Abstract
Background
The duration of prior hormonal treatment can predict responses to subsequent therapy in patients with metastatic castration-resistant prostate cancer (mCRPC).
Objective
To determine if prior endocrine therapy duration is an indicator of abiraterone acetate (AA) sensitivity.
Design, setting, and participants
Post-hoc exploratory analysis of randomised phase 3 studies examining post-docetaxel (COU-AA-301) or chemotherapy-naïve mCRPC (COU-AA-302) patients receiving AA. The treatment effect on overall survival (OS), radiographic progression-free survival (rPFS), and prostate-specific antigen (PSA) response analysed by quartile duration of prior gonadotropin-releasing hormone agonists (GnRHa) or androgen receptor (AR) antagonist.
Intervention
Patients were randomised to AA (1000 mg, orally once daily) plus prednisone (5 mg, orally twice daily) or placebo plus prednisone. Prior endocrine therapy was GnRHa (COU-AA-301, n = 1127 [94%]; COU-AA-302, n = 1057 [97%], 45.1 mo or 36.7 mo median duration, respectively) and/or orchiectomy (COU-AA-301, n = 78 [7%] COU-AA-302, n = 44 [4%]); castrated patients received prior AR antagonists (COU-AA-301, n = 1015 [85%]; COU-AA-302, n = 1078 [99%], 15.7 mo or 16.1 mo median duration, respectively).
Outcome measurements and statistical analysis
Cox model was used to obtain hazard ratio and associated 95% confidence interval with statistical inference by log rank statistic.
Results and limitations
Clinical benefit with AA was observed for OS, rPFS, and PSA response for nearly all quartiles with GnRHa or AR antagonists in both COU-AA-301 and COU-AA-302. In COU-AA-301, patients with a longer duration of prior endocrine therapy tended to have greater AA OS, rPFS, and PSA response benefit, with lead-time chemotherapy bias potentially impacting COU-AA-301 results. Time-to-castration-resistance was not captured. This analysis is limited as a post-hoc exploratory analysis.
Conclusions
In the COU-AA-301 and COU-AA-302 studies, AA produced clinical benefits regardless of prior endocrine therapy duration in patients with mCRPC.
Patient summary
Metastatic castration-resistant prostate cancer patients derived clinical benefits with abiraterone acetate regardless of prior endocrine therapy duration.
Keywords: Abiraterone acetate, Androgen receptor antagonists, Gonadotropin-releasing hormone, Prednisone, Prostate cancer
1. Introduction
Most tumours in men who present with metastatic disease at prostate cancer diagnosis or with disease recurrence after potentially curative local therapy respond to androgen deprivation [1] with luteinising hormone—releasing hormone agonists or antagonists or bilateral orchiectomy, and to first-line androgen receptor antagonists such as bicalutamide [2–5]. In most cases, however, the response is not durable and virtually all tumours eventually progress to a lethal castration-resistant phenotype [1,5].
Abiraterone acetate, a prodrug of abiraterone that is a selective inhibitor of CYP17 [6,7], administered in combination with prednisone/prednisolone (hereafter referred to as abiraterone) is one of several agents indicated for the treatment of patients with metastatic castration-resistant prostate cancer [8–17]. Abiraterone significantly improved overall survival and all secondary and tumour-specific endpoints [9,10], as well as patient-reported fatigue [18] and quality of life [19] in the phase 3 COU-AA-301 trial in patients with metastatic castration-resistant prostate cancer progressing after docetaxel chemotherapy. A similar survival benefit was observed in the pre-chemotherapy COU-AA-302 study along with a significant improvement in radiographic-free survival, all secondary endpoints, and patient-reported outcomes [8,11,16].
Previous data suggests that the duration of prior hormonal treatment predicts duration to subsequent hormone therapy [20,21]: the longer duration of the response to the first androgen depletion therapy, the longer the duration of response to the second therapy including CYP17 inhibitors [20] such as abiraterone and ketoconazole [21]. Here we report a post-hoc analysis to determine whether the duration of prior endocrine therapy with gonadotropin-releasing hormone (GnRH) agonists or first-generation androgen receptor antagonists was associated with overall survival, radiographic progression-free survival, or prostate-specific antigen (PSA) response rate in patients treated with abiraterone acetate plus prednisone in the post- or the pre-chemotherapy COU-AA-301 and COU-AA-302 trials.
