Abstract
The 2004 outbreaks of H5N1 influenza viruses in Vietnam and Thailand were highly lethal to humans and to poultry; therefore, newly emerging avian influenza A viruses pose a continued threat, not only to avian species but also to humans. We studied the pathogenicity of four human and nine avian H5N1/04 influenza viruses in ferrets (an excellent model for influenza studies). All four human isolates were fatal to intranasally inoculated ferrets. The human isolate A/Vietnam/1203/04 (H5N1) was the most pathogenic isolate; the severity of disease was associated with a broad tissue tropism and high virus titers in multiple organs, including the brain. High fever, weight loss, anorexia, extreme lethargy, and diarrhea were observed. Two avian H5N1/04 isolates were as pathogenic as the human viruses, causing lethal systemic infections in ferrets. Seven of nine H5N1/04 viruses isolated from avian species caused mild infections, with virus replication restricted to the upper respiratory tract. All chicken isolates were nonlethal to ferrets. A sequence analysis revealed polybasic amino acids in the hemagglutinin connecting peptides of all H5N1/04 viruses, indicating that multiple molecular differences in other genes are important for a high level of virulence. Interestingly, the human A/Vietnam/1203/04 isolate had a lysine substitution at position 627 of PB2 and had one to eight amino acid changes in all gene products except that of the M1 gene, unlike the A/chicken/Vietnam/C58/04 and A/quail/Vietnam/36/04 viruses. Our results indicate that viruses that are lethal to mammals are circulating among birds in Asia and suggest that pathogenicity in ferrets, and perhaps humans, reflects a complex combination of different residues rather than a single amino acid difference.
Influenza A viruses cause infections in humans, a limited range of mammals, and birds. Aquatic birds are considered to be the main source of gene segments that may reassort with circulating human strains to produce pandemic viruses. All 15 known influenza A virus hemagglutinin (HA) subtypes can be isolated from aquatic birds. Only the H5 and H7 HA subtypes are considered to be highly pathogenic in avian species, having shown pantropic dissemination within the host (1, 17).
Until the H5N1 influenza outbreak in Hong Kong in 1997, the H5 subtype was reported to circulate only among species of wild and domestic birds, causing mild respiratory disease or, on chicken farms, fatal epizootic disease. The direct transmission of avian H5N1 influenza viruses to humans in 1997 changed our understanding of the “rules” that govern the transmission of avian influenza viruses to humans and their virulence in humans (5, 33). Although the H5N1/97 viruses were eradicated by the wholesale slaughter of poultry, their precursors continued to circulate in southern China (2, 36). The reappearance of fatal human H5N1 disease in Hong Kong in 2003 showed that human H5N1 infection was not an isolated event (23). Avian H5N1, H6N1, and H9N2 influenza viruses are the possible ancestors of the viruses that were transmitted to humans in 1997 and 2003 (2, 9, 11, 15, 38). The emergence of multiple genotypes of avian H5N1 viruses in recent years indicated the continued cocirculation of these viruses in wild aquatic birds and poultry in China (9, 10, 23, 29). In 2004, highly pathogenic H5N1 avian influenza viruses were reported to be present in poultry in China, Japan, South Korea, Thailand, Vietnam, Indonesia, Cambodia, and Laos. Human H5N1 infections were identified in Vietnam and Thailand, and 34 cases were laboratory confirmed. Fifteen patients in Vietnam (35) and eight patients in Thailand died. The widespread isolation of the H5N1/04 viruses and their lethality warrant a thorough characterization of these viruses to elucidate the molecular correlates of their transmission and virulence.
The cleavability of the HA molecule plays a major role in the virulence of H5 and H7 subtypes in birds, although other genes also contribute (1, 17). The genetic factors responsible for the virulence of H5N1 viruses in humans are unknown, despite investigations conducted with a variety of mammalian animal models, including mice (7, 16), ferrets (39), pigs (27), and primates (26). Most of the currently available information on the pathogenicity of H5N1/97 Hong Kong isolates in mammals is based on experimental infections of BALB/c mice (16, 20, 22). The human H5N1/97 isolates possess differential pathogenicities in mice and can be divided into two groups, i.e., avirulent viruses that cause mild disease in mice without prior adaptation but that are nonlethal and virulent viruses that spread systemically and cause the death of animals (7, 13, 16). As in studies with chickens, a multibasic amino acid motif in the HA protein was found to be essential for the virulence of human H5N1/97 isolates in mice, although the lysine at position 627 of the polymerase subunit PB2 is also important (13).
Ferrets are an excellent mammalian host for studies of influenza virus pathogenicity and host immunity, and the manifestations of influenza virus infection in ferrets closely resemble those in humans. Previously, ferrets were used as an alternative model system to study the pathogenesis of H5N1/97 influenza virus infection (39). H5N1/97 viruses that differed in their pathogenicities in inbred BALB/c mice did not show similar differences in ferrets. Here we report that H5N1/04 influenza virus isolates differ in their pathogenicities in ferrets: human isolates were uniformly highly pathogenic, but most H5N1 isolates from lethal outbreaks among birds in Asia caused only mild illness, although some isolates were highly lethal. We identified molecular differences that may explain the observed differences in pathogenicity.
MATERIALS AND METHODS
Viruses.
The 13 different H5N1/04 influenza viruses isolated from humans and birds in Vietnam, Thailand, Hong Kong, and Indonesia were included in this study. Influenza viruses were propagated in the allantoic cavities of 9-day-old embryonated chicken eggs, and experiments were conducted under biosafety level 3+ conditions approved for work with these viruses.
Inoculation of ferrets.
Young adult male ferrets (Marshall's Farms, North Rose, N.Y.) that were 3 to 5 months of age and were seronegative by a hemagglutination inhibition test for exposure to currently circulating influenza B viruses and H1N1, H3N2, and H5N1 influenza A viruses were used for this study. Ferrets were lightly anesthetized with isoflurane and inoculated intranasally with 106 50% egg infective doses (EID50) of infectious virus in 0.5 ml of phosphate-buffered saline (PBS). Animals that showed signs of severe disease were sacrificed. Clinical signs of infection, relative inactivity indexes (25), weights, and temperatures were recorded daily. Body temperature was measured by the use of subcutaneous implantable temperature transponders. Each ferret's temperature was recorded for 3 days before inoculation, and the values were averaged to obtain a baseline value (range, 38.8 to 39.2°C). A body temperature of >3 standard deviations (SD) above the baseline was considered significant. All studies were conducted under applicable laws and guidelines and after approval from the St. Jude Children's Research Hospital Animal Care and Use Committee.
