Abstract
Dendritic cells (DCs) play a central role as major targets of dengue virus (DV) infections and initiators of antiviral immune responses. Previous observations showed that DCs are activated by infection, presumably acquiring the capacity to promote cell-mediated immunity. However, separate evaluations of the maturation profiles of infected and uninfected bystander cells show that infection impairs the ability of DCs to upregulate cell surface expression of costimulatory, maturation, and major histocompatibility complex molecules, resulting in reduced T-cell stimulatory capacity. Infected DCs failed to respond to tumor necrosis factor alpha as an additional maturation stimulus and were apoptotic. Interleukin 10 (IL-10) was detected in supernatants from cultures of DV-infected DCs and cocultures of DCs and T cells. Taken together, these results constitute an immune evasion strategy used by DV that directly impairs antigen-presenting cell function by maturation blockade and induction of apoptosis.
Dengue virus (DV) infection causes substantial morbidity and mortality in the tropics and subtropics (25, 32, 35), but at this time, there are no licensed, safe, and effective vaccines against DV. Data from several laboratories suggest that human dendritic cells (DCs) and Langerhans cells are the early, primary targets of DV in natural infections (13, 19, 21, 42). These studies show that DCs become activated upon exposure to live virus and express phenotypic changes characterized by enhanced cell surface expression of costimulatory molecules, major histocompatibility complex (MHC) class I and II molecules, and the maturation marker, CD83 (17). However, further examination of these data reveal heterogeneities in the expression of cell surface molecules on DCs exposed to DV (19), suggesting that only a fraction of DV-exposed cells become activated in response to infection. Data from Wu et al. (42) and Libraty et al. (19) support the viewpoint that the activation of DV-infected DCs is weaker than the activation of surrounding uninfected DCs; however, this issue has not been fully investigated.
An emerging characteristic of acute DV infection is diminished T-cell proliferative responses to mitogen and DV antigens (2, 22) resulting from defective antigen-presenting cells (22). Studies have also shown high levels of interleukin 10 (IL-10) circulating in the plasma of DV-infected individuals (2, 3, 9, 18) that could account for the reduced T-cell proliferation observed during acute viral infection. In addition to its suppressive effects on proliferation, elevated levels of IL-10 impair inflammatory immune responses by downregulating the synthesis of a wide range of inflammatory cytokines, including IL-12, and promoting the release of cytokine inhibitors (4, 24, 27, 30). The primary cellular source of IL-10 during DV infection is yet to be demonstrated, and at this time, it is unknown whether DV-infected DCs participate in the immunosuppressive process. Given the pivotal role that DCs play in the initiation of immune responses and modulation of DC function by many viruses (6, 8, 10, 12, 36), it is conceivable that DV also displays mechanisms to inhibit DC activation. In the present study, we show that after DV exposure, infected DCs undergo apoptosis, express a less mature phenotype than that of the surrounding, uninfected DCs, produce IL-10, and have reduced T-cell stimulatory capacity, suggesting a mechanism for previously observed clinical findings.
MATERIALS AND METHODS
Media and reagents.
Complete RPMI medium consisted of RPMI 1640 medium, 1% l-glutamine, 1% penicillin and streptomycin, 1% sodium pyruvate, 1% essential amino acids, 50 mM 2-mercaptoethanol, and 10% heat-inactivated fetal calf serum (all from GIBCO BRL, Gaithersburg, Md.). Recombinant human cytokines, specifically, recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF) (100 ng/ml) (Leukine; Immunex, Seattle, Wash.) and recombinant human IL-4 (rhIL-4) (50 ng/ml) (R&D Systems, Minneapolis, Minn.), were added to the medium.
Virus preparation.
DV type 2 (DV-2) (strain 16803) was grown and propagated in Mycoplasma-free Vero cell lines. The viral titer was 3 × 108 PFU/ml, as determined by limiting dilution plaque assays on Vero cells. The presence of contaminating lipopolysaccharide in the virus stock and culture supernatants was evaluated by the Limulus Amebocyte Lysate test (BioWhittaker, Inc., Walkersville, Md.). Mycoplasma contamination of the virus stocks and culture supernatants was evaluated by the Mycoplasma Rapid Detection System (Gen-Probe, San Diego, Calif.). All virus stocks and culture supernatants utilized in the present study were free from lipopolysaccharide and mycoplasma. Formalin-inactivated DV-2 (S16803; purified inactivated virus [PIV]) was kindly provided by Ken Eckels and Russ Olson, Department of Biologics, Walter Reed Army Institute of Research, Silver Spring, Md.
Generation of DCs.
Peripheral blood mononuclear cells were collected from whole-blood units from healthy, seronegative consenting donors under a protocol approved by the American Red Cross Institutional Review Board. CD14+ cells were isolated by positive selection according to the manufacturer's specifications using CD14 microbeads and a magnetic cell separator (MACS) (Miltenyi Biotech, Inc., Auburn, Calif.). Typically, the purity of monocytes isolated by this procedure was ≥95%. Enriched CD14+ cells were cultured for 6 days in six-well plates in complete RPMI medium (106 cells/ml) in the presence of rhGM-CSF and rhIL-4 at 37°C, 5% CO2, and 95% relative humidity. On day 3, half of the medium was replaced with fresh medium supplemented with cytokines. The majority of the gated DC population (>95%) was CD11c+ and CD1a+; <1% of the cells expressed CD14.