2. Patients and methods
COU-AA-301 (NCT00638690) [9,10] and COU-AA-302 (NCT00887198) [8,11,16] were phase 3, multinational, randomised, double-blind, placebo-controlled studies of post-docetaxel and chemotherapy-naïve patients, respectively, with progressive metastatic castration-resistant prostate cancer (Fig. 1). The review boards at all participating institutions approved the studies, which were conducted according to the principles of the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Conference on Harmonisation. All patients provided written informed consent to participate in the studies. In COU-AA-301 and COU-AA-302, patients were randomised 2:1 and 1:1, respectively, to oral abiraterone acetate 1 g daily and prednisone 5 mg twice daily versus placebo and prednisone 5 mg twice daily. Prednisolone at the same dose was used in place of prednisone at some sites. Patients received continuous GnRH agonist if they had not undergone a surgical orchiectomy to maintain serum testosterone <50 ng/dl. Prior endocrine therapies included GnRH agonists and androgen receptor antagonists as defined in Supplementary Table 1. Duration of prior endocrine therapy from the start of endocrine therapy to the date of randomisation as documented in the case report forms was recorded for each patient and categorised by quartiles as defined in Table 1 and 2 and Figure 2 and 3. Associations with clinical outcomes in the COU-AA-301 and COU-AA-302 studies were associated by quartiles. A study monitor had access to the patients’ medical records and was responsible for verifying adherence to the study protocols.
Fig.1.
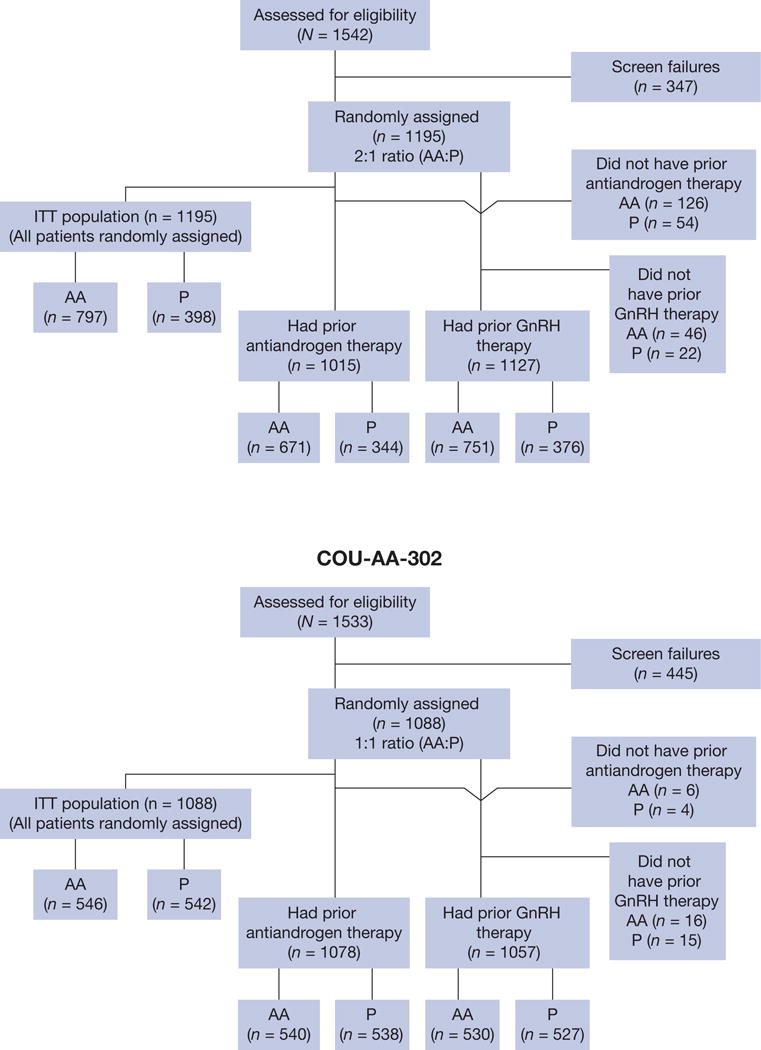
Consolidated Standards of Reporting Trials diagram for (A) COU-AA-301 and (B) COU-AA-302.
AA = abiraterone acetate; GnRH = gonadotropin-releasing hormone; ITT = intention-to-treat; P = prednisone.
Table 1.
