Abstract
Cataracts are the principal cause of treatable blindness worldwide. Inherited congenital cataract (CC) shows all types of inheritance patterns in a syndromic and nonsyndromic form. There are more than 100 genes associated with cataract with a predominance of autosomal dominant inheritance. A cataract is defined as an opacity of the lens producing a variation of the refractive index of the lens. This variation derives from modifications in the lens structure resulting in light scattering, frequently a consequence of a significant concentration of high-molecular-weight protein aggregates. The aim of this review is to introduce a guide to identify the gene involved in inherited CC. Due to the manifold clinical and genetic heterogeneity, we discarded the cataract phenotype as a cardinal sign; a 4-group classification with the genes implicated in inherited CC is proposed. We consider that this classification will assist in identifying the probable gene involved in inherited CC.
Key Words: Clinical heterogeneity, Clinical variability, Congenital cataract, Genetic heterogeneity
Cataracts cause half of all cases of blindness and one-third of the visual impairment cases worldwide. The term cataract comes from the Latin word “cataracta” that itself derives from the Greek “katarráktés” and refers to a waterfall. Apparently, Constantine the African (a Carthaginian monk from the Monte Cassino monastery and a member of the Salerno school of translators) borrowed this term for lens opacity from the translation of Arab medical writings into Latin. Cataract is a term that is still used today [Chance, 1939]. Since cataracts are the most common cause of vision loss in the world, surgical management of this has been documented since ancient times. Possibly, the first written evidence is from 600 years B.C.: “Uttara-Tantra of Sushruta Samshita,” wherein the recline method technique is described [Bhishagratna, 2005].
A cataract is defined as an opacity of the lens resulting from a variation of the refractive index of the lens. This variation derives from changes in the lens structure resulting in light scattering, frequently due to a significant concentration of high-molecular-weight protein aggregates. Congenital cataract (CC), one of the leading causes of treatable blindness worldwide, has a prevalence of 1–15/ 10,000 live births with a greater presence in developing countries than in developed countries [Apple et al., 2000; Gilbert and Foster, 2001]. Inherited CC is a clinically and genetically heterogeneous disease normally associated with the breakdown of the lens architecture. Inherited CC presents all types of inheritance patterns in a syndromic and nonsyndromic form with more than 100 causative genes (http://cat-map.wustl.edu/). For example, hereditary CC may be inherited as autosomal dominant, autosomal recessive, or X-linked traits, with autosomal dominant being the most prevalent form of inheritance pattern. Hereditary CC shows an important intrafamilial and interfamilial variability; the same mutation in one gene can result in distinct cataract phenotypes, whereas expression of different genes can produce the same cataract pattern. Clinical variability can even be observed in the same patient [Hejtmancik and Smaoui, 2003].
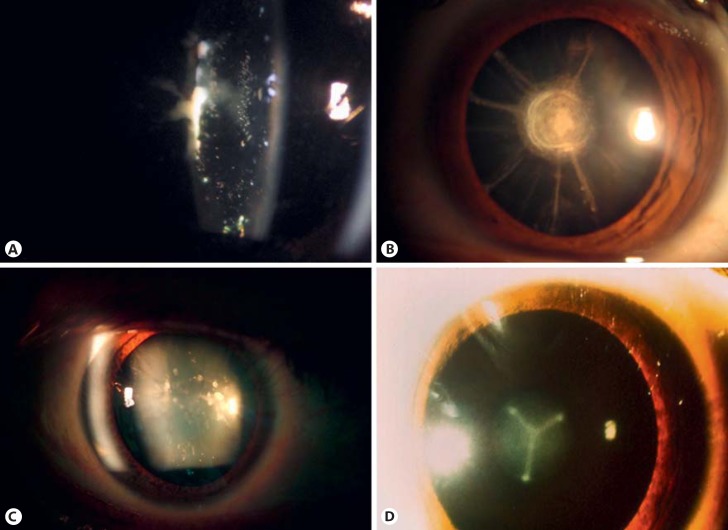
Several terms have been used to describe the different types of hereditary CC in the literature. CC classification has not been homogenous. In some cases, the description corresponds to the name of the author who first described the cataract, i.e., Marner cataract, a cataract with sutural opacities [Marner et al., 1989]; to the name of the affected family such as Coppock cataract, an embryonic nuclear cataract [Nettleship and Ogilvie, 1906]; the Volkman cataract, a central or zonular variety with opacities in the embryonic, fetal, and juvenile nucleus [Eiberg 1995], or to the affected community such as the Hutterite cataract [Boone et al., 2015]. In other cases, anatomic localization is used to establish the cataract definition, i.e., subcapsular, nuclear, sutural, cortical, fetal, embryonic, or capsular cataract (Fig. 1).
Fig. 1.
Some examples of inherited cataracts: anterior subcapsular (A), fetal nuclear (B), punctate in lens cortex (C), and embryonic nuclear Y-sutural (D).
There are excellent reviews of inherited cataracts in the literature. The purpose of this review is to provide a guide to the suspected genetic causes of inherited CC. Due to the great clinical and genetic heterogeneity, we separated the genes implicated in the process of cataractogenesis into 4 groups (Tables 1, 2, 3, 4). These groups contain the genes involved in syndromic cataracts, the genes implicated in syndromic cataracts but with reports of congenital cataracts only, the genes that present cataracts only, and finally, the genes with cataract and eye anomalies. We consider that this classification will assist in identifying the probable gene involved in inherited CC.
Table 1.
Syndromic cataract genes
| Locus | Gene | Inheritance | OMIM | Disease | Gene product |
|---|---|---|---|---|---|
| 1p36.32 | PEX10 | AR | 614870 | neonatal adrenoleukodystrophy, Zellweger syndrome | protein of peroxisomal matrix |
| 1p36.22 | PEX14 | AR | 614887 | Zellweger syndrome | peroxisomal import machinery |
| 1p36.1p34 | HSPG2 | AR | 224410/25800 | Schwartz-Jampel syndrome type 1/dyssegmental dysplasia | perlecan protein |
| 1p36p35 | GALE | AR | 230350 | galactose epimerase deficiency | UDP-galactose-4-epimerase |
| 1p34.1 | POMGNT1 | AR | 253280 | muscular dystrophy-dystroglycanopathy | type 2 transmembrane protein that resides in the Golgi apparatus |
| 1p22p21 | ABCD3 | AR | 616278 | Zellweger syndrome 2 | member of the superfamily of ATP-binding cassette transporters |
| 1p21 | COL11A1 | AD | 604841/154780 | Stickler syndrome type 2, Marshall syndrome cataracts, growth hormone deficiency, sensory neuropathy, sensorineural hearing loss, skeletal dysplasia | one of the 2 alpha chains of type XI collagen |
| 1q41 | IARS2 | AR | 616007 | Aminoacyl-tRNA synthetase | |
| 1q41 | RAB3GAP2 | AR | 212720/614225 | Martsolf syndrome, Warburg micro syndrome | RAB3 protein regulates exocytosis of neurotransmitters and hormones |
| 1q42 | GNPAT | AR | 222765 | rhizomelic chondrodysplasia punctata type 2 | enzyme in synthesis of ether phospholipids |
| 2p14p16 | PEX13 | AR | 614883 | Zellweger syndrome | peroxisomal membrane protein |
| 2q21.3 | RAB3GAP1 | AR | 600118 | Warburg micro syndrome | catalytic subunit of a Rab GTPase activating protein |
| 2q24q31 | LRP2 | AR | 222448 | Donnai-Barrow syndrome | low density lipoprotein-related protein 2 |
| 2q33qter | CYP27A1 | AR | 213700 | cerebrotendinous xanthomatosis | member of the cytochrome P450 superfamily |
| 2q37 | KCNJ13 | AD | 193230 | snowflake vitreoretinal degeneration | member of the inwardly rectifying potassium channel family |
| 3p21.1 | COL7A1 | AR | 226600 | epidermolysis bullosa dystrophica | alpha chain of type VII collagen |
| 3p14.3 | FLNB | AD, AR | 150250/272460 | Larsen syndrome/spondylocarpotarsal synostosis syndrome | member of the filamin family |
| 3q21q22 | CNBP | AD | 602668 | myotonic dystrophy type 2 | a nucleic-acid binding protein with 7 zinc-finger domains |
| 3q25 | SLC33A1 | AR | 614482 | congenital cataracts, hearing loss, neurodegeneration | required for the formation of O-acetylated (Ac) gangliosides |
| 4p16.1 | HMX1 | AR | 612109 | oculoauricular syndrome | transcription factor that belongs to the H6 family of homeobox proteins |
| 4p15.32 | CC2D2A | AR | 612285 | Joubert syndrome 9 | play a critical role in cilia formation |
| 4p12q12 | SRD5A3 | AR | 612713 | Kahirazi syndrome | steroid 5-alpha reductase family |
| 4q32q35 | ETFDH | AR | 231680 | glutaric acidemia | component of the electron-transfer system in mitochondria |
| 4q35.1 | TRAPPC11 | AR | 615356 | muscular dystrophy limb girdle 2S | a subunit of the TRAPP (transport protein particle) tethering complex |
| 5q12.1 | ERCC8 | AR | 216400 | Cockayne syndrome type A | a WD repeat protein |
| 5q14.3 | VCAN | AD | 143200 | Wagner syndrome 1 | a member of the aggrecan/versican proteoglycan family |
| 5q31 | SIL1 | AR | 248800 | Marinesco-Sjögren syndrome | resident endoplasmic reticulum N-linked glycoprotein |
| 6p24 | TFAP2A | AD | 113620 | branchiooculofacial syndrome | a transcription factor |
| 6p23 | GCM2 | AD | 146200 | hypoparathyroidism familial isolated | a homolog of the Drosophila glial cells missing gene |
| 6p21.3 | NEU1 | AR | 256550 | sialidosis type 2 | a lysosomal enzyme that cleaves terminal sialic acid residues |
| 6q21q23.2 | GJA1 | AD | 164200 | oculodentodigital dysplasia | a member of the connexin gene family |
| 6q22q24 | PEX7 | AR | 215100 | rhizomelic chondrodysplasia punctata type 1 | cytosolic receptor for the set of peroxisomal matrix enzymes |
| 6q24.2 | PEX3 | AR | 614882 | Zellweger syndrome | involved in peroxisome biosynthesis and integrity |
| 7p15.3 | FAM126A | AR | 610532 | hypomyelinating leukodystrophy 5 | part in the beta-catenin/Lef signaling pathway |
| 7q21.2 | PEX1 | AR | 214100 | Zellweger syndrome | a member of the AAA ATPase family |
| 7q31.1 | CAV1 | AD | 606721 | partial lipodystrophy, congenital cataracts, neurodegeneration syndrome | main component of the caveolae plasma membranes |
| 7q34 | AGK | AR | 212350 | Sengers syndrome | a mitochondrial membrane protein involved in lipid and glycerolipid metabolism |
| 8p21.1 | ESCO2 | AR | 268300 | Roberts syndrome | acetyltransferase activity may be required for the establishment of sister chromatid cohesion |
| 8q13.3 | EYA1 | AD | 601653 | branchiootorenal syndrome 1 | a member of the eyes absent (EYA) family of proteins |
| 8q21.1 | PXMP3 | AR | 614866 | Zellweger syndrome | an integral peroxisomal membrane protein required for peroxisome biogenesis |
| 8q21q22 | CNGB3 | AR | 262300 | achromatopsia 3 | the beta subunit of a cyclic nucleotide-gated ion channel |
| 8q24.3 | RECQL4 | AR | 268400 | Rothmund Thompson syndrome | DNA helicase that belongs to the RecQ helicase family |
| 9p13.13 | GALT | AR | 230400 | galatosemia | galactose-1-phosphate uridyl transferase |
| 9p24 | VLDLR | AR | 224050 | cerebellar hypoplasia and mental retardation with or without quadrupedal locomotion 1 | low-density lipoprotein receptor |
| 9q31q33 | FKTN | AR | 253800 | muscular dystrophy-dystroglycanopathy | a putative transmembrane protein localized to the cis-Golgi compartment |
| 9q34 | LMX1B | AD | 161200 | nail-patella syndrome | a member of LIM-homeodomain family of proteins |
| 9q34.1 | POMT1 | AR | 236670 | muscular dystrophy-dystroglycanopathy A1 | an O-mannosyltransferase |
| 10q11.23 | ERCC6 | AR | 214150/133540 | cerebrooculofacioskeletal syndrome I/Cockayne syndrome type B | a DNA-binding protein that is important in transcription-coupled excision repair |
| 10q23.31 | PTEN | AD | 158350 | Cowden disease | a tumor suppressor |
| 10q24.3 | ALDH18A1 | AD/AR | 616603/219150 | cutis laxa AD/cutis laxa AR | a member of the aldehyde dehydrogenase family |
| 10q26 | OAT | AR | 258870 | gyrate atrophy of choroid and retina | mitochondrial enzyme ornithine aminotransferase |
| 11p15.3p15.1 | PTH | AD/AR | 146200 | familial isolated hypoparathyroidism | a member of the parathyroid family of proteins |
| 11q13.4 | LRP5 | AR, AD | 601813/607634 | exudative vitreoretinopathy 4/osteopetrosis, autosomal dominant 1 | a transmembrane low-density lipoprotein receptor |
| 11q13.2q13.5 | DHCR7 | AR | 270400 | Smith-Lemli-Opitz syndrome | an enzyme that removes the C(7–8) double bond in the B ring of sterols |
| 11q13.4 | CLPB | AR | 616271 | 3-methylglutaconic aciduria with cataracts, neurologic involvement, neutropenia | member of the ATPases associated with diverse cellular activities (AAA+) superfamily |
| 11q14.2 | FZD4 | AD | 133780 | retinopathy of prematurity | a member of the frizzled gene family |
| 11q22.3 | MMP1 | AR | 226600 | epidermolysis bullosa dystrophic | a member of the peptidase M10 family of matrix metalloproteinases |
| 11q22.1q23.2 | CRYAB | AD | 608810 | myofibrillar myopathy | members of the small heat shock protein (HSP20) family |
| 11q23.3 | SC5DL | AR | 607330 | lathosterolosis | enzyme of cholesterol biosynthesis |
| 11q25 | JAM3 | AR | 613730 | hemorrhagic destruction of brain, subependymal calcification | immunoglobulin superfamily gene member |
| 12q13.12 | TUBA1A | AD | 611603 | lissencephaly | microtubule constituents of to the tubulin superfamily |
| 12p13.3 | PEX5 | AR | 214110 | Zellweger syndrome | protein essential for the assembly of functional peroxisomes |
| 12q24 | MVK | AR | 610377 | mevalonic aciduria | peroxisomal enzyme mevalonate kinase |
| 13q12 | GJB6 | AD | 129500 | Clouston syndrome | one of the connexin proteins |
| 13q12.3 | B3GALTL | AR | 261540 | Peters-plus syndrome | a beta-1,3-glucosyltransferase that transfers glucose to O-linked fucosylglycans on thrombospondin type-1 repeats |
| 13q14.3 | ITM2B | AD | 117300 | cerebral amyloid angiopathy | a transmembrane protein |
| 13q34 | COL4A1 | AD | 607595 | cerebral small vessel disease | a type IV collagen alpha protein |
| 14q21.1 | SEC23A | AR | 607812 | craniolenticulosutural dysplasia | a member of the SEC23 subfamily of the SEC23/SEC24 family |
| 14q24 | POMT2 | AR | 613150 | muscular dystrophy-dystroglycanopathy | an O-mannosyltransferase |
| 15q15 | BUB1B | AR | 257300 | mosaic variegated aneuploidy syndrome 1 | a kinase involved in spindle checkpoint function |
| 15q21.1 | FBN1 | AD | 608328 | Weill-Marchesani syndrome | a member of the fibrillin family of proteins |
| 15q25 | POLG | AD/AR | 157640 | progressive external ophthalmoplegia with mitochondrial DNA deletions 1 | the catalytic subunit of mitochondrial DNA polymerase |
| 16p13.3p13.2 | GFER | AR? | 613076 | progressive mitochondrial myopathy, sensorineural hearing loss, developmental delay | structural and functional homolog of the yeast scERV1 gene |
| 16q21 | NOD2 | AD | 186580 | Blau syndrome | a member of the Nod1/Apaf-1 family |
| 16q23 | ADAMTS18 | AR | 267750 | Knobloch syndrome | a member of the ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) protein family |
| 16q22q23 | MAF | AD | 601088 | Aymé-Gripp syndrome | a DNA-binding, leucine zipper-containing transcription factor |
| 17p13.3 | YWHAE | AD | 247200 | Miller-Dieker lissencephaly syndrome | member of the 14-3-3 family of proteins which mediate signal transduction by binding to phosphoserine-containing proteins |
| 17q12 | PEX12 | AR | 614859 | Zellweger syndrome | member of the peroxin-12 family |
| 17q21 | WNT3 | AR | 273395 | tetraamelia syndrome | secreted signaling proteins implicated in oncogenesis |
| 17q21.33 | XYLT2 | AR | 605822 | spondyloocular syndrome | an isoform of xylosyltransferase, which belongs to a family of glycosyltransferases |
| 18q12.3 | EPG5 | AR | 242840 | Vici syndrome (immunodeficiency, cleft lip/palate, cataract, hypopigmentation, absent corpus callosum) | a large coiled-coil domain-containing protein that functions in autophagy |
| 18q23 | CTDP1 | AR | 604168 | congenital cataracts, facial dysmorphism, neuropathy | a protein which interacts with the carboxy-terminus of the RAP74 subunit of transcription initiation factor TFIIF |
| 19q13.1 | MAN2B1 | AR | 248500 | alpha-mannosidosis | an enzyme that hydrolyzes terminal, nonreducing alpha-D-mannose residues in alpha-D-mannosides |
| 19q13.3 | DMPK | AD | 160900 | myotonic dystrophy 1 | a serine-threonine kinase |
| 19q13.32 | FKRP | AR | 613153 | muscular dystrophy-dystroglycanopathy | a protein which is targeted to the medial Golgi apparatus and is necessary for posttranslational modification of dystroglycan |
| 20p11.21q12 | ABHD12 | AR | 612674 | polyneuropathy, hearing loss, ataxia, retinitis pigmentosa, cataract | an enzyme that catalyzes the hydrolysis of 2-arachidonoyl glycerol |
| 20q13.13q13.2 | SALL4 | AD | 607323 | Duane-radial ray syndrome | a zinc finger transcription factor thought to play a role in the development of abducens motor neurons |
| 20q13.3 | GNAS | AD | 103580/612462/612463 | pseudohypoparathyroidism type 1A/pseudohypoparathyroidism type 1C/pseudohypoparathyroidism | multiple transcript variants encoding different isoforms |
| 21q22.3 | COL18A1 | AR | 267750 | Knobloch syndrome 1 | alpha chain of type XVIII collagen |
| 22q11.21 | PEX26 | AR | 614872 | Zellweger syndrome | member of the peroxin-26 gene family |
| 22q12.2 | NF2 | AD | 101000 | neurofibromatosis type 2 | a protein similar to ezrin, radixin, moesin family of proteins |
| 22q12.3 | LARGE | AR | 613154 | muscular dystrophy-dystroglycanopathy | a member of the N-acetylglucosaminyltransferase gene family |
| 22q13.1 | MYH9 | AD | 153640 | Fechtner syndrome | a conventional non-muscle myosin |
| Xp11.4 | BCOR | XL | 300166 | microphthalmia syncromic 2 | an interacting corepressor of BCL6 |
| Xp11.23 | PQBP1 | XL | 309500 | Renpenning syndrome | a nuclear polyglutamine-binding protein that is involved with transcription activation |
| Xp11.23p11.22 | EBP | XL | 302960 | chondrodysplasia punctata 2 | an integral membrane protein of the endoplasmic reticulum |
| Xp22.3 | ARSE | XL | 302950 | chondrodysplasia punctata 1 | a member of the sulfatase family |
| Xp22.3 | HCCS | XL | 309801 | linear skin defects with multiple congenital anomalies | an enzyme that covalently links a heme group to the apoprotein of cytochrome c |
| Xp22 | AIC | XL | 304050 | Aicardi syndrome | unknown |
| Xp22.13 | NHS | XL | 302350 | Nance-Horan syndrome | a protein containing 4 conserved nuclear localization signals |
| Xq22 | GLA | XL | 301500 | Fabry disease | a homodimeric glycoprotein that hydrolyzes the terminal alpha-galactosyl moieties from glycolipids and glycoproteins |
| Xq22 | COL4A5 | XL | 301050 | Alport syndrome | one of the 6 subunits of type IV collagen |
| Xq25q26.1 | OCRL | XL | 309000 | Lowe oculocerebrorenal syndrome | an inositol polyphosphate 5-phosphatase |
| Xq28 | IKBKG | XL | 308300 | incontinentia pigmenti | regulatory subunit of the inhibitor of kappaB kinase complex |
AD, autosomal dominant; AR, autosomal recessive; XL, X linked.