Infection of DCs with DV.
Unless stated otherwise, DCs were infected with DV-2 at a multiplicity of infection (MOI) of 1 for 2.5 h at 37°C and 5% CO2. Cells were washed twice to remove cell-free virus and cultured in complete RPMI medium (without cytokines) at a density of 106 cells/ml in 24-well plates. Cells were analyzed after 48 h unless stated otherwise. Control experiments included mock-infected DCs and DCs infected with PIV at the same MOI as that used for the wild-type virus.
MAbs for surface and intracellular staining.
For detection of surface markers, infected and uninfected (bystander) DCs were stained with the following phycoerythrin (PE)-conjugated monoclonal antibodies (MAbs) according to the manufacturer's recommendations (Pharmingen, San Diego, Calif.): CD40 (5C3), CD80 (L307.4), CD83 (HB15e), CD86 (IT2.2), HLA-A,B,C (G46-2.6), and HLA-DR (G46-6). Isotype-matched PE-labeled controls (Pharmingen) were included in each experiment.
Intracellular detection of virus.
Surface-labeled DCs were fixed and permeabilized with Cytofix and CytoPerm (Pharmingen) according to the manufacturer's recommendations. The cells were then stained for intracellular expression of viral envelope protein using a fluorescein isothiocyanate (FITC)-labeled MAb, 2H2. This allowed gating of 2H2-positive and -negative cells. The samples were analyzed on a FACScan instrument (Becton Dickinson, Mountain View, Calif.) using Cell Quest software.
Cytometric analyses of apoptotic cells.
The viability of DV-infected DCs was compared to mock-infected DCs at 24 and 48 h. Cells were stained with propidium iodide (PI)- and -conjugated annexin V using the Annexin-V FITC apoptosis detection kit I (Becton Dickinson) according to the manufacturer's recommendations. For intracellular detection of active caspase 3, DCs were fixed and permeabilized with Cytofix and CytoPerm (Pharmingen) and then stained with PE-conjugated polyclonal anti-caspase 3 antibody (Pharmingen). The samples were analyzed on a FACScan instrument using Cell Quest software.
Magnetic isolation of annexin-positive and -negative cells was performed using the apoptotic cell isolation kit (Miltenyi Biotech). Briefly, DCs were labeled with annexin V coupled to magnetic beads. The suspension of cells and beads was loaded onto a MACS column, and both positive and negative fractions were collected. Both populations were stained with MAb 2H2 to evaluate the proportion of infected (2H2-positive) cells in each fraction.
Cytokine production in culture supernatants.
Gamma interferon (IFN-α) was measured by an enzyme-linked immunosorbent assay (ELISA) performed according to the manufacturer's recommendations (PBL Biomedical Labs, Piscataway, N.J.). Samples were read on a SpectraMax 340 plate reader (Molecular Devices Corp., Sunnyvale, Calif.). All other cytokines were quantified by an inflammatory response cytometric bead array assay on a FACScan instrument using Cell Quest software (Becton Dickinson).
T-lymphocyte stimulation.
DCs were exposed for 24 h to live or PIV DV-2 at MOIs of 0.1 to 3.0 or to 1 μg of Staphylococcus aureus (Calbiochem, San Diego, Calif.) per ml. CD3+ T cells were positively selected from the peripheral blood mononuclear cells of adult donors using CD3+ microbeads (Miltenyi Biotech) and cocultured with DCs exposed to different agents in four replicate samples in complete RPMI medium for 5 days at stimulator/responder ratios of 1:10 and 1:100. Proliferation was measured by adding 0.5 μCi of [3H]thymidine per well for the last 18 h of culture. Plates were read on a 1450 Microbeta liquid scintillation and luminescence counter (Perkin-Elmer Life Sciences, Inc., Boston, Mass.).
RESULTS
Expression of cell surface molecules is downregulated on DV-infected, but not bystander, DCs.
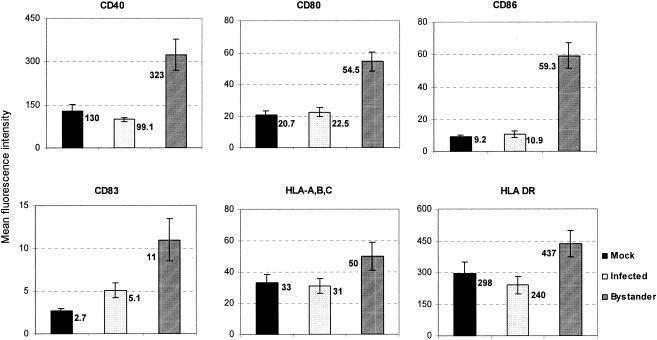
Previous studies described cell surface expression of costimulatory and other molecules on DV-exposed DCs regardless of their infectivity status (13, 19). By segregating DV-infected DCs from uninfected, bystander DCs using the 2H2 MAb, we found that bystander, but not DV-infected, DCs upregulated the cell surface expression of costimulatory, MHC, and activation molecules on their cell surfaces (Fig. 1), demonstrating different phenotypic outcomes related to infectivity status.