Titration of virus in upper respiratory tract.
On days 3, 5, and 7 after inoculation, ferrets were anesthetized with ketamine (25 mg/kg of body weight) injected intramuscularly, and 0.5 ml of sterile PBS containing antibiotics was introduced into each nostril and then collected in containers. Viruses were detected by infection of embryonated chicken eggs, and titers were expressed as log10 EID50 per milliliter.
Titration of virus in organs.
Organs were collected at 3 days postinfection or at the time of the animal's death. For virus titration, tissue samples were collected from each of the four lobes of the lungs (pooled), from anterior and sagittal sections of the middle part of the brain (pooled), and from the olfactory bulb, spleen, and small intestine. Tissue samples (∼0.5 g) were homogenized in 1 ml of sterile PBS with antibiotics. The homogenates were titrated in embryonated chicken eggs to determine the log10 EID50 per milliliter. Log-transformed virus titers were compared by a two-tailed t test.
Histopathologic analysis.
Two or three ferrets were sacrificed on days 6 and 7 after inoculation with H5N1/04 viruses and were processed for histological examination. Brain, lung, liver, spleen, and intestinal specimens were collected at the time of necropsy, fixed in 10% neutral buffered formalin, and embedded in paraffin. Five-micrometer-thick sections were stained with hematoxylin and eosin and studied by light microscopy.
Sequence analysis.
Viral RNAs were isolated directly from virus-containing allantoic fluid by use of an RNA isolation kit (RNeasy; Qiagen). A universal primer set for influenza A virus was used for cDNA preparations and PCRs (14). The Center for Biotechnology at St. Jude Children's Research Hospital determined the sequences of the template DNAs by using BigDye Terminator (v. 3) chemistry and synthetic oligonucleotides. Samples were analyzed on Applied Biosystem 3700 DNA analyzers.
Nucleotide sequence accession numbers.
The sequences determined for this study are available in GenBank under accession numbers AY818126 to AY818149.
RESULTS
Pathogenicity of human and avian H5N1/04 viruses in ferrets.
We inoculated ferrets with 13 different H5N1 influenza viruses isolated from humans in Vietnam and Thailand and with viruses isolated during fatal outbreaks in poultry in Asia. Three groups were identified on the basis of disease severity and type of host. One group included all four human H5N1/04 isolates, which caused acute severe infections. Two of these human viruses were fatal to both ferrets into which they were inoculated, and two killed one of two inoculated ferrets (Table 1). Three human isolates caused prolonged virus shedding and systemic spread of the virus. The second group comprised only two avian viruses, A/quail/Vietnam/36/04 and A/duck/Thailand/71.1/04, which were lethal to ferrets and caused clinical manifestations comparable to those caused by the human isolates. The third group consisted of seven avian viruses that caused mild infections and replicated only in the upper respiratory tracts of ferrets (Table 1). The relative inactivity indexes of ferrets inoculated with human H5N1/04 influenza viruses ranged from 1.4 to 2.7; the highest values were observed with the A/Vietnam/1203/04 virus. Ferrets inoculated with the avian A/quail/Vietnam/36/04 and A/duck/Thailand/71.1/04 viruses (which caused severe disease symptoms, as did the human isolates, but were in the avian host category) had relative inactivity indexes of 2.1 and 1.9. A third group comprised animals that remained alert and playful after inoculation with avian H5N1/04 viruses (Table 1). The magnitude of change in body temperature was considered to reflect the severity of disease, and ferrets infected with the human isolates and two of the avian viruses had significant increases in temperature (Table 1). Interestingly, all four isolates from chickens and all three viruses from China caused mild infections in ferrets. These results show that avian H5N1 viruses circulating among birds in Asia can be highly lethal to mammalian hosts and thus can potentially be transmitted from avian species to humans.
TABLE 1.
Pathogenicity of human and avian H5N1 influenza viruses in ferrets
| Virus and origin | Genotype | Severity of disease | No. dead/ total no. | Clinical signs
|
Virus titera
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Relative inactivity indexb | Weight change (%)c | T increase (°C)c | Nasal washes on indicated day postinfection
|
Internal organs
|
|||||||||
| 3 | 5 | 7 | Lungs | Brain | Spleen | Intestine | |||||||
| Human | |||||||||||||
| A/Vietnam/1203/04 | Z | Severe | 2/2 | 2.7 | −30.6 | 2.8 | 5.3 | 5.5d | NA | 5.5 | 6.0 | 2.0 | 3.3 |
| A/Thailand/1(Kan-1)/04 | Z | Severe | 2/2 | 1.9 | −19.4 | 2.2 | 3.6 | 4.6 | 5.3d | 3.0 | 5.3 | < | < |
| A/Vietnam/3062/04 | Z | Severe | 1/2 | 1.4 | −10.2 | 1.7 | 5.8 | 4.0d | 4.0d | 4.5 | 4.8 | 2.5 | 3.5 |
| A/Vietnam/3046/04 | Z | Severe | 1/2 | 1.9 | −18.8 | 2.2 | 3.8 | 1.5 | < | < | < | < | < |
| Avian | |||||||||||||
| A/quail/Vietnam/36/04 | Z | Severe | 2/2 | 2.1 | −17.3 | 1.4 | 4.3 | 4.8 | 5.8 | 3.5 | 1.0 | 2.3 | 2.3 |
| A/duck/Thailand/71.1/04 | Z | Severe | 1/2 | 1.9 | −14.8 | 1.6 | 4.1 | 3.5 | 3.0 | 3.3 | 5.5 | 2.5 | 3.3 |
| A/chicken/Vietnam/39/04 | Z | Mild | 0/2 | 1.0 | −9.3 | 0.6 | 3.4 | 1.8 | < | ND | ND | ND | ND |
| A/chicken/Vietnam/C58/04 | Z | Mild | 0/3 | 1.0 | −3.0 | 0.6 | 2.6 | < | < | < | < | < | < |
| A/chicken/Indonesia/5/04 | Z | Mild | 0/2 | 1.4 | −0.7 | 0.9 | 4.4 | 4.1 | 1.4 | < | < | < | < |
| A/chicken/Indonesia/MS/04 | Z | Mild | 0/2 | 1.0 | −8.0 | 0.5 | 4.2 | 3.4 | 1.0 | < | < | < | < |
| A/falcon/HK/D0028/04 | Z | Mild | 0/2 | 1.4 | +1.9 | 0.9 | 3.0 | 2.4 | < | ND | ND | ND | ND |
| A/duck/Hunan/1504/04 | Z | Mild | 0/2 | 1.4 | −1.8 | 0.6 | < | < | < | ND | ND | ND | ND |
| A/duck/Guanxi/1304/04 | Z | Mild | 0/2 | 1.7 | −8.3 | 0.8 | 3.0 | 3.5 | < | < | < | < | < |
Ferrets were inoculated with 106 EID50 of H5N1/04 viruses. Values are mean virus titers (log10 EID50/ml) for two to three ferrets, as determined in nasal washes or internal organs at three to seven days postinfection. Tissue samples (∼0.5 g) were collected and homogenized in 1 ml of sterile PBS with antibiotics. The lower limit of virus detection was <1 log10 EID50 per 1.0 ml of nasal wash or tissue homogenate. <, titer of <1.0 log10 EID50/ml; NA, not applicable (all ferrets in the group were dead); ND, not done, since there was no pathogenicity.