Table 2.
Syndromic genes only with cataract
| Locus | Gene | OMIM | Cataract inheritance | Syndrome inheritance | Syndrome | Reference of only cataract affection |
|---|---|---|---|---|---|---|
| 4p16.1 | WFS1 | 614296 | AD | AD | Wolfram-like syndrome (no cataract is associated with this syndrome) | Berry et al., 2013 |
| 7q34 | AGK | 212350 | AR | AR | Sengers syndrome | Aldahmesh et al., 2012 |
| 8q13.3 | EYA1 | 601653 | AD | AD | brachio-oto-renal syndrome-1, nystagmus | Azuma et al., 2000 |
| 11q22.1q23.2 | CRYAB | 608810 | AR/AD | AD | myofibrillar myopathy (alpha-B crystallinopathy) | Berry et al., 2001; Liu et al., 2006; Devi et al., 2008; Chen et al., 2009; Safieh et al., 2009; Sun et al., 2011b; Xia et al., 2014b; Jiaox et al., 2015; Khan et al., 2015; Ma AS et al., 2016 |
| 13q34 | COL4A1 | 607595 | AD | AD | familial porencephaly, brain small vessel disease with hemorrhage/Axenfeld-Rieger anomalies, hereditary angiopathy with nephropathy, aneurysms, and muscle cramps | Xia et al., 2014a |
| 15q21.1 | FBN1 | 608328 | AD | AD | Marfan syndrome, Weill-Marchesani syndrome | Li D et al., 2016 |
| 16q22q23 | MAF | 601088 | AD | AD | Aymé-Gripp syndrome | Jamieson et al., 2002; Vanita et al., 2006; Hansen et al., 2007, 2009; Narumi et al., 2014; Sun et al., 2014; Ma AS et al., 2016 |
| Xp22.13 | NHS | 302350 | XL | XL | Nance-Horan syndrome | Ramprasad et al., 2005; Florijn et al., 2006; Sharma et al., 2008; Coccia et al., 2009; Sun et al., 2014; Li D et al., 2016; Ma AS et al., 2016 |
AD, autosomal dominant; AR, autosomal recessive; XL, X linked.
Table 3.
Only cataract genes
Table 4.
Cataract genes and eye anomalies
| Locus | Gene | Inheritance | Cataract phenotype | Other phenotype | Gene product | Reference |
|---|---|---|---|---|---|---|
| 1p32 | FOXE3 | AR/AD | cerulean, congenital | microphthalmia, sclerocornea, aphakia, optic disc coloboma, aniridia plus, glaucoma, viteoretinal dysplasia (almost all reports) | the forkhead family of transcription factors | Semina et al., 2001; Ormestad et al., 2002; Valleix et al., 2006; Iseri et al., 2009; Ali et al., 2010; Anjum et al., 2010; Bremond-Gignac et al., 2010; Reis et al., 2010; Doucette et al., 2011; Gillespie et al., 2014; Li D et al., 2016 |
| 1q21.1 | GJA8 | AD/AR | nuclear, punctiform, pulverulent, jellyfish-like, star-shaped, full moon, Y-sutural, balloon-like, lamelar, zonular, nuclear, triangular, perinuclear | microcornea, glaucoma (about half of the reports) | a transmembrane connexin protein | Shiels et al., 1998; Berry et al., 1999; Polyakov et al., 2001; Willoughby et al., 2003; Zheng et al., 2005; Arora et al., 2006, 2008; Devi and Vijayalakshmi, 2006; Vanita et al., 2006; Hansen et al., 2007; Ponnam et al., 2007; Lin et al., 2008; Schmidt et al., 2008; Vanita et al., 2008; Yan et al., 2008; Graw et al., 2009; Hansen et al., 2009; Wang et al., 2009; Gao et al., 2010; Hu et al., 2010; He et al., 2011; Kumar at al., 2011; Sun et al., 2011a; Wang L et al., 2011; Li et al., 2013; Ponnam et al., 2013; Reis et al., 2013; Su et al., 2013; Chen et al., 2014; Ge et al., 2014; Gillespie et al., 2014; Mackay et al., 2014; Prokudin et al., 2014; Sun et al., 2014; Zhu et al., 2014; Chen et al., 2015; Liang et al., 2015; Yang Z et al., 2015; Ma AS et al., 2016; Min et al., 2016; Yu et al., 2016 |
| 9q21.12 | TRPM3 | AD | open-angle glaucoma | family of transient receptor potential channels | Bennett et al., 2014 | |
| 11p13 | PAX6 | AD | aniridia “plus” (iris and foveal hypoplasia, nystagmus, cataract, corneal abnormalities, glaucoma) | a homeobox and paired domain-containing protein that binds DNA | LOVD PAX6 Homepage | |
| 11q13 | BEST1 | AD | pulverulent-like | vitreoretinochoroidopathy, microcornea, rod-cone dystrophy, cataract, posterior staphyloma | a member of the bestrophin gene family | Boon et al., 2009 |
| 14q24.3 | VSX2 | AR | microphthalmia, anophthalmia, iris coloboma | a homeobox protein originally described as a retina-specific transcription factor | Ferda Percin et al., 2000 | |
| 15q22.32 | NR2E3 | AD, AR | retinitis pigmentosa-37, enhanced S-cone syndrome | a family of nuclear receptor transcription factors | Edwards et al., 2008 | |
| 15q25.1 | MIR184 | AD | congenital, early-onset anterior polar | endothelial dystrophy, iris hypoplasia, cataract and stromal thining (EDICT) syndrome, keratoconus. Corneal anomalies | microRNAs involved in post-transcriptional regulation of gene expression | Iliff et al., 2012; Bykhovskaya et al., 2015 |
| 17p13.1 | GUCY2D | AR | Leber congenital amaurosis-1 | a retina-specific guanylate cyclase | Gradstein et al., 2016 | |
| 21q22.3 | CRYAA | AR/AD | nuclear, laminar, posterior polar, punctate, spike-like, ring-like, fan-shaped, disc-like, Y-sutural, zonular, lamellar, polymorphic, perinuclear | microcornea, iris coloboma, axial elongation, macular hypoplasia, corneal opacity | chaperone-like small heat-shock protein family | Litt et al., 1998; Pras et al., 2000; Mackay et al., 2003; Graw et al., 2006; Santhiya et al., 2006; Vanita et al., 2006; Beby et al., 2007; Hansen et al., 2007; Khan et al., 2007; Devi et al., 2008; Gu et al., 2008; Richter et al., 2008; Hansen et al., 2009; Santana et al., 2009; Zhang et al., 2009a; Li et al., 2010; Sun et al., 2011a; Su et al., 2012; Wang and Zhu, 2012; Kondo et al., 2013; Laurie et al., 2013; Reis et al., 2013; Yang et al., 2013; Kong et al., 2015; Liang et al., 2015; Javadiyan et al., 2016; Li D et al., 2016; Ma AS et al., 2016 |
| 22q12.1 | CRYBA4 | AD | congenital nuclear, lamellar | microcornea, microphthalmia | soluble proteins in the human lens | Billingsley et al., 2006; Zhou G et al., 2010 |
For abbreviations, see Table 2.
Embryological Development of the Lens
Briefly, from mouse studies, the lens development starts at day 22 of gestation (4 mm embryonic stage) from the surface ectoderm. Pax6 and Sox2 genes, in the optic vesicle, induce the surface ectoderm to form the lens placode, which invaginates and forms the lens vesicle [Kamachi et al., 2001; Kondoh et al., 2004]. By day 40 of gestation (10 mm stage), the lens vesicle is completely separated from the surface ectoderm. Group B1 SOX proteins (SOX1, SOX2, and SOX3) activate γ-crystallin genes in the mouse lens, producing the crystallins, water-soluble proteins that comprise over 90% of the proteins of the lens [Hoehenwarter et al., 2006]. Elongating cells of the posterior end of the lens vesicle form the primary fibers, which become the embryonic nucleus in the mature lens. In the final phase of lens differentiation, several fibroblast growth factors seem to be required [McAvoy and Chamberlain, 1989; Lovicu and Overbeek, 1998]. The lens continues growing after birth, with the new secondary fibers generated from the equatorial cells of the lens epithelium (germinative zone). The lens epithelial cells synthesize crystallins and lose their nuclei to become mature lens fibers. Disruption of this delicate process due to toxic, metabolic, infectious, or genetic agents results in CC.
Syndromic and Nonsyndromic Cataracts
A rough division of hereditary cataracts comprises 2 parts: syndromic and nonsyndromic cataracts. If only the lens is implicated in the pathological process, it is defined as nonsyndromic cataracts. If there are more organs implicated, it results in syndromic cataracts. Around 100 genes throughout the genome are in the OMIM database explaining the different syndromes caused by different genes, either associated with cataracts or as part of them (Table 1). Nevertheless, genetically it is sometimes difficult to establish this difference because clinically one gene defect can result in either lens affection or systemic disease. Some examples of this variance (Table 2) are found in the affected AGK gene, which results in either an isolated cataract or in Senger syndrome (OMIM 212350) [Aldahmesh et al., 2012]; the MAF gene, in which a molecular defect can result in cataracts or in Aymé-Gripp syndrome (OMIM 601088) [Narumi et al., 2014; Sun et al., 2014; Ma AS et al., 2016], or in the NHS gene alteration which can lead to isolated cataracts or Nance-Horan syndrome (OMIM 302350) [Coccia et al., 2009; Li D et al., 2016]. The scope of this review does not include syndromic cataracts. It is only briefly referenced because some genes (WFS1, AGK, EYA1, CRYAB, COL4A1, FBN1, MAF, and NHS) are also described in previous reports with CC and no other anomalies (Table 2).
Nonsyndromic Cataracts
Around 35 genes have been strongly associated with inherited CC only and with no other systemic anomalies; they show autosomal dominant and autosomal recessive inheritance patterns (Table 3). However, in some of these cases (EPHA2, BFSP2, HSF4, CRYBA1, CRYBB1, and CRYBB3), hereditary CC can present both patterns of autosomal inheritance (dominant or recessive). A great variety of functions is described in all affected genes that produce nonsyndromic inherited CC. Protein functions encompass structural to enzymatic processes including channels, phagosomes, RNA processing, transcription factors, and regulation of intracellular signaling (Table 3).
The cataract phenotype by itself is not an efficient indicator of the affected gene or mutation, since identical cataracts can result from mutations at different loci and may have several inheritance patterns. Conversely, various cataract types can be found in the same gene affection, presenting clinical heterogeneity. In addition, some nonsyndromic cataracts could include the presence of lens opacities only or lens opacities with ocular abnormalities and with no systemic affection, i.e., microcornea, aniridia, vitreoretinochoroidopathy, microphthalmia, anophthalmia, iris hypoplasia, or rod-cone dystrophy (Table 4).