Clinical outcomes in COU-AA-301 patients with prior endocrine therapy exposure by duration in quartiles
| GnRH agonists | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||||||||
| ≤28 mo | ≥29 to ≤45 mo | ≥46 to ≤71 mo | >71 mo | |||||||||
| Treatment | AA + P | P | AA + P | P | AA + P | P | AA + P | P | ||||
| (n = 191) | (n = 87) | (n = 174) | (n = 109) | (n = 191) | (n = 91) | (n = 195) | (n = 89) | |||||
| Overall survival
| ||||||||||||
| HR | 0.99 | 0.61 | 0.73 | 0.68 | ||||||||
| (95% CI) | (0.71–1.37) | (0.46–0.82) | (0.53–1.00) | (0.48–0.97) | ||||||||
|
Radiographic progression-free survival | ||||||||||||
| HR | 0.84 | 0.65 | 0.56 | 0.68 | ||||||||
| (95% CI) | (0.62–1.13) | (0.49–0.86) | (0.41–0.76) | (0.50–0.91) | ||||||||
|
Androgen receptor antagonists | ||||||||||||
| Q1 | Q2 | Q3 | Q4 | |||||||||
| ≤7 mo | ≥8 to ≤16 mo | ≥17 to ≤36 mo | >36 mo | |||||||||
| Treatment | AA + P | P | AA + P | P | AA + P | P | AA + P | P | ||||
| (n = 155) | (n = 95) | (n = 179) | (n = 85) | (n = 167) | (n = 82) | (n = 170) | (n = 82) | |||||
| Overall survival
| ||||||||||||
| HR | 0.78 | 0.99 | 0.73 | 0.57 | ||||||||
| (95% CI) | (0.56–1.08) | (0.71–1.38) | (0.51–1.03) | (0.40–0.81) | ||||||||
|
Radiographic progression-free survival | ||||||||||||
| HR | 0.76 | 0.66 | 0.68 | 0.62 | ||||||||
| (95% CI) | (0.55–1.05) | (0.49–0.90) | (0.50–0.93) | (0.45–0.86) | ||||||||
AA = abiraterone acetate; CI = confidence interval; GnRH = gonadotropin-releasing hormone; HR = hazard ratio; P = prednisone; Q = quartile.
Table 2.
Clinical outcomes in COU-AA-302 patients with prior endocrine therapy exposure by duration in quartiles
| GnRH agonists | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||||||||
| ≤20 mo | ≥21 to ≤37 mo | ≥38 to ≤61 mo | >61 mo | |||||||||
| Treatment | AA + P | P | AA + P | P | AA + P | P | AA + P | P | ||||
| (n = 119) | (n = 133) | (n = 145) | (n = 137) | (n = 127) | (n = 127) | (n = 139) | (n = 130) | |||||
| Overall survival
| ||||||||||||
| HR | 0.79 | 0.69 | 1.05 | 0.78 | ||||||||
| (95% CI) | (0.59–1.04) | (0.52–0.91) | (0.76–1.43) | (0.57–1.06) | ||||||||
|
Radiographic progression-free survival | ||||||||||||
| HR | 0.52 | 0.46 | 0.67 | 0.46 | ||||||||
| (95% CI) | (0.37–0.72) | (0.33–0.62) | (0.48–0.93) | (0.34–0.64) | ||||||||
|
Androgen receptor antagonists | ||||||||||||
| Q1 | Q2 | Q3 | Q4 | |||||||||
| ≤7 mo | ≥8 to ≤16 mo | ≥17 to ≤33 mo | >33 mo | |||||||||
| Treatment | AA + P | P | AA + P | P | AA + P | P | AA + P | P | ||||
| (n = 138) | (n = 134) | (n = 132) | (n = 132) | (n = 138) | (n = 131) | (n = 132) | (n = 141) | |||||
| Overall survival
| ||||||||||||
| HR | 0.68 | 0.83 | 0.97 | 0.88 | ||||||||
| (95% CI) | (0.47–0.82) | (0.62–1.11) | (0.72–1.31) | (0.65–1.20) | ||||||||
|
Radiographic progression-free survival | ||||||||||||
| HR | 0.43 | 0.65 | 0.52 | 0.5 | ||||||||
| (95% CI) | (0.32–0.59) | (0.47–0.90) | (0.37–0.72) | (0.36–0.69) | ||||||||
AA = abiraterone acetate; CI = confidence interval; GnRH = gonadotropin-releasing hormone; HR = hazard ratio; P = prednisone; Q = quartile.
Fig. 2.
