Abstract
Solid organ transplantation provides life-saving therapy for patients with end-stage organ disease, and its outcomes have been improving dramatically over the past few decades. However, substantial morbidity results from chronic immunosuppressive therapy administered to prevent graft rejection. It predisposes patients to several life-threatening complications, such as opportunistic microbial infections and the development of different types of cancers. Here, we presented the case of a young man with probable Lynch syndrome, who developed an aggressive colon carcinoma after long-term immunosuppressive therapy due to a prior liver transplantation. Based on this case report, we attempt to find an answer to the question about the risk of cancer development or recurrence in patients with familial syndromes receiving long-term immunosuppressive therapy and to find out how it can be minimized. Answering these questions is particularly important, given the facts that disease course is substantially more aggressive among transplanted patients and that prognosis is poor due to lack of immunocompetence, especially in the setting of Lynch syndrome.
Key Words: Rapid disease progression, Liver metastases, Resection, Liver-transplanted patient, Lynch syndrome
Introduction
Solid organ transplantation provides life-saving therapy for patients with end-stage organ disease, and its outcomes have been improving dramatically over the past few decades. However, substantial morbidity results from chronic immunosuppressive therapy administered to prevent graft rejection [1]. It predisposes patients to several life-threatening complications, such as opportunistic microbial infections and the development of different types of cancers [2, 3].
Approximately 10–30% of all deaths among transplant recipients are attributed to posttransplant malignancy, with an upward trend in this incidence due to prolonged immunosuppression. It has been estimated that solid organ transplant recipients have a 3-fold excess risk of cancer relative to the age- and sex-matched general population [3]. Although the risk is higher for malignancies caused by viral infections (lymphomas, Kaposi sarcoma, hepatocellular carcinoma, and anogenital cancers), several other malignancies have been reported in transplant recipients, such as cancers of the kidney, lung, skin, and thyroid cancer [1]. An increased risk of colorectal cancer (CRC) among solid organ transplant recipients has also been found in several retrospective studies [4, 5, 6, 7, 8, 9], as well as in a meta-analysis [10], where the risk of developing CRC was 2.6 times higher in liver transplant recipients when compared to nontransplant patients.
Hereditary nonpolyposis CRC, also commonly referred to as Lynch syndrome, has been defined as a familial syndrome with an increased incidence of CRC and/or other extracolonic tumors [11, 12], such as cancers of the endometrium, upper gastrointestinal tract, urinary tract, ovarium, hepatobiliary tract, pancreas, and brain [13]. Approximately half of all hereditary nonpolyposis CRC cases are caused by DNA mismatch repair (MMR) pathway defects [14, 15]. Germline mutations in MMR genes (MLH1, MSH2, MSH6, and PMS2) are responsible for these cases, which comprises about 2–4% of all CRC cancers. Most commonly, in about 90% of the cases, mutations in the MLH1 and MSH2 genes are detected [14, 15]. These mutations lead to microsatellite instability (MSI). Distinct from the majority of CRCs with chromosomal instability which harbor allelic imbalance from chromosomal aneuploidy or polyploidy, MSI tumors retain intact chromosomal numbers. However, they contain microsatellite repeats due to deficiency in MMR which are thought to contribute to the early steps of tumorigenesis in CRC.
Most colorectal tumors in Lynch syndrome are found in the right colon [15, 16] and have an earlier median age of onset than the general population (45 vs. 63 years) [13]. They also demonstrate accelerated carcinogenesis, as adenomas can develop into carcinomas within 2–3 years [13]. On histology, they are typically poorly differentiated, with mucinous or signet ring cell features [17].
The epidemiology and the course of posttransplantation de novo neoplasia in the context of Lynch syndrome are completely unknown. Here, we report the case of a young patient with probable Lynch syndrome (his family declined germline test) who developed a right colon cancer after 14 years of immunosuppressive therapy following liver transplantation and had a very aggressive course of the disease, which was probably worsened by decreased immunosurveillance.
Case Report
A 39-year-old man had undergone hepatic transplantation due to liver insufficiency secondary to hepatitis A virus infection in 2002 at the age of 25. Since then, he was started on immunosuppressive therapy (cyclosporine 75 mg twice daily and azathioprine 50 mg once daily). After the transplant, he underwent an irregular follow-up. Family history was positive for colon cancer in his brother at the age of 40.
