Abstract
Since animal models for studying human cytomegalovirus (HCMV) replication in vivo and pathogenesis are not available, severe combined immunodeficiency mice into which human tissues were implanted (SCID-hu mice) provide an alternative and valuable model for such studies. The HCMV clinical isolates, including those of the Toledo strain, replicate to high titers in human tissue implanted into SCID mice; however, the attenuated AD169 strain has completely lost this ability. The major difference between Toledo and AD169 is a 15-kb segment, encoding 19 open reading frames, which is present in all virulent strains but deleted from attenuated strains. This fact suggests that crucial genes required for HCMV replication in vivo are localized to this region. In this study, the importance of this 15-kb segment for HCMV replication in vivo was determined. First, ToledoBAC virus (produced from a Toledo bacterial artificial chromosome) and AD169 virus were tested for growth in SCID-hu mice. ToledoBAC, like Toledo, grew to high titers in implanted human thymus and liver tissues, while AD169 did not. This outcome showed that the Toledo genome propagated in bacteria (ToledoBAC) retained its virulence. The 15-kb segment was then deleted from ToledoBAC, and the resulting virus, ToledoΔ15kb, was tested for growth in both human foreskin fibroblast (HFF) cells and SCID-hu mice. ToledoΔ15kb had a minor growth defect in HFF but completely failed to replicate in human thymus and liver implants. This failure to grow was rescued when the 15-kb region was inserted back into the ToledoΔ15kb genome. These results directly demonstrated that the genes located in the 15-kb segment are crucial for HCMV replication in vivo.
Human cytomegalovirus (HCMV), a member of the beta-herpesvirus family, is a ubiquitous human pathogen. After a primary infection, HCMV establishes lifelong latency in myeloid lineage cells in peripheral blood (35, 36). HCMV rarely causes symptomatic disease in an immunocompetent host; however, it is a major cause of infectious morbidity and mortality in immunocompromised individuals and developing fetuses (27).
HCMV strains that have been characterized at the molecular level are often extensively passaged in cultured human fibroblast cells. The two most commonly used laboratory strains, AD169 and Towne, were developed for use as live, attenuated vaccine strains by repeated propagation in tissue culture cells (14, 30). The AD169 and Towne strains have lost their abilities to replicate and disseminate in vivo and are virulent for humans. Indeed, in HCMV vaccine trials, several hundred seronegative individuals were inoculated with 103 to 105 PFU of AD169 or Towne and no infection was detected (14, 15, 18, 29). In addition, inoculated viruses were not reisolated from immunized individuals, including those who later underwent immunosuppression regimens for renal transplantation (3, 31). Low-passage clinical isolates were used as challenge viruses in human clinical studies, and the Toledo strain was the most populous of these isolates (32, 34). In contrast to the effects of the AD169 and Towne strains, after 10 to 100 PFU of the Toledo strain was inoculated into each of six HCMV-seronegative volunteers, symptoms of primary HCMV infection were observed (32), indicating that the Toledo strain retained its virulence.
The pathogenic differences between high-passage strains (i.e., AD169 and Towne) and low-passage strains (i.e., Toledo) are thought to be due to genetic differences of the viruses. Comparisons of the nucleotide sequences of viral genomes indicate that at the 3′ end of the unique long region, Toledo and five other clinical HCMV genomes carried a DNA segment of ∼15 kb that was absent in the AD169 genome and a segment of ∼13 kb that was absent in the Towne genome (10, 26). Although this region is dispensable for viral replication in human fibroblast cells, some of these genes are likely to play an essential role in viral replication in vivo and in human pathogenesis. DNA sequence analysis predicts 19 open reading frames (ORFs), named UL133 through UL151, in this region. These ORFs are completely missing in AD169, and only one (UL147) is present in Towne (10).