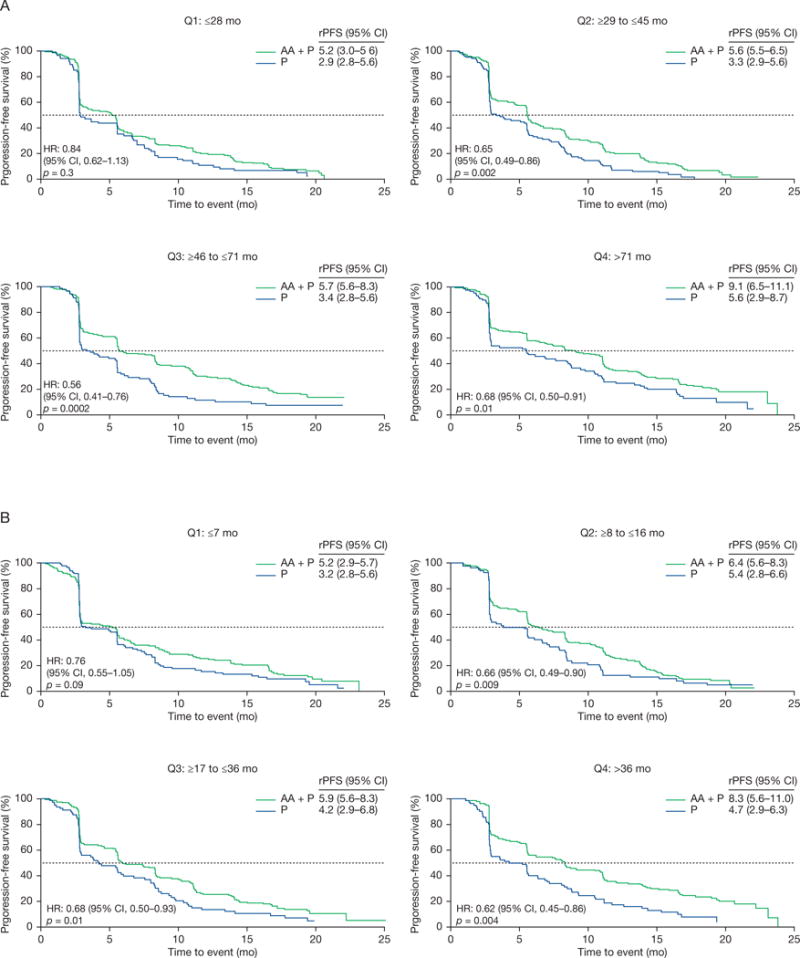
Radiographic progression-free survival Kaplan-Meier estimates in COU-AA-301 patients with prior endocrine therapy exposure by duration in quartiles for (A) gonadotropin-releasing hormone agonists and (B) androgen receptor antagonists.
AA = abiraterone acetate; CI = confidence interval; HR = hazard ratio; P = prednisone; rPFS = radiographic progression-free survival; Q = quartile.
Fig. 3.
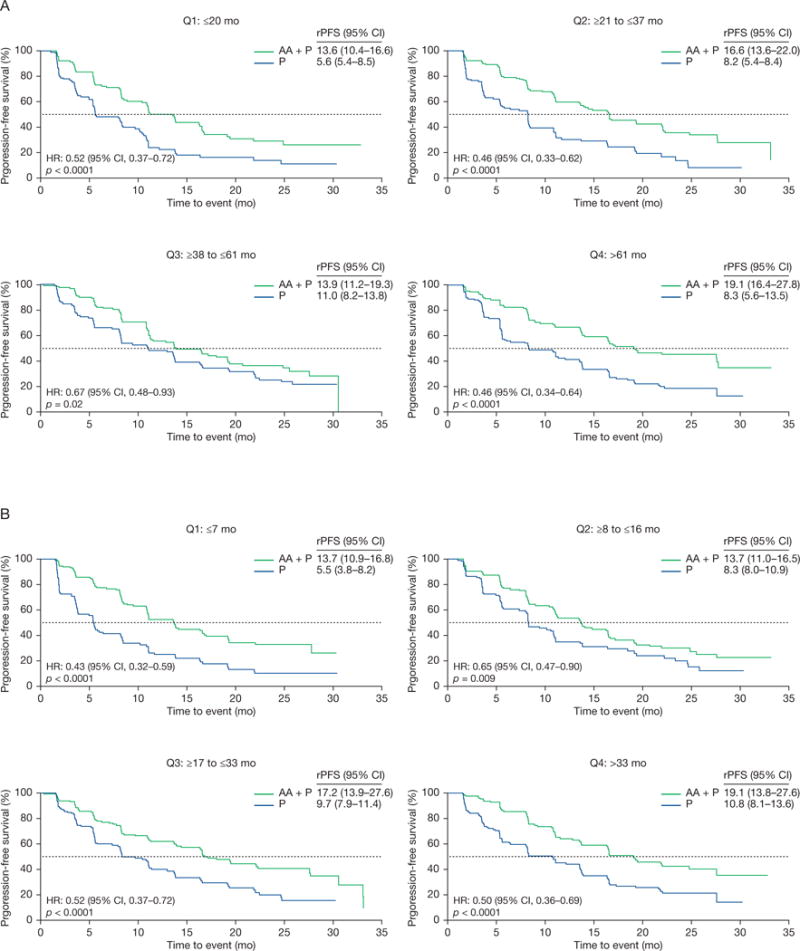
Radiographic progression-free survival Kaplan-Meier estimates in COU-AA-302 patients with prior endocrine therapy exposure by duration in quartiles for (A) gonadotropin-releasing hormone agonists and (B) androgen receptor antagonists.
