Abstract
Background/Aims
Cardiovascular disease and protein-energy wasting are among the strongest predictors of the high mortality of dialysis patients. In the general population, the novel cardiovascular and wasting biomarker, growth differentiation factor 15 (GDF15), is associated with decreased survival. However, little is known about GDF15 in dialysis patients.
Methods
Among prevalent hemodialysis patients participating in a prospective study (October 2011 to August 2015), we examined the association of baseline GDF15 levels with all-cause mortality using unadjusted and case mix-adjusted death hazard ratios (HRs) that controlled for age, sex, race, ethnicity, diabetes, and dialysis vintage.
Results
The mean age ± SD of the 203 patients included in the study was 53.2 ± 14.5 years, and the cohort included 41% females, 34% African-Americans, and 48% Hispanics. GDF15 levels (mean ± SD 5.94 ± 3.90 ng/mL; range 1.58-39.8 ng/mL) were higher among older patients and were inversely associated with serum creatinine concentrations as a surrogate for muscle mass. Each 1.0 ng/mL increase in GDF15 was associated with an approximately 17-18% higher mortality risk in the unadjusted and case mix models (p < 0.05). Increments of about 1 SD (a 4.0 ng/mL increase in GDF15) were associated with a nearly 2-fold higher death risk. The highest GDF15 tertile was associated with higher mortality risk (reference: lowest tertile): the HRs (95% CI) were 3.19 (1.35-7.55) and 2.45 (1.00-6.00) in the unadjusted and the case mix-adjusted model, respectively. These incremental death trends were confirmed in cubic spline models.
Conclusion
Higher circulating GDF15 levels are associated with higher mortality risk in hemodialysis patients. Future studies are needed to determine whether GDF15 may represent a novel therapeutic target for cardiovascular disease, wasting, and death in this population.
Key Words: Dialysis, Growth differentiation factor 15, Wasting, Cardiovascular disease, Mortality
Introduction
Hemodialysis patients have an exceedingly high mortality risk compared to the general population, largely due to cardiovascular causes (40% of deaths) [1]. However, conventional risk stratification tools remain imprecise in establishing cardiovascular prognoses in this population [2]. Furthermore, traditional Framingham risk factors explain only a fraction of the high frequency of cardiovascular disease and death among hemodialysis patients [3]. Hence, there has been increasing interest in identifying novel prognostic biomarkers that can more effectively identify high-risk individuals who may benefit from more intensive prevention and treatment.
In the general population, growth differentiation factor 15 (GDF15) is a protein (molecular weight 28 kDa) in the transforming growth factor-β superfamily that has emerged as a potential marker of cardiovascular disease and death [4,5,6,7]. Under normal physiologic conditions, GDF15 is weakly expressed by most tissues [8,9]. However, following injury, ischemia, and other forms of oxidative and/or metabolic stress, its production is potently upregulated by a wide range of tissues including activated macrophages, cardiomyocytes, and vascular smooth muscle cells [10,11,12]. In large population-based studies, GDF15 has been associated with endothelial dysfunction, atherosclerosis, left ventricular hypertrophy, and impaired systolic function, independent of cardiovascular risk factors such as inflammatory markers (e.g., C-reactive protein) [13,14]. In studies of healthy community-dwelling adults, as well as of those who have sustained recent myocardial infarction, elevated GDF15 levels have been linked with a higher incidence of congestive heart failure, myocardial infarction, stroke, or other cardiovascular events [15,16,17]. Furthermore, among patients with preexisting heart failure, higher GDF15 levels have been associated with higher risk of heart failure events and death [18,19].
Notably, GDF15 has also been identified as a novel appetite regulator that causes anorexia and weight loss when overexpressed in malignancy [20]. Indeed, in cancer-associated cachexia, circulating GDF15 levels correlate with weight loss, lower lean body and fat mass, weaker handgrip strength, and worse survival [21]. These findings bear particular relevance to the hemodialysis population, in whom weight loss and protein-energy wasting are among the most potent predictors of mortality, including cardiovascular death [22,23,24,25,26].
While limited data suggest that circulating GDF15 levels are higher in kidney dysfunction [20], there have been few studies that have examined the prognostic significance of this marker in hemodialysis patients [27,28]. Thus, to better inform the field, we sought to examine the association between GDF15 levels and mortality risk in a large, racially/ethnically diverse cohort of hemodialysis patients from the prospective, multicenter Malnutrition, Diet, and Racial Disparities in Chronic Kidney Disease (MADRAD) study who underwent rigorous, protocolized measurements of clinical and laboratory characteristics.
Subjects and Methods
Source Population
The study population was recruited from the MADRAD cohort (Clinicaltrials.gov No. NCT01415570) examining racial and ethnic differences in dietary factors and nutritional status among hemodialysis patients [29]. In this substudy, patients were enrolled from 6 outpatient dialysis units in Los Angeles County over the period of October 2011 to November 2011 (i.e., the first semester of the MADRAD study). Patients were included provided that they had undergone serum GDF15 measurements, were aged 18-85 years at the time of study entry (i.e., the date of serum GDF15 measurement), had received thrice-weekly in-center hemodialysis for at least 4 consecutive weeks, and signed a local institutional review board approved consent form. Patients were excluded if they were actively receiving peritoneal dialysis, had a life expectancy of less than 6 months (e.g., stage IV cancer), or were unable to provide consent without a proxy (e.g., suffering from dementia). The study was approved by the Institutional Review Boards of the Los Angeles Biomedical Research Institute at Harbor-UCLA (Torrance, CA, USA) and the University of California Irvine Medical Center (Orange, CA, USA).