Crystallins
Crystallins are divided in α, β, and γ and constitute around 90% of the soluble proteins of the lens with an asymmetric and biphasic distribution [Augusteyn, 2010]. α-crystallins (αA-crystallin and αB-crystallin) belong to the chaperone-like small heat shock protein family (sHSPs). They block the formation of stress protein aggregates to avoid toxic effects [Clark et al., 2012]. The sHSPs are large, polydisperse, and do not crystallize, thus playing an important role in the lens. It is likely that the high refractive index of a lens with no compromise of transparency is due to the ability to form extended lattices of the water-insoluble α-crystallin [Slingsby et al., 2013]. Outside of the lens, α-crystallin is expressed principally in the retina, the muscle, and the brain. In the retina, it seems to be implicated in cytoprotection, cell survival, inflammation, and autophagy [Kannan et al., 2012]. A great diversity of mutations associated with cataracts has been reported in the CRYAA and CRYAB genes, whose products are αA-crystallin and αB-crystallin, respectively. In approximately half of the cases, CRYAA gene defects are associated with eye anomalies, whereas CRYAB gene defects are associated with myofibrillar myopathy (Tables 1, 4); in both cases, the rest of the reports connect lens opacities with great clinical heterogeneity (Table 4). Most cases show an autosomal dominant pattern, and in a few cases, a recessive autosomal inheritance.
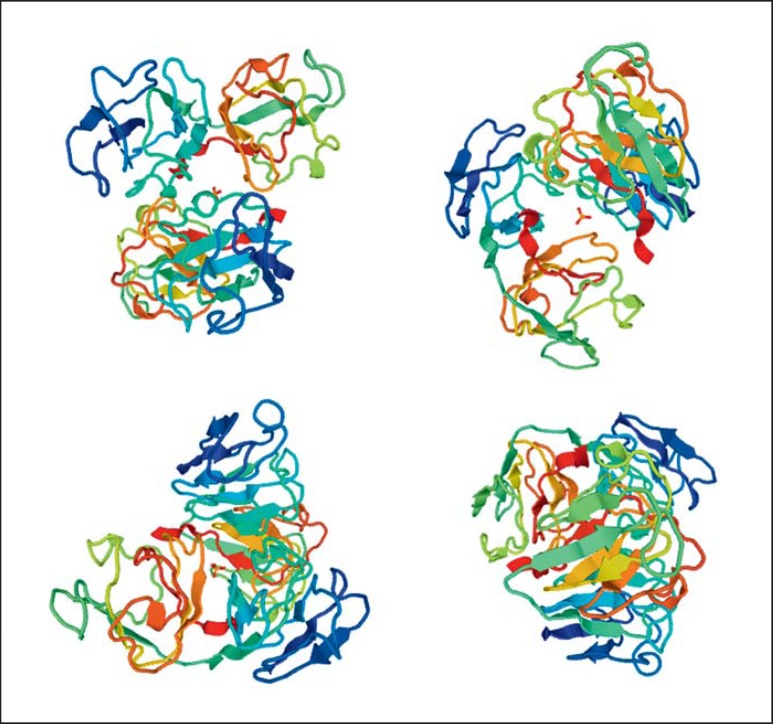
The β- and γ-crystallins consist of 4 similarly folded Greek key motifs organized into 2 domains. The Greek key motif is a structure of 4 β-sheets, folded in an antiparallel form that generates a folded compact domain [Richardson, 1977]. The values and gradients in the refractive index of lens proteins seem to be related to the Greek key motif [Zhao et al., 2011]. The central portion and the embryonic nuclear region of the lens are rich in β- and γ-crystallins. In humans, γ-crystallins comprise from γA to γF. Now, γS-crystallin (βS-crystallin) is also included in this group; γE and γF are pseudogenes. Interestingly, CRYBA, CRYBB, CRYGA, CRYGB, CRYGC, CRYGD, and CRYGS gene mutations are only associated with inherited CC and no other systemic or eye anomalies (Table 3). In Figure 2, the normal CYGD crystallin structure is shown.
Fig. 2.
The structure of CRYGD, from https://swissmodel.expasy.org/.
EPH2
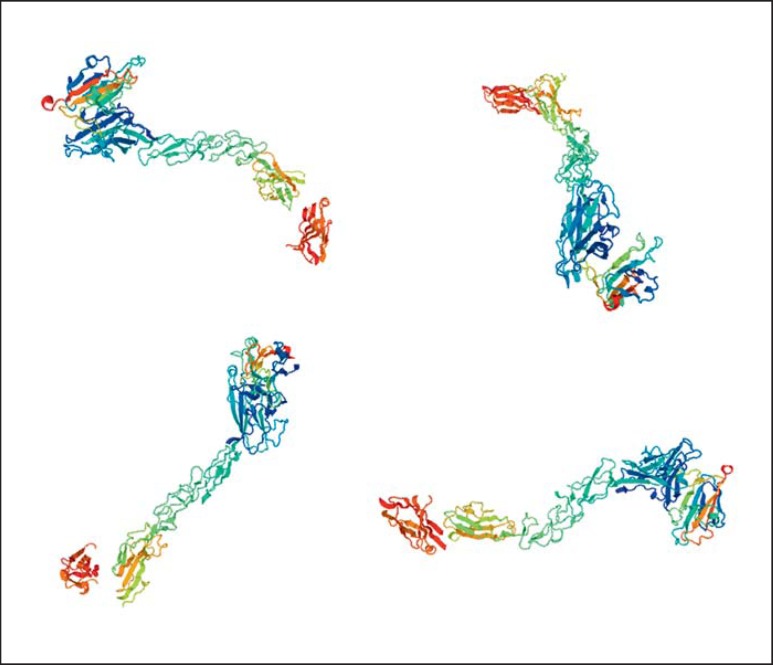
The EPHA2 (ephrin receptor A2) gene encodes a transmembrane tyrosine kinase receptor (epithelial cell) which contains an extracellular ligand-binding domain and is expressed in the human lens [Pasquale, 2010]. EPH2, with its ligands ephrin-A1 or ephrin-A5, has a role in cell adhesion and cell repulsion [Miao and Wang, 2009]. Inhibition of EPHA2 induces apoptosis and abrogates tumorigenic growth of tumor cells [Amato et al., 2014]. Apparently, the downstream signaling of activated EPHA2 promotes the antioxidative capacity of lens epithelial cells to eradicate the overproduction of reactive oxygen species [Yang J et al., 2015]. It is probable that the loss of EPHA2 function could affect the structural stability of the cell, cell-to-cell crosstalk, protein folding and transcriptional activation [Park et al., 2013]. Thus, the cytoprotective and antiapoptotic functions of EPHA2 in the lens indicate the possible role of EPHA2 in avoiding lens opacity. Practically, all mutations reported in the EPH2 gene are associated exclusively with inherited CC and no other anomalies (some cataracts are age related, as well). Only one study reports microcornea with mild dysmorphic features [Gillespie et al., 2014]. Both autosomal recessive and dominant patterns as well as clinical heterogeneity are present (Table 3). Figure 3 shows the structure of the EPH2 protein.
Fig. 3.
The structure of EPHA2, from https://swissmodel.expasy.org/.
GJA3 and GJA8
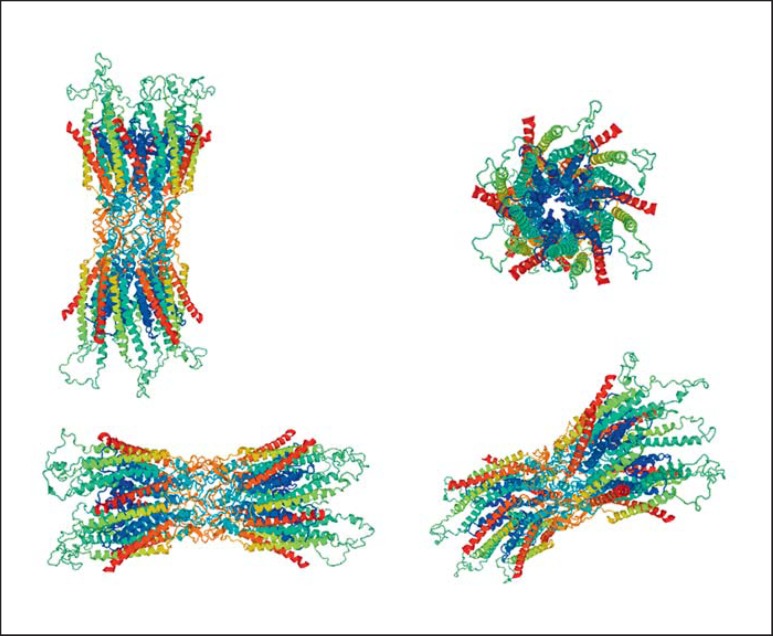
The GJA3 (gap junction alpha-3) gene encodes connexin 46, whereas the GJA8 (gap junction alpha-8) gene encodes connexin 50, gap junction channel proteins that play an important role in lens cell homeostasis. Connexin proteins have 4 transmembrane domains, 3 intracellular regions, and 2 extracellular loops. Six connexin subunits form 1 connexon. Connexin 46 and connexin 50 are expressed in the lens fiber cells and are essential for the coupling of mature fibers in the central core of the lens [Gong et al., 1997; Martinez-Wittinghan et al., 2004]. Connexins allow the interchange of ions and low-molecular-weight molecules between contiguous cells. Connexin 46 and connexin 50 are the major components of human lens fiber cells, and their molecular defects result in about 20% of nonsyndromic cataract reports [Shiels et al., 2010]. GJA3 gene mutations produce only cataracts with no other manifestations, unlike GJA8 that includes the presence of microcornea in half of the reports (Tables 3, 4). The structure of connexin 46 is shown in Figure 4.
Fig. 4.
The structure of GJA3, from https://swissmodel.expasy.org/.
MIP
The MIP (major intrinsic protein) gene encodes aquaporin 0 (AQP0), the most abundant protein in the fiber cell membrane that functions as a water channel and belongs to the superfamily of AQPs [Shiels et al., 2001]. AQPs create a microcirculation in the avascular lens to nourish central fiber cells, maintaining their transparency and homeostasis [Gao et al., 2013]. AQP0 accounts for about 45% of the total plasma proteins [Bassnett et al., 2009]. Homozygous or heterozygous loss of AQP0 will reduce the water permeability of lens fiber cells producing lens opacities in mice [Shiels et al., 2001]. Apart from the intermolecular contacts between AQP0 monomers, AQP0 interacts with other proteins in lens fiber cells as crystallins and connexins [Liu and Liang 2008; Liu et al. 2011]. Mutations in the MIP gene result only in cataracts with no other manifestations, and all of them are inherited in an autosomal dominant pattern (Table 3). The structure of the AQP0 channel is shown in Figure 5.
Fig. 5.
The structure of MIP, from https://swissmodel.expasy.org/.
FYCO1
FYCO (FYVE and coiled-coil domain containing 1), a PI(3)P-, Rab7-, and LC3-binding protein, mediates microtubule plus end-directed vesicle transport of autophagosomes, a required process for autolysosome formation [Pankiv et al., 2010]. FYCO1 is expressed in the lens epithelium and fiber cells (principally in nuclear fibers) in newborn mice, suggesting that autophagy is important in lens fiber cell differentiation [Brennan et al., 2012]. When FYCO1 is lacking, phagosomes stay p40phox+ longer and produce more reactive oxygen. This represents FYCO1's participation in the immunity role of regulating the phagosome maturation process and production of reactive oxygen, processes necessary for handling extracellular pathogens [Ma et al., 2014]. All mutations reported in the FYCO1 gene are inherited in an autosomal recessive pattern and have no other systemic or eye anomalies (Table 3). Figure 6 shows the structure of the FYCO1 protein.
Fig. 6.
The structure of FYCO1, from https://swissmodel.expasy.org/.
BFSP1 and BFSP2
BFSP1 (filesin, CP115) and BFSP2 (phakinin, CP49), lens-specific proteins, are the principal components of beaded filaments, which are unique cytoskeletal lens structures [Alizadeh et al., 2003]. BFSP1 and BFSP2 genes (beaded filament structural protein 1 and 2) encode these proteins. Apparently, the beaded filament is required to maintain cell morphology, 3-dimensional membrane architecture and lens transparency during fetal development and fiber cell differentiation [Alizadeh et al., 2002]. Both genes are associated with cataracts with no other anomalies: the BFS1 gene inherited in an autosomal recessive pattern and the BFS2 gene inherited in autosomal dominant and recessive patterns. Figure 7 shows the BFSP1 structure.
Fig. 7.
The structure of BFSP1, from https://swissmodel.expasy.org/.
Nonsyndromic Cataracts with Eye Anomalies
In some cases, nonsyndromic cataracts are associated with eye anomalies; about 15 genes have been reported in the literature with this condition (Tables 3, 4), but only a few genes have consistently presented this association (BEST1, VSX2, NR2E3, MIR184, and GUCY2D). The rest of the genes (CRYGB, CRYGA, FYCO1, BFSP2, CRYGS, WDR36, GCNT2, RRAGA, CTPL1, TDRD7, VIM, MIP, GJA3, TMEM114, HSF4, CRYBA1, UNC45B, LONP1, SIPA1L3, WDR87, LIM2, BFSP1, CHMP4B, and LSS) have also been reported with inherited CC exclusively. The principal eye anomalies correspond to microcornea, aniridia, microphthalmia, or vitreoretinal dysplasia.
IRE, GALK1 and GCNT2
The hyperferritinaemia-cataract syndrome (OMIM 600886), an autosomal dominant condition, presents high serum ferritin levels and bilateral CCs. Mutations in the I-ferritin gene that encodes the iron-responsive element (IRE) results in hyperferritinaemia-cataract syndrome (http://www.hgmd.cf.ac.uk/ac/gene.php?gene=FTL). The IRE in the ferritin mRNAs is a structure in the 5′-untranslated region. The increase of iron diminishes the binding of trans-acting iron-regulatory proteins with the ferritin mRNA structure, resulting in increased ferritin mRNA translation. Mutations in the IRE block this IRE-mediated regulation of ferritin production with the subsequent hyperferritinemia [Brooks et al., 2009]; this gene defect produces CC. Galactokinase 1 converts galactose into galactose-1-phosphate, when galactokinase deficiency is present, the accumulating galactose is converted to galactitol by aldose reductase and the cataractogenesis is observed. In this case, only CC with no other manifestations is present (Table 3). The human blood i and I antigens are linear and branched repeats of N-acetyllactosamine, respectively. The I-branching beta-1,6-N-acetylglucosaminyltransferase enzyme catalyzes the conversion of the i to the I antigen. Null phenotype of I (the adult i phenotype) is associated with CC. The gene defect in the I locus leads to the formation of CC with no other clinical manifestations (Table 3).
Diagnosis of Inherited Cataracts
As previously described, hereditary CC is a difficult condition to diagnose genetically due to more than 100 genes that have been associated with it. This task is even more difficult if no specific inherited pattern is observed in the pedigree. When systemic anomalies are present in the clinical symptomatology, syndromic cataracts are a possible diagnosis. The presence of cataracts with no other alterations reduces the genes involved to 35. Fortunately, the new molecular tools are very useful in identifying the gene affection with the support of clinical data. Next-generation sequencing (NGS) certainly represents an excellent tool for analyzing inherited cataracts. Some physicians believe the technique should not be used in cases of monogenic diseases due to high costs and the fact that it generates volumes of data which can be difficult to interpret. The analysis would be easier to interpret if the potentially affected gene is preselected. NGS enables identifying mutations throughout the human genome by facilitating the genetic diagnosis of hereditary diseases, of course including CCs, and has opened a new pathway in genetic diagnosis.
Disclosure Statement
The authors report no conflicts of interest.
