Abstract
A 61-year-old Caucasian male with severe plaque psoriasis without joint involvement was initiated on adalimumab therapy. Shortly thereafter he presented to the emergency room with acute loss of vision in the right eye. A comprehensive systemic workup was instituted which included magnetic resonance imaging (MRI) with and without gadolinium of the brain and orbits. MRI revealed findings that were consistent with CNS demyelination and retrobulbar optic neuritis. Immediate cessation of adalimumab was instituted without any other systemic therapy. Complete return of vision occurred within 6 weeks. No additional psoriatic or neurologic treatment was instituted, and the patient has remained stable now for 14 months.
Key Words: Adalimumab, Psoriasis, Optic neuritis, Demyelination, Loss of vision
Case Report
The patient is a 61-year-old Caucasian male with plaque psoriasis without joint involvement who presented to the emergency room with a chief complaint of a 5-day history of progressive vision loss in the right eye associated with pain on eye movements. His past ocular history, past medical history, and review of symptoms were noncontributory. His past dermatologic history was significant for failed treatment of his psoriasis with etanercept. Adalimumab therapy was initiated 8 weeks prior to presentation with clinical improvement of his psoriasis.
Emergent ophthalmologic examination was performed and was significant for decreased visual acuity to 20/50 in the right eye with an afferent pupillary defect, decreased color vision on the right side, and a normal funduscopic examination. Formal visual field testing revealed an arcuate visual field defect in the right eye and was full in the left eye (Fig. 1). Comprehensive laboratory evaluation inclusive of, FTA-ABS and RPR was within normal limits. A magnetic resonance imaging (MRI) with and without gadolinium of the brain and orbits was performed and revealed no orbital abnormality, but multiple nonspecific, bihemispheric white matter signal hyperintensities on FLAIR and T2-weighted sequences without mass effect or edema. These findings were consistent with CNS demyelination and retrobulbar optic neuritis. Immediate cessation of adalimumab was instituted. Formal neurologic consultation was obtained, and despite a recommendation for initiation of systemic steroid therapy, the patient refused.
Fig. 1.
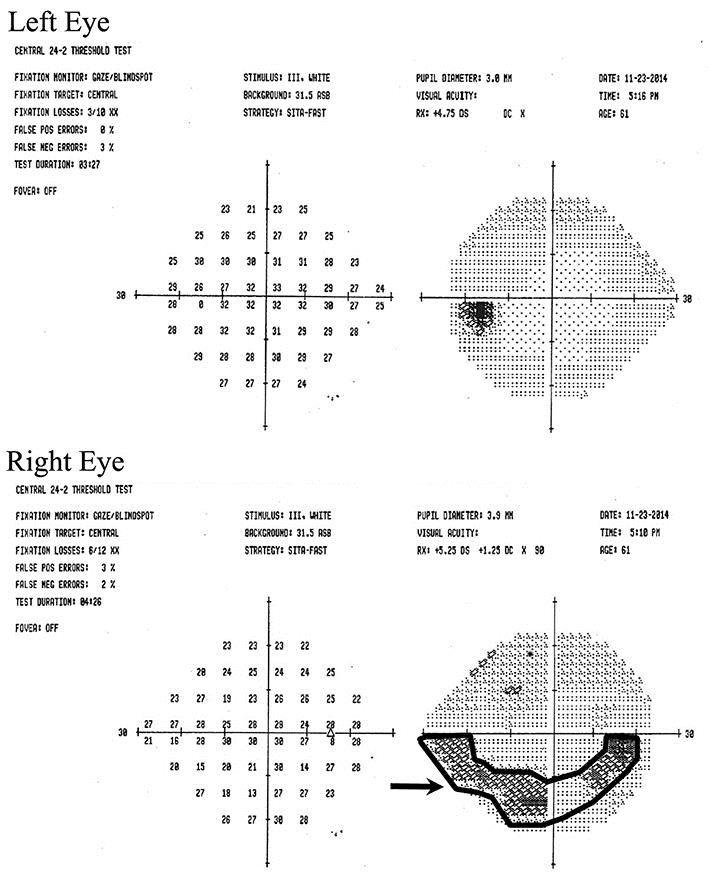
Visual field testing (SITA 24–2) at initial presentation revealed superior arcuate defect (arrow) in the right eye.
Follow-up ophthalmologic examination 6 weeks later demonstrated a return of visual acuity and visual field (Fig. 2) and has remained stable for 14 months. No systemic therapy was reinstituted for his psoriasis.
Fig. 2.

Visual field testing (SITA 24–2) at the 6 week follow-up visit demonstrated full visual fields bilaterally.
Discussion
Adalimumab is a recombinant, human monoclonal antibody that neutralizes tumor necrosis factor-alpha (TNF-α). It is approved by the Food and Drug Administration (FDA) as a therapy for moderate and severe psoriasis as well as for a number of other autoimmune and rheumatologic conditions. The use of anti-TNF-α agents, including adalimumab, has been associated with various demyelinating disorders of both central and peripheral nervous system. Cases of optic neuritis, multiple sclerosis, transverse myelitis, as well as of Guillain-Barre syndrome associated with the use of anti-TNF-α agents in various rheumatologic and inflammatory gastrointestinal conditions have been previously reported [1, 2, 3]. Here, we report the first case of retrobulbar optic neuritis and CNS demyelination in a patient treated with adalimumab for plaque psoriasis without joint involvement.
The use of biological therapies targeting T cells and immune cytokines has become more prevalent in the recent years for the treatment of psoriasis and psoriatic arthritis due to their clinical efficacy and relative safety [4]. TNF-α inhibitors, such as adalimumab, are the most commonly used biologic agents for the treatment of these conditions. Besides being associated with an increased risk of infections, these agents carry a potential risk of, new onset of, or exacerbation of underlying CNS or peripheral demyelinating disorders [1, 2, 3]. A recent study analyzed all reported cases of demyelinating disorders associated with TNF-α therapy in patients with psoriasis and psoriatic arthritis and concluded that such complications are quite rare with only 21 other cases reported to date during and after clinical trials [5]. Herein, we reported the first case describing adalimumab associated with retrobulbar optic neuritis and CNS demyelination in a patient with plaque psoriasis without psoriatic arthritis. Continuous monitoring of psoriatic patients who are being treated with adalimumab for possible neurologic side effects may be warranted.
Statement of Ethics
This work complies with the guidelines for human studies.
Disclosure Statement
The authors have no conflicts of interest to disclose. No funding was received for this work.
References
- 1.Bensouda-Grimaldi L, et al. Adalimumab-associated multiple sclerosis. J Rheumatol. 2007;34:239–240. discussion 240. [PubMed] [Google Scholar]
- 2.Shin IS, et al. Guillain-Barre and Miller Fisher syndromes occurring with tumor necrosis factor alpha antagonist therapy. Arthritis Rheum. 2006;54:1429–1434. doi: 10.1002/art.21814. [DOI] [PubMed] [Google Scholar]
- 3.Chung JH, et al. Adalimumab-associated optic neuritis. J Neurol Sci. 2006;244:133–136. doi: 10.1016/j.jns.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Weger W. Current status and new developments in the treatment of psoriasis and psoriatic arthritis with biological agents. Br J Pharmacol. 2010;160:810–820. doi: 10.1111/j.1476-5381.2010.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu TH, et al. Demyelinating disorders secondary to TNF-inhibitor therapy for the treatment of psoriasis: a review. J Dermatolog Treat. 2016;27:406–413. doi: 10.3109/09546634.2015.1136385. [DOI] [PubMed] [Google Scholar]