Exposure Ascertainment
The exposure of interest was the serum GDF15 level ascertained at study entry. GDF15 levels were measured from thawed serum samples that were obtained before dialysis during weekday hemodialysis treatments at the time of study entry and that chronologically coincided with routine blood tests conducted at outpatient dialysis facilities, and they were immediately stored at −80°C. GDF15 was measured using enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, MN, USA). In primary analyses, we examined the association between serum GDF15 levels, categorized into tertiles (categorized as <4.21, 4.21 to <6.13, and ≥6.13 ng/mL for tertiles 1, 2, and 3, respectively) and all-cause mortality. In secondary analyses, GDF15 was considered as a continuous variable and scaled to a 1.0 and 4.0 ng/mL (approx. 1 SD) change. To flexibly model the association between continuous GDF15 levels and mortality, we also conducted analyses in which GDF15 was examined as a restricted cubic spline with knots corresponding to the 33rd and 66th percentiles of observed GDF15 values (4.18 and 6.08 ng/mL, respectively).
Outcome Ascertainment
The primary outcome of interest was all-cause mortality. At-risk time began the day after serum GDF15 measurement, and the patients were censored for kidney transplantation, transfer to a nonaffiliated outpatient dialysis unit or peritoneal dialysis, or at the end of the study (August 27, 2015). Each semester, information regarding mortality, censoring events, and associated dates from the preceding 6 months was collected from event forms completed by the MADRAD research coordinators and reviewed by 2 MADRAD study nephrologists (C.M.R. and K.K.-Z.) [29].
Sociodemographic, Comorbidity, and Laboratory Characteristics
Information on sociodemographics, comorbid conditions, and dialysis treatment characteristics were collected at study entry and every semester thereafter by MADRAD research coordinators [29]. Dialysis vintage was defined as the time between the date of study entry and the date of hemodialysis initiation. Routine dialysis laboratory measurements were performed by the outpatient dialysis laboratories on a monthly or quarterly basis using automated methods. In the present study, dialysis laboratory measurements (serum albumin, creatinine, phosphorus, and hemoglobin) were conducted on the date of or within 30 days of GDF15 measurement.
Statistical Analyses
Baseline characteristics between exposure groups were compared using p for trend tests calculated by ANOVA or Cochran-Armitage trend tests. We first examined the relationship of relevant clinical characteristics to high serum GDF15 levels at study entry (defined as GDF15 >66th percentile) using logistic regression. We then estimated the association between serum GDF15 tertiles and all-cause mortality using Cox proportional-hazard models. Logistic regression and the Cox regression models were analyzed using 2 incremental levels of covariate adjustment:
Unadjusted model: included serum GDF15 level as the primary exposure of interest
Case mix analyses: adjusted for covariates in the unadjusted model, as well as for age, sex, race, ethnicity, diabetes, and dialysis vintage
Given that higher GDF15 levels may be representative of both cardiovascular disease and wasting, we sought to parse out these potential pathways by conducting sensitivity analyses that incrementally adjusted for body mass index (BMI) and normalized protein catabolic rate (nPCR) in addition to case mix covariates in sensitivity analyses.
The proportional-hazards assumption was confirmed graphically. There were no missing values for age, sex, race, ethnicity, diabetes, and dialysis vintage. The analyses and figures were generated using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA), Stata version 13.1 (Stata Corporation, College Station, TX, USA), and SigmaPlot version 12.5 (Systat Software, San Jose, CA, USA).
Results
Study Population
Among 203 patients meeting the eligibility criteria, the mean ± SD, median (IQR), and minimum-to-maximum of the obtained GDF15 values were 5.94 ± 3.90, 5.05 (3.84, 6.67), and 1.58-39.8 ng/mL, respectively. Compared with patients in the lowest GDF15 tertile, patients in the highest tertile were older, were less likely to be of Black race, were more likely to be of Hispanic ethnicity, and had lower serum creatinine levels (Table 1). Patients in the highest tertile were also more likely to be male and to have underlying diabetes, a longer dialysis vintage, and lower serum phosphorus and higher hemoglobin levels, although the differences across tertiles were not statistically significant.
Table 1.
Baseline characteristics according to growth differentiation factor 15 (GDF15) levels categorized as tertiles in maintenance hemodialysis patients
| Overall | Tertile 1 <4.21 ng/mL | Tertile 2 4.21 to <6.13 ng/mL | Tertile 3 ≥6.13 ng/mL | p valuea | |
|---|---|---|---|---|---|
| Patients, % (n) | 100.0 (203) | 33.0 (67) | 33.5 (68) | 33.5 (68) | N/A |
| Case mix characteristics | |||||
| Mean age ± SD, years | 53.2±14.5 | 47.0±14.1 | 53.7±14.9 | 58.9±11.9 | <0.001 |
| Female, % | 41 | 45 | 43 | 35 | 0.26 |
| Black race, % | 34 | 42 | 38 | 21 | 0.009 |
| Hispanic ethnicity, % | 48 | 40 | 47 | 57 | 0.05 |
| Diabetes, % | 54 | 46 | 54 | 62 | 0.07 |
| Mean dialysis vintage ± SD, months | 55.6±53.4 | 46.8±49.2 | 68.7±64.3 | 51.1±42.6 | 0.65 |
| Laboratory testsb | |||||
| Serum albumin, g/dL | 4.1 (3.8, 4.3) | 4.1 (3.9, 4.3) | 4.1 (3.8, 4.3) | 4.0 (3.8, 4.3) | 0.25 |
| Creatinine, mg/dL | 9.5 (7.5, 11.9) | 10.1 (7.6, 13.3) | 9.6 (8.0, 11.7) | 8.9 (7.3, 10.6) | 0.01 |
| Phosphorus, mg/dL | 4.8 (4.1, 5.7) | 4.9 (4.1, 6.3) | 4.8 (4.0, 5.8) | 4.7 (4.1, 5.5) | 0.28 |
| Hemoglobin, g/dL | 10.6 (9.9, 11.0) | 10.4 (9.9, 10.9) | 10.6 (9.9, 11.0) | 10.7 (10.0, 11.2) | 0.18 |
| Body mass index | 26.2 (23.5, 31.0) | 27.1 (23.1, 34.5) | 26.9 (23.6, 31.5) | 25.5 (23.6, 28.7) | 0.003 |
| Normalized protein catabolic rate, g/kg/day | 1.04 (0.85, 1.22) | 0.97 (0.83, 1.16) | 1.06 (0.11, 0.86) | 1.05 (0.86, 1.23) | 0.27 |
p for trend estimated by ANOVA or Cochran-Armitage trend test.