References
- Addison PK, Berry V, Holden KR, Espinal D, Rivera B, et al. A novel mutation in the connexin 46 gene (GJA3) causes autosomal dominant zonular pulverulent cataract in a Hispanic family. Mol Vis. 2006;12:791–795. [PubMed] [Google Scholar]
- Aldahmesh MA, Khan AO, Mohamed J, Alkuraya FS. Novel recessive BFSP2 and PITX3 mutations: insights into mutational mechanisms from consanguineous populations. Genet Med. 2011;11:978–981. doi: 10.1097/GIM.0b013e31822623d5. [DOI] [PubMed] [Google Scholar]
- Aldahmesh MA, Khan AO, Mohamed JY, Hijazi H, Al-Owain M, et al. Genomic analysis of pediatric cataract in Saudi Arabia reveals novel candidate disease genes. Genet Med. 2012;14:955–962. doi: 10.1038/gim.2012.86. [DOI] [PubMed] [Google Scholar]
- AlFadhli S, Abdelmoaty S, Al-Hajeri A, Behbehani A, Alkuraya F. Novel crystallin gamma B mutations in a Kuwaiti family with autosomal dominant congenital cataracts reveal genetic and clinical heterogeneity. Mol Vis. 2012;18:2931–2936. [PMC free article] [PubMed] [Google Scholar]
- Ali M, Buentello-Volante B, McKibbin M, Rocha-Medina JA, Fernandez-Fuentes N, et al. Homozygous FOXE3 mutations cause non-syndromic, bilateral, total sclerocornea, aphakia, microphthalmia and optic disc coloboma. Mol Vis. 2010;16:1162–1168. [PMC free article] [PubMed] [Google Scholar]
- Alizadeh A, Clark JI, Seeberger T, Hess J, Blankenship T, et al. Targeted genomic deletion of the lens-specific intermediate filament protein CP49. Invest Ophthalmol Vis Sci. 2002;43:3722–3727. [PubMed] [Google Scholar]
- Alizadeh A, Clark J, Seeberger T, Hess J, Blankenship T, FitzGerald PG. Targeted deletion of the lens fiber cell-specific intermediate filament protein filensin. Invest Ophthalmol Vis Sci. 2003;44:5252–5258. doi: 10.1167/iovs.03-0224. [DOI] [PubMed] [Google Scholar]
- Amato KR, Wang S, Hastings AK, Youngblood VM, Santapuram PR, et al. Genetic and pharmacologic inhibition of EPHA2 promotes apoptosis in NSCLC. J Clin Invest. 2014;124:2037–2049. doi: 10.1172/JCI72522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjum I, Eiberg H, Baig SM, Tommerup N, Hansen L. A mutation in the FOXE3 gene causes congenital primary aphakia in an autosomal recessive consanguineous Pakistani family. Mol Vis. 2010;16:549–555. [PMC free article] [PubMed] [Google Scholar]
- Apple DJ, Ram J, Foster A, Peng Q. Elimination of cataract blindness: a global perspective entering the new millenium. Surv Ophthalmol 45 Suppl. 2000;1:S1–196. [PubMed] [Google Scholar]
- Arora A, Minogue PJ, Liu X, Reddy MA, Ainsworth JR, et al. A novel GJA8 mutation is associated with autosomal dominant lamellar pulverulent cataract: further evidence for gap junction dysfunction in human cataract. J Med Genet. 2006;43:e2. doi: 10.1136/jmg.2005.034108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora A, Minogue PJ, Liu X, Addison PK, Russel-Eggitt I, et al. A novel connexin50 mutation associated with congenital nuclear pulverulent cataracts. J Med Genet. 2008;45:155–160. doi: 10.1136/jmg.2007.051029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada M, Okano Y, Imamura T, Suyama I, Hase Y, Isshiki G. Molecular characterization of galactokinase deficiency in Japanese patients. J Hum Genet. 1999;44:377–382. doi: 10.1007/s100380050182. [DOI] [PubMed] [Google Scholar]
- Augusteyn RC. On the growth and internal structure of human lens. Exp Eye Res. 2010;90:643–654. doi: 10.1016/j.exer.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma N, Hirakiyama A, Inoue T, Asaka A, Yamada M. Mutations of a human homologue of the Drosophila eyes absent gene (EYA1) detected in patients with congenital cataracts and ocular anterior segment anomalies. Hum Mol Genet. 2000;9:363–366. doi: 10.1093/hmg/9.3.363. [DOI] [PubMed] [Google Scholar]
- Bassnett S, Wilmarth PA, David LL. The membrane proteome of the mouse lens fiber cell. Mol Vis. 2009;15:2448–2463. [PMC free article] [PubMed] [Google Scholar]
- Bateman JB, Geyer DD, Flodman P, Johannes M, Sikela J, et al. A new betaA1-crystallin splice junction mutation in autosomal dominant cataract. Invest Ophthalmol Vis Sci. 2000;41:3278–3285. [PubMed] [Google Scholar]
- Bateman JB, von-Bischhoffshaunsen FR, Richter L, Flodman P, Burch D, Spence MA. Gene conversion mutation in crystallin, beta-B2 (CRYBB2) in a Chilean family with autosomal dominant cataract. Ophthalmology. 2007;114:425–432. doi: 10.1016/j.ophtha.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Beby F, Commeaux C, Bozon M, Denis P, Edery P, Morlé L. New phenotype associated with an Arg116Cys mutation in the CRYAA gene: nuclear cataract, iris coloboma, and microphthalmia. Arch Ophthalmol. 2007;125:213–216. doi: 10.1001/archopht.125.2.213. [DOI] [PubMed] [Google Scholar]
- Behnam M, Imagawa E, Chaleshtori AR, Ronasian F, Salehi M, et al. A novel homozygous mutation in HSF4 causing autosomal recessive congenital cataract. J Hum Genet. 2016;61:177–179. doi: 10.1038/jhg.2015.127. [DOI] [PubMed] [Google Scholar]
- Bennett TM, Shiels A. A recurrent missense mutation in GJA3 associated with autosomal dominant cataract linked to chromosome 13q. Mol Vis. 2011;17:2255–2262. [PMC free article] [PubMed] [Google Scholar]
- Bennett TM, Mackay DS, Knopf HL, Shiels A. A novel missense mutation in the gene for gap-junction protein alpha3 (GJA3) associated with autosomal dominant “nuclear punctate” cataracts linked to chromosome 13q. Mol Vis. 2004;10:376–382. [PubMed] [Google Scholar]
- Bennett TM, Mackay DS, Siegfried CJ, Shiels A. Mutation of the melastatin-related cation channel, TRPM3, underlies inherited cataract and glaucoma. PLoS One. 2014;9:e104000. doi: 10.1371/journal.pone.0104000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry V, Mackay D, Khaliq S, Francis PJ, Hameed A, et al. Connexin 50 mutation in a family with congenital “zonular nuclear” pulverulent cataract of Pakistani origin. Hum Genet. 1999;105:168–170. doi: 10.1007/s004399900094. [DOI] [PubMed] [Google Scholar]
- Berry V, Francis P, Kaushal S, Moore A, Bhattacharya S. Missense mutations in MIP underlie autosomal dominant “polymorphic” and lamellar cataracts linked to 12q. Nat Genet. 2000;25:15–17. doi: 10.1038/75538. [DOI] [PubMed] [Google Scholar]
- Berry V, Francis P, Reddy MA, Collyer D, Vithana E, et al. Alpha-B crystallin gene (CRYAB) mutation causes dominant congenital posterior polar cataract in humans. Am J Hum Genet. 2001;69:1141–1145. doi: 10.1086/324158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry V, Gregory-Evans C, Emmett W, Waseem N, Raby J, et al. Wolfram gene (WFS1) mutation causes autosomal dominant congenital nuclear cataract in humans. Eur J Hum Genet. 2013;21:1356–1360. doi: 10.1038/ejhg.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhishagratna K. Chowkhamba Sanskrit Series Office. XVII. Vol. 3. Calcutta, Varanasi: 2005. An English translation of the SushrutaSamshitá: Based on original Sanskrit text, with a full and comprehensive introduction, additional texts, different readings, notes, comparative views, index, glossary and plates; pp. 206–210. [Google Scholar]
- Billingsley G, Santhiya ST, Paterson AD, Ogata K, Wodak S, et al. CRYBA4, a novel human cataract gene, is also involved in microphthalmia. Am J Hum Genet. 2006;79:702–709. doi: 10.1086/507712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon CJ, Klevering BJ, Leroy BP, Hoyng CB, Keunen JE, den Hollander AI. The spectrum of ocular phenotypes caused by mutations in the BEST1 gene. Prog Retin Eye Res. 2009;28:187–205. doi: 10.1016/j.preteyeres.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Boone PM, Yuan B, Gu S, Ma Z, Gambin T, et al. Hutterite-type cataract maps to chromosome 6p21.32-p21.31, cosegregates with a homozygous mutation in LEMD2, and is associated with sudden cardiac death. Mol Genet Genomic Med. 2015;4:77–94. doi: 10.1002/mgg3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borck G, Kakar N, Hoch J, Friedrich K, Freudenberg J, et al. An Alu repeat-mediated genomic GCNT2 deletion underlies congenital cataracts and adult i blood group. Hum Genet. 2012;131:209–216. doi: 10.1007/s00439-011-1062-1. [DOI] [PubMed] [Google Scholar]
- Brémond-Gignac D, Bitoun P, Reis LM, Copin H, Murray JC, Semina EV. Identification of dominant FOXE3 and PAX6 mutations in patients with congenital cataract and aniridia. Mol Vis. 2010;16:1705–1711. [PMC free article] [PubMed] [Google Scholar]
- Brennan LA, Kantorow WL, Chauss D, McGreal R, He S, et al. Spatial expression patterns of autophagy genes in the eye lens and induction of autophagy in lens cells. Mol Vis. 2012;18:1773–1786. [PMC free article] [PubMed] [Google Scholar]
- Broooks DG, Manova-Todorova K, Farmer J, Lobmayr L, Wilson RB, et al. Ferritin crystal cataracts in hereditary hyperferritinemia cataract syndrome. Invest Ophthalmol Vis Sci. 2002;43:1121–1126. [PubMed] [Google Scholar]
- Bu J, He S, Wang L, Li J, Liu J, Zhang X. A novel splice donor site mutation in EPHA2 caused congenital cataract in a Chinese family. Indian J Ophthalmol. 2016;64:364–368. doi: 10.4103/0301-4738.185597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu L, Jin Y, Shi Y, Chu R, Ban A, Eiberg H, et al. Mutant DNA-binding domain of HSF4 is associated with autosomal dominant lamellar and Marner cataract. Nat Genet. 2002;31:276–278. doi: 10.1038/ng921. [DOI] [PubMed] [Google Scholar]
- Burdon KP, Wirth MG, Mackey DA, Russell-Eggitt IM, Craig JE, et al. A novel mutation in the Connexin 46 gene causes autosomal dominant congenital cataract with incomplete penetrance. J Med Genet. 2004;41:e106. doi: 10.1136/jmg.2004.018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bykhovskaya Y, Caiado Canedo AL, Wright KW, Rabinowitz YS. C.57C>T mutation in MIR 184 is responsible for congenital cataracts and corneal abnormalities in a five-generation family from Galicia, Spain. Ophthalmic Genet. 2015;36:244–247. doi: 10.3109/13816810.2013.848908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacon-Camacho OF, Buentello-Volante B, Velázquez-Montoya R, Ayala-Ramirez R, Zenteno JC. Homozygosity mapping identifies a GALK1 mutation as the cause of autosomal recessive congenital cataracts in 4 adult siblings. Gene. 2014;534:218–221. doi: 10.1016/j.gene.2013.10.057. [DOI] [PubMed] [Google Scholar]
- Chance B. Clio Medica: A Series of Primers on the History of Medicine. In: Krumbhaar EB, editor. Cloth. XX. New York: Paul B. Hoeber Inc.; 1939. [Google Scholar]
- Chen C, Sun Q, Gu M, Liu K, Sun Y, Xu X. A novel Cx50 (GJA8) p.H277Y mutation associated with autosomal dominant congenital cataract identified with targeted next-generation sequencing. Graefes Arch Clin Exp Ophthalmol. 2015;253:915–924. doi: 10.1007/s00417-015-3019-x. [DOI] [PubMed] [Google Scholar]
- Chen J, Ma Z, Jiao X, Fariss R, Kantorow WL, et al. Mutations in FYCO1 cause autosomal-recessive congenital cataracts. Am J Hum Genet. 2011;88:827–838. doi: 10.1016/j.ajhg.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JH, Qiu J, Chen H, Pang CP, Zhang M. Rapid and cost-effective molecular diagnosis using exome sequencing of one proband with autosomal dominant congenital cataract. Eye (Lond) 2014;28:1511–1516. doi: 10.1038/eye.2014.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JH, Huang C, Zhang B, Yin S, Liang J, et al. Mutations of RagA GTPase in mTORC1 pathway are associated with autosomal dominant cataracts. PLoS Genet. 2016;12:e1006090. doi: 10.1371/journal.pgen.1006090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Ma J, Yan M, Mothobi ME, Liu Y, Zheng F. A novel mutation in CRYAB associated with autosomal dominant congenital nuclear cataract in a Chinese family. Mol Vis. 2009;15:1359–1365. [PMC free article] [PubMed] [Google Scholar]
- Chen W, Chen X, Hu Z, Lin H, Zhou F, et al. A missense mutation in CRYBB2 leads to progressive congenital membranous cataract by impacting the solubility and function of βB2-crystallin. PLoS One. 2013;8:e81290. doi: 10.1371/journal.pone.0081290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AR, Lubsen NH, Slingsby C. sHSP in the eye lens: crystallin mutations, cataract and proteostasis. Int J Biochem Cell Biol. 2012;44:1687–1697. doi: 10.1016/j.biocel.2012.02.015. [DOI] [PubMed] [Google Scholar]
- Coccia M, Brooks SP, Webb TR, Christodoulou K, Wozniak IO, et al. X-linked cataract and Nance-Horan syndrome are allelic disorders. Hum Mol Genet. 2009;18:2643–2655. doi: 10.1093/hmg/ddp206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D, Bar-Yosef U, Levy J, Gradstein L, Belfair N, et al. Homozygous CRYBB1 deletion mutation underlies autosomal recessive congenital cataract. Invest Ophthalmol Vis Sci. 2007;48:2208–2213. doi: 10.1167/iovs.06-1019. [DOI] [PubMed] [Google Scholar]
- Conley YP, Erturk D, Keverline A, Mah TS, Keravala A, et al. A juvenile-onset, progressive cataract locus on chromosome 3q21-q22 is associated with a missense mutation in the beaded filament structural protein-2. Am J Hum Genet. 2000;66:1426–1431. doi: 10.1086/302871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Gao L, Jin Y, Zhang Y, Bai J, et al. The E233del mutation in BFSP2 causes a progressive autosomal dominant congenital cataract in a Chinese family. Mol Vis. 2007;13:2023–2029. [PubMed] [Google Scholar]
- Dave A, Laurie K, Staffieri SE, Taranath D, Mackey DA, et al. Mutations in the EPHA2 gene are a major contributor to inherited cataracts in South-Eastern Australia. PLoS One. 2013;8:e72518. doi: 10.1371/journal.pone.0072518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi RR, Vijayalakshmi P. Novel mutations in GJA8 associated with autosomal dominant congenital cataract and microcornea. Mol Vis. 2006;12:190–195. [PubMed] [Google Scholar]
- Devi RR, Reena C, Vijayalakshmi P. Novel mutations in GJA3 associated with autosomal dominant congenital cataract in the Indian population. Mol Vis. 2005;11:846–852. [PubMed] [Google Scholar]
- Devi RR, Yao W, Vijayalakshmi P, Sergeev YV, Sundaresan P, Hejtmancik JF. Crystallin gene mutations in Indian families with inherited pediatric cataract. Mol Vis. 2008;14:1157–1170. [PMC free article] [PubMed] [Google Scholar]
- Ding X, Wang B, Luo Y, Hu S, Zhou G, et al. A novel mutation in the connexin 46 (GJA3) gene associated with congenital cataract in a Chinese pedigree. Mol Vis. 2011;17:1343–1349. [PMC free article] [PubMed] [Google Scholar]