AA = abiraterone acetate; CI = confidence interval; HR = hazard ratio; P = prednisone; rPFS = radiographic progression-free survival; Q = quartile.
Distributions of time-to-event variables were estimated using the Kaplan-Meier product limits method. The log-rank statistic was used as the primary analysis for treatment comparison. Cox model analysis was used to obtain the hazard ratio and its associated 95% confidence interval. Data shown for COU-AA-301 represent the final analysis of the study before patient crossover from prednisone to abiraterone (775 of the expected 797 death events), with a median follow-up for overall survival of 20.2 mo. Data shown for COU-AA-302 (ie, radiographic progression-free survival and PSA response rate) represent mature data obtained at the third interim analysis conducted at 56% of the expected death events, whereas mature overall survival data were obtained at the final analysis. Results were considered significant if p ≤ 0.05; no multiplicity adjustments were made for this hypothesis generating post-hoc analysis. An interaction test was performed to assess whether the effect of abiraterone acetate was dependent on prior endocrine therapy duration. This analysis was performed for GnRH agonists given that the majority of patients received prior GnRH agonists (Supplementary Table 2).
3. Results
3.1. Patient characteristics
Patients received prior endocrine therapy with GnRH agonists (COU-AA-301, n = 1127 [94%]; COU-AA-302, n = 1057 [97%]) and/or orchiectomy (COU-AA-301, n = 78 [6.5%]; COU-AA-302, n = 44 [4.1%]) (Fig. 1). Pure androgen receptor antagonists (COU-AA-301, n = 1015 [85%]; COU-AA-302, n = 1078 [99%]) were also used in COU-AA-302. In COU-AA-301, the median duration of prior GnRH agonist and androgen receptor antagonist exposure was 45.1 mo and 15.7 mo, respectively. Median durations of prior GnRH agonist and androgen receptor antagonist exposure in COU-AA-302 were 36.7 mo and 16.1 mo, respectively. These durations represent the duration of prior endocrine therapies, not a single exposure to one form of manipulation.
3.2. Outcomes
Overall survival was improved in the abiraterone group versus the prednisone group in all quartiles of duration of prior endocrine therapy studied in COU-AA-301 (Table 1 and Supplementary Fig. 1) and all except quartile 3 in COU-AA-302 (Table 2 and Supplementary Fig. 2). However, there were inconsistencies across quartiles in demonstrating a significant treatment benefit with abiraterone acetate in this post-hoc exploratory analysis. In both trials, patients who experienced a longer duration (quartile 4 equals the longest duration) of prior endocrine therapy had a longer overall survival, whether measured against quartile exposure of GnRH agonists or androgen receptor antagonists. This was observed regardless of assignment with few exceptions for both the abiraterone and prednisone groups.
Radiographic progression-free survival was significantly improved in the abiraterone group versus the prednisone group in patients for all quartiles of prior GnRH agonists or androgen receptor antagonists treatment in both COU-AA-301 (Table 1 and Fig. 2) and COU-AA-302 (Table 2 and Fig. 3). The PSA response proportions were also superior independent of the type and duration of prior endocrine therapy (Supplementary Fig. 3).
Results from an interaction analysis to examine whether the effect of abiraterone acetate was dependent on prior endocrine therapy duration were not significant in both COU-AA-301 and COU-AA-302 for both overall survival and radiographic progression-free survival (Table 3). Analysis by GnRH agonist quartiles yielded similar results, with none of the interaction tests on outcome measures showing significance.
Table 3.