Laboratory test results are presented as median (IQR).
Predictors of GDF15 Level
In the unadjusted and case mix-adjusted logistic regression analyses, patients of older age had a higher risk of having a high GDF15 level at study entry, whereas patients with a higher BMI had a lower likelihood of high GDF15 levels (Table 2). In the unadjusted analyses, patients of Black race and with higher serum creatinine levels had a lower likelihood of high GDF15 levels, but these associations were attenuated to null following adjustment for case mix covariates.
Table 2.
Clinical characteristics associated with high growth differentiation factor 15 (GDF15) levels (>66th percentile) using logistic regression
| Variable | Unadjusted |
Case mix adjusted |
||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Case mix characteristics | ||||
| Age (Δ10 years) | 1.57 (1.25–1.98) | <0.001 | 1.58 (1.23–2.02) | <0.001 |
| Female | 0.70 (0.39–1.28) | 0.25 | 0.62 (0.33–1.19) | 0.15 |
| Black race | 0.39 (0.20–0.77) | 0.007 | 0.43 (0.17–1.07) | 0.07 |
| Hispanic | 1.73 (0.96–3.12) | 0.07 | 1.12 (0.48–2.58) | 0.80 |
| Diabetes | 1.59 (0.88–2.88) | 0.13 | 1.00 (0.49–2.02) | 0.99 |
| Dialysis vintage (Δ1 year) | 0.97 (0.90–1.04) | 0.40 | 0.99 (0.92–1.07) | 0.79 |
| Laboratory tests | ||||
| Serum albumin, Δ0.5 g/dL | 0.76 (0.49–1.17) | 0.21 | 0.77 (0.47–1.26) | 0.30 |
| Creatinine (mg/dL) | 0.89 (0.80–0.98) | 0.02 | 0.96 (0.85–1.10) | 0.56 |
| Phosphorus (mg/dL) | 0.92 (0.75–1.11) | 0.37 | 1.03 (0.84–1.27) | 0.75 |
| Hemoglobin (g/dL) | 1.18 (0.89–1.57) | 0.25 | 1.21 (0.88–1.68) | 0.24 |
| Body mass index | 0.75 (0.58, 0.96) | 0.02 | 0.75 (0.56, 0.99) | 0.02 |
| Normalized protein catabolic rate (g/kg/day) | 1.05 (0.86, 1.28) | 0.61 | 0.99 (0.78, 1.24) | 0.90 |
Case mix analyses adjusted for age, sex, race, ethnicity, diabetes, and dialysis vintage. Bold values indicate significant associations with the highest GDF15 levels.
Association of GDF15 Levels with All-Cause Mortality
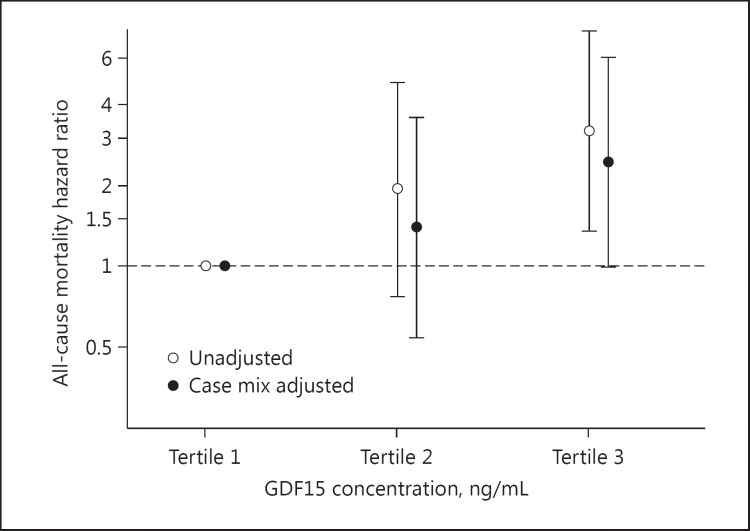
The patients contributed a total of 7,633 person-months of follow-up, during which time 40 all-cause death events occurred. The median (IQR) at-risk time was 45.8 (29.7, 46.0) months. In the unadjusted analyses, incrementally higher tertiles were associated with increasingly higher numerical risk, but this did not reach statistical significance for the middle tertile (reference: lowest tertile; hazard ratios [HRs] [95% CI] 1.94 [0.77-4.86] and 3.19 [1.35-7.55] for the middle and highest tertiles, respectively) (Fig. 1; Table 3). Upon adjustment for case mix covariates, incrementally higher tertiles were again associated with higher numerical risk, but this did not achieve statistical significance for the middle tertile (reference: lowest tertile; adjusted HRs [95% CI] 1.40 [0.54-3.59] and 2.45 [1.00-6.00] for the middle and highest tertiles, respectively). A similar pattern of findings was observed in the sensitivity analyses that were adjusted for case mix covariates + BMI + nPCR (adjusted HRs [95% CI] 1.57 [0.60-4.10] and 2.62 [1.03-6.63] for the middle and highest tertiles, respectively; p for trend = 0.03).
Fig. 1.