FIG. 1.
The levels of expression of costimulatory, maturation, and MHC molecules on the cell surface are downregulated in DV-infected DCs. DV-infected or mock-infected (uninfected) controls were cultured for 48 h and then stained with PE-conjugated cell surface MAb and FITC-conjugated 2H2 MAb specific for viral premembrane and envelope glycoprotein, respectively. Gating was performed on 2H2-positive (infected) DCs or 2H2-negative cells (bystander and uninfected) DCs. The results are expressed as mean fluorescence intensities ± standard errors of the means (error bars) and are from eight independent cultures with a mean infection rate of 66.6% ± 9.29%.
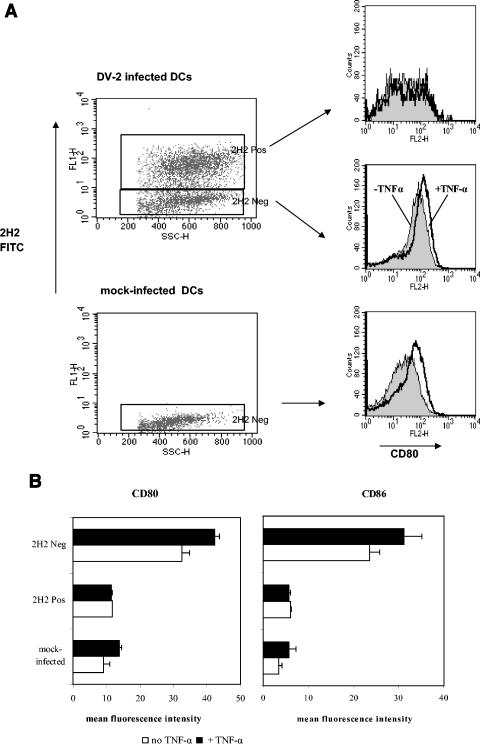
DC maturation can be further regulated by other stimuli, including tumor necrosis factor alpha (TNF-α). In subsequent experiments, DV-infected DCs and bystander cells were exposed to TNF-α and assessed for upregulation of costimulatory molecules CD80 and CD86 (Fig. 2). Exposure of mock-infected or bystander DCs to TNF-α resulted in increased cell surface expression of CD80 with less pronounced enhancement of CD86. In contrast, on DV-infected DCs, the levels of both molecules remained relatively unchanged after exposure to TNF-α, suggesting that the ability to upregulate these markers was impaired in DV-infected DCs.
FIG. 2.
DV-infected DCs fail to upregulate CD80 and CD86 after exposure to TNF-α. Infected or mock-infected (uninfected) DCs were cultured for 24 h and then exposed to TNF-α for an additional 24 h. Cells were stained with PE-conjugated cell surface MAb and FITC-conjugated 2H2 MAb (2H2 FITC), and gating was performed on 2H2-positive and -negative DCs. (A) Histograms illustrate changes in CD80 expression after exposure to TNF-α (+TNF-α). (B) Fluorescence intensities of cell surface expression of CD80 and CD86 in the presence or absence of TNF-α. Values are the means ± standard errors of the means (error bars) for three independent experiments. Neg, negative; Pos, positive.
Infection with DV induces apoptosis of infected DCs.
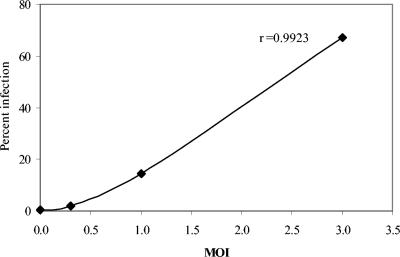
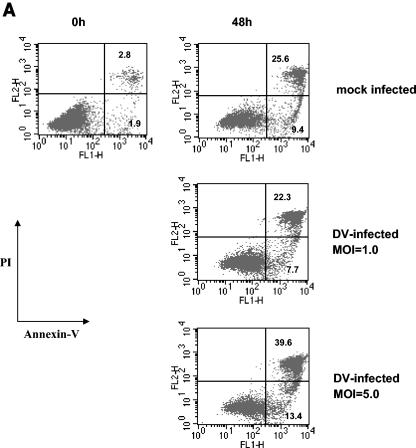
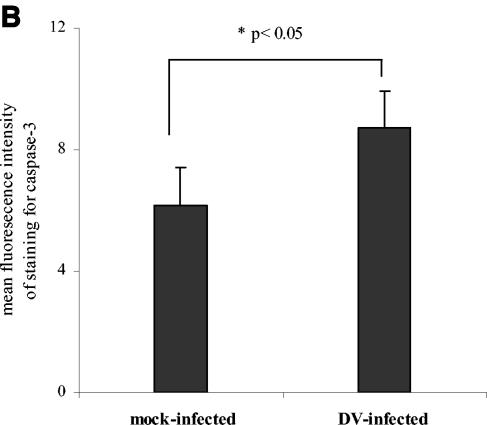
We speculated that similar to DV infection of endothelial cells (1), infection of DCs might induce apoptosis, contributing to the failure of the cells to upregulate surface molecules. In initial experiments, cells exposed to DV were stained with annexin V and PI to detect viable, early and late apoptotic cells. Since exposure of DCs to increasing MOIs was associated with increased infection levels (Fig. 3), apoptosis was evaluated in cultures infected with DV at increasing MOIs. At a low MOI (MOI of 1.0), we observed similar levels of apoptosis in DV- and mock-infected cultures (Fig. 4A). However, an increase in the numbers of apoptotic cells was observed at 48 h postinfection in cultures infected at a higher MOI (MOI of 5.0) (Fig. 4A). Since DC infection levels increased with increasing MOIs (Fig. 3), virus burden might be an important trigger for apoptosis. Further confirmation of apoptosis in cultures infected with DV at a high MOI was obtained from intracellular staining experiments showing significantly higher levels of caspase 3, an intracellular cysteine protease activated during the early stages of apoptosis (Fig. 4B).
