Abstract
This report presents the case of a 60-year-old woman who was diagnosed with stage IV lung adenocarcinoma with asymptomatic brain metastases and commenced chemotherapy with cisplatin/pemetrexed (CDDP/Pem). She experienced tonic-clonic convulsions on day 9 of the first cycle, which were accompanied by increased blood pressure (173/69 mm Hg) and headache. Therefore, brain MRI was performed to check for stroke or progression of brain metastatic foci. T2-weighted, FLAIR, and ADC map images showed high-intensity areas in the subcortical region of the bilateral parieto-occipital lobes, leading to a diagnosis of posterior reversible encephalopathy syndrome (PRES). The symptoms improved after treatment with antihypertensive and antiepileptic drugs. Clinicians should keep it in mind that central nervous system symptoms during anticancer therapy containing Pem may indicate possible PRES.
Key Words: Posterior reversible encephalopathy syndrome, Pemetrexed, Cisplatin
Introduction
There have been various reports of posterior reversible encephalopathy syndrome (PRES) since it was first reported by Hinchey et al. in 1996 [1]. Radiologically, it is characterized by a reversible MRI finding of high-intensity areas in the subcortical region of the parieto-occipital lobe on T2-weighted, FLAIR, and ADC map images. Pathologically, it is a reversible edematous change rather than an infarct. Clinical findings include headache, alterations in consciousness, epilepsy, and nausea, which peak in severity within 12–48 h of onset and then resolve [2]. Here we report a patient with pulmonary adenocarcinoma complicated by PRES following systemic chemotherapy with the pemetrexed (Pem) regimen.
Case Report
The case was a 60-year-old woman who presented to our hospital with increasing cervical lymphadenopathy. Detailed examination revealed stage IV lung adenocarcinoma with asymptomatic brain metastases and cervical lymph node metastases (T4N3M1b, epidermal growth factor receptor mutation-negative, anaplastic lymphoma kinase translocation-negative). She had a history of hypertension and diabetes, which were well controlled, and a performance status of 0. She commenced first-line chemotherapy with a combination of cisplatin (CDDP; 80 mg/m2, day 1, q3w) and Pem (500 mg/m2, day 1, q3w). Tonic-clonic convulsions suddenly occurred on day 9 of the first cycle of chemotherapy. The convulsions were accompanied by increased blood pressure (173/69 mm Hg) and headache, indicating possible stroke or progression of brain metastatic foci. Therefore, brain MRI was performed, showing high-intensity areas in the subcortical region of the bilateral parieto-occipital lobes on T2-weighted, FLAIR, and ADC map images (Fig. 1). There was no clear evidence of hemorrhage, infarction, or increased metastatic foci. A diagnosis of PRES was made based on the seizures and brain MRI findings. Both hyperthyroidism and autoimmune disease, which can induce PRES, were ruled out. The symptoms decreased after treatment with antiepileptic (phenytoin and carbamazepine) and antihypertensive drugs (amlodipine). Eight days after onset, brain MRI showed that the areas with abnormal intensity had disappeared. Therefore, she was successfully discharged from the hospital 11 days after onset.
Fig. 1.
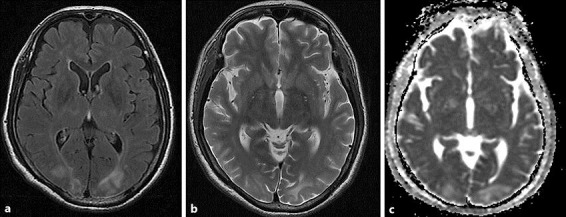
MRI showing high-intensity areas in the subcortical region of the bilateral parieto-occipital lobes on T2-weighted (a), FLAIR (b), and ADC map images (c).
Discussion
PRES is triggered by various factors, including hypertension, renal failure, drugs, and autoimmune disease, but no precise mechanism has been identified. Plausible hypotheses include reduced cerebral autoregulation and blood-brain barrier impairment due to vascular endothelial cell damage, which may result in edematous change [3, 4]. Various drugs are causative, including cytocidal anticancer drugs, molecularly targeted drugs, immunosuppressants, long-term steroids, immunoglobulin preparations, erythropoietin preparations, and IFN-α [4, 5]. PRES has been reported with both monotherapy and multiple drug therapy with cytocidal anticancer drugs, including CDDP and methotrexate, which is a folate antagonist [6, 7, 8].
Since the present case of PRES was a patient treated with CDDP/Pem, it is difficult to determine which anticancer drug was responsible. In 2016, Xie and Jones [9] reported a case of PRES associated with CDDP/Pem, which was concluded to be caused by CDDP based on the absence of previous reports of PRES related to Pem alone. In 2009, Nguyen et al. [10] also reported a case of PRES associated with CDDP/Pem, which was attributed to neurotoxicity of CDDP enhanced by dexamethasone for treating nausea and vomiting. On the other hand, Chen et al. [11] reported 3 cases of PRES in a phase II clinical study conducted with CDDP/Pem/ziv-aflibercept and considered PRES to be due to vascular endothelial cell damage caused by the VEGF inhibitor ziv-aflibercept.