Due to HCMV's restricted host specificity (it rarely infects nonhuman tissues productively), there is no animal model that entirely reproduces HCMV growth and disease. This deficiency greatly complicates studies of viral pathogenesis, tissue tropism, latency, and evaluation of anti-HCMV compounds in organisms. Studies of HCMV are thus largely limited to human cultured cells, and in vivo models have concentrated on the related nonhuman CMV homologs, including mouse, rat, and guinea pig CMVs (37). A better in vivo model is needed for rapid progress in understanding mechanisms of HCMV pathogenesis and control.
Severe combined immunodeficiency mice into which human tissues were implanted (SCID-hu mice) were developed as a preclinical model for the analysis of human physiology and disease and, more recently, for studying human-restricted viruses. SCID-hu mice into which T cells were implanted have been widely used as in vivo models for human immunodeficiency virus type 1 pathogenesis and therapeutic intervention (6, 16). In addition, the SCID-hu model has been used for the in vivo evaluation of a number of other human viral pathogens, including measles virus (1, 40), varicella-zoster virus (24, 25), and HCMV (9, 19, 23). Mocarski et al. (23) first demonstrated that conjoint implants of human fetal thymus and liver, as well as of human fetal lung and colon, in SCID mice supported the replication of HCMV Toledo. In addition, treatment of infected SCID-hu mice with the anti-HCMV drug ganciclovir blocked viral growth in the implanted human tissues. Interestingly, subsequent experiments showed that the clinical Toledo strain grew to high levels but that the attenuated AD169 virus strain (obtained from the American Type Culture Collection) failed to grow at all. These results demonstrated that the SCID-hu model, in which human T-cell xenografts are infected in vivo, makes it possible to define the effects of nonlethal HCMV mutations on virulence in these differentiated human cells within their unique tissue microenvironments. SCID mice into which fetal human retinal tissue was implanted also provide an excellent model for evaluation of HCMV pathogenesis in an ocular structure in vivo and therapeutic treatment of HCMV retinitis in AIDS patents (4, 12, 19).
The major difference between the attenuated strains (AD169 and Towne) and the virulent strains (including Toledo) of HCMV is a 15-kb deletion from the HCMV genome. We hypothesized that ORFs in this region are necessary for HCMV replication in vivo. However, there is no direct evidence for this assumption. Smaller changes in other regions of AD169 and Towne may also contribute to their attenuated phenotype, and these changes cannot be specifically identified by sequencing analysis, because there are many genetic variations between HCMV strains (26). To directly demonstrate that the 15-kb segment was necessary for HCMV replication in vivo, two genetic approaches were considered. One option was to insert the 15-kb segment back into AD169 and test whether it restored the virulence of AD169 in vivo. However, this approach was discarded because of difficulty manipulating such large pieces of DNA and because a failure to restore virulence would not be a conclusive result. There is a strong possibility that AD169 harbors unknown mutations in its genome that affect replication in vivo. Thus, we chose the alternative approach, to delete the 15-kb segment from the wild-type Toledo strain and test whether this recombinant virus was able to replicate in vivo. Indeed, we showed that the genes located in the 15-kb segment are required for HCMV replication in T-cell xenografts in SCID-hu mice.
MATERIALS AND METHODS
Cells and viruses.
Escherichia coli strain DY380 was obtained from Neal Copeland and Craig Stranthdee and is described in references 38 and 42. Primary human foreskin fibroblast (HFF) cells were isolated from skin and grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 U of penicillin-streptomycin, and 2.5 μg of amphotericin B/ml. The HCMV AD169 strain was obtained from the American Type Culture Collection. The low-passage Toledo strain was a gift from E. Mocarski (Stanford University).
Plasmid and BAC clones.
The HCMV tegument protein expression plasmid pCMV-pp71 (2) and the ToledoBAC clone (produced from a Toledo bacterial artificial chromosome [BAC]) (26) were gifts from T. Shenk. Cre recombinase expression plasmid pGS403 was a gift from L. Enquist.
Concentration of HCMV.