FIG. 3.
Exposure of DCs to increasing MOIs is associated with increased infection levels. The plot represents the mean infection levels from two separate experiments.
FIG. 4.
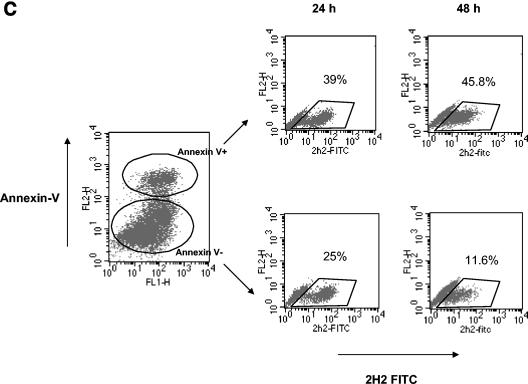
DV-infected DCs undergo apoptosis. (A) FITC-conjugated annexin V and PI staining of DV-infected and mock-infected (uninfected) DCs. The percentages of annexin V-positive, PI-negative cells representing early stage apoptosis and the percentages of annexin V-positive, PI-positive labeling representing end-stage apoptosis are shown. (B) Active caspase 3 levels in DV- and mock-infected cultures. The values are the means ± standard errors of the means (error bars) for three experiments. The values for mock- and DV-infected cultures (asterisk) were significantly different (P ≤ 0.03) by a paired t test. (C) At 24 and 48 h postinfection, DV-infected DCs were stained with annexin V MAb, and the annexin V-positive and -negative cells (Annexin V+ and Annexin V−, respectively) were subsequently isolated using an apoptotic cell isolation kit. Both populations were fixed, permeabilized, and stained with FITC-conjugated MAb 2H2 (2H2 FITC). The percentage of 2H2-positive cells in each fraction is shown. The data are representative of two separate experiments.
To directly evaluate the proportion of infected (2H2-positive) DCs undergoing apoptosis, infected DC cultures were sorted into annexin V-positive and -negative fractions by magnetic selection and then stained with 2H2 MAb. Morphological alterations that occurred after fixation or permeabilization permitted clear delineation of only the 2H2-positive population (Fig. 4C). The data show an increase in the number of infected (2H2-positive) DCs in the annexin-positive fraction (39 to 45.8%) and a concurrent decline in the annexin-negative fraction in cultures 48 h after infection (25 to 11.6%) (Fig. 4C). These results support the possibility that infected DCs constitute the bulk of the apoptotic cells in DV-infected cultures.
DV-infected DCs show impaired stimulation of allogeneic T cells.
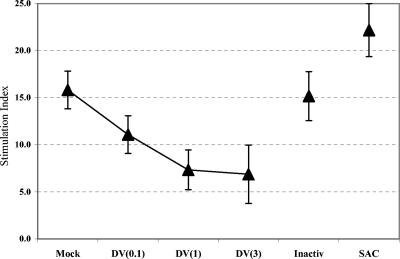
Reduced expression of costimulatory and maturation markers on infected DCs might compromise their ability to stimulate T cells. To investigate this possibility, we cocultured DV-infected cultures with allogeneic T cells and assessed T-cell proliferation by [3H]thymidine incorporation. The data show reduced T-cell stimulatory capabilities of DV-infected DC cultures (Fig. 5). However, T-cell responses were not entirely suppressed, raising the possibility of involvement of activated bystander DCs that were also present in infected cultures. In these cultures, infectivity levels increased at high MOIs (Fig. 3). Although these studies did not distinguish bystander DCs from DV-infected DCs, the detection of decreased proliferation with increasing MOI in a closed culture system suggests that the degree of stimulation achieved was partially dependent on the availability of bystander cells for T-cell activation. The nonlinearity of proliferation responses at high MOIs (Fig. 5) suggest that additional factors, including secreted cytokines, might also influence the T-cell stimulatory capacities of the DCs in these cultures.
FIG. 5.
DV-infected cultures display reduced stimulatory capacity for T cells in mixed-lymphocyte reaction assays. DCs were infected with DV at MOIs of 0.1, 1, and 3 [DV(0.1), DV(1), and DV(3), respectively], PIV (Inactiv), or S. aureus (SAC) for 24 h prior to coculturing with allogeneic CD3+ T cells. Mock-infected (uninfected) DCs were included as controls. Proliferation was measured via tritiated thymidine uptake as counts per minute (cpm). Stimulation indices for cultures containing DC and T cells at a ratio of 1:100 are shown. The stimulation index was calculated as follows: (cpm + number of T cells)/(cpm − number of T cells). Values are means ± standard errors of the means (error bars). Results are representative of five independent experiments.