- Ding X, Zhou N, Lin H, Chen J, Zhao C, et al. A novel MIP gene mutation analysis in a Chinese family affected with congenital progressive punctate cataract. PLoS One. 2014;9:e102733. doi: 10.1371/journal.pone.0102733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucette L, Green J, Fernandez B, Johnson GJ, Parfrey P, Young TL. A novel, non-stop mutation in FOXE3 causes an autosomal dominant form of variable anterior segment dysgenesis including Peters anomaly. Eur J Hum Genet. 2011;19:293–299. doi: 10.1038/ejhg.2010.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AO. Clinical features of the congenital vitreoretinopathies. Eye (Lond) 2008;22:1233–1242. doi: 10.1038/eye.2008.38. [DOI] [PubMed] [Google Scholar]
- Eiberg H, Lund AM, Warburg M, Rosenberg T. Assignment of congenital cataract Volkmann type (CCV) to chromosome 1p36. Hum Genet. 1995;96:33–38. doi: 10.1007/BF00214183. [DOI] [PubMed] [Google Scholar]
- Faletra F, d'Adamo AP, Pensiero S, Athanasakis E, Catalano D, et al. A novel CRYBB2 missense mutation causing congenital autosomal dominant cataract in an Italian family. Ophthalmic Genet. 2013;34:115–117. doi: 10.3109/13816810.2012.707273. [DOI] [PubMed] [Google Scholar]
- Ferda Percin E, Ploder LA, Yu JJ, Arici K, Horsford DJ, et al. Human microphthalmia associated with mutations in the retinal homeobox gene CHX10. Nat Genet. 2000;25:397–401. doi: 10.1038/78071. [DOI] [PubMed] [Google Scholar]
- Ferrini W, Schorderet DF, Othenin-Girard P, Uffer S, Héon E, Munier FL. CRYBA3/A1 gene mutation associated with suture-sparing autosomal dominant congenital nuclear cataract: a novel phenotype. Invest Ophthalmol Vis Sci. 2004;45:1436–1441. doi: 10.1167/iovs.03-0760. [DOI] [PubMed] [Google Scholar]
- Florijn RJ, Loves W, Maillette de Buy Wenniger-Prick LJ, Mannens MM, Tijmes N, et al. New mutations in the NHS gene in Nance-Horan Syndrome families from the Netherlands. Eur J Hum Genet. 2006;14:986–990. doi: 10.1038/sj.ejhg.5201671. [DOI] [PubMed] [Google Scholar]
- Forshew T, Johnson CA, Khaliq S, Pasha S, Willis C, et al. Locus heterogeneity in autosomal recessive congenital cataracts: linkage to 9q and germline HSF4 mutations. Hum Genet. 2005;117:452–459. doi: 10.1007/s00439-005-1309-9. [DOI] [PubMed] [Google Scholar]
- Gao J, Wang H, Sun X, Varadaraj K, Li L, et al. The effects of age on lens transport. Invest Ophthalmol Vis Sci. 2013;54:7174–7187. doi: 10.1167/iovs.13-12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Huang S, Li J, Zou Y, Xu P, et al. A novel pathogenic mutation of CRYGD gene in a congenital cataract family (in Chinese) Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2016;4:515–518. doi: 10.3760/cma.j.issn.1003-9406.2016.04.020. [DOI] [PubMed] [Google Scholar]
- Gao X, Cheng J, Lu C, Li X, Li F, et al. A novel mutation in the connexin 50 gene (GJA8) associated with autosomal dominant congenital nuclear cataract in a Chinese family. Curr Eye Res. 2010;35:597–604. doi: 10.3109/02713681003725831. [DOI] [PubMed] [Google Scholar]
- Garnai SJ, Huyghe JR, Reed DM, Scott KM, Liebmann JM, et al. Congenital cataracts: de novo gene conversion event in CRYBB2. Mol Vis. 2014;20:1579–1593. [PMC free article] [PubMed] [Google Scholar]
- Ge XL, Zhang Y, Wu Y, Lv J, Zhang W, et al. Identification of a novel GJA8 (Cx50) point mutation causes human dominant congenital cataracts. Sci Rep. 2014;4:4121. doi: 10.1038/srep04121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer DD, Spence MA, Johannes M, Flodman P, Clancy KP, et al. Novel single-base deletional mutation in major intrinsic protein (MIP) in autosomal dominant cataract. Am J Ophthalmol. 2006;141:761–763. doi: 10.1016/j.ajo.2005.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C, Foster A. Childhood blindness in the context of VISION 2020 – the right to sight. Bull World Health Organ. 2001;79:227–232. [PMC free article] [PubMed] [Google Scholar]
- Gill D, Klose R, Munier FL, McFadden M, Priston M, et al. Genetic heterogeneity of the Coppock-like cataract: a mutation in CRYBB2 on chromosome 22q11.2. Invest Ophthalmol Vis Sci. 2000;41:159–165. [PubMed] [Google Scholar]
- Gillespie RL, O'Sullivan J, Ashworth J, Bhaskar S, Williams S, et al. Personalized diagnosis and management of congenital cataract by next-generation sequencing. Ophthalmology. 2014;121:2124–2137. doi: 10.1016/j.ophtha.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Gong X, Li E, Klier G, Huang Q, Wu Y, et al. Disruption of alpha3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell. 1997;91:833–843. doi: 10.1016/s0092-8674(00)80471-7. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Huerta LM, Messina-Baas OM, Cuevas-Covarrubias SA. A family with autosomal dominant primary congenital cataract associated with a CRYGC mutation: evidence of clinical heterogeneity. Mol Vis. 2007;13:1333–1338. [PubMed] [Google Scholar]
- Gradstein L, Zolotushko J, Sergeev YV, Lavy I, Narkis G, et al. Novel GUCY2D mutation causes phenotypic variability of Leber congenital amaurosis in a large kindred. BMC Med Genet. 2016;17:52. doi: 10.1186/s12881-016-0314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graw J, Klopp N, Illig T, Preising MN, Lorenz B. Congenital cataract and macular hypoplasia in humans associated with a de novo mutation in CRYAA and compound heterozygous mutations in P. Graefes Arch Clin Exp Ophthalmol. 2006;244:912–919. doi: 10.1007/s00417-005-0234-x. [DOI] [PubMed] [Google Scholar]
- Graw J, Schmidt W, Minogue PJ, Rodriguez J, Tong JJ, et al. The GJA8 allele encoding CX50I247M is a rare polymorphism, not a cataract-causing mutation. Mol Vis. 2009;15:1881–1885. [PMC free article] [PubMed] [Google Scholar]
- Greenlees R, Mihelec M, Yousoof S, Speidel D, Wu SK, et al. Mutations in SIPA1L3 cause eye defects through disruption of cell polarity and cytoskeleton organization. Hum Mol Genet. 2015;24:5789–5804. doi: 10.1093/hmg/ddv298. [DOI] [PubMed] [Google Scholar]
- Gu F, Li R, Ma XX, Shi LS, Huang SZ, Ma X. A missense mutation in the gammaD-crystallin gene CRYGD associated with autosomal dominant congenital cataract in a Chinese family. Mol Vis. 2006;12:26–31. [PubMed] [Google Scholar]
- Gu F, Zhai H, Li D, Zhao L, Li C, et al. A novel mutation in major intrinsic protein of the lens gene (MIP) underlies autosomal dominant cataract in a Chinese family. Mol Vis. 2007;13:1651–1656. [PubMed] [Google Scholar]
- Gu F, Luo W, Li X, Wang Z, Lu S, et al. A novel mutation in AlphaA-crystallin (CRYAA) caused autosomal dominant congenital cataract in a large Chinese family. Hum Mutat. 2008;29:769. doi: 10.1002/humu.20724. [DOI] [PubMed] [Google Scholar]
- Gu J, Qi Y, Wang L, Wang J, Shi L, et al. A new congenital nuclear cataract caused by a missense mutation in the gammaD-crystallin gene (CRYGD) in a Chinese family. Mol Vis. 2005;11:971–976. [PubMed] [Google Scholar]
- Gu Z, Ji B, Wan C, He G, Zhang J, et al. A splice site mutation in CRYBA1/A3 causing autosomal dominant posterior polar cataract in a Chinese pedigree. Mol Vis. 2010;16:154–160. [PMC free article] [PubMed] [Google Scholar]
- Guleria K, Sperling K, Singh D, Varon R, Singh JR, Vanita V. A novel mutation in the connexin 46 (GJA3) gene associated with autosomal dominant congenital cataract in an Indian family. Mol Vis. 2007;13:1657–1665. [PubMed] [Google Scholar]
- Guo Y, Su D, Li Q, Yang Z, Ma Z, et al. A nonsense mutation of CRYGC associated with autosomal dominant congenital nuclear cataracts and microcornea in a Chinese pedigree. Mol Vis. 2012;18:1874–1880. [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Yuan L, Yi J, Xiao J, Xu H, et al. Identification of a GJA3 mutation in a Chinese family with congenital nuclear cataract using exome sequencing. Indian J Biochem Biophys. 2013;50:253–258. [PubMed] [Google Scholar]
- Hansen L, Yao W, Eiberg H, Funding M, Riise R, et al. The congenital ‘ant-egg’ cataract phenotype is caused by a missense mutation in connexin46. Mol Vis. 2006;12:1033–1039. [PubMed] [Google Scholar]
- Hansen L, Eiberg H, Rosenberg T. Novel MAF mutation in a family with congenital cataract-microcornea syndrome. Mol Vis. 2007;13:2019–2022. [PubMed] [Google Scholar]
- Hansen L, Mikkelsen A, Nürnberg P, Nürnberg G, Anjum I, et al. Comprehensive mutational screening in a cohort of Danish families with hereditary congenital cataract. Invest Ophthalmol Vis Sci. 2009;50:3291–3303. doi: 10.1167/iovs.08-3149. [DOI] [PubMed] [Google Scholar]
- Hansen L, Comyn S, Mang Y, Lind-Thomsen A, Myhre L, et al. The myosin chaperone UNC45B is involved in lens development and autosomal dominant juvenile cataract. Eur J Hum Genet. 2014;22:1290–1297. doi: 10.1038/ejhg.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happ H, Weh E, Costakos D, Reis LM, Semina EV. Case report of homozygous deletion involving the first coding exons of GCNT2 isoforms A and B and part of the upstream region of TFAP2A in congenital cataract. BMC Med Genet. 2016;17:64. doi: 10.1186/s12881-016-0316-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Li X, Chen J, Xu L, Zhang F, et al. Genetic linkage analyses and Cx50 mutation detection in a large multiplex Chinese family with hereditary nuclear cataract. Ophthalmic Genet. 2011;32:48–53. doi: 10.3109/13816810.2010.535886. [DOI] [PubMed] [Google Scholar]
- Hejtmancik JF, Smaoui N. Molecular genetics of cataract. Dev Ophthalmol. 2003;37:67–82. doi: 10.1159/000072039. [DOI] [PubMed] [Google Scholar]
- Héon E, Priston M, Schorderet DF, Billingsley GD, Girard PO, et al. The gamma-crystallins and human cataracts: a puzzle made clearer. Am J Hum Genet. 1999;65:1261–1277. doi: 10.1086/302619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Héon E, Paterson AD, Fraser M, Billingsley G, Priston M, et al. A progressive autosomal recessive cataract locus maps to chromosome 9q13-q22. Am J Hum Genet. 2001;68:772–777. doi: 10.1086/318798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehenwarter W, Klose J, Jungblut PR. Eye lens proteomics. Amino Acids. 2006;30:369–389. doi: 10.1007/s00726-005-0283-9. [DOI] [PubMed] [Google Scholar]
- Hu S, Wang B, Zhou Z, Zhou G, Wang J, et al. A novel mutation in GJA8 causing congenital cataract-microcornea syndrome in a Chinese pedigree. Mol Vis. 2010;16:1585–1592. [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Gao L, Feng Y, Yang T, Huang S, et al. Identification of a novel mutation of the gene for gap junction protein α3 (GJA3) in a Chinese family with congenital cataract. Mol Biol Rep. 2014;41:4753–4758. doi: 10.1007/s11033-014-3346-8. [DOI] [PubMed] [Google Scholar]
- Hunter M, Angelicheva D, Levy HL, Pueschel SM, Kalaydjieva L. Novel mutations in the GALK1 gene in patients with galactokinase deficiency. Hum Mutat. 2001;17:77–78. doi: 10.1002/1098-1004(2001)17:1<77::AID-HUMU20>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Iliff BW, Riazuddin SA, Gottsch JD. A single-base substitution in the seed region of miR-184 causes EDICT syndrome. Invest Ophthalmol Vis Sci. 2012;53:348–353. doi: 10.1167/iovs.11-8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba N, Hiruma T, Togayachi A, Iwasaki H, Wang XH, et al. A novel I-branching beta-1,6-N-acetylglucosaminyltransferase involved in human blood group I antigen expression. Blood. 2003;101:2870–2876. doi: 10.1182/blood-2002-09-2838. [DOI] [PubMed] [Google Scholar]
- Iseri SU, Osborne RJ, Farrall M, Wyatt AW, Mirza G, et al. Seeing clearly: the dominant and recessive nature of FOXE3 in eye developmental anomalies. Hum Mutat. 2009;30:1378–1386. doi: 10.1002/humu.21079. [DOI] [PubMed] [Google Scholar]
- Jakobs PM, Hess JF, FitzGerald PG, Kramer P, Weleber RG, Litt M. Autosomal-dominant congenital cataract associated with a deletion mutation in the human beaded filament protein gene BFSP2. Am J Hum Genet. 2000;66:1432–1436. doi: 10.1086/302872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson RV, Perveen R, Kerr B, Carette M, Yardley J, et al. Domain disruption and mutation of the bZIP transcription factor, MAF, associated with cataract, ocular anterior segment dysgenesis and coloboma. Hum Mol Genet. 2002;11:33–42. doi: 10.1093/hmg/11.1.33. [DOI] [PubMed] [Google Scholar]
- Jamieson RV, Farrar N, Stewart K, Perveen R, Mihelec M, et al. Characterization of a familial t(16;22) balanced translocation associated with congenital cataract leads to identification of a novel gene, TMEM114, expressed in the lens and disrupted by the translocation. Hum Mutat. 2007;28:968–977. doi: 10.1002/humu.20545. [DOI] [PubMed] [Google Scholar]
- Javadiyan S, Craig JE, Souzeau E, Sharma S, Lower KM, et al. Recurrent mutation in the crystallin alpha A gene associated with inherited paediatric cataract. BMC Res Notes. 2016;9:83. doi: 10.1186/s13104-016-1890-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X, Zhang F, Bai J, Gao L, Zhang X, et al. Combinational analysis of linkage and exome sequencing identifies the causative mutation in a Chinese family with congenital cataract. BMC Med Genet. 2013;14:107. doi: 10.1186/1471-2350-14-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Jin Y, Bu L, Zhang W, Liu J, Cui B, et al. A novel mutation in GJA3 (connexin46) for autosomal dominant congenital nuclear pulverulent cataract. Mol Vis. 2003;9:579–583. [PubMed] [Google Scholar]
- Jiang J, Jin C, Wang W, Tang X, Shentu X, et al. Identification of a novel splice-site mutation in MIP in a Chinese congenital cataract family. Mol Vis. 2009;15:38–44. [PMC free article] [PubMed] [Google Scholar]
- Jiaox X, Khan SY, Irum B, Khan AO, Wang Q, et al. Missense mutations in CRYAB are liable for recessive congenital cataracts. PLoS One. 2015;10:e0137973. doi: 10.1371/journal.pone.0137973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaydjieva L, Perez-Lezaun A, Angelicheva D, Onengut S, Dye D, et al. A founder mutation in the GK1 gene is responsible for galactokinase deficiency in Roma (Gypsies) Am J Hum Genet. 1999;65:1299–1307. doi: 10.1086/302611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamachi Y, Uchikawa M, Tanouchi A, Sekido R, Kondoh H. Pax6 and SOX2 form a co-DNA-binding partner complex that regulates initiation of lens development. Genes Dev. 2001;15:1272–1286. doi: 10.1101/gad.887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannabiran C, Rogan PK, Olmos L, Basti S, Rao GN, et al. Autosomal dominant zonular cataract with sutural opacities is associated with a splice mutation in the betaA3/A1-crystallin gene. Mol Vis. 1998;4:21. [PubMed] [Google Scholar]
- Kannan R, Sreekumar PG, Hinton DR. Novel roles for alpha-crystallins in retinal function and disease. Prog Retin Eye Res. 2012;31:576–604. doi: 10.1016/j.preteyeres.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul H, Riazuddin SA, Shahid M, Kousar S, Butt NH, et al. Autosomal recessive congenital cataract linked to EPHA2 in a consanguineous Pakistani family. Mol Vis. 2010;16:511–517. [PMC free article] [PubMed] [Google Scholar]