Association of growth differentiation factor 15 (GDF15) levels with all-cause mortality. Case mix analyses adjusted for age, sex, race, ethnicity, diabetes, and dialysis vintage.
Table 3.
Association of growth differentiation factor 15 (GDF15) levels with all-cause mortality
| Unadjusted |
Case mix adjusted |
Case mix + BMI + nPCR adjusted |
||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p valuea | HR (95% CI) | p valueb | HR (95% CI) | p valueb | |
| Categorical GDF15 analyses | ||||||
| GDF15 tertile 1 (<4.21 ng/mL) | 1 (Ref.) | N/A | 1 (Ref.) | N/A | 1 (Ref.) | N/A |
| GDF15 tertile 2 (4.21 to <6.13 ng/mL) | 1.94 (0.77–4.86) | 0.16 | 1.40 (0.54–3.59) | 0.49 | 1.57 (0.60–4.10) | 0.36 |
| GDF15 tertile 3 (≥6.13 ng/mL) | 3.19 (1.35–7.55) | 0.008 | 2.45 (1.00–6.00) | 0.05 | 2.62 (1.03–6.63) | 0.04 |
| Continuous GDF15 analyses | ||||||
| GDF15 by 1.0 ng/mL | 1.18 (1.11–1.26) | <0.001 | 1.17 (1.09–1.26) | <0.001 | 1.18 (1.10–1.27) | <0.001 |
| GDF15 by 4.0 ng/mL | 1.96 (1.51–2.55) | <0.001 | 1.90 (1.43–2.52) | <0.001 | 1.92 (1.44–2.57) | <0.001 |
Case mix-adjusted models adjusted for age, sex, race, ethnicity, diabetes, and dialysis vintage. BMI, body mass index; nPCR, normalized protein catabolic rate. a p for trend = 0.006. b p for trend = 0.03.
In additional analyses, we observed that a 1.0 ng/mL increase in GDF15 level was associated with an approximately 17-18% higher mortality risk in the unadjusted and case mix models (Table 3). Increments of approximately 1 SD (4.0 ng/mL increase in GDF15 level) were associated with a nearly 2-fold higher death risk. Similar estimates were observed in sensitivity analyses that adjusted for case mix covariates + BMI + nPCR.
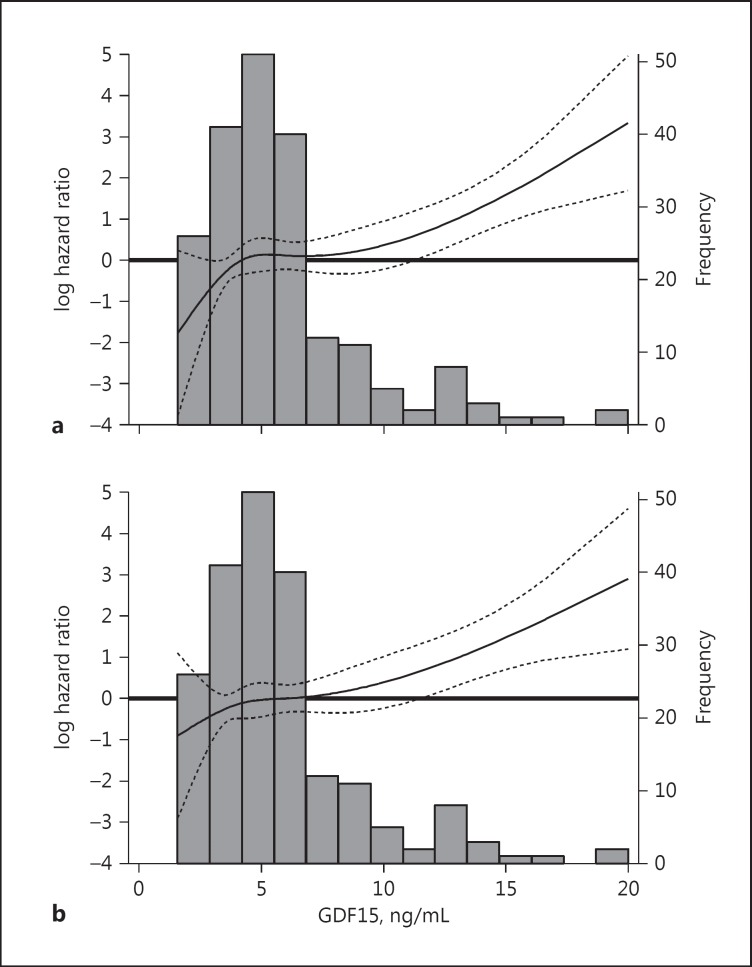
In analyses examining the association between continuous GDF15 levels and all-cause mortality using a cubic spline function, we observed that there was a monotonic increase in death risk across higher GDF15 levels exceeding 10 ng/mL in both unadjusted and case mix-adjusted analyses (Fig. 2).
Fig. 2.
Association between continuous growth differentiation factor 15 (GDF15) gradations and all-cause mortality using unadjusted (a) and case mix-adjusted (b) restricted cubic splines. The figures present log hazard ratios (short-dashed lines indicate 95% CIs) for GDF15 analyzed as a spline with knots at the 33rd and 66th percentiles of observed values (GDF15 values of 4.18 and 6.08 ng/mL, respectively). A histogram of the observed GDF15 values and a log hazard reference ratio of 0 (horizontal solid line) is overlaid. The case mix-adjusted model is adjusted for age, sex, race, ethnicity, diabetes, and dialysis vintage.
Discussion
In this prospective contemporary cohort of maintenance hemodialysis patients from the multicenter MADRAD study with a median follow-up of approximately 4 years, we found that higher circulating levels of GDF15 were associated with higher mortality risk independent of sociodemographic and comorbidity characteristics. In cross-sectional analyses adjusted for case mix covariates, we also observed that older age was directly associated with higher GDF15 levels, whereas higher BMI was inversely associated with higher GDF15 levels.