In September 2015, he noticed a hardened mass in the right iliac fossa as well as a 6-kg weight loss. An abdominal CT was requested and showed an irregular and circumferential thickening involving the cecum and the middle and distal thirds of the ascending colon with an extension of approximately 13.5 cm (Fig. 1). There was also a hypodense nodule in segment IV measuring 1.2 cm (Fig. 2). He then underwent a colonoscopy which confirmed an ulcerated mass at the cecum and ascending colon (Fig. 3). Biopsy was consistent with a poorly differentiated adenocarcinoma with MLH1 and PMS2 expression loss, consistent with microsatellite instability. On November 5th, 2015, he underwent right hemicolectomy, intraoperative liver ultrasound, and resection of segments IV and V (a new lesion was found during liver ultrasound). Pathology confirmed a poorly differentiated adenocarcinoma pT3 pN2a pM1a. Both liver lesions were consistent with metastatic deposits. On November 11th, 2015, postoperative CT scan showed no evidence of new nodules (Fig. 4).
Fig. 1.
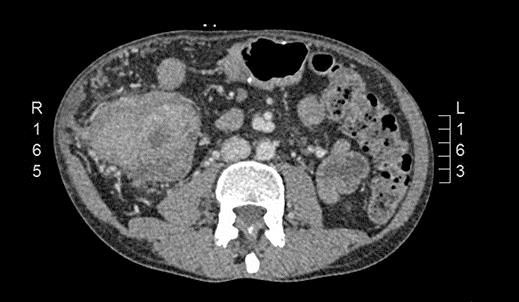
CT scan showing an irregular and circumferential thickening involving the cecum and the middle and distal thirds of the ascending colon with an extension of approximately 13.5 cm.
Fig. 2.
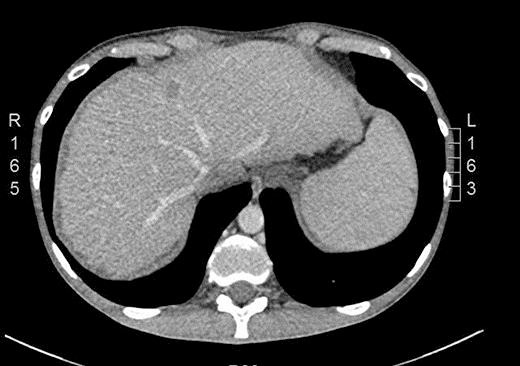
CT scan showing a hypodense nodule in segment IV measuring 1.2 cm.
Fig. 3.
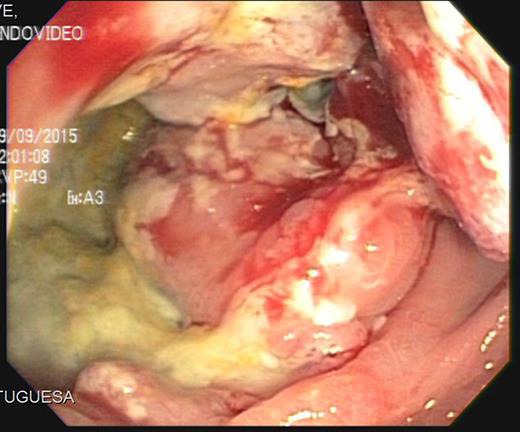
Colonoscopy showing an ulcerated mass at the cecum and ascending colon.
Fig. 4.
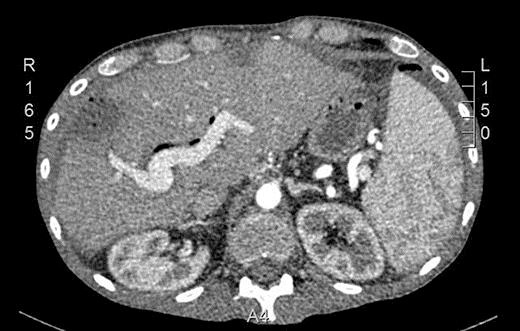
Postoperative CT scan showing no evidence of macroscopic liver lesions.