DV induces TNF-α, IFN-α, and IL-10 from DCs, with enhancement of IL-10 in cocultures with T cells.
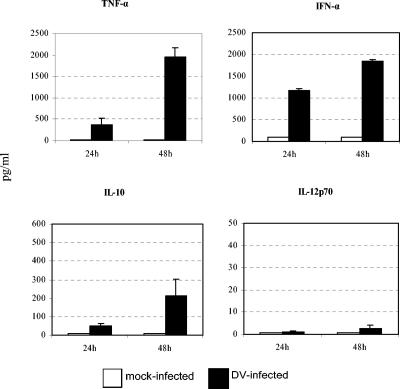
Similar to other investigators (13, 19), we detected large amounts of TNF-α and IFN-α and minimal amounts of IL-12p70 in DC-infected cultures compared to uninfected control cultures (Fig. 6). Peak production of TNF-α and IFN-α occurred at 48 h, a time period that coincided with maximum expression of surface molecules on bystander cells (Fig. 6).
FIG. 6.
DV-infected cultures secrete large amounts of TNF-α and IFN-α and increased but variable amounts of IL-10. DV-infected DCs or mock-infected (uninfected) controls were cultured for 48 h and were analyzed 24 or 48 h postinfection. Cell-free supernatants were removed and assessed by an ELISA (for IFN-α) or cytometric bead array analysis (for TNF-α, IL-10, and IL-12p70). The levels (in picograms per milliliter) of TNF-α, IFN-α, IL-10, and IL-12p70 in mock- and DV-infected DCs are shown. The values are means ± standard errors of the means (error bars) from three independent experiments.
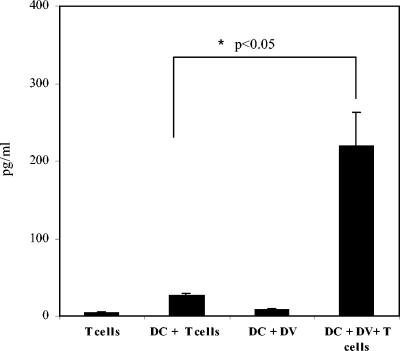
Levels of IL-10 were also increased in DV-infected cultures than in uninfected control cultures; however, cultures infected at the same MOI produced variable amounts of this cytokine (range 35 to >300 pg/ml [Fig. 6]). Peak production of IL-10 occurred at 48 h (Fig. 6) and subsided thereafter to minimal levels in cells cultured for 5 days (Fig. 7). Peak levels of IL-10 coincided with peak production of both TNF-α and IFN-α, raising the possibility that after the initial DC-DV encounter, the production of IL-10 may mitigate DC activation or maturation by TNF-α and IFN-α or may suppress the overstimulation of the immune response by proinflammatory cytokines (4, 5, 24). It is also likely that elevation of IL-10 levels in DC-T-cell cocultures might have contributed to the suppressed alloresponses detected in these cultures. Therefore, we measured levels of this cytokine in supernatants collected from cocultures of DCs and alloreactive T cells. We detected significant increases in IL-10 levels in cells cultured for 5 days, indicating that IL-10 is a prominent cytokine produced after DC-T-cell interaction during DV infection (Fig. 7).
FIG. 7.
Enhanced levels of IL-10 are detected in cocultures of DV-infected DCs and allogeneic T cells. DV-infected or mock-infected (uninfected) DCs were cultured for 5 days with T cells at a DC/T-cell ratio of 1:100. Cell-free supernatants were removed and assessed by cytometric bead array analyses for IL-10. The levels (in picograms per milliliter) of IL-10 in different cultures are shown. The values are means ± standard errors of the means (error bars) from three independent experiments. The values for the coculture of DCs and T cells (DC + T cells) and the coculture of DV-infected DCs and T cells (DC + DV + T cells) were significantly different (P < 0.05) (asterisk).
DISCUSSION
DCs are the main initiators of immune responses in naïve persons (7, 23), but their ability to fulfill this function could be compromised when they become targets of invading pathogens (6, 8, 10, 12, 36). To date, this phenomenon has not been fully described for DCs infected with DV. Although previous studies investigated the outcome of DV infection of DCs, they did not specifically distinguish infected DCs from bystander cells after exposure to DV or address the issue of T-cell activation (13, 19). By evaluating phenotypic changes in these two populations in the present study, we found that DV infection induces apoptosis of infected DCs and impairs their ability to upregulate cell surface costimulatory, maturation, and MHC molecules.
In contrast to DV-infected DCs, bystander DCs upregulated their expression of cell surface costimulatory and other molecules. Their activation was likely induced by TNF-α and IFN-α secreted by DV-infected DCs. DC activation by TNF-α alone or in synergy with IFN-α has been described in canarypox (14) and adenovirus (31) infections and under various culture conditions (20, 34). TNF-α mediates its effects via NF-κB, resulting in the regulation of many genes involved in immune and inflammatory responses. One outcome is increased expression of cell surface costimulatory and activation molecules on DCs and enhancement of their antigen-presenting capacities (20, 31). In our studies, exposure to exogenous TNF-α induced upregulation of CD80 and CD86 but only on bystander cells. DV-infected DCs failed to upregulate these molecules, suggesting an impairment in their ability to respond to a maturation stimulus.