To date, there have been 2 studies that have examined the relationship between GDF15 levels and mortality in end-stage renal disease patients [27,28]. In one study of 381 prevalent US hemodialysis patients by Breit et al. [27], incrementally higher GDF15 levels (i.e., every 10 ng/mL increase) were independently associated with higher mortality risk. In the same study, among 98 incident hemodialysis patients from Sweden, higher GDF15 levels were independently associated with higher mortality in the first 3 years of dialysis. In the Swedish subcohort, higher GDF15 levels were also associated with worse self-reported nutrition as ascertained by Subjective Global Assessment questionnaires, as well as with lower BMIs (<25). In a subsequent study of 87 hemodialysis patients from Turkey without preexisting cardiovascular disease by Yilmaz et al. [28], higher GDF15 levels (i.e., every 10 ng/mL increase) were independently associated with higher mortality risk as well as subclinical atherosclerosis as assessed by carotid intima media thickness.
Our study expands upon the existing literature on GDF15 in end-stage renal disease patients across several fronts. In this racially/ethnically diverse cohort of hemodialysis patients (approx. 33% Black and 50% Hispanic patients), we observed that Black patients were less likely to have high GDF15 levels compared to those of non-Black race in unadjusted cross-sectional analyses. Conversely, there was a trend towards Hispanics having a higher likelihood of high GDF15 levels, although the estimates did not achieve statistical significance. In addition, we observed a persistent association between higher GDF15 levels and higher mortality risk in this racially and ethnically heterogeneous study population. While the small sample size of our cohort precluded us from separately examining GDF15-mortality associations across individual racial/ethnic subgroups, our findings indicate that GDF15 is an adverse prognostic marker even among minority kidney disease patients despite their established differences in underlying cardiovascular risk [30,31,32] and body anthropometry [33,34,35].
Another noteworthy finding of our study was the remarkably similar distribution of GDF15 levels in comparison to that found in the aforementioned study by Breit et al. [27] (ranges of 1.4-34.4 and 3.0-25.1 ng/mL and medians of 7.1 and 7.4 ng/mL in the Swedish and US cohorts, respectively). In non-ESRD populations, an incrementally higher prevalence of elevated GDF15 levels has been observed across the cardiovascular continuum, including those patients with stable coronary artery disease, congestive heart failure, and terminal heart failure [10]. Our data add to the growing body of literature showing that, similar to cardiovascular and cancer patients, the hemodialysis population displays higher GDF15 concentrations [28]. While a consistent association between higher GDF15 levels and kidney dysfunction has been observed [10], further studies are needed to establish the sources of GDF15 production in hemodialysis patients. Although the renal clearance of GDF15 has not yet been documented, animal studies suggest that GDF15 may undergo hepatic clearance via scavenger receptors expressed on liver sinusoidal cells [36].
At this time, the specific pathways by which GDF15 adversely impacts the survival of hemodialysis patients remain undefined. In terms of cardiovascular mechanisms, GDF15 is a product of activated macrophages [33] - which plays a prominent role in the pathogenesis of atherosclerosis and vascular thrombosis, as the final endpoint of atherosclerotic disease [37,38]. Two recent studies have in fact shown an antiatherogenic feature of GDF15-deficient mice, suggesting that low levels of GDF15 may be cardioprotective [39,40]. Cardiomyocytes also produce and secrete GDF15 in response to oxidative stress, ischemia, mechanical stretch, angiotensin II, and proinflammatory cytokines [41]. As cardiovascular disease models have shown that GDF15 may have apoptotic, antihypertrophic, and anti-inflammatory actions, it remains unclear as to whether GDF15 may have a counterregulatory role in cardiac injury [10]. With respect to protein-energy wasting pathways, GDF15 acts directly upon the hypothalamus to reduce food intake and energy expenditure [20]. In animal models, GDF15 administration has been shown to lead to satiety and weight reduction, which has been reversed or prevented by the administration of anti-GDF15 neutralizing antibodies [20,26,42]. Notably, in the sensitivity analyses we found that the relationship between higher GDF15 levels and higher mortality risk persisted following adjustment for body size (i.e., BMI) and protein intake (i.e., nPCR), suggesting that cardiovascular pathways may be implicated. However, given the large body of evidence showing the interrelationships between higher GDF15 levels, cardiovascular disease, anorexia, muscle and fat wasting, and mortality across multiple chronic disease cohorts, prospective controlled studies are needed to elucidate the causal implications of GDF15, and whether GDF15 may represent a novel therapeutic target for the fatal complications of cardiovascular disease and protein-energy wasting in these populations.
The strengths of our study include the following: its examination of a racially/ethnically diverse, multicenter study population; the uniform collection of GDF15 serum specimens in an outpatient setting and measurements within a single laboratory; and the rigorous, protocolized collection of sociodemographic, comorbidity, and laboratory data. However, several limitations of our study should be acknowledged. First, GDF15 levels were based upon measurements obtained at a single point in time upon study entry, and changes in levels over time were not considered. However, it bears mentioning that in certain analogous chronic disease populations (i.e., acute coronary syndrome patients without acute heart failure), GDF15 levels have been shown to remain stable over time [15,43]. Second, due to data limitations, we lacked information on cause-specific death. Third, due to the modest sample size of our cohort, we had limited power to examine associations within subgroups or to adjust for a large number of confounders. Lastly, given the observational nature of our study, our findings do not confirm a causal association between elevated GDF15 levels and higher death risk.
In conclusion, our study supports an independent association between higher GDF15 levels and mortality risk in a racially/ethnically diverse cohort of hemodialysis patients. As an emerging biomarker, further studies are needed to confirm the findings, to define the pathways by which GDF15 adversely impacts survival among hemodialysis patients, and to determine whether the reduction of GDF15 concentrations favorably impacts cardiovascular and metabolic health in this population.