Determined from observations over 7 days, as described previously (25). The relative inactivity index before H5N1/04 virus infection was 0; all ferrets were alert and playful.
Maximal change in weight or temperature.
Value for one animal.
Disease signs caused by human and avian H5N1/04 isolates.
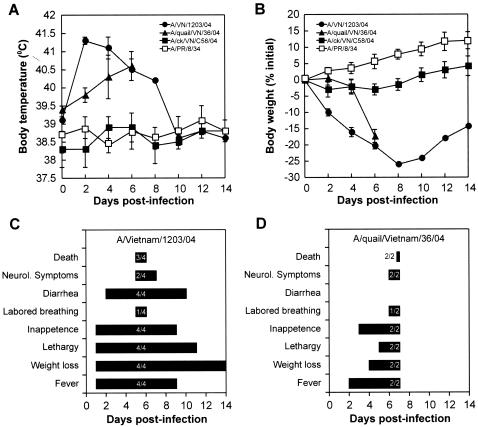
To characterize the disease caused by H5N1 infection and to identify characteristics that may explain the severity of infection, we examined one representative of each of the three pathogenicity groups in more detail. We included the human influenza virus A/PR/8/34 (H1N1), which has been well characterized in ferrets (30), for comparison. In ferrets infected with the human A/Vietnam/1203/04 (H5N1) virus, all clinical symptoms began acutely and progressed during the course of infection. We observed high fevers (40.3 to 42.3°C), substantial weight loss (17.6 to 20.3% by day 6 postinfection), anorexia, and extreme lethargy (Fig. 1A, B, and C). Labored breathing was observed during late infection in one of the four ferrets. Diarrhea was one of the most frequently observed clinical symptoms, as all four ferrets exhibited yellow diarrhea (Fig. 1C). Two of the four showed neurological symptoms (uncontrolled movements and hindlimb paralysis) during the late stage of infection, at 5 to 6 days postinfection. In two independent experiments, the infection of seven ferrets with the A/Vietnam/1203/04 virus consistently caused severe disease and death of the animals by 5 to 7 days postinfection.
FIG. 1.
Clinical signs of infection with H5N1/04 influenza viruses in ferrets. (A) Changes in body temperatures of ferrets infected with H5N1/04 influenza viruses. Ferrets were inoculated with 106 EID50 of the A/Vietnam/1203/04, A/quail/Vietnam/36/04, A/chicken/Vietnam/C58/04, or A/PR/8/34 (H1N1) influenza virus, and body temperatures were monitored daily by the use of subcutaneous implantable temperature transponders for 12 days postinfection. Each data point represents the mean value ± SD for two to four ferrets. (B) Changes in weights of ferrets infected with H5N1/04 influenza viruses. The weights of ferrets were measured on days 0, 2, 4, 6, 8, 10, and 12 after inoculation. The loss or gain of weight was calculated for each ferret as the percent change in the initial mean starting weight on day 0. Values are the averages ± SD for two to four ferrets for each group. The clinical signs of infection with A/Vietnam/1203/04 (C) or A/quail/Vietnam/36/04 (D) virus were noted daily. The numbers on the bars represent the numbers of ferrets showing signs/total number of ferrets. The ferrets infected with the A/quail/Vietnam/36/04 influenza virus showed clinical signs of severe infection and were sacrificed on day 7.
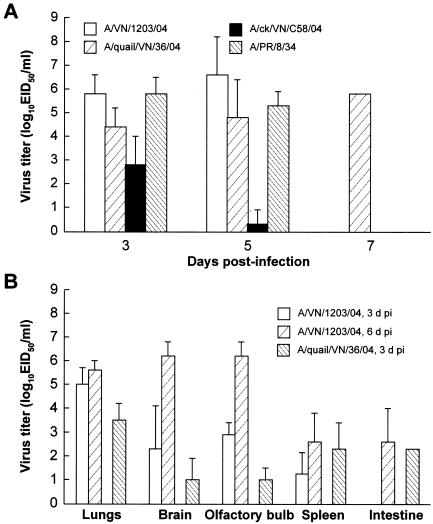
The two ferrets inoculated with the A/quail/Vietnam/36/04 virus showed disease signs later than the ferrets inoculated with the human isolate, but their disease progressed very rapidly between 5 and 7 days postinfection (Fig. 1A, B, and D). The animals had body temperatures of 39.6 to 41.3°C, a loss of 17.3% of their initial weight, extreme lethargy, and neurological signs, and both died at 7 days postinfection (Fig. 1D). The four ferrets inoculated with the A/chicken/Vietnam/C58/04 virus showed milder signs of infection. The titers of the A/Vietnam/1203/04 and A/quail/Vietnam/36/04 viruses in the upper respiratory tract did not differ significantly from those of A/PR/8/34 (P < 0.05; two-tailed t test), but infection with A/chicken/Vietnam/C58/04 produced virus titers that were significantly lower than those of A/PR/8/34 (P < 0.05) at both 3 and 5 days postinfection (Fig. 2A). Although virus was only detected in ferrets infected with the A/quail/Vietnam/36/04 virus on day 7 postinfection, only one ferret inoculated with the A/Vietnam/1203/04 virus remained alive on that day.