- Ke T, Wang QK, Ji B, Wang X, Liu P, et al. Novel HSF4 mutation causes congenital total white cataract in a Chinese family. Am J Ophthalmol. 2006;142:298–303. doi: 10.1016/j.ajo.2006.03.056. [DOI] [PubMed] [Google Scholar]
- Khan AO, Aldahmesh MA, Meyer B. Recessive congenital total cataract with microcornea and heterozygote carrier signs caused by a novel missense CRYAA mutation (R54C) Am J Ophthalmol. 2007;144:949–952. doi: 10.1016/j.ajo.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Khan AO, Aldahmesh MA, Ghadhfan FE, Al-Mesfer S, Alkuraya FS. Founder heterozygous P23T CRYGD mutation associated with cerulean (and coralliform) cataract in 2 Saudi families. Mol Vis. 2009;15:1407–1411. [PMC free article] [PubMed] [Google Scholar]
- Khan AO, Aldahmesh MA, Mohamed JY, Alkuraya FS. Clinical and molecular analysis of children with central pulverulent cataract from the Arabian Peninsula. Br J Ophthalmol. 2012;96:650–655. doi: 10.1136/bjophthalmol-2011-301053. [DOI] [PubMed] [Google Scholar]
- Khan AO, Aldahmesh MA, Alkuraya FS. Phenotypes of recessive pediatric cataract in a cohort of children with identified homozygous gene mutations (An American Ophthalmological Society Thesis). Trans Am Ophthalmol Soc. 2015;113:T7. [PMC free article] [PubMed] [Google Scholar]
- Kmoch S, Brynda J, Asfaw B, Bezouska K, Novák P, et al. Link between a novel human gammaD-crystallin allele and a unique cataract phenotype explained by protein crystallography. Hum Mol Genet. 2000;9:1779–1786. doi: 10.1093/hmg/9.12.1779. [DOI] [PubMed] [Google Scholar]
- Kolosha V, Anoia E, de Cespedes C, Gitzelmann R, Shih L, et al. Novel mutations in 13 probands with galactokinase deficiency. Hum Mutat. 2000;15:447–453. doi: 10.1002/(SICI)1098-1004(200005)15:5<447::AID-HUMU6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Saitsu H, Miyamoto T, Lee BJ, Nishiyama K, et al. Pathogenic mutations in two families with congenital cataract identified with whole-exome sequencing. Mol Vis. 2013;19:384–389. [PMC free article] [PubMed] [Google Scholar]
- Kondoh H, Uchikawa M, Kamachi Y. Interplay of Pax6 and SOX2 in lens development as a paradigm of genetic switch mechanisms for cell differentiation. Int J Dev Biol. 2004;48:819–827. doi: 10.1387/ijdb.041868hk. [DOI] [PubMed] [Google Scholar]
- Kong XD, Liu N, Shi HR, Dong JM, Zhao ZH, et al. A novel 3-base pair deletion of the CRYAA gene identified in a large Chinese pedigree featuring autosomal dominant congenital perinuclear cataract. Genet Mol Res. 2015;14:426–432. doi: 10.4238/2015.January.23.16. [DOI] [PubMed] [Google Scholar]
- Kumar M, Agarwal T, Khokhar S, Kumar M, Kaur P, et al. Mutation screening and genotype phenotype correlation of α-crystallin, γ-crystallin and GJA8 gene in congenital cataract. Mol Vis. 2011;17:693–707. [PMC free article] [PubMed] [Google Scholar]
- Lachke SA, Alkuraya FS, Kneeland SC, Ohn T, Aboukhalil A, et al. Mutations in the RNA granule component TDRD7 cause cataract and glaucoma. Science. 2011;331:1571–1576. doi: 10.1126/science.1195970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie KJ, Dave A, Straga T, Souzeau E, Chataway T, et al. Identification of a novel oligomerization disrupting mutation in CRYΑA associated with congenital cataract in a South Australian family. Hum Mutat. 2013;34:435–438. doi: 10.1002/humu.22260. [DOI] [PubMed] [Google Scholar]
- Li B, Liu Y, Liu Y, Guo H, Hu Z, et al. Identification of a GJA3 mutation in a large family with bilateral congenital cataract. DNA Cell Biol. 2016;35:135–139. doi: 10.1089/dna.2015.3125. [DOI] [PubMed] [Google Scholar]
- Li D, Wang S, Ye H, Tang Y, Qiu X, et al. Distribution of gene mutations in sporadic congenital cataract in a Han Chinese population. Mol Vis. 2016;22:589–598. [PMC free article] [PubMed] [Google Scholar]
- Li FF, Zhu SQ, Wang SZ, Gao C, Huang SZ, et al. Nonsense mutation in the CRYBB2 gene causing autosomal dominant progressive polymorphic congenital coronary cataracts. Mol Vis. 2008;14:750–755. [PMC free article] [PubMed] [Google Scholar]
- Li FF, Yang M, Ma X, Zhang Q, Zhang M, et al. Autosomal dominant congenital nuclear cataracts caused by a CRYAA gene mutation. Curr Eye Res. 2010;35:492–498. doi: 10.3109/02713681003624901. [DOI] [PubMed] [Google Scholar]
- Li J, Wang Q, Fu Q, Zhu Y, Zhai Y, et al. A novel connexin 50 gene (gap junction protein, alpha 8) mutation associated with congenital nuclear and zonular pulverulent cataract. Mol Vis. 2013;19:767–774. [PMC free article] [PubMed] [Google Scholar]
- Li XQ, Cai HC, Zhou SY, Yang JH, Xi YB, et al. A novel mutation impairing the tertiary structure and stability of γC-crystallin (CRYGC) leads to cataract formation in humans and zebrafish lens. Hum Mutat. 2012;2:391–401. doi: 10.1002/humu.21648. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang J, Dong B, Man H. A novel connexin46 (GJA3) mutation in autosomal dominant congenital nuclear pulverulent cataract. Mol Vis. 2004;10:668–671. [PubMed] [Google Scholar]
- Liang C, Liang H, Yang Y, Ping L, Jie Q. Mutation analysis of two families with inherited congenital cataracts. Mol Med Rep. 2015;12:3469–3475. doi: 10.3892/mmr.2015.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Hejtmancik JF, Qi Y. A substitution of arginine to lysine at the COOH-terminus of MIP caused a different binocular phenotype in a congenital cataract family. Mol Vis. 2007;13:1822–1827. [PubMed] [Google Scholar]
- Lin Y, Liu NN, Lei CT, Fan YC, Liu XQ, et al. A novel GJA8 mutation in a Chinese family with autosomal dominant congenital cataract (in Chinese) Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2008;25:59–62. [PubMed] [Google Scholar]
- Litt M, Carrero-Valenzuela R, LaMorticella DM, Schultz DW, Mitchell TN, et al. Autosomal dominant cerulean cataract is associated with a chain termination mutation in the human beta-crystallin gene CRYBB2. Hum Mol Genet. 1997;6:665–668. doi: 10.1093/hmg/6.5.665. [DOI] [PubMed] [Google Scholar]
- Litt M, Kramer P, LaMorticella DM, Murphey W, Lovrien EW, Weleber RG. Autosomal dominant congenital cataract associated with a missense mutation in the human alpha crystallin gene CRYAA. Hum Mol Genet. 1998;7:471–474. doi: 10.1093/hmg/7.3.471. [DOI] [PubMed] [Google Scholar]
- Liu BF, Liang JJ. Confocal fluorescence microscopy study of interaction between lens MIP26/AQP0 and crystallins in living cells. J Cell Biochem. 2008;104:51–58. doi: 10.1002/jcb.21598. [DOI] [PubMed] [Google Scholar]
- Liu J, Xu J, Gu S, Nicholson BJ, Jiang JX. Aquaporin 0 enhances gap junction coupling via its cell adhesion function and interaction with connexin 50. J Cell Sci. 2011;124:198–206. doi: 10.1242/jcs.072652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhang Q, Zhou LX, Tang ZH. A novel HSF4 mutation in a Chinese family with autosomal dominant congenital cataract. J Huazhong Univ Sci Technolog Med Sci. 2015;35:316–318. doi: 10.1007/s11596-015-1430-5. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wang KJ, Zhu SQ. A novel p.G112E mutation in BFSP2 associated with autosomal dominant pulverulent cataract with sutural opacities. Curr Eye Res. 2014;39:1013–1019. doi: 10.3109/02713683.2014.891749. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang X, Luo L, Wu M, Zeng R, et al. A novel alphaB-crystallin mutation associated with autosomal dominant congenital lamellar cataract. Invest Ophthalmol Vis Sci. 2006;47:1069–1075. doi: 10.1167/iovs.05-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou D, Tong JP, Zhang LY, Chiang SW, Lam DS, Pang CP. A novel mutation in CRYBB2 responsible for inherited coronary cataract. Eye (Lond) 2009;23:1213–1220. doi: 10.1038/eye.2008.222. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, Overbeek PA. Overlapping effects of different members of the FGF family on lens fiber differentiation in transgenic mice. Development. 1998;125:3365–3377. doi: 10.1242/dev.125.17.3365. [DOI] [PubMed] [Google Scholar]
- Lu S, Zhao C, Jiao H, Kere J, Tang X, et al. Two Chinese families with pulverulent congenital cataracts and deltaG91 CRYBA1 mutations. Mol Vis. 2007;13:1154–1160. [PubMed] [Google Scholar]
- Lv H, Huang C, Zhang J, Liu Z, Zhang Z, et al. A novel HSF4 gene mutation causes autosomal-dominant cataracts in a Chinese family. G3 (Bethesda) 2014;4:823–828. doi: 10.1534/g3.113.009860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma AS, Grigg JR, Ho G, Prokudin I, Farnsworth E, et al. Sporadic and familial congenital cataracts: mutational spectrum and new diagnoses using next-generation sequencing. Hum Mutat. 2016;37:371–384. doi: 10.1002/humu.22948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Becker C, Reyes C, Underhill DM. Cutting edge: FYCO1 recruitment to dectin-1 phagosomes is accelerated by light chain 3 protein and regulates phagosome maturation and reactive oxygen production. J Immunol. 2014;192:1356–1360. doi: 10.4049/jimmunol.1302835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma MF, Li LB, Pei YQ, Cheng Z. Use of high-throughput targeted exome sequencing in genetic diagnosis of Chinese family with congenital cataract. Int J Ophthalmol. 2016;9:650–654. doi: 10.18240/ijo.2016.05.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Li FF, Wang SZ, Gao C, Zhang M, Zhu SQ. A new mutation in BFSP2 (G1091A) causes autosomal dominant congenital lamellar cataracts. Mol Vis. 2008;14:1906–1911. [PMC free article] [PubMed] [Google Scholar]
- Ma ZW, Zheng JQ, Li J, Li XR, Tang X, et al. Two novel mutations of connexin genes in Chinese families with autosomal dominant congenital nuclear cataract. Br J Ophthalmol. 2005;89:1535–1537. doi: 10.1136/bjo.2005.075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay D, Ionides A, Kibar Z, Rouleau G, Berry V, et al. Connexin46 mutations in autosomal dominant congenital cataract. Am J Hum Genet. 1999;64:1357–1364. doi: 10.1086/302383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay DS, Andley UP, Shiels A. Cell death triggered by a novel mutation in the alphaA-crystallin gene underlies autosomal dominant cataract linked to chromosome 21q. Eur J Hum Genet. 2003;11:784–793. doi: 10.1038/sj.ejhg.5201046. [DOI] [PubMed] [Google Scholar]
- Mackay DS, Andley UP, Shiels A. A missense mutation in the gammaD crystallin gene (CRYGD) associated with autosomal dominant ‘coral-like’ cataract linked to chromosome 2q. Mol Vis. 2004;10:155–162. [PubMed] [Google Scholar]
- Mackay DS, Bennett TM, Culican SM, Shiels A. Exome sequencing identifies novel and recurrent mutations in GJA8 and CRYGD associated with inherited cataract. Hum Genomics. 2014;8:19. doi: 10.1186/s40246-014-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marner E, Rosenberg T, Eiberg H. Autosomal dominant congenital cataract: morphology and genetic mapping. Acta Ophthalmol (Copenh) 1989;67:151–158. doi: 10.1111/j.1755-3768.1989.tb00745.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Wittinghan FJ, Sellitto C, White TW, Mathias RT, Paul D, Goodenough DA. Lens gap junctional coupling is modulated by connexin identity and the locus of gene expression. Invest Ophthalmol Vis Sci. 2004;45:3629–3637. doi: 10.1167/iovs.04-0445. [DOI] [PubMed] [Google Scholar]
- McAvoy JW, Chamberlain CG. Fibroblast growth factor (FGF) induces different responses in lens epithelial cells depending on its concentration. Development. 1989;107:221–228. doi: 10.1242/dev.107.2.221. [DOI] [PubMed] [Google Scholar]
- Messina-Baas OM, Gonzalez-Huerta LM, Cuevas-Covarrubias SA. Two affected siblings with nuclear cataract associated with a novel missense mutation in the CRYGD gene. Mol Vis. 2006;12:995–1000. [PubMed] [Google Scholar]
- Messina-Baas OM, Gonzalez-Garay ML, González-Huerta LM, Toral-López J, Cuevas-Covarrubias SA. Whole exome sequencing reveals a mutation in CRYBB2 in a large Mexican family with autosomal dominant pulverulent cataract. Mol Syndromol. 2016;7:87–92. doi: 10.1159/000445669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer E, Rahman F, Owens J, Pasha S, Morgan NV, et al. Initiation codon mutation in betaB1-crystallin (CRYBB1) associated with autosomal recessive nuclear pulverulent cataract. Mol Vis. 2009;15:1014–1019. [PMC free article] [PubMed] [Google Scholar]
- Miao H, Wang B. Eph/ephrin signaling in epithelial development and homeostasis. Int J Biochem Cell Biol. 2009;41:762–770. doi: 10.1016/j.biocel.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min HY, Qiao PP, Yan ZH, Jiang HF, et al. Targeted genes sequencing identified a novel 15 bp deletion on GJA8 in a Chinese family with autosomal dominant congenital cataracts. Chin Med J (Engl) 2016;129:860–867. doi: 10.4103/0366-6999.178966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothobi ME, Guo S, Liu Y, Chen Q, Yussuf AS, et al. Mutation analysis of congenital cataract in a Basotho family identified a new missense allele in CRYBB2. Mol Vis. 2009;15:1470–1475. [PMC free article] [PubMed] [Google Scholar]
- Müller M, Bhattacharya SS, Moore T, Prescott Q, Wedig T, et al. Dominant cataract formation in association with a vimentin assembly disrupting mutation. Hum Mol Genet. 2009;18:1052–1057. doi: 10.1093/hmg/ddn440. [DOI] [PubMed] [Google Scholar]
- Nandrot E, Slingsby C, Basak A, Cherif-Chefchaouni M, Benazzouz B, et al. Gamma-D crystallin gene (CRYGD) mutation causes autosomal dominant congenital cerulean cataracts. J Med Genet. 2003;40:262–267. doi: 10.1136/jmg.40.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narumi Y, Nishina S, Tokimitsu M, Aoki Y, Kosaki R, et al. Identification of a novel missense mutation of MAF in a Japanese family with congenital cataract by whole exome sequencing: a clinical report and review of literature. Am J Med Genet A. 2014;164A:1272–1276. doi: 10.1002/ajmg.a.36433. [DOI] [PubMed] [Google Scholar]
- Nettleship E, Ogilvie FM. A peculiar form of hereditary congenital cataract. Trans Ophthal Soc UK. 1906;26:191–206. [Google Scholar]
- Ormestad M, Blixt A, Churchill A, Martinsson T, Enerbäck S, Carlsson P. Foxe3 haploinsufficiency in mice: a model for Peters' anomaly. Invest Ophthalmol Vis Sci. 2002;43:1350–1357. [PubMed] [Google Scholar]