Interaction analysis of abiraterone acetate treatment and prior endocrine therapy duration for overall survival and radiographic progression-free survival in COU-AA-301 and COU-AA-302
| COU-AA-301 | |
|---|---|
| Parameter | p value |
| Overall survival | |
| Treatment | 0.1 |
| Duration | 0.009 |
| Treatment × duration | 0.4 |
| Radiographic progression-free survival | |
| Treatment | 0.0006 |
| Duration | <0.0001 |
| Treatment × duration | 0.7 |
| COU-AA-302 | |
| Parameter | p value |
| Overall survival | |
| Treatment | 0.4 |
| Duration | 0.002 |
| Treatment × duration | 0.6 |
| Radiographic progression-free survival | |
| Treatment | <0.0001 |
| Duration | 0.04 |
| Treatment × duration | 0.7 |
Treatment with abiraterone acetate and prednisone was well tolerated by patients, as previously reported for both COU-AA-301 [9,10] and COU-AA-302 [8,11,16].
4. Discussion
The clinical benefit of abiraterone was maintained regardless of type and duration of prior endocrine therapy at nearly all quartiles examined, as shown in this post-hoc analysis of the phase 3 COU-AA-301 and COU-AA-302 studies in patients with metastatic castration-resistant prostate cancer progressing post-docetaxel or without prior chemotherapy, respectively. The clinical benefit of abiraterone was maintained despite the fact that longer exposure of prior endocrine therapy in COU-AA-301 and COU-AA-302 was associated with a longer time-to-death and radiographic progression-free survival regardless of treatment assignment. The results show the importance of considering the duration of prior hormone therapy in trial design, both as a stratification factor and a predictive factor in the evaluation of patients with castration-resistant prostate cancer who are progressing in the pre- or post-chemotherapy setting. When interpreting these results, it should be evident that prior endocrine therapy exposure in the setting of this post-hoc analysis equates with the duration of prior hormone therapy and not with hormone sensitivity or hormone response.
Previous data using other hormonal agents suggested that a short response to first-line androgen deprivation therapy (ADT) predicts poor response both in frequency and duration to a subsequent hormone therapy [20–23]. In one retrospective study of 57 patients with progressing castration-resistant prostate cancer treated with post-docetaxel enzalutamide from the AFFIRM trial, the median time-to-progression-free survival was significantly shorter (2.8 mo vs 8.6 mo, p = 0.002) and PSA response rate was significantly lower (8% vs 58%, p < 0.001) in patients with a less than 12-mo median duration versus greater than 12-mo median duration of response to first-line ADT [22]. The results are consistent with the current analysis which showed that the patients in the lowest quartile of duration of prior endocrine therapy had the shortest overall survival and radiographic progression-free survival. The effects of abiraterone acetate and prednisone, however, were seen in patients with short and long durations of exposure by quartile with the exception of the lowest quartile for overall survival and radiographic progression-free survival in COU-AA-301. This is consistent with results shown in a single-site analysis limited to 37 patients with metastatic castration-resistant prostate cancer post-docetaxel with varying duration of enzalutamide therapy, in which PSA response to subsequent abiraterone was similar for patients who received enzalutamide for ≤3 mo or >3 mo [24]. As reported recently [23], earlier treatment with docetaxel might not have a large impact on the subsequent activity of hormonal treatment, as comparable outcomes from enzalutamide after abiraterone were observed irrespective of prior docetaxel use [25]. Cabazitaxel was also shown to significantly improve overall survival compared with mitoxantrone regardless of the duration of prior ADT separated by tertiles of <2.5 yr, 2.5–5.0 yr, and ≥5 yr [26]. Although beyond the scope of this study, it would be of clinical value to examine whether patients with a particular duration of prior endocrine therapy before developing castration-resistance might be optimally sequenced with a particular second-line treatment of abiraterone acetate plus prednisone versus enzalutamide versus docetaxel.
The current study has several important limitations. Some patients might have received short courses of androgen receptor antagonists to prevent tumour flare in the castrate setting. This short course of therapy would not necessarily be expected to affect outcomes. There is also uncertainty with respect to the analysis of the lowest quartile with presumably more aggressive disease as evidenced by a short duration of 0–12 mo of prior GnRH agonist therapy, as the number of patients in this group was too low to analyse definitively. An additional concern is whether duration of exposure is an appropriate surrogate for sensitivity, given that there are no standards for reporting the response to ADT. Time-to-castration-resistance, which probably better describes sensitivity to ADT, could not be tested as a potential predictor of abiraterone clinical benefit in this study because this parameter was not available in the database. It should be noted that in the current study duration of prior hormonal treatment comprised time-to-castration-resistance and time-with-castration-resistance on hormonal treatment. Moreover, the onset of castration-resistance could have started earlier than indicated by the addition of abiraterone acetate and prednisone, reflecting individual physicians’ management philosophy and preferences. The effects of abiraterone acetate and prednisone on further outcomes are valid given that patients were randomised between the two treatment groups, as this analysis is reporting phase 3 randomised trials.