To prepare concentrated virions, intracellular and extracellular viruses were centrifuged at 8,000 × g for 20 min at room temperature to pellet cell debris. The virus supernatant was transferred into SW27 ultracentrifuge tubes (30 ml/tube) and underlaid with 7 ml of 20% sorbitol buffer (20% d-sorbitol, 50 mM Tris-HCl [pH 7.2], 1 mM MgCl2). This mixture was centrifuged at 55,000 × g for 1 h at room temperature to pellet the virus. Pellets were then resuspended in a small volume of phosphate-buffered saline (PBS), aliquoted, and stored at −80°C.
Generation of the ToledoΔ15kb BAC clone and virus.
To generate a Toledo deletion mutant (ToledoΔ15kb) (i.e., without ORFs UL136 through UL150), the 15-kb segment from ToledoBAC was replaced with a zeocin resistance marker by homologous recombination. The zeocin expression cassette was amplified from plasmid pCMV/zeo (Invitrogen, Inc., Carlsbad, Calif.) by PCR using primers Zeo-F (5′-TTG AAC GGA GCT ATG TAC TAC GGC AGC GGC TGT CGC TTC GAC ACG GTG GAG GAA CGG TGC ATT GGA ACG GAC) and Zeo-R (5′-ACG TCT CGT ACA CTA CCC GAT ACG ATT TTG GCA GTG AAA CGC CGT TCC GTC AAG TTT CGA GGT CGA GTG TCA G). These primers have 22 to 23 bp at the 3′ ends to amplify the zeocin expression cassette and 50-bp tails on the 5′ ends that are homologous to the HCMV sequences of UL136 and UL150. HotStar Taq DNA polymerase (QIAGEN, Inc., Valencia, Calif.) was used for PCR amplification. The resulting 400-bp PCR product was purified from agarose by use of the QIAEX II gel extraction kit (QIAGEN), and the concentration of DNA was determined with a mass ladder (Invitrogen). Approximately 200 ng of DNA was electroporated into E. coli DY380 harboring ToledoBAC. This highly efficient homologous recombination system was described previously (38, 41, 42). ToledoΔ15kb clones were selected at 32°C on Luria-Bertani (LB) agar containing 12.5 μg of chloramphenicol/ml and 50 μg of zeocin/ml and then characterized by PCR and Southern blot analyses as described in reference 41. To produce ToledoΔ15kb virus, the BAC DNA was transfected into HFF cells as described elsewhere (20). Plasmids expressing HCMV pp71 protein, pCMV-pp71, and phage P1 Cre recombinase, pGS403, were cotransfected with the BAC DNA. The pp71 protein was used to enhance viral gene expression (2), and Cre removed the BAC vector sequences and zeocin marker from the viral genome.
Generation of the ToledoOK BAC clone.
To rescue the ToledoΔ15kb deletion clone, we first constructed a recombinant Toledo virus with a kanamycin resistance gene to facilitate the screening of rescued viruses. An oriV/Kanr cassette was amplified from the EZ:TNTM<oriV/KAN-2> transposon (Epicentre, Madison, Wis.) with primers OK-Lox-F (5′-GTC CGG CAG GAT AGC GGT TAA GGA TTC GGT GCT AAG GCC GAT AAC TTC GTA TAA TGT ATG CTA TAC GAA GTT ATC TGT CTC TTA TAC ACA TCT C) and OK-Lox-R (5′-TAT CTG CGT GGG TCT AAT CAT GGG TGT CAC CGT GAT CGC GAT AAC TTC GTA TAG CAT ACA TTA TAC GAA GTT ATC TGT CTC TTA TAC ACA TCT C). In addition to the sequences required for amplifying the oriV/Kanr cassette, these primers contain 40-bp tails homologous to the sequences between the noncoding region of Toledo UL130 and UL149 (see Fig. 3A) and 34-bp loxP sites. The 2-kb oriV/Kanr PCR product was electroporated into E. coli DY380 carrying ToledoBAC (as described above). The recombinant clone, ToledoOK, was selected at 32°C on LB agar with 12.5 μg of chloramphenicol/ml and 50 μg of kanamycin/ml. The insertion of oriV/Kanr in the resulting clones was confirmed by PCR using primers OK-Lox-F and OK-Lox-R. Two primers, Tol-a (upstream of the insertion, 5′-GGA CTA CGG AGG TCA TGA CCA) and Tol-b (downstream of the insertion, 5′-AAC ATC TAG TCG CGG AGA AGG), were used to check the correct insertion position in the viral genome. This insertion was further verified by sequencing the junctions. The left junction was confirmed with primers Tol-a and Kan-a (inside of the oriV/Kanr cassette, 5′-TGG CGG CCG TCT ATG GCC CTG), resulting in a 700-bp product. The right junction was confirmed with primers Kan-b (inside of the oriV/Kanr cassette, 5′-TGC AAC CGG CGC AGG AAC ACT) and Tol-b, which resulted in a 600-bp product.