- Pankiv S, Alemu EA, Brech A, Bruun JA, Lamark T, et al. FYCO1 is a Rab7 effector that binds to LC3 and PI3P to mediate microtubule plus end-directed vesicle transport. Cell Biol. 2010;188:253–269. doi: 10.1083/jcb.200907015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JE, Son AI, Zhou R. Roles of EphA2 in development and disease. Genes (Basel) 2013;4:334–357. doi: 10.3390/genes4030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer. 2010;10:165–180. doi: 10.1038/nrc2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli S, Söker T, Klopp N, Illig T, Engel W, Graw J. Mutation analysis in a German family identified a new cataract-causing allele in the CRYBB2 gene. Mol Vis. 2007;13:962–967. [PMC free article] [PubMed] [Google Scholar]
- Plotnikova OV, Kondrashov FA, Vlasov PK, Grigorenko AP, Ginter EK, Rogaev EI. Conversion and compensatory evolution of the gamma-crystallin genes and identification of a cataractogenic mutation that reverses the sequence of the human CRYGD gene to an ancestral state. Am J Hum Genet. 2007;81:32–43. doi: 10.1086/518616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyakov AV, Shagina IA, Khlebnikova OV, Evgrafov OV. Mutation in the connexin 50 gene (GJA8) in a Russian family with zonular pulverulent cataract. Clin Genet. 2001;60:476–478. doi: 10.1034/j.1399-0004.2001.600614.x. [DOI] [PubMed] [Google Scholar]
- Ponnam SP, Ramesha K, Tejwani S, Ramamurthy B, Kannabiran C. Mutation of the gap junction protein alpha 8 (GJA8) gene causes autosomal recessive cataract. J Med Genet. 2007;44:e85. doi: 10.1136/jmg.2007.050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnam SP, Ramesha K, Tejwani S, Matalia J, Kannabiran C. A missense mutation in LIM2 causes autosomal recessive congenital cataract. Mol Vis. 2008;14:1204–1208. [PMC free article] [PubMed] [Google Scholar]
- Ponnam SP, Ramesha K, Matalia J, Tejwani S, Ramamurthy B, Kannabiran C. Mutational screening of Indian families with hereditary congenital cataract. Mol Vis. 2013;19:1141–1148. [PMC free article] [PubMed] [Google Scholar]
- Pras E, Frydman M, Levy-Nissenbaum E, Bakhan T, Raz J, et al. A nonsense mutation (W9X) in CRYAA causes autosomal recessive cataract in an inbred Jewish Persian family. Invest Ophthalmol Vis Sci. 2000;41:3511–3515. [PubMed] [Google Scholar]
- Pras E, Raz J, Yahalom V, Frydman M, Garzozi HJ, et al. A nonsense mutation in the glucosaminyl (N-acetyl) transferase 2 gene (GCNT2): association with autosomal recessive congenital cataracts. Invest Ophthalmol Vis Sci. 2004;45:1940–1945. doi: 10.1167/iovs.03-1117. [DOI] [PubMed] [Google Scholar]
- Prokudin I, Simons C, Grigg JR, Storen R, Kumar V, et al. Exome sequencing in developmental eye disease leads to identification of causal variants in GJA8 CRYGC PAX6 and CYP1B1. Eur J Hum Genet. 2014;22:907–915. doi: 10.1038/ejhg.2013.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Jia H, Huang S, Lin H, Gu J, et al. A deletion mutation in the betaA1/A3 crystallin gene (CRYBA1/A3) is associated with autosomal dominant congenital nuclear cataract in a Chinese family. Hum Genet. 2004;114:192–197. doi: 10.1007/s00439-003-1049-7. [DOI] [PubMed] [Google Scholar]
- Qin L, Guo L, Wang H, Li T, Lou G, et al. A novel MIP mutation in familial congenital nuclear cataracts. Eur J Med Genet. 2016;59:488–491. doi: 10.1016/j.ejmg.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Ramachandran RD, Perumalsamy V, Hejtmancik JF. Autosomal recessive juvenile onset cataract associated with mutation in BFSP1. Hum Genet. 2007;121:475–482. doi: 10.1007/s00439-006-0319-6. [DOI] [PubMed] [Google Scholar]
- Ramprasad VL, Thool A, Murugan S, Nancarrow D, Vyas P, et al. Truncating mutation in the NHS gene: phenotypic heterogeneity of Nance-Horan syndrome in an Asian Indian family. Invest Ophthalmol Vis Sci. 2005;46:17–23. doi: 10.1167/iovs.04-0477. [DOI] [PubMed] [Google Scholar]
- Reddy MA, Bateman OA, Chakarova C, Ferris J, Berry V, et al. Characterization of the G91del CRYBA1/3-crystallin protein: a cause of human inherited cataract. Hum Mol Genet. 2004;13:945–953. doi: 10.1093/hmg/ddh110. [DOI] [PubMed] [Google Scholar]
- Rees MI, Watts P, Fenton I, Clarke A, Snell RG, et al. Further evidence of autosomal dominant congenital zonular pulverulent cataracts linked to 13q11 (CZP3) and a novel mutation in connexin 46 (GJA3) Hum Genet. 2000;106:206–209. doi: 10.1007/s004390051029. [DOI] [PubMed] [Google Scholar]
- Reich S, Hennermann J, Vetter B, Neumann LM, Shin YS, et al. An unexpectedly high frequency of hypergalactosemia in an immigrant Bosnian population revealed by newborn screening. Pediatr Res. 2002;51:598–601. doi: 10.1203/00006450-200205000-00009. [DOI] [PubMed] [Google Scholar]
- Reis LM, Tyler RC, Schneider A, Bardakjian T, Stoler JM, et al. FOXE3 plays a significant role in autosomal recessive microphthalmia. Am J Med Genet A. 2010;152A:582–590. doi: 10.1002/ajmg.a.33257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis LM, Tyler RC, Muheisen S, Raggio V, Salviati L, et al. Whole exome sequencing in dominant cataract identifies a new causative factor, CRYBA2, and a variety of novel alleles in known genes. Hum Genet. 2013;132:761–770. doi: 10.1007/s00439-013-1289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis LM, Tyler RC, Semina EV. Identification of a novel C-terminal extension mutation in EPHA2 in a family affected with congenital cataract. Mol Vis. 2014;20:836–842. [PMC free article] [PubMed] [Google Scholar]
- Ren Z, Li A, Shastry BS, Padma T, Ayyagari R, et al. A 5-base insertion in the gammaC-crystallin gene is associated with autosomal dominant variable zonular pulverulent cataract. Hum Genet. 2000;106:531–537. doi: 10.1007/s004390000289. [DOI] [PubMed] [Google Scholar]
- Riazuddin SA, Yasmeen A, Yao W, Sergeev YV, Zhang Q, et al. Mutations in betaB3-crystallin associated with autosomal recessive cataract in two Pakistani families. Invest Ophthalmol Vis Sci. 2005;46:2100–2106. doi: 10.1167/iovs.04-1481. [DOI] [PubMed] [Google Scholar]
- Richardson JS. beta-Sheet topology and the relatedness of proteins. Nature. 1977;268:495–500. doi: 10.1038/268495a0. [DOI] [PubMed] [Google Scholar]
- Richter L, Flodman P, Barria F, Burch D, Brown S, et al. Clinical variability of autosomal dominant cataract, microcornea and corneal opacity and novel mutation in the alpha A crystallin gene (CRYAA) Am J Med Genet A. 2008;146A:833–842. doi: 10.1002/ajmg.a.32236. [DOI] [PubMed] [Google Scholar]
- Roshan M, Vijaya PH, Lavanya GR, Shama PK, Santhiya ST, et al. A novel human CRYGD mutation in a juvenile autosomal dominant cataract. Mol Vis. 2010;16:887–896. [PMC free article] [PubMed] [Google Scholar]
- Safieh LA, Khan AO, Alkuraya FS. Identification of a novel CRYAB mutation associated with autosomal recessive juvenile cataract in a Saudi family. Mol Vis. 2009;15:980–984. [PMC free article] [PubMed] [Google Scholar]
- Sajjad N, Goebel I, Kakar N, Cheema AM, Kubisch C, Ahmad J. A novel HSF4 gene mutation (p.R405X) causing autosomal recessive congenital cataracts in a large consanguineous family from Pakistan. BMC Med Genet. 2008;9:99. doi: 10.1186/1471-2350-9-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana A, Waiswol M, Arcieri ES, Cabral de Vasconcellos JP, Barbosa de Melo M. Mutation analysis of CRYAA CRYGC, and CRYGD associated with autosomal dominant congenital cataract in Brazilian families. Mol Vis. 2009;15:793–800. [PMC free article] [PubMed] [Google Scholar]
- Santhiya ST, Shyam M, Rawlley D, Vijayalakshmi P, Namperumalsamy P, et al. Novel mutations in the gamma-crystallin genes cause autosomal dominant congenital cataracts. J Med Genet. 2002;39:352–358. doi: 10.1136/jmg.39.5.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhiya ST, Manisastry SM, Rawlley D, Malathi R, Anishetty S, et al. Mutation analysis of congenital cataracts in Indian families: identification of SNPS and a new causative allele in CRYBB2 gene. Invest Ophthalmol Vis Sci. 2004;45:3599–3607. doi: 10.1167/iovs.04-0207. [DOI] [PubMed] [Google Scholar]
- Santhiya ST, Soker T, Klopp N, Illig T, Prakash MV, et al. Identification of a novel, putative cataract-causing allele in CRYAA (G98R) in an Indian family. Mol Vis. 2006;12:768–737. [PubMed] [Google Scholar]
- Santhiya ST, Kumar GS, Sudhakar P, Gupta N, Klopp N, et al. Molecular analysis of cataract families in India: new mutations in the CRYBB2 and GJA3 genes and rare polymorphisms. Mol Vis. 2010;16:1837–1847. [PMC free article] [PubMed] [Google Scholar]
- Schmidt W, Klopp N, Illig T, Graw J. A novel GJA8 mutation causing a recessive triangular cataract. Mol Vis. 2008;14:851–856. [PMC free article] [PubMed] [Google Scholar]
- Semina EV, Brownell I, Mintz-Hittner HA, Murray JC, Jamrich M. Mutations in the human forkhead transcription factor FOXE3 associated with anterior segment ocular dysgenesis and cataracts. Hum Mol Genet. 2001;10:231–236. doi: 10.1093/hmg/10.3.231. [DOI] [PubMed] [Google Scholar]
- Senthil Kumar G, Kyle JW, Minogue PJ, Dinesh Kumar K, Vasantha K, et al. An MIP/AQP0 mutation with impaired trafficking and function underlies an autosomal dominant congenital lamellar cataract. Exp Eye Res. 2013;110:136–141. doi: 10.1016/j.exer.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Burdon KP, Dave A, Jamieson RV, Yaron Y, et al. Novel causative mutations in patients with Nance-Horan syndrome and altered localization of the mutant NHS-A protein isoform. Mol Vis. 2008;14:1856–1864. [PMC free article] [PubMed] [Google Scholar]
- Shentu X, Yao K, Xu W, Zheng S, Hu S, Gong X. Special fasciculiform cataract caused by a mutation in the gammaD-crystallin gene. Mol Vis. 2004;10:233–239. [PubMed] [Google Scholar]
- Shentu X, Zhao SJ, Zhang L, Miao Q. A novel p.R890C mutation in EPHA2 gene associated with progressive childhood posterior cataract in a Chinese family. Int J Ophthalmol. 2013;6:34–38. doi: 10.3980/j.issn.2222-3959.2013.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shentu X, Miao Q, Tang X, Yin H, Zhao Y. Identification and functional analysis of a novel MIP gene mutation associated with congenital cataract in a Chinese family. PLoS One. 2015;10:e0126679. doi: 10.1371/journal.pone.0126679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels A, Mackay D, Ionides A, Berry V, Moore A, Bhattacharya S. A missense mutation in the human connexin50 gene (GJA8) underlies autosomal dominant “zonular pulverulent” cataract, on chromosome 1q. Am J Hum Genet. 1998;62:526–532. doi: 10.1086/301762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels A, Bassnett S, Varadaraj K, Mathias R, Al-Ghoul K, et al. Optical dysfunction of the crystalline lens in aquaporin-0-deficient mice. Physiol Genomics. 2001;7:179–186. doi: 10.1152/physiolgenomics.00078.2001. [DOI] [PubMed] [Google Scholar]
- Shiels A, Bennett TM, Knopf HL, Yamada K, Yoshiura K, et al. CHMP4B, a novel gene for autosomal dominant cataracts linked to chromosome 20q. Am J Hum Genet. 2007;81:596–606. doi: 10.1086/519980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels A, Bennett TM, Knopf HL, Maraini G, Li A, et al. The EPHA2 gene is associated with cataracts linked to chromosome 1p. Mol Vis. 2008;14:2042–2055. [PMC free article] [PubMed] [Google Scholar]
- Shiels A, Bennett TM, Hejtmancik JF. Cat-Map: putting cataract on the map. Mol Vis. 2010;16:2007–2015. [PMC free article] [PubMed] [Google Scholar]
- Singh R, Ram J, Kaur G, Prasad R. Galactokinase deficiency induced cataracts in Indian infants: identification of 4 novel mutations in GALK gene. Curr Eye Res. 2012;37:949–954. doi: 10.3109/02713683.2012.688162. [DOI] [PubMed] [Google Scholar]
- Slingsby C, Wistow GJ, Clark AR. Evolution of crystallins for a role in the vertebrate eye lens. Protein Sci. 2013;22:367–380. doi: 10.1002/pro.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaoui N, Beltaief O, BenHamed S, M'Rad R, Maazoul F, et al. A homozygous splice mutation in the HSF4 gene is associated with an autosomal recessive congenital cataract. Invest Ophthalmol Vis Sci. 2004;45:2716–2721. doi: 10.1167/iovs.03-1370. [DOI] [PubMed] [Google Scholar]
- Song Z, Wang L, Liu Y, Xiao W. A novel nonsense mutation in the MIP gene linked to congenital posterior polar cataracts in a Chinese family. PLoS One. 2015;10:e0119296. doi: 10.1371/journal.pone.0119296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambolian D, Ai Y, Sidjanin D, Nesburn K, Sathe G, et al. Cloning of the galactokinase cDNA and identification of mutations in two families with cataracts. Nat Genet. 1995;10:307–312. doi: 10.1038/ng0795-307. [DOI] [PubMed] [Google Scholar]
- Stephan DA, Gillanders E, Vanderveen D, Freas-Lutz D, Wistow G, et al. Progressive juvenile-onset punctate cataracts caused by mutation of the gammaD-crystallin gene. Proc Natl Acad Sci USA. 1999;96:1008–1012. doi: 10.1073/pnas.96.3.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su D, Guo Y, Li Q, Guan L, Zhu S, Ma X. A novel mutation in CRYAA is associated with autosomal dominant suture cataracts in a Chinese family. Mol Vis. 2012;18:3057–3063. [PMC free article] [PubMed] [Google Scholar]
- Su D, Yang Z, Li Q, Guan L, Zhang H, et al. Identification and functional analysis of GJA8 mutation in a Chinese family with autosomal dominant perinuclear cataracts. PLoS One. 2013;8:e59926. doi: 10.1371/journal.pone.0059926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Ma Z, Li Y, Liu B, Li Z, et al. Gamma-S crystallin gene (CRYGS) mutation causes dominant progressive cortical cataract in humans. J Med Genet. 2005;42:706–710. doi: 10.1136/jmg.2004.028274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Xiao X, Li S, Guo X, Zhang Q. Mutational screening of six genes in Chinese patients with congenital cataract and microcornea. Mol Vis. 2011a;17:1508–1513. [PMC free article] [PubMed] [Google Scholar]
- Sun W, Xiao X, Li S, Guo X, Zhang Q. Mutation analysis of 12 genes in Chinese families with congenital cataracts. Mol Vis. 2011b;17:2197–2206. [PMC free article] [PubMed] [Google Scholar]
- Sun W, Xiao X, Li S, Guo X, Zhang Q. Exome sequencing of 18 Chinese families with congenital cataracts: a new sight of the NHS gene. PLoS One. 2014;9:e100455. doi: 10.1371/journal.pone.0100455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valleix S, Niel F, Nedelec B, Algros MP, Schwartz C, et al. Homozygous nonsense mutation in the FOXE3 gene as a cause of congenital primary aphakia in humans. Am J Hum Genet. 2006;79:358–364. doi: 10.1086/505654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanita V, Singh D. A missense mutation in CRYGD linked with autosomal dominant congenital cataract of aculeiform type. Mol Cell Biochem. 2012;368:167–172. doi: 10.1007/s11010-012-1355-2. [DOI] [PubMed] [Google Scholar]