Statement of Ethics
This study was approved by the Institutional Review Boards of the Los Angeles Biomedical Research Institute at Harbor-UCLA (Torrance, CA, USA) and the University of California Irvine Medical Center (Orange, CA, USA). All participants provided written informed consent.
Disclosure Statement
K.K.-Z. has received honoraria/support from AVEO Oncology.
Acknowledgements
The authors are supported by the research grants from the NIH/NIDDK including: K23-DK102903 (C.M.R.), K24-DK091419 (K.K.-Z.), and R01-DK092232 (D.V.N.); NIH/NCATS UL1-TR001414 (D.V.N., F.Z.); and philanthropist grants from Mr. Harold Simmons, Mr. Louis Chang, and Dr. Joseph Lee.
Portions of these data were presented as an abstract at the 2016 National Kidney Foundation Spring Clinical Meeting, April 27 to May 1, 2016, Boston, MA, USA.
References
- 1.United States Renal Data System . USRDS 2014 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda: USRDS; 2014. [Google Scholar]
- 2.Ortiz A, Massy ZA, Fliser D, Lindholm B, Wiecek A, Martínez-Castelao A, Covic A, Goldsmith D, Süleymanlar G, London GM, Zoccali C. Clinical usefulness of novel prognostic biomarkers in patients on hemodialysis. Nat Rev Nephrol. 2012;8:141–150. doi: 10.1038/nrneph.2011.170. [DOI] [PubMed] [Google Scholar]
- 3.Wheeler DC, Haynes R, Landray MJ, Baigent C. Cardiovascular aspects of kidney disease. In: Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu AS, Brenner BM, editors. Brenner and Rector's The Kidney. ed 9. Philadelphia: Elsevier Saunders; 2012. pp. 2060–2075. [Google Scholar]
- 4.Baskin HJ, Cobin RH, Duick DS, Gharib H, Guttler RB, Kaplan MM, Segal RL. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Endocr Pract. 2002;8:457–469. [PubMed] [Google Scholar]
- 5.Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, Zhang HP, Donnellan M, Mahler S, Pryor K, Walsh BJ, Nicholson RC, Fairlie WD, Por SB, Robbins JM, Breit SN. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci USA. 1997;94:11514–11519. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi C, Shi J, Xu Z. A review of murine models of latent tuberculosis infection. Scand J Infect Dis. 2011;43:848–856. doi: 10.3109/00365548.2011.603745. [DOI] [PubMed] [Google Scholar]
- 7.Wollert KC, Kempf T. Growth differentiation factor 15 in heart failure: an update. Curr Heart Fail Rep. 2012;9:337–345. doi: 10.1007/s11897-012-0113-9. [DOI] [PubMed] [Google Scholar]
- 8.Breit SN, Johnen H, Cook AD, Tsai VW, Mohammad MG, Kuffner T, Zhang HP, Marquis CP, Jiang L, Lockwood G, Lee-Ng M, Husaini Y, Wu L, Hamilton JA, Brown DA. The TGF-β superfamily cytokine, MIC-1/GDF15: a pleotrophic [sic!] cytokine with roles in inflammation, cancer and metabolism. Growth Factors. 2011;29:187–195. doi: 10.3109/08977194.2011.607137. [DOI] [PubMed] [Google Scholar]
- 9.Wiklund FE, Bennet AM, Magnusson PK, Eriksson UK, Lindmark F, Wu L, Yaghoutyfam N, Marquis CP, Stattin P, Pedersen NL, Adami HO, Grönberg H, Breit SN, Brown DA. Macrophage inhibitory cytokine-1 (MIC-1/GDF15): a new marker of all-cause mortality. Aging Cell. 2010;9:1057–1064. doi: 10.1111/j.1474-9726.2010.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bermúdez B, López S, Pacheco YM, Villar J, Muriana FJ, Hoheisel JD, Bauer A, Abia R. Influence of postprandial triglyceride-rich lipoproteins on lipid-mediated gene expression in smooth muscle cells of the human coronary artery. Cardiovasc Res. 2008;79:294–303. doi: 10.1093/cvr/cvn082. [DOI] [PubMed] [Google Scholar]
- 11.Kempf T, Eden M, Strelau J, Naguib M, Willenbockel C, Tongers J, Heineke J, Kotlarz D, Xu J, Molkentin JD, Niessen HW, Drexler H, Wollert KC. The transforming growth factor-β superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ Res. 2006;98:351–360. doi: 10.1161/01.RES.0000202805.73038.48. [DOI] [PubMed] [Google Scholar]
- 12.Schlittenhardt D, Schober A, Strelau J, Bonaterra GA, Schmiedt W, Unsicker K, Metz J, Kinscherf R. Involvement of growth differentiation factor-15/macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in oxLDL-induced apoptosis of human macrophages in vitro and in arteriosclerotic lesions. Cell Tissue Res. 2004;318:325–333. doi: 10.1007/s00441-004-0986-3. [DOI] [PubMed] [Google Scholar]
- 13.Lind L, Wallentin L, Kempf T, Tapken H, Quint A, Lindahl B, Olofsson S, Venge P, Larsson A, Hulthe J, Elmgren A, Wollert KC. Growth-differentiation factor-15 is an independent marker of cardiovascular dysfunction and disease in the elderly: results from the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) Study. Eur Heart J. 2009;30:2346–2353. doi: 10.1093/eurheartj/ehp261. [DOI] [PubMed] [Google Scholar]