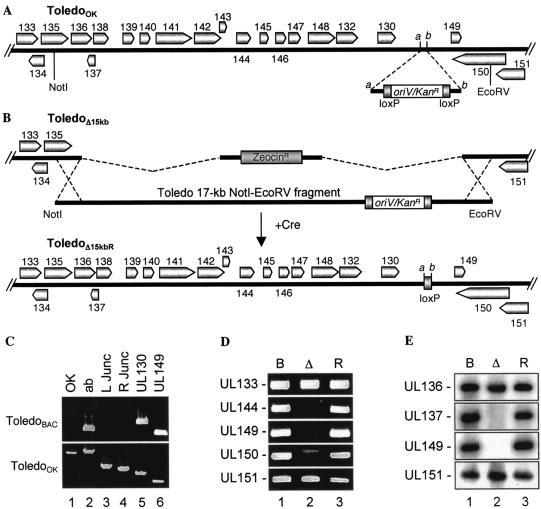
FIG. 3.
Construction of the ToledoΔ15kbR clone. (A) Construction of a ToledoOK clone. An oriV/Kanr cassette flanked by two loxP sites was inserted between UL130 and UL149 of ToledoBAC. This resulting clone was kanamycin resistant and also contained a 100-bp deletion between points a and b. (B) ToledoOK DNA was digested with NotI and EcoRV. The 17-kb NotI-EcoRV fragment with the oriV/Kanr cassette (as indicated) was purified and electroporated into E. coli DY380 harboring ToledoΔ15kb. Homologous recombination allowed the 17-kb fragment to replace the zeocin cassette, and the resulting clone was a ToledoΔ15kbR clone that was resistant to kanamycin but sensitive to zeocin. To produce ToledoΔ15kbR virus, ToledoΔ15kbR DNA and a Cre expression plasmid were cotransfected into HFF cells. The BAC vector and the oriV/Kanr cassette were removed from the viral genome. (C) The ToledoOK clone was verified by PCR analysis. ToledoBAC and ToledoOK DNAs were used as templates and amplified using primers for the oriV/Kanr cassette (OK; lane 1), the a-to-b site (ab; lane 2), the left junction (L Junc; lane 3), the right junction (R Junc; lane 4), UL130 (lane 5), and UL149 (lane 6). (D) ToledoBAC (lane B), ToledoΔ15kb (lane Δ), and ToledoΔ15kbR (lane R) were used as templates for PCRs. Amplified ORFs are indicated to the left of each panel. (E) ToledoBAC (lane B), ToledoΔ15kb (lane Δ), and ToledoΔ15kbR (lane R) were digested with EcoRI and used for Southern blot analyses. The probes are indicated to the left of each panel.
Generation of the ToledoΔ15kbR BAC clone and virus.