5. Conclusion
In general, the efficacy outcomes favoured the abiraterone treatment groups compared with prednisone groups regardless of prior endocrine therapy exposure in metastatic castration-resistant prostate cancer patients either post-docetaxel or without prior chemotherapy. Consistent with other studies, a longer duration of prior endocrine therapy in less pre-treated patients (ie, chemotherapy-naïve) tended to have a greater benefit. There were too few patients in the subgroup with a short initial sensitivity to androgen deprivation (eg, 6–12 mo) to draw the definitive conclusion highlighting the need of further studies in this specific patient population.
Supplementary Material
Acknowledgments
Funding/Support and role of the sponsor: This work was supported by Janssen Research and Development (formerly Ortho Biotech Research and Development, unit of Cougar Biotechnology). Employees of Janssen Research and Development participated in trial design and oversight, data monitoring and collection, data analysis, data interpretation, and writing of the report. The study sponsor was involved in trial design and provided grants to trial sites, but had no involvement in the conduct of the trial. Analyses done by Janssen for this report were funded by Janssen Global Services.
Writing assistance was provided by Ira Mills, PhD, of PAREXEL and was funded by Janssen Global Services.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Joaquim Bellmunt had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bellmunt, Oudard, Yu, Kheoh, Molina, Griffin. Acquisition of data: Kheoh, Yu, Molina, De Porre, Griffin.
Analysis and interpretation of data: Bellmunt, Oudard, Saad, Schrijvers, Yu, Kheoh, Molina, De Porre, Griffin.
Drafting of the manuscript: Bellmunt, Kheoh, Yu, Smith, Small, Mulders, Fizazi, Rathkopf, Saad, Scher, Taplin, Davis, Schrijvers, Protheroe, Molina, De Porre, Griffin, de Bono, Ryan, Oudard.
Critical revision of the manuscript for important intellectual content: Bellmunt, Kheoh, Yu, Smith, Small, Mulders, Fizazi, Rathkopf, Saad, Scher, Taplin, Davis, Schrijvers, Protheroe, Molina, De Porre, Griffin, de Bono, Ryan, Oudard.
Statistical analysis: Kheoh.
Obtaining funding: Molina.
Administrative, technical, or material support: Molina.
Supervision: Molina.
Other: None.
Financial disclosures: Joaquim Bellmunt certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: all authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Bellmunt has received honoraria and has served as a consultant for Janssen Research and Development. Kheoh, Yu, Molina, De Porre, and Griffin are employees of Janssen Research and Development and hold stock options in Johnson & Johnson. Smith served as a consultant to Janssen Research and Development. Small and Protheroe have no disclosures or potential conflicts of interest to report. Mulders has served as a consultant and has received research support from Janssen Research and Development, Astellas, and Bayer. Fizazi has participated in advisory boards and served as a speaker for Janssen. Rathkopf has received research funding from Janssen Research and Development. Saad has served as a consultant and has received research funding from Janssen Research and Development. Scher has served as an uncompensated consultant to Janssen Research and Development and reports support to Memorial Sloan Kettering Cancer Centre from Janssen Research and Development related to this work. He has also served as an uncompensated consultant to Astra Zeneca, Bristol Myers Squibb, Celgene, Endocyte, Exelixis, Foundation Medicine, Genentech, Medivation, Novartis, Pfizer, Takeda-Millennium and Ventana — Member of Roche; a consultant to Astellas, BIND Pharmaceuticals, Chugai Academy for Advanced Oncology, Endo/Orion Pharmaceuticals, OncologySTAT, Palmetto GBA, LLC, Sanofi Aventis, and WCG Oncology; has received an honorarium from Chugai Academy for Advanced Science; and has received support for Memorial Sloan Kettering Cancer Centre from BIND Pharmaceuticals, Epic Sciences, Exelixis, and Medivation. Dr. Taplin has received institutional (Dana-Farber Cancer Institute) funding for clinical trials involving abiraterone. Davis has served as a consultant without remunerations from Janssen Research and Development, Medivation, BMS, Sanofi, Bayer, Astellas, and Ipsen. Schrijvers has participated in studies and served as a consultant and speaker boards for Janssen Research and Development and Sanofi. de Bono is a paid employee of The Institute of Cancer Research, which has a commercial interest in abiraterone, and has served as a paid consultant for Johnson & Johnson. Ryan has received honoraria from Janssen Research and Development. Oudard has received honoraria from Janssen Research and Development.