- Vanita V, Sarhadi V, Reis A, Jung M, Singh D, et al. A unique form of autosomal dominant cataract explained by gene conversion between beta-crystallin B2 and its pseudogene. J Med Genet. 2001;38:392–396. doi: 10.1136/jmg.38.6.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanita V, Singh D, Robinson PN, Sperling K, Singh JR. A novel mutation in the DNA-binding domain of MAF at 16q23.1 associated with autosomal dominant “cerulean cataract” in an Indian family. Am J Med Genet A. 2006;140:558–566. doi: 10.1002/ajmg.a.31126. [DOI] [PubMed] [Google Scholar]
- Vanita V, Singh JR, Singh D, Varon R, Sperling K. A mutation in GJA8 (p.P88Q) is associated with “balloon-like” cataract with Y-sutural opacities in a family of Indian origin. Mol Vis. 2008;14:1171–1175. [PMC free article] [PubMed] [Google Scholar]
- Vanita V, Singh JR, Singh D, Varon R, Sperling K. Novel mutation in the gamma-S crystallin gene causing autosomal dominant cataract. Mol Vis. 2009;15:476–481. [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang T, Wu D, Zhang J. A novel beaded filament structural protein 1 (BFSP1) gene mutation associated with autosomal dominant congenital cataract in a Chinese family. Mol Vis. 2013;19:2590–2595. [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ma X, Gu F, Liu NP, Hao XL, et al. A missense mutation S228P in the CRYBB1 gene causes autosomal dominant congenital cataract. Chin Med J (Engl) 2007;120:820–824. [PubMed] [Google Scholar]
- Wang KJ, Zhu SQ. A novel p.F206I mutation in Cx46 associated with autosomal dominant congenital cataract. Mol Vis. 2012;18:968–973. [PMC free article] [PubMed] [Google Scholar]
- Wang KJ, Li SS, Yun B, Ma WX, Jiang TG, Zhu SQ. A novel mutation in MIP associated with congenital nuclear cataract in a Chinese family. Mol Vis. 2011a;17:70–77. [PMC free article] [PubMed] [Google Scholar]
- Wang KJ, Wang BB, Zhang F, Zhao Y, Ma X, Zhu SQ. Novel beta-crystallin gene mutations in Chinese families with nuclear cataracts. Arch Ophthalmol. 2011b;129:337–343. doi: 10.1001/archophthalmol.2011.11. [DOI] [PubMed] [Google Scholar]
- Wang L, Lin H, Gu J, Su H, Huang S, Qi Y. Autosomal-dominant cerulean cataract in a Chinese family associated with gene conversion mutation in beta-B2-crystallin. Ophthalmic Res. 2009;41:148–153. doi: 10.1159/000209668. [DOI] [PubMed] [Google Scholar]
- Wang L, Luo Y, Wen W, Zhang S, Lu Y. Another evidence for a D47N mutation in GJA8 associated with autosomal dominant congenital cataract. Mol Vis. 2011;17:2380–2385. [PMC free article] [PubMed] [Google Scholar]
- Wang L, Chen Y, Chen X, Sun X. Further evidence for P59L mutation in GJA3 associated with autosomal dominant congenital cataract. Indian J Ophthalmol. 2016;64:508–512. doi: 10.4103/0301-4738.190139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Jiang J, Zhu Y, Li J, Jin C, et al. A novel mutation in the major intrinsic protein (MIP) associated with autosomal dominant congenital cataracts in a Chinese family. Mol Vis. 2010;16:534–539. [PMC free article] [PubMed] [Google Scholar]
- Weisschuh N, Aisenbrey S, Wissinger B, Riess A. Identification of a novel CRYBB2 missense mutation causing congenital autosomal dominant cataract. Mol Vis. 2012;18:174–180. [PMC free article] [PubMed] [Google Scholar]
- Willoughby CE, Arab S, Gandhi R, Zeinali S, Arab S, et al. A novel GJA8 mutation in an Iranian family with progressive autosomal dominant congenital nuclear cataract. J Med Genet. 2003;40:e124. doi: 10.1136/jmg.40.11.e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby CE, Shafiq A, Ferrini W, Chan LL, Billingsley G, et al. CRYBB1 mutation associated with congenital cataract and microcornea. Mol Vis. 2005;11:587–593. [PubMed] [Google Scholar]
- Wu Q, Shi H, Liu N, Lu N, Jiang M, et al. Mutation analysis of CRYBB1 gene and prenatal diagnosis for a Chinese kindred featuring autosomal dominant congenital nuclear cataract (in Chinese) Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2013;30:266–269. doi: 10.3760/cma.j.issn.1003-9406.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Wussuki-Lior O, Abu-Horowitz A, Netzer I, Almer Z, Morad Y, et al. Hematologic biomarkers in childhood cataracts. Mol Vis. 2011;17:1011–1015. [PMC free article] [PubMed] [Google Scholar]
- Xia XY, Li N, Cao X, Wu QY, Li TF, et al. A novel COL4A1 gene mutation results in autosomal dominant non-syndromic congenital cataract in a Chinese family. BMC Med Genet. 2014a;15:97. doi: 10.1186/s12881-014-0097-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XY, Wu QY, An LM, Li WW, Li N, et al. A novel P20R mutation in the alpha-B crystallin gene causes autosomal dominant congenital posterior polar cataracts in a Chinese family. BMC Ophthalmol. 2014b;14:108. doi: 10.1186/1471-2415-14-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Li W, Wang P, Li L, Li S, et al. Cerulean cataract mapped to 12q13 and associated with a novel initiation codon mutation in MIP. Mol Vis. 2011;17:2049–2055. [PMC free article] [PubMed] [Google Scholar]
- Xu WZ, Zheng S, Xu SJ, Huang W, Yao K, Zhang SZ. Autosomal dominant coralliform cataract related to a missense mutation of the gammaD-crystallin gene. Chin Med J (Engl) 2004;117:727–732. [PubMed] [Google Scholar]
- Yan M, Xiong C, Ye SQ, Chen Y, Ke M, et al. A novel connexin 50 (GJA8) mutation in a Chinese family with a dominant congenital pulverulent nuclear cataract. Mol Vis. 2008;14:418–424. [PMC free article] [PubMed] [Google Scholar]
- Yang G, Xing B, Liu G, Lu X, Jia X, et al. A novel mutation in the GJA3 (connexin46) gene is associated with autosomal dominant congenital nuclear cataract in a Chinese family. Mol Vis. 2011a;17:1070–1073. [PMC free article] [PubMed] [Google Scholar]
- Yang G, Zhang G, Wu Q, Zhao J. A novel mutation in the MIP gene is associated with autosomal dominant congenital nuclear cataract in a Chinese family. Mol Vis. 2011b;17:1320–1323. [PMC free article] [PubMed] [Google Scholar]
- Yang G, Chen Z, Zhang W, Liu Z, Zhao J. Novel mutations in CRYGD are associated with congenital cataracts in Chinese families. Sci Rep. 2016;6:18912. doi: 10.1038/srep18912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhu Y, Gu F, He X, Cao Z, et al. A novel nonsense mutation in CRYBB1 associated with autosomal dominant congenital cataract. Mol Vis. 2008;14:727–731. [PMC free article] [PubMed] [Google Scholar]
- Yang J, Li D, Fan Q, Cai L, Qiu X, et al. The polymorphisms with cataract susceptibility impair the EPHA2 receptor stability and its cytoprotective function. J Ophthalmol. 2015;2015:401894. doi: 10.1155/2015/401894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Li Q, Ma Z, Guo Y, Zhu S, Ma X. A G→T splice site mutation of CRYBA1/A3 associated with autosomal dominant suture cataracts in a Chinese family. Mol Vis. 2011;17:2065–2071. [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Su D, Li Q, Yang F, Ma Z, et al. A novel T→G splice site mutation of CRYBA1/A3 associated with autosomal dominant nuclear cataracts in a Chinese family. Mol Vis. 2012;18:1283–1288. [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Su D, Li Q, Ma Z, Yang F, et al. A R54L mutation of CRYAA associated with autosomal dominant nuclear cataracts in a Chinese family. Curr Eye Res. 2013;38:1221–1228. doi: 10.3109/02713683.2013.811260. [DOI] [PubMed] [Google Scholar]
- Yang Z, Li Q, Ma X, Zhu SQ. Mutation analysis in Chinese families with autosomal dominant hereditary cataracts. Curr Eye Res. 2015;40:1225–1231. doi: 10.3109/02713683.2014.997885. [DOI] [PubMed] [Google Scholar]
- Yao K, Tang X, Shentu X, Wang K, Rao H, Xia K. Progressive polymorphic congenital cataract caused by a CRYBB2 mutation in a Chinese family. Mol Vis. 2005;11:758–763. [PubMed] [Google Scholar]
- Yao K, Jin C, Zhu N, Wang W, Wu R, et al. A nonsense mutation in CRYGC associated with autosomal dominant congenital nuclear cataract in a Chinese family. Mol Vis. 2008;14:1272–1276. [PMC free article] [PubMed] [Google Scholar]
- Yao K, Li J, Jin C, Wang W, Zhu Y, et al. Characterization of a novel mutation in the CRYBB2 gene associated with autosomal dominant congenital posterior subcapsular cataract in a Chinese family. Mol Vis. 2011a;17:144–152. [PMC free article] [PubMed] [Google Scholar]
- Yao K, Wang W, Zhu Y, Jin C, Shentu X, et al. A novel GJA3 mutation associated with congenital nuclear pulverulent and posterior polar cataract in a Chinese family. Hum Mutat. 2011b;32:1367–1370. doi: 10.1002/humu.21552. [DOI] [PubMed] [Google Scholar]
- Yasmeen A, Riazuddin SA, Kaul H, Mohsin S, Khan M, et al. Autosomal recessive congenital cataract in consanguineous Pakistani families is associated with mutations in GALK1. Mol Vis. 2010;16:682–688. [PMC free article] [PubMed] [Google Scholar]
- Yu LC, Twu YC, Chang CY, Lin M. Molecular basis of the adult i phenotype and the gene responsible for the expression of the human blood group I antigen. Blood. 2001;98:3840–3845. doi: 10.1182/blood.v98.13.3840. [DOI] [PubMed] [Google Scholar]
- Yu Y, Li J, Xu J, Wang Q, Yu Y, Yao K. Congenital polymorphic cataract associated with a G to A splice site mutation in the human beta-crystallin gene CRYβA3/A1. Mol Vis. 2012;18:2213–2220. [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Yu Y, Chen P, Li J, Zhu Y, et al. A novel MIP gene mutation associated with autosomal dominant congenital cataracts in a Chinese family. BMC Med Genet. 2014;15:6. doi: 10.1186/1471-2350-15-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Wu M, Chen X, Zhu Y, Gong X, Yao K. Identification and functional analysis of two novel connexin 50 mutations associated with autosome dominant congenital cataracts. Sci Rep. 2016;6:26551. doi: 10.1038/srep26551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Guo Y, Yi J, Xiao J, Yuan J, et al. Identification of a novel GJA3 mutation in congenital nuclear cataract. Optom Vis Sci. 2015;92:337–342. doi: 10.1097/OPX.0000000000000518. [DOI] [PubMed] [Google Scholar]
- Yuan L, Yi J, Lin Q, Xu H, Deng X, et al. Identification of a PRX variant in a Chinese family with congenital cataract by exome sequencing. QJM. 2016;109:731–735. doi: 10.1093/qjmed/hcw058. [DOI] [PubMed] [Google Scholar]
- Zeng L, Liu W, Feng W, Wang X, Dang H, et al. A novel donor splice-site mutation of major intrinsic protein gene associated with congenital cataract in a Chinese family. Mol Vis. 2013;19:2244–2249. [PMC free article] [PubMed] [Google Scholar]
- Zenteno JC, Morales ME, Moran-Barroso V, Sanchez-Navarro A. CRYGD gene analysis in a family with autosomal dominant congenital cataract: evidence for molecular homogeneity and intrafamilial clinical heterogeneity in aculeiform cataract. Mol Vis. 2005;11:438–442. [PubMed] [Google Scholar]
- Zhai Y, Li J, Zhu Y, Xia Y, Wang W, et al. A nonsense mutation of γD-crystallin associated with congenital nuclear and posterior polar cataract in a Chinese family. Int J Med Sci. 2014;11:158–163. doi: 10.7150/ijms.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang Y, Fang F, Mu W, Zhang N, et al. Congenital cataracts due to a novel 2-bp deletion in CRYBA1/A3. Mol Med Rep. 2014;10:1614–1618. doi: 10.3892/mmr.2014.2324. [DOI] [PubMed] [Google Scholar]
- Zhang L, Gao L, Li Z, Qin W, Gao W, et al. Progressive sutural cataract associated with a BFSP2 mutation in a Chinese family. Mol Vis. 2006;12:1626–1631. [PubMed] [Google Scholar]
- Zhang L, Qu X, Su S, Guan L, Liu P. A novel mutation in GJA3 associated with congenital Coppock-like cataract in a large Chinese family. Mol Vis. 2012;18:2114–2118. [PMC free article] [PubMed] [Google Scholar]
- Zhang LY, Yam GH, Fan DS, Tam PO, Lam DS, Pang CP. A novel deletion variant of gammaD-crystallin responsible for congenital nuclear cataract. Mol Vis. 2007;13:2096–2104. [PubMed] [Google Scholar]
- Zhang LY, Yam GH, Tam PO, Lai RY, Lam DS, et al. An alphaA-crystallin gene mutation, Arg12Cys, causing inherited cataract-microcornea exhibits an altered heat-shock response. Mol Vis. 2009a;15:1127–1138. [PMC free article] [PubMed] [Google Scholar]
- Zhang LY, Gong B, Tong JP, Fan DS, Chiang SW, et al. A novel gammaD-crystallin mutation causes mild changes in protein properties but leads to congenital coralliform cataract. Mol Vis. 2009b;15:1521–1529. [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Guo X, Xiao X, Yi J, Jia X, Hejtmancik JF. Clinical description and genome wide linkage study of Y-sutural cataract and myopia in a Chinese family. Mol Vis. 2004;10:890–900. [PubMed] [Google Scholar]
- Zhao H, Brown PH, Magone MT, Schuck P. The molecular refractive function of lens γ-crystallins. J Mol Biol. 2011;411:680–699. doi: 10.1016/j.jmb.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Chen XJ, Zhu J, Xi YB, Yang X, et al. Lanosterol reverses protein aggregation in cataracts. Nature. 2015;523:607–611. doi: 10.1038/nature14650. [DOI] [PubMed] [Google Scholar]
- Zheng JQ, Ma ZW, Sun HM. A heterozygous transversion of connexin 50 in a family with congenital nuclear cataract in the northeast of China (in Chinese). Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2005;22:76–78. [PubMed] [Google Scholar]
- Zhou D, Ji H, Wei Z, Guo L, Li Y, et al. A novel insertional mutation in the connexin 46 (gap junction alpha 3) gene associated with autosomal dominant congenital cataract in a Chinese family. Mol Vis. 2013;19:789–795. [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Zhou N, Hu S, Zhao L, Zhang C, Qi Y. A missense mutation in CRYBA4 associated with congenital cataract and microcornea. Mol Vis. 2010;16:1019–1024. [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Hu S, Wang B, Zhou N, Zhou S, et al. Mutation analysis of congenital cataract in a Chinese family identified a novel missense mutation in the connexin 46 gene (GJA3) Mol Vis. 2010;16:713–719. [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Shentu X, Wang W, Li J, Jin C, Yao K. A Chinese family with progressive childhood cataracts and IVS3+1G>A CRYBA3/A1 mutations. Mol Vis. 2010;16:2347–2353. [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Yu H, Wang W, Gong X, Yao K. A novel GJA8 mutation (p.V44A) causing autosomal dominant congenital cataract. PLoS One. 2014;9:e115406. doi: 10.1371/journal.pone.0115406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Wang L, Song Z, Xiao W. A novel insertion variant of CRYGD is associated with congenital nuclear cataract in a Chinese family. PLoS One. 2015;10:e0131471. doi: 10.1371/journal.pone.0131471. [DOI] [PMC free article] [PubMed] [Google Scholar]