FIG. 2.
Replication of H5N1/04 influenza viruses in upper respiratory tracts (A) and internal organs (B) of ferrets. (A) Virus titers were determined at 3, 5, and 7 days postinfection in nasal washes of ferrets infected with 106 EID50 of the A/Vietnam/1203/04, A/quail/Vietnam/36/04, A/chicken/Vietnam/C58/04, or A/PR/8/34 (H1N1) virus. Values (log10 EID50/ml) are the mean virus titers ± SD of all live ferrets on the indicated days. The lower limit of virus detection was <1 log10 EID50 per 1.0 ml of nasal wash or tissue homogenate. For calculations of the means, influenza virus-positive samples with virus titers of <1 log10 EID50/ml were assigned a value of 0.5 log10 EID50/ml. (B) Virus titers were determined for the lungs, brains, olfactory bulbs, spleens, and intestines of ferrets at days 3 and 6 postinfection for animals infected with A/Vietnam/1203/04 and at day 3 postinfection for animals infected with A/quail/Vietnam/36/04. Values (log10 EID50/ml) are the means ± SD for two to four animals.
The severe clinical signs, including neurological symptoms and diarrhea, induced by infection with A/Vietnam/1203/04 indicated a rapid spread of the virus. The rapid spread was confirmed by virus detection in multiple organs, including the brain (Fig. 2B). There was a clear tendency toward a late increase in virus titers: at 6 to 7 days postinfection, virus titers in the lungs were 4.5 to 6.0 log10 EID50/ml, and titers in the brains and olfactory bulbs were even higher (5.5 to 6.5 log10 EID50/ml). A systemic spread of virus was also observed after inoculation with the A/quail/Vietnam/36/04 virus (Fig. 2B). In contrast, after inoculation with either A/chicken/Vietnam/C58/04 or A/PR/8/34, virus was detectable only in the upper respiratory tracts of ferrets. These results indicate that the severity of disease caused by the A/Vietnam/1203/04 virus was associated with broad tissue tropism and with high virus titers in multiple organs, particularly the brain.
Histopathology in ferrets infected with A/Vietnam/1203/04 influenza virus.
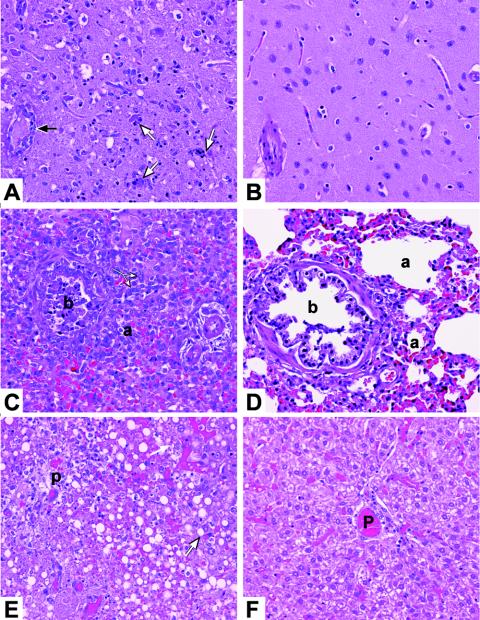
We histologically examined the tissues of ferrets that were infected with the human H5N1 isolate A/Vietnam/1203/04 to investigate the lesions associated with virus replication in the infected organs and to obtain more information about the local inflammatory response. On days 6 and 7 postinfection, a spectrum of histologic alterations was observed in the lungs, brains, and livers of ferrets that had been inoculated with the A/Vietnam/1203/04 virus (Fig. 3). In the neuropil of the olfactory bulb, cerebrum, and brain stem, there were scattered foci of marked neuronal degeneration and neuronophagia associated with inflammatory cell infiltrates. Mononuclear cell infiltrates were also observed in the meninges and the perivascular spaces of the neuropil (Fig. 3A). Bronchitis, bronchiolitis, and pneumonia were observed on days 6 and 7 postinfection. Our findings included bronchial and bronchiolar epithelial necrosis, hypertrophy, and/or hyperplasia, with intraluminal aggregates of necrotic cellular debris and mononuclear inflammatory cells. In the alveoli, we observed epithelial hyperplasia and various proportions of intra-alveolar fibrin and mixed inflammatory cells (predominantly mononuclear cells) (Fig. 3C). In the liver, there was a diffuse vacuolization of the hepatocellular cytoplasm, consistent with fat, portal tract biliary duct necrosis, mononuclear infiltrates, periportal hemorrhage, and hepatocellular necrosis (Fig. 3E). No pathological changes were seen in the spleen or intestine. The brains, lungs, and livers of ferrets that had been inoculated with A/chicken/Vietnam/C58/04 showed no apparent changes (Fig. 3B, D, and F).
FIG. 3.