Recent studies show that IFN-α alone also stimulates DC maturation (20, 26, 35, 37), and many viruses have evolved strategies to avoid this effect (29). DV encodes three viral proteins that inhibit IFN-α/β signaling in infected cells (28), presumably blocking their ability to initiate IFN-α/β-mediated maturational events, including the upregulation of MHC and costimulatory molecules (20, 26). Thus, the ability of infected cells to become activated is impaired, but the cells are still able to induce the maturation of neighboring, uninfected cells through their production of IFN-α.
We detected increased apoptosis of DV-infected DCs that might also serve as maturation stimuli for the bystander cells. Bystander DC maturation resulting from engulfment of infected DCs has been described for a number of pathogens, including canarypox (14), measles (38), human immunodeficiency virus (HIV) (16), and salmonella (40). The apoptotic bodies might also serve as sources of antigen and provide a reservoir of antigens that could be presented by bystander DCs as demonstrated in HIV, salmonella, and toxoplasma infections (16, 40, 41). The cross-presentation of antigens by these cells may provide an alternative route for initiating immune responses in the absence of functional DV-infected DCs (11).
Clinical data show that antigen-presenting cells in patients suffering from acute DV infections are unable to stimulate T-cell responses to mitogens and DV antigens (22). Although the cell type mediating this suppression was not identified in this study, the central roles played by DCs both as major replication targets of DV and as initiators of immunity make them obvious targets for immune evasion by the virus. Our in vitro data suggest that the suppression induced by DV infection reduced the ability of DCs to fully stimulate T cells in a mixed-lymphocyte reaction. The reduction in T-cell stimulation was most pronounced in cultures exposed to high MOIs with increased numbers of infected DCs. This finding suggests a requirement for mature bystander DCs for stimulation and further demonstrates a potential role for these cells in eliciting immune responses against DV.
DCs mature from a processing to presenting stage promoted by interaction with pathogens. Factors that inhibit the maturation of antigen-presenting cells include IL-10; IL-10 levels are enhanced in patients with dengue fever (2, 3, 9). In our studies, IL-10 was detected after exposing DCs to DV. IL-10 plays a role in immune suppression and can be inhibitory in a variety of ways: the secretion of IL-10 downregulates the expression of costimulatory, MHC, and other cell surface molecules, resulting in depressed antigen presentation and suppressed immune responses (5, 24, 27, 30, 39). IL-10 also has counterregulatory properties to a number of proinflammatory cytokines, including IL-12 (4, 15, 28). In mice and humans, IL-10 can induce antigen-specific anergy in CD4+ T cells (27, 39), and the detection of enhanced IL-10 levels in cocultures of DV-exposed DCs and T cells raises the possibility that secretion of this cytokine during DV infection could suppress immune responses against the virus.
Our findings provide evidence of a strategy utilized by DV to evade immune recognition and could link in vitro observations to clinical findings described in earlier studies (2, 3, 9, 17, 22).
Acknowledgments
We thank Robert Putnak, Ken Eckels, and Russ Olson, Walter Reed Army Institute of Research, Silver Spring, Md., for providing the PIV.
This work was supported by work unit number WUN 61102A.870.S.A0015 and by DAMD17-02-0005.
The opinions and assertions contained herein are not to be construed as official or as reflecting the views of the U.S. Army, U.S. Navy, or the naval services at large.
REFERENCES
- 1.Avirutnan, P., P. Malasit, B. Seliger, S. Bhakdi, and M. Husmann. 1998. Dengue virus infection of human endothelial cells leads to chemokine production, complement activation, and apoptosis. J. Immunol. 161:6338-6346. [PubMed] [Google Scholar]
- 2.Azeredo, E. L., S. M. Zagne, M. A. Santiago, A. S. Gouvea, A. A. Santana, P. C. Neves-Souza, R. M. Noguiera, M. P. Miagostovich, and C. F. Kubelka. 2001. Characterisation of lymphocyte response and cytokine patterns in patients with dengue fever. Immunobiology 204:494-507. [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi, U. C., R. Agarwal, E. A. Elbishbishi, and A. S. Mustafa. 2000. Cytokine cascade in dengue hemorrhagic fever: implications for pathogenesis. FEMS Immunol. Med. Microbiol. 28:183-188. [DOI] [PubMed] [Google Scholar]