- 14.Rohatgi A, Patel P, Das SR, Ayers CR, Khera A, Martinez-Rumayor A, Berry JD, McGuire DK, de Lemos JA. Association of growth differentiation factor-15 with coronary atherosclerosis and mortality in a young, multiethnic population: observations from the Dallas Heart Study. Clin Chem. 2012;58:172–182. doi: 10.1373/clinchem.2011.171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonaca MP, Morrow DA, Braunwald E, Cannon CP, Jiang S, Breher S, Sabatine MS, Kempf T, Wallentin L, Wollert KC. Growth differentiation factor-15 and risk of recurrent events in patients stabilized after acute coronary syndrome: observations from PROVE IT-TIMI 22. Arterioscler Thromb Vasc Biol. 2011;31:203–210. doi: 10.1161/ATVBAHA.110.213512. [DOI] [PubMed] [Google Scholar]
- 16.Brown DA, Breit SN, Buring J, Fairlie WD, Bauskin AR, Liu T, Ridker PM. Concentration in plasma of macrophage inhibitory cytokine-1 and risk of cardiovascular events in women: a nested case-control study. Lancet. 2002;359:2159–2163. doi: 10.1016/S0140-6736(02)09093-1. [DOI] [PubMed] [Google Scholar]
- 17.Wang TJ, Wollert KC, Larson MG, Coglianese E, McCabe EL, Cheng S, Ho JE, Fradley MG, Ghorbani A, Xanthakis V, Kempf T, Benjamin EJ, Levy D, Vasan RS, Januzzi JL. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation. 2012;126:1596–1604. doi: 10.1161/CIRCULATIONAHA.112.129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anand IS, Kempf T, Rector TS, Tapken H, Allhoff T, Jantzen F, Kuskowski M, Cohn JN, Drexler H, Wollert KC. Serial measurement of growth-differentiation factor-15 in heart failure: relation to disease severity and prognosis in the Valsartan Heart Failure Trial. Circulation. 2010;122:1387–1395. doi: 10.1161/CIRCULATIONAHA.109.928846. [DOI] [PubMed] [Google Scholar]
- 19.Kempf T, von Haehling S, Peter T, Allhoff T, Cicoira M, Doehner W, Ponikowski P, Filippatos GS, Rozentryt P, Drexler H, Anker SD, Wollert KC. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1054–1060. doi: 10.1016/j.jacc.2007.04.091. [DOI] [PubMed] [Google Scholar]
- 20.Johnen H, Lin S, Kuffner T, Brown DA, Tsai VW, Bauskin AR, Wu L, Pankhurst G, Jiang L, Junankar S, Hunter M, Fairlie WD, Lee NJ, Enriquez RF, Baldock PA, Corey E, Apple FS, Murakami MM, Lin EJ, Wang C, During MJ, Sainsbury A, Herzog H, Breit SN. Tumor-induced anorexia and weight loss are mediated by the TGF-β superfamily cytokine MIC-1. Nat Med. 2007;13:1333–1340. doi: 10.1038/nm1677. [DOI] [PubMed] [Google Scholar]
- 21.Lerner L, Hayes TG, Tao N, Krieger B, Feng B, Wu Z, Nicoletti R, Chiu MI, Gyuris J, Garcia JM. Plasma growth differentiation factor 15 is associated with weight loss and mortality in cancer patients. J Cachexia Sarcopenia Muscle. 2015;6:317–324. doi: 10.1002/jcsm.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Mutsert R, Grootendorst DC, Axelsson J, Boeschoten EW, Krediet RT, Dekker FW. Excess mortality due to interaction between protein-energy wasting, inflammation and cardiovascular disease in chronic dialysis patients. Nephrol Dial Transplant. 2008;23:2957–2964. doi: 10.1093/ndt/gfn167. [DOI] [PubMed] [Google Scholar]
- 23.Kalantar-Zadeh K, Rhee C, Sim JJ, Stenvinkel P, Anker SD, Kovesdy CP. Why cachexia kills: examining the causality of poor outcomes in wasting conditions. J Cachexia Sarcopenia Muscle. 2013;4:89–94. doi: 10.1007/s13539-013-0111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mak RH, Ikizler AT, Kovesdy CP, Raj DS, Stenvinkel P, Kalantar-Zadeh K. Wasting in chronic kidney disease. J Cachexia Sarcopenia Muscle. 2011;2:9–25. doi: 10.1007/s13539-011-0019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obi Y, Qader H, Kovesdy CP, Kalantar-Zadeh K. Latest consensus and update on protein-energy wasting in chronic kidney disease. Curr Opin Clin Nutr Metab Care. 2015;18:254–262. doi: 10.1097/MCO.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lerner L, Tao J, Liu Q, Nicoletti R, Feng B, Krieger B, Mazsa E, Siddiquee Z, Wang R, Huang L, Shen L, Lin J, Vigano A, Chiu MI, Weng Z, Winston W, Weiler S, Gyuris J. MAP3K11/GDF15 axis is a critical driver of cancer cachexia. J Cachexia Sarcopenia Muscle. 2016;7:467–482. doi: 10.1002/jcsm.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breit SN, Carrero JJ, Tsai VW, Yagoutifam N, Luo W, Kuffner T, Bauskin AR, Wu L, Jiang L, Barany P, Heimburger O, Murikami MA, Apple FS, Marquis CP, Macia L, Lin S, Sainsbury A, Herzog H, Law M, Stenvinkel P, Brown DA. Macrophage inhibitory cytokine-1 (MIC-1/GDF15) and mortality in end-stage renal disease. Nephrol Dial Transplant. 2012;27:70–75. doi: 10.1093/ndt/gfr575. [DOI] [PubMed] [Google Scholar]
- 28.Yilmaz H, Çelik HT, Gurel OM, Bilgic MA, Namuslu M, Bozkurt H, Ayyildiz A, Inan O, Bavbek N, Akcay A. Increased serum levels of GDF-15 associated with mortality and subclinical atherosclerosis in patients on maintenance hemodialysis. Herz. 2015;40(suppl 3):305–312. doi: 10.1007/s00059-014-4139-5. [DOI] [PubMed] [Google Scholar]