ToledoOK DNA was isolated from E. coli and digested with NotI and EcoRV. A 17.8-kb fragment, which includes the 15-kb sequence deleted from ToledoΔ15kb and the oriV/Kanr selectable marker (Fig. 3), was purified from agarose gel by use of QIAEX II. This 17.8-kb fragment was electroporated into E. coli DY380 harboring ToledoΔ15kb for homologous recombination. The rescue clones were selected at 32°C on LB agar with 12.5 μg of chloramphenicol/ml and 50 μg of kanamycin/ml. The correct clones were sensitive to zeocin, and they were further verified by PCR and Southern blot analyses. The ToledoΔ15kbR rescue virus was produced in the same manner as ToledoΔ15kb. Both the BAC vector and the oriV/Kanr cassette were removed from the viral genome by use of Cre recombinase.
Infection of human T-cell xenografts in the SCID-hu mouse model.
Thymus-liver conjoint implants were engrafted in male C.B-17 scid/beige mice. The construction and use of thymus-liver implants are described in detail elsewhere (5, 21). Briefly, human fetal thymus and liver were placed together under the mouse kidney capsule, where a thymus-like organ developed over 2 to 4 months. The thymus-liver implants were surgically exposed and directly injected with exactly 2 × 10 μl (2 × 105 PFU) of HCMV virus suspension from a 1-ml syringe fitted with a 27-gauge needle attached to a volumetric stepper (Tridak, Brookfield, Conn.). The incisions were closed with sutures and staples, and the virus infection progressed for 10 to 14 days. It has been shown that HCMV infection was restricted to the human thymus-liver implant, and HCMV was detected neither in the peripheral blood leukocytes nor in the surrounding kidneys of the inoculated mice (23). The thymus-liver implants were harvested and divided for analysis. One portion was fixed in formalin (3.7% formaldehyde in PBS) for histology, and the remainder was used for the detection of infectious viruses by plaque assay. The genotype of recovered viruses was confirmed by PCR and restriction enzyme analyses. Human fetal tissues were obtained from Advanced Bioscience Resources (Alameda, Calif.) with informed consent according to local, state, and federal regulations.
Growth curve and plaque assays.
HFF cells in six-well plates were infected at a low multiplicity of infection (MOI; 0.1) for extended growth curves or at a high multiplicity (MOI = 5) for single-step growth curves. At the times indicated in Fig. 4, infected cells and culture media were collected and centrifuged to separate the supernatant and the pellet. The pellet was resuspended in 50 μl of medium, and the cells were lysed by 2 cycles of rapid freezing in ethanol cooled with frozen CO2 (dry ice) and thawing in water at 37°C. The supernatant and the pellet were then combined, and the cell debris was removed by centrifugation. The viral titers were determined by using HFF cells. To determine the titers of HCMV in thymus-liver implants, the tissues were minced and placed into 1 ml of PBS on ice. The human tissues and cell suspension were sonicated for 10 s at output 5 with a Sonifier cell disruptor (Ultrasonics, Inc.). Virus titers were determined in triplicate by standard plaque assay on monolayers of HFF cells.
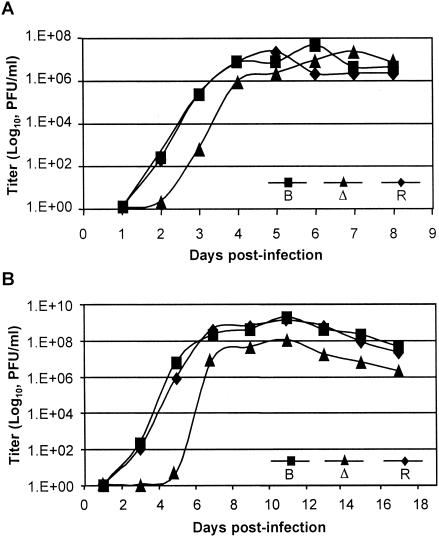
FIG. 4.