References
- 1.Oudard S. Progress in emerging therapies for advanced prostate cancer. Cancer Treat Rev. 2013;39:275–89. doi: 10.1016/j.ctrv.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Cookson MS, Roth BJ, Dahm P, et al. Castration-resistant prostate cancer: AUA Guideline. J Urol. 2013;190:429–38. doi: 10.1016/j.juro.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Cookson MS, Lowrance WT, Murad MH, Kibel AS. Castration-resistant prostate cancer: AUA guideline amendment. J Urol. 2015;193:491–9. doi: 10.1016/j.juro.2014.10.104. [DOI] [PubMed] [Google Scholar]
- 4.Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65:467–79. doi: 10.1016/j.eururo.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Lam JS, Leppert JT, Vemulapalli SN, Shvarts O, Belldegrun AS. Secondary hormonal therapy for advanced prostate cancer. J Urol. 2006;175:27–34. doi: 10.1016/S0022-5347(05)00034-0. [DOI] [PubMed] [Google Scholar]
- 6.Barrie SE, Potter GA, Goddard PM, Haynes BP, Dowsett M, Jarman M. Pharmacology of novel steroidal inhibitors of cytochrome P450(17) alpha (17 alpha-hydroxylase/C17-20 lyase) J Steroid Biochem Mol Biol. 1994;50:267–73. doi: 10.1016/0960-0760(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 7.O’Donnell A, Judson I, Dowsett M, et al. Hormonal impact of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br J Cancer. 2004;90:2317–25. doi: 10.1038/sj.bjc.6601879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–92. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 11.Rathkopf DE, Smith MR, de Bono JS, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302) Eur Urol. 2014;66:815–25. doi: 10.1016/j.eururo.2014.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 13.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 14.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 15.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 16.Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–60. doi: 10.1016/S1470-2045(14)71205-7. [DOI] [PubMed] [Google Scholar]
- 17.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sternberg CN, Molina A, North S, et al. Effect of abiraterone acetate on fatigue in patients with metastatic castration-resistant prostate cancer after docetaxel chemotherapy. Ann Oncol. 2013;24:1017–25. doi: 10.1093/annonc/mds585. [DOI] [PubMed] [Google Scholar]
- 19.Harland S, Staffurth J, Molina A, et al. Effect of abiraterone acetate treatment on the quality of life of patients with metastatic castration-resistant prostate cancer after failure of docetaxel chemotherapy. Eur J Cancer. 2013;49:3648–57. doi: 10.1016/j.ejca.2013.07.144. [DOI] [PubMed] [Google Scholar]
- 20.Nakabayashi M, Werner L, Oh WK, Regan MM, Kantoff PW, Taplin ME. Secondary hormonal therapy in men with castration-resistant prostate cancer. Clin Genitourin Cancer. 2011;9:95–103. doi: 10.1016/j.clgc.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Nakabayashi M, Xie W, Regan MM, Jackman DM, Kantoff PW, Oh WK. Response to low-dose ketoconazole and subsequent dose escalation to high-dose ketoconazole in patients with androgen-independent prostate cancer. Cancer. 2006;107:975–81. doi: 10.1002/cncr.22085. [DOI] [PubMed] [Google Scholar]
- 22.Loriot Y, Eymard JC, Patrikidou A, et al. Prior long response to androgen deprivation predicts response to next-generation androgen receptor axis targeted drugs in castration resistant prostate cancer. Eur J Cancer. 2015;51:1946–52. doi: 10.1016/j.ejca.2015.06.128. [DOI] [PubMed] [Google Scholar]
- 23.Orsola A, Bellmunt J. The “artificial” docetaxel space: the evolving treatment paradigm of metastatic castration-resistant prostate cancer. Eur Urol. 2015;67:30–2. doi: 10.1016/j.eururo.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 24.Loriot Y, Bianchini D, Ileana E, et al. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100) Ann Oncol. 2013;24:1807–12. doi: 10.1093/annonc/mdt136. [DOI] [PubMed] [Google Scholar]
- 25.Azad AA, Eigl BJ, Murray RN, Kollmannsberger C, Chi KN. Efficacy of enzalutamide following abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer patients. Eur Urol. 2015;67:23–9. doi: 10.1016/j.eururo.2014.06.045. [DOI] [PubMed] [Google Scholar]
- 26.Oudard S, de Bono JS, Ozguroglu M, et al. Impact of cabazitaxel (CBZ) + prednisone (P; CBZP) on overall survival (OS) at 2 yrs and in patients (pts) with aggressive disease: post-hoc analyses of TROPIC trial [abstract] Ann Oncol. 2012;23(Suppl 9) Abstract 933P. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