On December 21th, 2015, he was started on “adjuvant” chemotherapy with 5-fluorouracil and oxaliplatin (FOLFOX). After 5 cycles, the serum CEA significantly increased to 52.5 ng/mL, and a restaging PET scan was ordered on February 26th, 2016. It revealed multiple new liver nodules (Fig. 5). Due to disease progression and increased bilirubin levels, it was decided to start him on 5-fluorouracil and bevacizumab. However, a few days later, he needed to be re-admitted to the hospital due to extreme fatigue and mental confusion. Laboratory exams revealed acute kidney injury as well as liver failure. After 4 days of hospitalization he died.
Fig. 5.
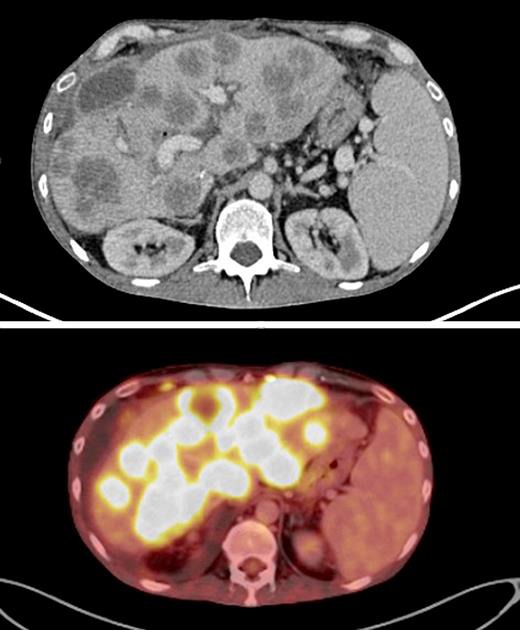
PET-CT scans showing rapid development of multiple liver lesions.
Discussion
In this paper, we presented the case of a young man with probable Lynch syndrome, who developed an aggressive colon carcinoma after long-term immunosuppressive therapy due to a prior liver transplantation. It has been previously reported that transplant patients experience worse outcomes than the general population [18]. In a retrospective study with 635 patients who developed malignancy following solid organ transplantation, compared with data from 1,282,984 adults (from the Surveillance, Epidemiology, and End Results database) in the general population, disease-specific survival was worse in the transplant population for colon cancer (all stages), non-small-cell lung cancer (stage II), breast cancer (stage III), prostate cancer (stage II, III, and IV), bladder cancer (stage III), and renal cell carcinoma (stage IV). Multivariate analyses demonstrated transplantation to be a negative risk factor for survival for each cancer studied. These data suggest that cancers in transplant recipients are more aggressive biologically at the time of diagnosis [18].
On the other hand, MSI CRCs have been linked to increased overall survival. Enhancement of the immune response has emerged as an explanation for the better prognosis observed in individuals with MSI cancers. The mutation frequency is 100-fold higher in MSI tumors than in cells proficient in repair [19]. Therefore, it has been postulated that mutations in surface molecules on the tumor might trigger immune responses that ultimately assist the host in attacking the tumor [20, 21]. This hypothesis became even more attractive when one characteristic of these tumors was found to be strong lymphocytic infiltration [22]. This improved survival among MSI tumors has been nicely demonstrated in early-stage CRC [23, 24]. However, patients with metastatic MSI CRC have worse overall survival when compared to patients without MSI [25].
A possible explanation for the worse outcomes among patients with metastatic MSI CRC is that those tumors have developed mechanisms to overcome immunosurveillance. In nonmetastatic MSI CRCs, tumor-specific neo-antigens induce strong local and systemic anti-tumor immune responses that correlate with a favorable prognosis [26]. In order to progress with systemic spread, suppression or evasion of these immune responses is required for MSI CRC. This may lead to increased malignant potential and poor prognosis. We postulate that long-term immunosuppression in our patient with probable Lynch syndrome could be associated with the very aggressive disease course of his metastatic CRC.
Recent clinical findings with the use of immune checkpoint inhibitors in MSI CRC render the screening of this characteristic particularly important [27]. A phase 2 study evaluated the clinical activity of pembrolizumab, an anti-programmed death 1 immune checkpoint inhibitor, in 41 patients with progressive metastatic carcinoma with or without MMR deficiency. There were 3 cohorts of patients: MMR-deficient CRCs, MMR-proficient CRCs, and MMR-deficient cancers that were not CRC. The immune-related objective response rate and immune-related progression-free survival rate were 40 and 78%, respectively, for MMR-deficient CRCs and 0 and 11% for MMR-proficient CRCs. This was the first study to show that tumors harboring MSI derive benefit from immunotherapy [27]. Unfortunately, our presented patient could not receive pembrolizumab, given the fact that he had previously received a liver transplant and needed chronic immunosuppression.