- 4.Demangel, C., P. Bertolino, and W. J. Britton. 2002. Autocrine IL-10 impairs dendritic cell (DC)-derived immune responses to mycobacterial infection by suppressing DC trafficking to draining lymph nodes and local IL-12 production. Eur. J. Immunol. 32:994-1002. [DOI] [PubMed] [Google Scholar]
- 5.De Smedt, T., M. Van Mechelen, G. De Becker, J. Urbain, O. Leo, and M. Moser. 1997. Effect of interleukin-10 on dendritic cell maturation and function. Eur. J. Immunol. 27:1229-1235. [DOI] [PubMed] [Google Scholar]
- 6.Fugier-Vivier, I., C. Servet-Delprat, P. Rivailler, M. C. Rissoan, Y. J. Liu, and C. Rabourdin-Combe. 1997. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J. Exp. Med. 186:813-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grabbe, S., E. Kampgen, and G. Schuler. 2002. Dendritic cells: multi-linear and multi-functional. Trends Immunol. Today 21:431-433. [DOI] [PubMed] [Google Scholar]
- 8.Granelli-Piperno, A., A. Golebiowska, C. Trumpfheller, F. Siegal, and R. M. Steiman. 2004. HIV-1-infected monocyte-derived dendritic cells do not undergo maturation but elicit IL-10 production and T cell regulation. Proc. Natl. Acad. Sci. USA 101:7669-7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green, S., D. W. Vaughn, S. Kalayanarooj, S. Nimmannitya, S. Suntayakorn, A. Nisalak, A. L. Rothman, and F. A. Ennis. 1999. Elevated plasma interleukin-10 levels in acute dengue correlate with disease severity. J. Med. Virol. 59:329-334. [PubMed] [Google Scholar]
- 10.Grigoleit, U., S. Riegler, H. Einsele, K. Sampaio, G. Jahn, H. Hebart, P. Brossart, F. Frank, and C. Sinzger. 2002. Human cytomegalovirus induces a direct inhibitory effect on antigen presentation by monocyte-derived immature dendritic cells. Br. J. Haematol. 119:189-198. [DOI] [PubMed] [Google Scholar]
- 11.Heath, W. R., and F. R. Carbone. 2001. Cross-presentation, dendritic cell, tolerance and immunity. Annu. Rev. Immunol. 19:7-64. [DOI] [PubMed] [Google Scholar]
- 12.Hertel, L., V. G. Lacaille, H. Strobl, E. D. Mellins, and E. S. Mocarski. 2003. Susceptibility of immature and mature Langerhans cell-type dendritic cells to infection and immunomodulation by human cytomegalovirus. J. Virol. 77:7563-7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho, L. J., J. J. Wang, M. F. Shaio, C. L. Kao, D. M. Chang, S. W. Han, and J. H. Lai. 2001. Infection of human dendritic cells by dengue virus causes cell maturation and cytokine production. J. Immunol. 166:1499-1506. [DOI] [PubMed] [Google Scholar]
- 14.Ignatius, R., M. Marovich, E. Mehlhop, L. Villamide, K. Mahnke, W. I. Cox, F. Isdell, S. S. Frankel, J. R. Mascola, R. M. Steinman, and M. Pope. 2000. Canarypox virus-induced maturation of dendritic cells is mediated by apoptotic cell death and tumor necrosis factor alpha secretion. J. Virol. 74:11329-11338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komastu, T., D. Ireland, and C. S. Reiss. 1998. IL-12 and viral infections. Cytokine Growth Factor Rev. 9:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsson, M., J. F. Fonteneau, M. Lirvall, P. Haslett, J. D. Lifson, and N. Bhardwaj. 2002. Activation of HIV-1 specific CD4 and CD8 T cells by human dendritic cells: roles for cross-presentation and noninfectious HIV-1 virus. AIDS 16:1319-1329. [DOI] [PubMed] [Google Scholar]
- 17.Lechmann, M., S. Berchtold, J. Hauber, and A. Steinkasserer. 2002. CD83 on dendritic cells: more than just a marker for maturation. Trends Immunol. 23:273-275. [DOI] [PubMed] [Google Scholar]
- 18.Libraty, D. H., T. P. Endy, H. S. Huong, S. Green, S. Kalayanarooj, S. Suntayakorn, W. Chansiriwongs, D. W. Vaughn, A. Nisalak, F. A. Ennis, and A. L. Rothman. 2002. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J. Infect. Dis. 185:1213-1221. [DOI] [PubMed] [Google Scholar]
- 19.Libraty, D. H., S. Pichyangkul, C. Ajariyakhajorn, T. P. Endy, and F. A. Ennis. 2001. Human dendritic cells are activated by dengue virus infection: enhancement by gamma interferon and implications for disease pathogenesis. J. Virol. 75:3501-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luft, T., K. C. Pang, E. Thomas, P. Hertzog, D. N. Hart, J. Trapani, and J. Cebon. 1998. Type I IFNs enhance the terminal differentiation of dendritic cells. J. Immunol. 161:1947-1953. [PubMed] [Google Scholar]
- 21.Marovich, M., G. Grouard-Vogel, M. Louder, M. Eller, W. Sun, S. J. Wu, R. Putvatana, G. Murphy, B. Tassaneetrithep, T. Burgess, D. Birx, C. Hayes, S. Schlesinger-Frankel, and J. Mascola. 2001. Human dendritic cells as targets of dengue virus infection. J. Soc. Investig. Dermatol. Symp. Proc. 6:219-224. [DOI] [PubMed] [Google Scholar]