However, it should be noted that Pem is a folate antagonist, a drug of the same class as methotrexate, which induces PRES. The pharmacological mechanism of methotrexate is based on its inhibition of cellular dihydrofolate reductase, resulting in inhibition of the formation of tetrahydrofolic acid, which is necessary for DNA synthesis, to exhibit an antitumor effect [12]. In addition, methotrexate sometimes causes central neurotoxicity, which is characterized by convulsion and focal neurological deficits [7]. Pem also exhibits an antitumor effect by inhibiting multiple folate-metabolizing enzymes, including cellular dihydrofolate reductase [13]. Therefore, we are of the opinion that Pem, like methotrexate, could at least partially cause PRES.
At present, Pem is an important therapeutic option for stage IV nonsquamous non-small-cell lung cancer and is increasingly used as monotherapy. Clinicians should keep it in mind that central nervous system symptoms such as seizure during anticancer therapy containing Pem may indicate possible PRES.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no financial disclosures to make.
References
- 1.Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, Pessin MS, Lamy C, Mas JL, Caplan LR. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 2.Roth C, Ferbert A. The posterior reversible encephalopathy syndrome: what's certain, what's new? Pract Neurol. 2011;11:136–144. doi: 10.1136/practneurol-2011-000010. [DOI] [PubMed] [Google Scholar]
- 3.Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29:1043–1049. doi: 10.3174/ajnr.A0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Legriel S, Pico F, Azoulay E. Understanding posterior reversible encephalopathy syndrome. In: Vincent JL, editor. Annual Update in Intensive Care and Emergency Medicine. Berlin/Heidelberg: Springer; 2011. pp. 631–653. [Google Scholar]
- 5.Rajasekhar A, George TJ., Jr Gemcitabine-induced reversible posterior leukoencephalopathy syndrome: a case report and review of the literature. Oncologist. 2007;12:1332–1335. doi: 10.1634/theoncologist.12-11-1332. [DOI] [PubMed] [Google Scholar]
- 6.Zahir MN, Masood N, Shabbir-Moosajee M. Cisplatin-induced posterior reversible encephalopathy syndrome and successful re-treatment in a patient with non-seminomatous germ cell tumor: a case report. J Med Case Rep. 2012;6:409. doi: 10.1186/1752-1947-6-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dicuonzo F, Salvati A, Palma M, Lefons V, Lasalandra G, De Leonardis F, Santoro N. Posterior reversible encephalopathy syndrome associated with methotrexate neurotoxicity: conventional magnetic resonance and diffusion-weighted imaging findings. J Child Neurol. 2009;24:1013–1018. doi: 10.1177/0883073809332705. [DOI] [PubMed] [Google Scholar]
- 8.Aradillas E, Arora R, Gasperino J. Methotrexate-induced posterior reversible encephalopathy syndrome. J Clin Pharm Ther. 2011;36:529–536. doi: 10.1111/j.1365-2710.2010.01207.x. [DOI] [PubMed] [Google Scholar]
- 9.Xie C, Jones VT. Reversible posterior leukoencephalopathy syndrome following combinatorial cisplatin and pemetrexed therapy for lung cancer in a normotensive patient: a case report and literature review. Oncol Lett. 2016;11:1512–1516. doi: 10.3892/ol.2015.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen MT, Virk IY, Chew L, Villano JL. Extended use dexamethasone-associated posterior reversible encephalopathy syndrome with cisplatin-based chemotherapy. J Clin Neurosci. 2009;16:1688–1690. doi: 10.1016/j.jocn.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Modiano MR, Neal JW, Brahmer JR, Rigas JR, Jotte RM, Leighl NB, Riess JW, Kuo CJ, Liu L, Gao B, DiCioccio AT, Adjei AA, Wakelee HA. A phase II multicentre study of ziv-aflibercept in combination with cisplatin and pemetrexed in patients with previously untreated advanced/metastatic non-squamous non-small cell lung cancer. Br J Cancer. 2014;110:602–608. doi: 10.1038/bjc.2013.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jolivet J, Chabner BA. Intracellular pharmacokinetics of methotrexate polyglutamates in human breast cancer cells. Selective retention and less dissociable binding of 4-NH2-10-CH3-pteroylglutamate4 and 4-NH2-10-CH3-pteroylglutamate5 to dihydrofolate reductase. J Clin Invest. 1983;72:773–778. doi: 10.1172/JCI111048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shih C, Chen VJ, Gossett LS, Gates SB, MacKellar WC, Habeck LL, Shackelford KA, Mendelsohn LG, Soose DJ, Patel VF, Andis SL, Bewley JR, Rayl EA, Moroson BA, Beardsley GP, Kohler W, Ratnam M, Schultz RM. LY231514, a pyrrolo[2,3-d]pyrimidine-based antifolate that inhibits multiple folate-requiring enzymes. Cancer Res. 1997;57:1116–1123. [PubMed] [Google Scholar]