Colonic surveillance reduces the life-time risk of CRC in patients with Lynch syndrome from 60 to 80 to 10% and confers a 7-year survival advantage [28]. Surveillance colonoscopies have been a key modality toward preventing or catching colorectal malignancies at early stages. The recommendations for patients with Lynch syndrome include a screening colonoscopy every 1–2 years starting at age 20–25 [29]. Here, we propose that cancer screening should be even stricter in posttransplant patients with Lynch syndrome.
Based on this case report, we attempted to find an answer to the question about the risk of cancer development or recurrence in patients with familial syndromes receiving long-term immunosuppressive therapy and to find out how it can be minimized. Answering these questions is particularly important given the fact that disease course is substantially more aggressive among transplanted patients and that prognosis is poor due to lack of immunocompetence, especially in the setting of Lynch syndrome.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Engels EA, Pfeiffer RM, Fraumeni JF. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891–1901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapman JR, Webster AC, Wong G. Cancer in the transplant recipient. Cold Spring Harb Perspect Med. 2013 doi: 10.1101/cshperspect.a015677. pii: a015677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vajdic CM, van Leeuwen MT. Cancer incidence and risk factors after solid organ transplantation. Int J Cancer. 2009;125:1747–1754. doi: 10.1002/ijc.24439. [DOI] [PubMed] [Google Scholar]
- 4.Haagsma EB, Hagens VE, Schaapveld M, van den Berg AP, de Vries EG, Klompmaker IJ, Slooff MJ, Jansen PL. Increased cancer risk after liver transplantation: a population-based study. J Hepatol. 2001;34:84–91. doi: 10.1016/s0168-8278(00)00077-5. [DOI] [PubMed] [Google Scholar]
- 5.Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. Am J Transplant. 2010;10:1889–1896. doi: 10.1111/j.1600-6143.2010.03181.x. [DOI] [PubMed] [Google Scholar]
- 6.Herrero JI, Alegre F, Quiroga J, Pardo F, Iñarrairaegui M, Sangro B, Rotellar F, Montiel C, Prieto J. Usefulness of a program of neoplasia surveillance in liver transplantation. A preliminary report. Clin Transplant. 2009;23:532–536. doi: 10.1111/j.1399-0012.2008.00927.x. [DOI] [PubMed] [Google Scholar]
- 7.Carenco C, Faure S, Herrero A, Assenat E, Duny Y, Danan G, Bismuth M, Chanques G, Ursic-Bedoya J, Jaber S, Larrey D, Navarro F, Pageaux GP. Incidence of solid organ cancers after liver transplantation: comparison with regional cancer incidence rates and risk factors. Liver Int. 2015;35:1748–1755. doi: 10.1111/liv.12758. [DOI] [PubMed] [Google Scholar]
- 8.Maggi U, Consonni D, Manini MA, Gatti S, Cuccaro F, Donato F, Conte G, Bertazzi PA, Rossi G. Early and late de novo tumors after liver transplantation in adults: the late onset of bladder tumors in men. PLoS One. 2013;8:e65238. doi: 10.1371/journal.pone.0065238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang Y, Villeneuve PJ, Fenton SS, Schaubel DE, Lilly L, Mao Y. Liver transplantation and subsequent risk of cancer: findings from a Canadian cohort study. Liver Transpl. 2008;14:1588–1597. doi: 10.1002/lt.21554. [DOI] [PubMed] [Google Scholar]
- 10.Sint Nicolaas J, de Jonge V, Steyerberg EW, Kuipers EJ, van Leerdam ME, Veldhuyzen-van Zanten SJ. Risk of colorectal carcinoma in post-liver transplant patients: a systematic review and meta-analysis. Am J Transplant. 2010;10:868–876. doi: 10.1111/j.1600-6143.2010.03049.x. [DOI] [PubMed] [Google Scholar]