- 22.Mathew, A., I. Kurane, S. Green, D. W. Vaughn, S. Kalayanarooj, S. Suntayakorn, F. A. Ennis, and A. L. Rothman. 1999. Impaired T cell proliferation in acute dengue infection. J. Immunol. 162:5609-5615. [PubMed] [Google Scholar]
- 23.Mellman, I., and R. M. Steinman. 2001. Dendritic cells: specialized and regulated antigen-processing machines. Cell 106:255-258. [DOI] [PubMed] [Google Scholar]
- 24.Mocellin, S., M. Panelli, E. Wang, D. Nagorsen, and F. M. Marincola. 2003. The dual role of IL-10. Trends Immunol. 24:36-43. [DOI] [PubMed] [Google Scholar]
- 25.Monath, T. 1994. Dengue: the risk to developed and developing countries. Proc. Natl. Acad. Sci. USA 91:2395-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montoya, M., G. Schiavoni, F. Mattei, I. Gresser, F. Belardelli, P. Borrow, and D. F. Tough. 2002. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood 99:3263-3271. [DOI] [PubMed] [Google Scholar]
- 27.Moore, K. W., R. de Waal Malefyt, R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683-765. [DOI] [PubMed] [Google Scholar]
- 28.Munoz-Jordan, J. L., G. G. Sanchez-Burgos, M. Laurent-Rolle, and A. Garcia-Sastre. 2003. Inhibition of interferon signaling by dengue virus. Proc. Natl. Acad. Sci. USA 100:14333-14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noraz, N., J. L. Lathey, and S. A. Spector. 1997. Human cytomegalovirus-associated immunosuppression is mediated through interferon-α. Blood 89:2443-2452. [PubMed] [Google Scholar]
- 30.Payvandi, F., S. Amrute, and P. Fitzgerald-Bocarsly. 1998. Exogenous and endogenous IL-10 regulate IFN-alpha production by peripheral blood mononuclear cells in response to viral stimulation. J. Immunol. 160:5861-5868. [PubMed] [Google Scholar]
- 31.Philpott, N. J., M. Nociari, K. B. Elkon, and E. Falck-Pederson. 2004. Adenovirus-induced maturation of dendritic cells through a PI3 kinase-mediated TNF-alpha induction pathway. Proc. Natl. Acad. Sci. USA 101:6200-6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinheiro, F. P., and S. J. Corber. 1997. Global situation of dengue and dengue haemorrhagic fever, and its emergence in the Americas. World Health Stat. Q. 50:161-169. [PubMed] [Google Scholar]
- 33.Qi, H., V. Popov, and L. Soong. 2001. Leishmania amazonensis-dendritic cell interactions in vitro and the priming of parasite-specific CD4+ T cells in vivo. J. Immunol. 167:4534-4542. [DOI] [PubMed] [Google Scholar]
- 34.Reddy, A., M. Sapp, M. Feldman, M. Subklewe, and N. Bhardwaj. 1997. A monocyte conditioned medium is more effective than defined cytokines in mediating the terminal maturation of human dendritic cells. Blood 90:3640-3646. [PubMed] [Google Scholar]
- 35.Rosen, L. 1986. Dengue hemorrhagic fever: a critical appraisal of current hypotheses. Jpn. J. Trop. Med. Hyg. 14:117-122. [Google Scholar]
- 36.Salio, M., M. Cella, M. Suter, and A. Lanzavecchia. 1999. Inhibition of dendritic cell maturation by herpes simplex virus. Eur. J. Immunol. 29:3245-3253. [DOI] [PubMed] [Google Scholar]
- 37.Santini, S. M., D. Pucchio, C. Lapenta, S. Parlato, M. Logozzi, and F. Belardelli. 2002. The natural alliance between type I interferon and dendritic cells and its role in linking innate and adaptive immunity. J. Interferon Cytokine Res. 22:1071-1080. [DOI] [PubMed] [Google Scholar]
- 38.Servet-Delprat, C., P. O. Vidalain, O. Azocar, F. Le Deist, A. Fischer, and C. Rabourdin-Combe. 2000. Consequences of Fas-mediated human dendritic cell apoptosis induced by measles virus. J. Virol. 74:4387-4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steinbrink, K., M. Wolfl, H. Jonuleit, J. Knop, and A. H. Enk. 1997. Induction of tolerance by IL-10-treated dendritic cells. J. Immunol. 159:4772-4780. [PubMed] [Google Scholar]
- 40.Sundquist, M., A. Rydstrom, and M. J. Wick. 2004. Immunity to Salmonella from a dendritic point of view. Cell. Microbiol. 6:1-11. [DOI] [PubMed] [Google Scholar]
- 41.Wei, S., F. Marches, J. Borvak, W. Zou, J. Channon, M. White, J. Radke, M.-F. Cesbron-Delauw, and T. J. Curiel. 2002. Toxoplasma gondii-infected human myeloid dendritic cells induce T-lymphocyte dysfunction and contact-dependent apoptosis. Infect. Immun. 70:1750-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu, S.-J., G. Grouard-Vogel, W. Sun, J. R. Mascola, E. Brachtel, R. Putvatana, M. K. Louder, L. Filgueira, M. A. Marovich, H. K. Wong, A. Blauvelt, G. S. Murphy, M. L. Robb, B. L. Innes, D. L. Birx, C. G. Hayes, and S. S. Frankel. 2000. Human skin Langerhans cells are targets of dengue virus infection. Nat. Med. 6:816-820. [DOI] [PubMed] [Google Scholar]