Histologic changes in brains, lungs, and livers of ferrets infected with H5N1/04 viruses. The images shown are hematoxylin-and-eosin-stained sections of tissue from ferrets inoculated with A/Vietnam/1203/04 (isolated from a human) (A, C, and E) and A/chicken/Vietnam/C58/04 (isolated from a chicken) (B, D, and F) obtained on day 6 after inoculation. (A) Hypercellular brain tissue showing infiltrate of mononuclear inflammatory cells, neuronal degeneration, neuronophagia (white arrows), and perivascular mononuclear infiltrate (black arrow). (C) Lung showing bronchiole (b) with epithelial necrosis, intraluminal debris, and inflammatory cells. Alveoli (a) are lined with proliferating hypertrophic pneumocytes (white arrows) and are filled with a mononuclear cell infiltrate. (E) Liver showing portal tract (P) with biliary duct necrosis and inflammatory cell infiltration. The tissue in this area is necrotic and hemorrhagic, unlike normal liver tissue (white arrow). The brain, lung, and liver (B, D, and F) of a ferret inoculated with A/chicken/Vietnam/C58/04 were normal. Magnification, ×20.
Sequence analysis of human and avian H5N1/04 isolates.
To characterize the molecular basis of the high level of virulence of the A/Vietnam/1203/04 (H5N1) influenza virus, we sequenced the complete genomes of the A/Vietnam/1203/04, A/quail/Vietnam/36/04, and A/chicken/Vietnam/C58/04 viruses. Within these three representatives of lethal and nonlethal viruses, we found changes in multiple amino acids (Table 2). The NS gene of A/Vietnam/1203/04 encoded an NS1 polypeptide of 215 amino acids, whereas those of A/quail/Vietnam/36/04 and A/chicken/Vietnam/C58/04 encoded a 225-amino-acid protein. In addition to the length variation in NS1, six to seven mutations were identified. No amino acid substitution was found in the M1 gene. One conservative change (M→ L) was found in the nucleoprotein. The HAs of all three viruses had identical polybasic amino acids (PQRERRRKKRG) in the connecting peptide. The HA and NA segments encoded amino acid substitutions at six positions. Three to five amino acid changes were found in the polymerase subunit proteins PB1, PB2, and PA. Interestingly, PB2 had a lysine at position 627 in the human strain, whereas the chicken and quail viruses had glutamic acids. Lysine 627 in PB2 was previously found in the H5N1/97 viruses and in the H7N7 virus isolated from a fatal human infection in 2003 (6, 7). These results show that multiple changes in seven genes are candidate determinants of the high pathogenicity level of A/Vietnam/1203/04 (H5N1) in ferrets and that the M1 protein can be excluded as a determinant of pathogenicity in ferrets.
TABLE 2.
Comparison of amino acid sequences encoded by gene segments of representative lethal and nonlethal H5N1/04 influenza viruses
| Gene | Amino acid positiona | Virusb
|
||
|---|---|---|---|---|
| A/Vietnam/ 1203/04 | A/chicken/ Vietnam/ C58/04 | A/quail/ Vietnam/ 36/04 | ||
| PB1 | 3 | V | A | V |
| 328 | N | K | N | |
| 375 | N | S | N | |
| PB2 | 368 | R | Q | R |
| 391 | Q | E | E | |
| 430 | P | P | T | |
| 447 | Q | H | Q | |
| 627 | K | E | E | |
| PA | 142 | E | K | K |
| 151 | T | I | T | |
| 421 | I | S | S | |
| 595 | M | L | M | |
| HA | 3 | K | R | K |
| 52 (36) | K | T | T | |
| 191 (175) | L | L | M | |
| 250 (234) | K | R | K | |
| 292 (276) | T | A | T | |
| 544 | A | V | A | |
| NP | 331 | M | L | M |
| NA | 26 | I | I | V |
| 38 | I | T | I | |
| 46 | S | A | A | |
| 180 | N | S | N | |
| 264 | N | D | D | |
| 309 | N | D | N | |
| M | ||||
| M1 | ||||
| M2 | 61 | R | R | I |
| 65 | T | M | T | |
| NS | ||||
| NS1 | 71 | G | E | E |
| 89 | T | N | T | |
| 101 | M | I | M | |
| 132 | I | I | T | |
| 148 | E | E | G | |
| 200 | N | S | S | |
| 202 | D | N | D | |
| 216 | * | K | K | |
| 226 | * | * | ||
| NS2/NEP | 48 | T | A | A |
| 115 | A | A | T | |
Numbering is based on the deduced amino acid sequence; numbers in parentheses refer to the numbering of the three-dimensional structure of A/duck/Singapore/97 (H5N1) (12).
*, stop codon.
DISCUSSION
The direct transmission of avian H5N1 influenza viruses to several humans in Vietnam and Thailand has underscored the pandemic potential of these viruses and has heightened the urgency of identifying the mechanisms of avian-human transmission and the genetic determinants of a high level of virulence. In the present study, ferrets infected with H5N1 viruses that were isolated from humans in 2004 experienced extreme lethargy, diarrhea, severe neurological impairment, and death. Similar clinical signs were observed in 7 of 10 human patients with confirmed H5N1 influenza virus infections in Vietnam, who showed not only fever and respiratory symptoms, but also diarrhea (35). Multiple organ failure was observed in patients who died of H5N1 infections in 1997 (34). The H5N1 influenza viruses A/HK/483/97 and A/HK/486/97, which were isolated from humans, were also reported to cause lethargy, fever, and weight loss in ferrets and to replicate in the upper and lower respiratory tracts and the brain (39). Here we showed that avian H5N1/04 viruses differ in their pathogenicity in ferrets and can be divided into two groups, one that causes a mild, nonlethal disease and one that causes systemic spread of the virus and death. Interestingly, viruses that were lethal to ferrets were isolated from quails and ducks (36), but not from chickens. Quails and chickens are susceptible to infection by H5N1 viruses, and quails are considered to act as an intermediate host in facilitating the interspecies spread of influenza viruses (24). Our understanding of the pathogenicity of H5N1 influenza viruses in ducks has changed in recent years, and the isolates from 2002 were reported to be highly pathogenic to ducks (31). Our results indicate that among avian H5N1/04 viruses circulating in Asia, some are able to cause lethal infections in ferrets, which are an excellent mammalian model. H5N1 influenza viruses are continually evolving (3, 9, 10, 19), and our results support the hypothesis that the H5N1/04 viruses have undergone multiple genetic changes. Although our study did not directly address potential human-to-human transmission, the continual evolution of these viruses may increase the likelihood of such transmission and is therefore of concern.