- 11.Watson P, Lynch HT. Extracolonic cancer in hereditary nonpolyposis colorectal cancer. Cancer. 2013;71:677–685. doi: 10.1002/1097-0142(19930201)71:3<677::aid-cncr2820710305>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Lynch HT, Smyrk T, Lynch JF. Overview of natural history, pathology, molecular genetics and management of HNPCC (Lynch syndrome). Int J Cancer. 1996;69:38–43. doi: 10.1002/(SICI)1097-0215(19960220)69:1<38::AID-IJC9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 13.Lanspa SJ, Jenkins JX, Cavalieri RJ, et al. Surveillance in Lynch syndrome: how aggressive. Am J Gastroenterol. 1994;89:1978–1980. [PubMed] [Google Scholar]
- 14.Peltomaki P, Vasen H. Mutations associated with HNPCC predisposition – update of ICG-HNPCC/INSiGHT mutation database. Dis Markers. 2014;20:269–276. doi: 10.1155/2004/305058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lynch HT, de la Chapelle A. Genetic susceptibility to nonpolyposis colorectal cancer. J Med Genet. 1999;36:801–818. [PMC free article] [PubMed] [Google Scholar]
- 16.Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348:919–932. doi: 10.1056/NEJMra012242. [DOI] [PubMed] [Google Scholar]
- 17.Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044–2058. doi: 10.1053/j.gastro.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miao Y, Everly JJ, Gross TG, et al. De novo cancers arising in organ transplant recipients are associated with adverse outcomes compared with the general population. Transplantation. 2009;87:1347–1359. doi: 10.1097/TP.0b013e3181a238f6. [DOI] [PubMed] [Google Scholar]
- 19.Parsons R, Li GM, Longley MJ, Fang WH, Papadopoulos N, Jen J, de la Chapelle A, Kinzler KW, Vogelstein B, Modrich P. Hypermutability and mismatch repair deficiency in RER+ tumor cells. Cell. 1993;75:1227–1236. doi: 10.1016/0092-8674(93)90331-j. [DOI] [PubMed] [Google Scholar]
- 20.Branch P, Bicknell DC, Rowan A, Bodmer WF, Karran P. Immune surveillance in colorectal carcinoma. Nat Genet. 1995;9:231–232. doi: 10.1038/ng0395-231. [DOI] [PubMed] [Google Scholar]
- 21.Bodmer W, Bishop T, Karran P. Genetic steps in colorectal cancer. Nat Genet. 1994;6:217–219. doi: 10.1038/ng0394-217. [DOI] [PubMed] [Google Scholar]
- 22.Lynch HT, Drescher KM, de la Chapelle A. Immunology and the Lynch syndrome. Gastroenterology. 2008;134:1246–1249. doi: 10.1053/j.gastro.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lothe RA, Peltomäki P, Meling GI, Aaltonen LA, Nyström-Lahti M, Pylkkänen L, Heimdal K, Andersen TI, Møller P, Rognum TO. Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res. 1993;53:5849–5852. [PubMed] [Google Scholar]
- 24.Gryfe R, Kim H, Hsieh ET, Aronson MD, Holowaty EJ, Bull SB, Redston M, Gallinger S. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med. 2000;342:69. doi: 10.1056/NEJM200001133420201. [DOI] [PubMed] [Google Scholar]
- 25.Lanza G, Gafà R, Santini A, Maestri I, Guerzoni L, Cavazzini L. Immunohistochemical test for MLH1 and MSH2 expression predicts clinical outcome in stage II and III colorectal cancer patients. J Clin Oncol. 2006;24:2359. doi: 10.1200/JCO.2005.03.2433. [DOI] [PubMed] [Google Scholar]
- 26.Kim CG, Ahn JB, Jung M, Beom SH, Kim C, Kim JH, Heo SJ, Park HS, Kim JH, Kim NK, Min BS, Kim H, Koom WS, Shin SJ. Effects of microsatellite instability on recurrence patterns and outcomes in colorectal cancers. Br J Cancer. 2016;115:25–33. doi: 10.1038/bjc.2016.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newton K, Green K, Lalloo F, Evans DG, Hill J. Colonoscopy screening compliance and outcomes in patients with Lynch syndrome. Colorectal Dis. 2015;17:38–46. doi: 10.1111/codi.12778. [DOI] [PubMed] [Google Scholar]
- 29.Zhang T, Boswell EL, McCall SJ, Hsu DS. Mismatch repair gone awry: management of Lynch syndrome. Crit Rev Oncol Hematol. 2015;93:170–179. doi: 10.1016/j.critrevonc.2014.10.005. [DOI] [PubMed] [Google Scholar]