- 29.Rhee CM, Nguyen DV, Moradi H, Brunelli SM, Dukkipati R, Jing J, Nakata T, Kovesdy CP, Brent GA, Kalantar-Zadeh K. Association of adiponectin with body composition and mortality in hemodialysis patients. Am J Kidney Dis. 2015;66:313–321. doi: 10.1053/j.ajkd.2015.02.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arce CM, Goldstein BA, Mitani AA, Winkelmayer WC. Trends in relative mortality between Hispanic and non-Hispanic whites initiating dialysis: a retrospective study of the US Renal Data System. Am J Kidney Dis. 2013;62:312–321. doi: 10.1053/j.ajkd.2013.02.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fischer MJ, Go AS, Lora CM, Ackerson L, Cohan J, Kusek JW, Mercado A, Ojo A, Ricardo AC, Rosen LK, Tao K, Xie D, Feldman HI, Lash JP. CKD in Hispanics: baseline characteristics from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic-CRIC studies. Am J Kidney Dis. 2011;58:214–227. doi: 10.1053/j.ajkd.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lash JP, Ricardo AC, Roy J, Deo R, Fischer M, Flack J, He J, Keane M, Lora C, Ojo A, Rahman M, Steigerwalt S, Tao K, Wolf M, Wright JT, Jr, Go AS. Race/ethnicity and cardiovascular outcomes in adults with CKD: findings from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic CRIC studies. Am J Kidney Dis. 2016;68:545–553. doi: 10.1053/j.ajkd.2016.03.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noori N, Kovesdy CP, Dukkipati R, Feroze U, Molnar MZ, Bross R, Nissenson AR, Kopple JD, Norris KC, Kalantar-Zadeh K. Racial and ethnic differences in mortality of hemodialysis patients: role of dietary and nutritional status and inflammation. Am J Nephrol. 2011;33:157–167. doi: 10.1159/000323972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park J, Jin DC, Molnar MZ, Dukkipati R, Kim YL, Jing J, Levin NW, Nissenson AR, Lee JS, Kalantar-Zadeh K. Mortality predictability of body size and muscle mass surrogates in Asian vs white and African American hemodialysis patients. Mayo Clin Proc. 2013;88:479–486. doi: 10.1016/j.mayocp.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ricks J, Molnar MZ, Kovesdy CP, Kopple JD, Norris KC, Mehrotra R, Nissenson AR, Arah OA, Greenland S, Kalantar-Zadeh K. Racial and ethnic differences in the association of body mass index and survival in maintenance hemodialysis patients. Am J Kidney Dis. 2011;58:574–582. doi: 10.1053/j.ajkd.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schledzewski K, Géraud C, Arnold B, Wang S, Gröne HJ, Kempf T, Wollert KC, Straub BK, Schirmacher P, Demory A, Schönhaber H, Gratchev A, Dietz L, Thierse HJ, Kzhyshkowska J, Goerdt S. Deficiency of liver sinusoidal scavenger receptors stabilin-1 and −2 in mice causes glomerulofibrotic nephropathy via impaired hepatic clearance of noxious blood factors. J Clin Invest. 2011;121:703–714. doi: 10.1172/JCI44740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ross R. Atherosclerosis - an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 38.Taddei S, Virdis A. Growth differentiation factor-15 and cardiovascular dysfunction and disease: malefactor or innocent bystander? Eur Heart J. 2010;31:1168–1171. doi: 10.1093/eurheartj/ehq077. [DOI] [PubMed] [Google Scholar]
- 39.Bonaterra GA, Zügel S, Thogersen J, Walter SA, Haberkorn U, Strelau J, Kinscherf R. Growth differentiation factor-15 deficiency inhibits atherosclerosis progression by regulating interleukin-6-dependent inflammatory response to vascular injury. J Am Heart Assoc. 2012;1:e002550. doi: 10.1161/JAHA.112.002550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jager KJ, van Dijk PC, Zoccali C, Dekker FW. The analysis of survival data: the Kaplan-Meier method. Kidney Int. 2008;74:560–565. doi: 10.1038/ki.2008.217. [DOI] [PubMed] [Google Scholar]
- 41.Widera C, Giannitsis E, Kempf T, Korf-Klingebiel M, Fiedler B, Sharma S, Katus HA, Asaumi Y, Shimano M, Walsh K, Wollert KC. Identification of follistatin-like 1 by expression cloning as an activator of the growth differentiation factor 15 gene and a prognostic biomarker in acute coronary syndrome. Clin Chem. 2012;58:1233–1241. doi: 10.1373/clinchem.2012.182816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsai VW, Macia L, Johnen H, Kuffner T, Manadhar R, Jørgensen SB, Lee-Ng KK, Zhang HP, Wu L, Marquis CP, Jiang L, Husaini Y, Lin S, Herzog H, Brown DA, Sainsbury A, Breit SN. TGF-β superfamily cytokine MIC-1/GDF15 is a physiological appetite and body weight regulator. PLoS One. 2013;8:e55174. doi: 10.1371/journal.pone.0055174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eggers KM, Kempf T, Lagerqvist B, Lindahl B, Olofsson S, Jantzen F, Peter T, Allhoff T, Siegbahn A, Venge P, Wollert KC, Wallentin L. Growth-differentiation factor-15 for long-term risk prediction in patients stabilized after an episode of non-ST-segment-elevation acute coronary syndrome. Circ Cardiovasc Genet. 2010;3:88–96. doi: 10.1161/CIRCGENETICS.109.877456. [DOI] [PubMed] [Google Scholar]