Our findings suggest that broad tissue tropism, high replication efficiencies, and neurovirulence are among the possible causes of the high rates of lethality of H5N1/04 viruses in ferrets. H5N1/04 virus infections that are lethal to ferrets are characterized by prolonged virus shedding. The molecular basis for the increased virulence of these H5N1 viruses in ferrets is unresolved. The NS1 gene product is an interferon antagonist (18), and this gene has been associated with cytokine imbalances in mice and humans (4, 10, 27). It remains to be determined whether this gene is associated with severe disease in ferrets. Multiple changes detected in the NS1 protein of H5N1/04 viruses and the established role of NS1 as a virulence factor support this hypothesis (Table 2). As in human H5N1 cases (37), the ability of H5N1/04 viruses to replicate in multiple organs of ferrets was associated with the severity of disease.
In ferrets infected with human H5N1/04 viruses, virus was consistently detected in the brain and olfactory bulb at even higher titers than those in the lungs. This finding led us to conclude that these viruses are highly neurotropic, as reported for some H5N1 viruses in a mouse model (20, 22). Whether the high virus titers in tissues of the nervous system are the result of generally higher replication efficiencies or a specific cell tropism remains to be investigated. The detection of virus in the olfactory bulbs of ferrets early in infection suggests that the olfactory nerves and ethmoid plate may be routes of entry into the central nervous system for the H5N1 viruses after intranasal inoculation, as shown previously in mice (22).
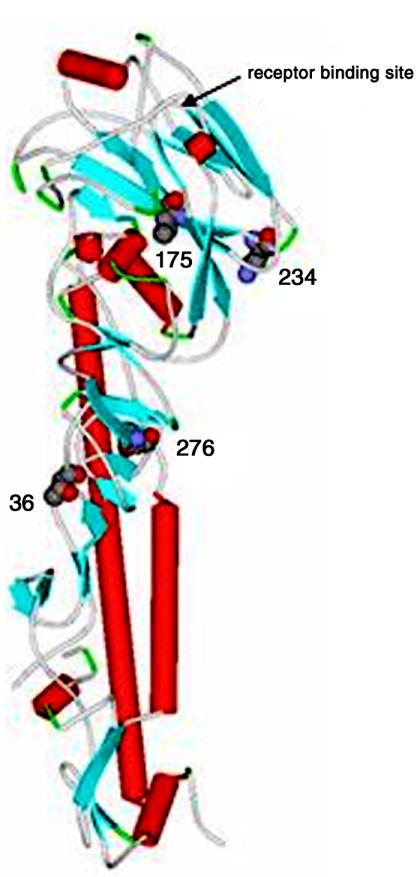
The sequence differences detected between viruses that differ in pathogenicity are the first steps to understanding the molecular basis of virulence and host range restriction of the H5N1/04 viruses. Our results showed amino acid differences in the coding region of multiple genes (Table 2). These residues may play important roles in virus pathogenicity and lethality in ferrets. Among six amino acid differences observed between the HA proteins of the A/Vietnam/1203/04, A/quail/Vietnam/36/04, and A/chicken/Vietnam/C58/04 influenza viruses, one (K250R) is located at the globular head of the molecule and may indirectly affect the binding of the virus (Fig. 4). However, there were no substitutions in the HA receptor-binding site or differences in the number of glycosylation sites between HAs of avian and human origin. Our results suggest that HA receptor specificity is not the only factor that restricts initial infection and are consistent with earlier reports that altered receptor preference may not be required for primary human infections by avian influenza viruses (12, 21). Among six changes in the NA protein coding sequence, two (N180 and N309) in both the human and quail isolates and one (N264) in the human isolate only resulted in the acquisition of asparagine, a more basic amino acid. Those changes did not result in any new glycosylation site and were not located within the catalytic site of the NA protein. The specific sequences identified in the genes encoding the polymerase subunit proteins suggest that those residues may be important for the high replication efficiency observed in the ferret. H5N1/97 viruses with lysine at position 627 of the PB2 protein caused a lethal disease in mice (7, 13). An amino acid substitution at residue 627 influenced the replication efficiency of the virus in different mouse organs but did not affect the cell tropism of the virus (28). Our findings showed that lysine 627 of PB2 is not essential for the high lethality rate of the H5N1/04 viruses in ferrets, as the quail isolate, which caused severe disease, had glutamic acid at this position. This result is consistent with previous findings that lysine 627 of PB2 is important for host range (28, 32). It is noteworthy that A/Vietnam/1203/04 (H5N1) had unique amino acids in PB2 (Q391) and NS1 (G71); these residues were not found in A/quail/Vietnam/36/04 or any other influenza A virus. Some of the amino acid changes we observed may be important for the severity of the disease in ferrets and humans.
FIG. 4.
Location of amino acid changes in three-dimensional structure of HA. A schematic representation of the HA monomer shows residues that differ between the A/Vietnam/1203/04, A/chicken/Vietnam/C58/04, and A/quail/Vietnam/36/04 viruses, based on the three-dimensional structure of H5 from A/duck/Singapore/3/97 (H5N3).
The occurrence of lethal H5N1 influenza in humans in Vietnam and Thailand during the summer of 2004 is of great concern. No human cases of H5N1 infection have previously been reported during the summer months. An overall increase in the number of cases raises the odds that the virus may acquire the attributes that allow human spread (19).
Acknowledgments
This study was supported by grant AI 95357 and Cancer Center Support (CORE) grant CA21765 from the National Institutes of Health, by the American Lebanese Syrian Associated Charities (ALSAC), by grant 067072/D/02/Z from The Wellcome Trust, by the Ellison Foundation, by the Li Ka Shing Foundation, and by grants HKU7334/01 M, HKU/7225/02 M, and HKU/7335/02 M from the Research Grants Council of Hong Kong.
We gratefully acknowledge the excellent technical assistance of Jennifer Humberd, Jon P. Seiler, and David Walker, the editorial assistance of Sharon Naron, and the excellent technical support of the Center for Biotechnology.
