnik, and J. Lieberman. 2000. Impaired function of circulating HIV-specific CD8(+) T cells in chronic human immunodeficiency virus infection. Blood 96:3094-3101. [PubMed] [Google Scholar]
- 70.Shapiro, V. S., K. E. Truitt, J. B. Imboden, and A. Weiss. 1997. CD28 mediates transcriptional upregulation of the interleukin-2 (IL-2) promoter through a composite element containing the CD28RE and NF-IL-2B AP-1 sites. Mol. Cell. Biol. 17:4051-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shimizu, Y. K., A. Iwamoto, M. Hijikata, R. H. Purcell, and H. Yoshikura. 1992. Evidence for in vitro replication of hepatitis C virus genome in a human T-cell line. Proc. Natl. Acad. Sci. USA 89:5477-5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shoukry, N. H., A. Grakoui, M. Houghton, D. Y. Chien, J. Ghrayeb, K. A. Reimann, and C. M. Walker. 2003. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J. Exp. Med. 197:1645-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shrivastava, A., S. K. Manna, R. Ray, and B. B. Aggarwal. 1998. Ectopic expression of hepatitis C virus core protein differentially regulates nuclear transcription factors. J. Virol. 72:9722-9728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soguero, C., M. Joo, K. A. Chianese-Bullock, D. T. Nguyen, K. Tung, and Y. S. Hahn. 2002. Hepatitis C virus core protein leads to immune suppression and liver damage in a transgenic murine model. J. Virol. 76:9345-9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Su, B., E. Jacinto, M. Hibi, T. Kallunki, M. Karin, and Y. Ben-Neriah. 1994. JNK is involved in signal integration during costimulation of T lymphocytes. Cell 77:727-736. [DOI] [PubMed] [Google Scholar]
- 76.Sung, V. M.-H., S. Shimodaira, A. L. Doughty, G. R. Picchio, H. Can, T. S. B. Yen, K. L. Lindsay, A. M. Levine, and M. M. C. Lai. 2003. Establishment of B-cell lymphoma cell lines persistently infected with hepatitis C virus in vivo and in vitro: the apoptotic effects of virus infection. J. Virol. 77:2134-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Szabo, S. J., J. S. Gold, T. L. Murphy, and K. M. Murphy. 1993. Identification of cis-acting regulatory elements controlling interleukin-4 gene expression in T cells: roles for NF-Y and NF-ATc. Mol. Cell. Biol. 13:4793-4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Taylor, D. R., S. T. Shi, P. R. Romano, G. N. Barber, and M. M. Lai. 1999. Inhibition of the interferon-inducible protein kinase PKR by HCV E2 protein. Science 285:107-110. [DOI] [PubMed] [Google Scholar]
- 79.Thimme, R., J. Bukh, H. C. Spangenberg, S. Wieland, J. Pemberton, C. Steiger, S. Govindarajan, R. H. Purcell, and F. V. Chisari. 2002. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc. Natl. Acad. Sci. USA 99:15661-15668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thimme, R., D. Oldach, K. M. Chang, C. Steiger, S. C. Ray, and F. V. Chisari. 2001. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 194:1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tsai, S. L., Y. F. Liaw, M. H. Chen, C. Y. Huang, and G. C. Kuo. 1997. Detection of type 2-like T-helper cells in hepatitis C virus infection: implications for hepatitis C virus chronicity. Hepatology 25:449-458. [DOI] [PubMed] [Google Scholar]
- 82.Tsuchihara, K., M. Hijikata, K. Fukuda, T. Kuroki, N. Yamamoto, and K. Shimotohno. 1999. Hepatitis C virus core protein regulates cell growth and signal transduction pathway transmitting growth stimuli. Virology 258:100-107. [DOI] [PubMed] [Google Scholar]
- 83.Ulsenheimer, A., J. T. Gerlach, N. H. Gruener, M. C. Jung, C. A. Schirren, W. Schraut, R. Zachoval, G. R. Pape, and H. M. Diepolder. 2003. Detection of functionally altered hepatitis C virus-specific CD4 T cells in acute and chronic hepatitis C. Hepatology 37:1189-1198. [DOI] [PubMed] [Google Scholar]
- 84.Wedemeyer, H., X. S. He, M. Nascimbeni, A. R. Davis, H. B. Greenberg, J. H. Hoofnagle, T. J. Liang, H. Alter, and B. Rehermann. 2002. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J. Immunol. 169:3447-3458. [DOI] [PubMed] [Google Scholar]
- 85.Werlen, G., E. Jacinto, Y. Xia, and M. Karin. 1998. Calcineurin preferentially synergizes with PKC-theta to activate JNK and IL-2 promoter in T lymphocytes. EMBO J. 17:3101-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Woitas, R. P., M. Lechmann, G. Jung, R. Kaiser, T. Sauerbruch, and U. Spengler. 1997. CD30 induction and cytokine profiles in hepatitis C virus core-specific peripheral blood T lymphocytes. J. Immunol. 159:1012-1018. [PubMed] [Google Scholar]
- 87.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]