REFERENCES
- 1.Bosch, F. X., W. Garten, H. D. Klenk, and R. Rott. 1981. Proteolytic cleavage of influenza virus hemagglutinins: primary structure of the connecting peptide between HA1 and HA2 determines proteolytic cleavability and pathogenicity of avian influenza viruses. Virology 113:725-735. [DOI] [PubMed] [Google Scholar]
- 2.Cauthen, A. N., D. E. Swayne, S. Schultz-Cherry, M. L. Perdue, and D. L. Suarez. 2000. Continued circulation in China of highly pathogenic avian influenza viruses encoding the hemagglutinin gene associated with the 1997 H5N1 outbreak in poultry and humans. J. Virol. 74:6592-6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, H., G. Deng, Z. Li, G. Tian, Y. Li, P. Jiao, L. Zhang, Z. Liu, R. G. Webster, and K. Yu. 2004. The evolution of H5N1 influenza viruses in ducks in southern China. Proc. Natl. Acad. Sci. USA 101:10452-10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung, C. Y., L. L. Poon, A. S. Lau, W. Luk, Y. L. Lau, K. F. Shortridge, S. Gordon, Y. Guan, and J. S. Peiris. 2002. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet 360:1831-1837. [DOI] [PubMed] [Google Scholar]
- 5.Claas, E. C., J. C. de Jong, R. van Beek, G. F. Rimmelzwaan, and A. D. Osterhaus. 1998. Human influenza virus A/HongKong/156/97 (H5N1) infection. Vaccine 16:977-978. [DOI] [PubMed] [Google Scholar]
- 6.Fouchier, R. A., P. M. Schneeberger, F. W. Rozendaal, J. M. Broekman, S. A. Kemink, V. Munster, T. Kuiken, G. F. Rimmelzwaan, M. Schutten, G. J. Van Doornum, G. Koch, A. Bosman, M. Koopmans, and A. D. Osterhaus. 2004. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc. Natl. Acad. Sci. USA 101:1356-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao, P., S. Watanabe, T. Ito, H. Goto, K. Wells, M. McGregor, A. J. Cooley, and Y. Kawaoka. 1999. Biological heterogeneity, including systemic replication in mice, of H5N1 influenza A virus isolates from humans in Hong Kong. J. Virol. 73:3184-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Sastre, A. 2001. Inhibition of interferon-mediated antiviral responses by influenza A viruses and other negative-strand RNA viruses. Virology 279:375-384. [DOI] [PubMed] [Google Scholar]
- 9.Guan, Y., M. Peiris, K. F. Kong, K. C. Dyrting, T. M. Ellis, T. Sit, L. J. Zhang, and K. F. Shortridge. 2002. H5N1 influenza viruses isolated from geese in Southeastern China: evidence for genetic reassortment and interspecies transmission to ducks. Virology 292:16-23. [DOI] [PubMed] [Google Scholar]
- 10.Guan, Y., L. L. Poon, C. Y. Cheung, T. M. Ellis, W. Lim, A. S. Lipatov, K. H. Chan, K. M. Sturm-Ramirez, C. L. Cheung, Y. H. Leung, K. Y. Yuen, R. G. Webster, and J. S. Peiris. 2004. H5N1 influenza: a protean pandemic threat. Proc. Natl. Acad. Sci. USA 101:8156-8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan, Y., K. F. Shortridge, S. Krauss, and R. G. Webster. 1999. Molecular characterization of H9N2 influenza viruses: were they the donors of the “internal” genes of H5N1 viruses in Hong Kong? Proc. Natl. Acad. Sci. USA 96:9363-9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ha, Y., D. J. Stevens, J. J. Skehel, and D. C. Wiley. 2001. X-ray structures of H5 avian and H9 swine influenza virus hemagglutinins bound to avian and human receptor analogs. Proc. Natl. Acad. Sci. USA 98:11181-11186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatta, M., P. Gao, P. Halfmann, and Y. Kawaoka. 2001. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science 293:1840-1842. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann, E., J. Stech, Y. Guan, R. G. Webster, and D. R. Perez. 2001. Universal primer set for the full-length amplification of all influenza A viruses. Arch. Virol. 146:2275-2289. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann, E., J. Stech, I. Leneva, S. Krauss, C. Scholtissek, P. S. Chin, M. Peiris, K. F. Shortridge, and R. G. Webster. 2000. Characterization of the influenza A virus gene pool in avian species in southern China: was H6N1 a derivative or a precursor of H5N1? J. Virol. 74:6309-6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz, J. M., X. Lu, T. M. Tumpey, C. B. Smith, M. W. Shaw, and K. Subbarao. 2000. Molecular correlates of influenza A H5N1 virus pathogenesis in mice. J. Virol. 74:10807-10810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawaoka, Y., A. Nestorowicz, D. J. Alexander, and R. G. Webster. 1987. Molecular analyses of the hemagglutinin genes of H5 influenza viruses: origin of a virulent turkey strain. Virology 158:218-227. [DOI] [PubMed] [Google Scholar]
- 18.Krug, R. M., W. Yuan, D. L. Noah, and A. G. Latham. 2003. Intracellular warfare between human influenza viruses and human cells: the roles of the viral NS1 protein. Virology 309:181-189. [DOI] [PubMed] [Google Scholar]
- 19.Li, K. S., Y. Guan, J. Wang, G. J. Smith, K. M. Xu, L. Duan, A. P. Rahardjo, P. Puthavathana, C. Buranathai, T. D. Nguyen, A. T. Estoepangestie, A. Chaisingh, P. Auewarakul, H. T. Long, N. T. Hanh, R. J. Webby, L. L. Poon, H. Chen, K. F. Shortridge, K. Y. Yuen, R. G. Webster, and J. S. Peiris. 2004. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature 430:209-213. [DOI] [PubMed] [Google Scholar]
- 20.Lipatov, A. S., S. Krauss, Y. Guan, M. Peiris, J. E. Rehg, D. R. Perez, and R. G. Webster. 2003. Neurovirulence in mice of H5N1 influenza virus genotypes isolated from Hong Kong poultry in 2001. J. Virol. 77:3816-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matrosovich, M., N. Zhou, Y. Kawaoka, and R. Webster. 1999. The surface glycoproteins of H5 influenza viruses isolated from humans, chickens, and wild aquatic birds have distinguishable properties. J. Virol. 73:1146-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park, C. H., M. Ishinaka, A. Takada, H. Kida, T. Kimura, K. Ochiai, and T. Umemura. 2002. The invasion routes of neurovirulent A/Hong Kong/483/97 (H5N1) influenza virus into the central nervous system after respiratory infection in mice. Arch. Virol. 147:1425-1436. [DOI] [PubMed] [Google Scholar]
- 23.Peiris, J. S., W. C. Yu, C. W. Leung, C. Y. Cheung, W. F. Ng, J. M. Nicholls, T. K. Ng, K. H. Chan, S. T. Lai, W. L. Lim, K. Y. Yuen, and Y. Guan. 2004. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet 363:617-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez, D. R., R. J. Webby, E. Hoffmann, and R. G. Webster. 2003. Land-based birds as potential disseminators of avian mammalian reassortant influenza A viruses. Avian Dis. 47:1114-1117. [DOI] [PubMed] [Google Scholar]
- 25.Reuman, P. D., S. Keely, and G. M. Schiff. 1989. Assessment of signs of influenza illness in the ferret model. J. Virol. Methods 24:27-34. [DOI] [PubMed] [Google Scholar]
- 26.Rimmelzwaan, G. F., T. Kuiken, G. van Amerongen, T. M. Bestebroer, R. A. Fouchier, and A. D. Osterhaus. 2001. Pathogenesis of influenza A (H5N1) virus infection in a primate model. J. Virol. 75:6687-6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seo, S. H., E. Hoffmann, and R. G. Webster. 2002. Lethal H5N1 influenza viruses escape host anti-viral cytokine responses. Nat. Med. 8:950-954. [DOI] [PubMed] [Google Scholar]
- 28.Shinya, K., S. Hamm, M. Hatta, H. Ito, T. Ito, and Y. Kawaoka. 2004. PB2 amino acid at position 627 affects replicative efficiency, but not cell tropism, of Hong Kong H5N1 influenza A viruses in mice. Virology 320:258-266. [DOI] [PubMed] [Google Scholar]
- 29.Shortridge, K. F., N. N. Zhou, Y. Guan, P. Gao, T. Ito, Y. Kawaoka, S. Kodihalli, S. Krauss, D. Markwell, K. G. Murti, M. Norwood, D. Senne, L. Sims, A. Takada, and R. G. Webster. 1998. Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology 252:331-342. [DOI] [PubMed] [Google Scholar]
- 30.Smith, H., and C. Sweet. 1988. Lessons for human influenza from pathogenicity studies with ferrets. Rev. Infect. Dis. 10:56-75. [DOI] [PubMed] [Google Scholar]
- 31.Sturm-Ramirez, K. M., T. Ellis, B. Bousfield, L. Bissett, K. Dyrting, J. E. Rehg, L. Poon, Y. Guan, M. Peiris, and R. G. Webster. 2004. Reemerging H5N1 influenza viruses in Hong Kong in 2002 are highly pathogenic to ducks. J. Virol. 78:4892-4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subbarao, E. K., W. London, and B. R. Murphy. 1993. A single amino acid in the PB2 gene of influenza A virus is a determinant of host range. J. Virol. 67:1761-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subbarao, K., A. Klimov, J. Katz, H. Regnery, W. Lim, H. Hall, M. Perdue, D. Swayne, C. Bender, J. Huang, M. Hemphill, T. Rowe, M. Shaw, X. Xu, K. Fukuda, and N. Cox. 1998. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science 279:393-396. [DOI] [PubMed] [Google Scholar]
- 34.To, K. F., P. K. Chan, K. F. Chan, W. K. Lee, W. Y. Lam, K. F. Wong, N. L. Tang, D. N. Tsang, R. Y. Sung, T. A. Buckley, J. S. Tam, and A. F. Cheng. 2001. Pathology of fatal human infection associated with avian influenza A H5N1 virus. J. Med. Virol. 63:242-246. [DOI] [PubMed] [Google Scholar]
- 35.Tran, T. H., T. L. Nguyen, T. D. Nguyen, T. S. Luong, P. M. Pham, V. C. Nguyen, T. S. Pham, C. D. Vo, T. Q. Le, T. T. Ngo, B. K. Dao, P. P. Le, T. T. Nguyen, T. L. Hoang, V. T. Cao, T. G. Le, D. T. Nguyen, H. N. Le, K. T. Nguyen, H. S. Le, V. T. Le, D. Christiane, T. T. Tran, J. de Menno, C. Schultsz, P. Cheng, W. Lim, P. Horby, and J. Farrar. 2004. Avian influenza A (H5N1) in 10 patients in Vietnam. N. Engl. J. Med. 350:1179-1188. [DOI] [PubMed] [Google Scholar]
- 36.Webster, R. G., Y. Guan, M. Peiris, D. Walker, S. Krauss, N. N. Zhou, E. A. Govorkova, T. M. Ellis, K. C. Dyrting, T. Sit, D. R. Perez, and K. F. Shortridge. 2002. Characterization of H5N1 influenza viruses that continue to circulate in geese in southeastern China. J. Virol. 76:118-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuen, K. Y., P. K. Chan, M. Peiris, D. N. Tsang, T. L. Que, K. F. Shortridge, P. T. Cheung, W. K. To, E. T. Ho, R. Sung, and A. F. Cheng. 1998. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet 351:467-471. [DOI] [PubMed] [Google Scholar]
- 38.Xu, X., K. Subbarao, N. J. Cox, and Y. Guo. 1999. Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology 261:15-19. [DOI] [PubMed] [Google Scholar]
- 39.Zitzow, L. A., T. Rowe, T. Morken, W. J. Shieh, S. Zaki, and J. M. Katz. 2002. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. J. Virol. 76:4420-4429. [DOI] [PMC free article] [PubMed] [Google Scholar]