Growth curve analyses of ToledoΔ15kb and ToledoΔ15kbR clones. The ToledoBAC (lane B), ToledoΔ15kb (lane Δ), and ToledoΔ15kbR (lane R) viruses were used to infect HFF cells at MOIs of 5 (A) and 0.1 (B). At the indicated days, the samples were collected and the titers of the viruses were determined.
Histology.
Thymus-liver implants were fixed in buffered formalin (3.7% formaldehyde in PBS), embedded in paraffin, cut into 6-μm-thick sections, deparaffinized in xylene, rehydrated in graded ethanol washes, and stained with hematoxylin and eosin. Coverslips were affixed with Permount (Fisher), and the sections were examined by light microscopy. Images were captured by digital photomicrography with an Olympus IX50 microscope and Optronics' MagnaFire imaging system.
HCMV microarray.
HFF cells were infected with ToledoBAC and ToledoΔ15kb viruses at an MOI of 3 for 3 days, and total RNA was isolated with TRIZOL reagent (Gibco BRL). The cDNAs were synthesized and labeled with Cy3 and Cy5 fluorescent dyes as described previously (39). The HCMV oligonucleotide microarray was executed as described in reference 11.
RESULTS
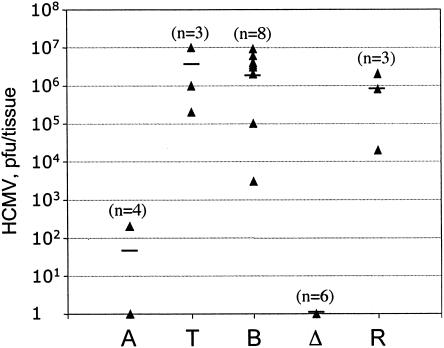
The SCID-hu mouse is a unique model for studying the differences between attenuated and virulent strains. To confirm the attenuation of strain AD169 and the virulence of strain Toledo in vivo, we first performed a pilot experiment using SCID-hu mice with thymus-liver xenografts. Small pieces of thymus and liver from the same donor were placed together under the kidney capsule, where they developed over 4 months into thymus-like organs that contained human T cells, stem cells, and epithelial cells. The implants were directly injected with 2 × 10 μl (2 × 105 PFU) of HCMV strain AD169 (four mice) or Toledo (three mice). After 13 days, the implants were then divided for measurement of the virus yield and histological analysis. This time point was chosen because previous studies showed that peak replication occurs between 12 and 14 days after inoculation (9, 23). To measure the viral yield, the tissues were sonicated and the titers of the viruses were determined. Similar to results found previously (9, 19, 23), the Toledo virus replicated to a high level, reaching an average of 4.9 × 106 PFU/implant (range, 106 to 107 PFU), whereas AD169 was recovered from only one of four implants and the titer was very low (200 PFU) (Fig. 5).
FIG. 5.
Replication of HCMV in SCID-hu thymus-liver implants. Concentrations of 2 × 105 PFU of AD169 (A), Toledo (T), ToledoBAC (B), ToledoΔ15kb (Δ), and ToledoΔ15kbR (R) were injected into thymus-liver implants in SCID mice. Thirteen days postinoculation, the tissues were collected and the titers in HFF cells were individually determined. The titer from each implant (filled triangle), the average titers (bars), and the number of mice in each experimental group (n) are indicated.
Portions of the infected implants were sectioned and stained with hematoxylin and eosin to determine the histological effects of HCMV replication (Fig. 1). Examination by light microscopy showed that implants infected with AD169, like uninfected tissue (Fig. 1A and B), appeared as dark-blue lobes due to the uptake of the hematoxylin stain in the T-cell nuclei (Fig. 1C). At a higher magnification of the area within the box, T cells were found to be densely packed (Fig. 1D), which is the normal appearance of uninfected thymus-liver implants. In contrast, implants infected with Toledo showed extensive areas where proteins were stained red with eosin and cell nuclei were absent (Fig. 1E). These necrotic areas were depleted of thymocytes due to HCMV infection (Fig. 1F). In thymus-liver implants, HCMV infects stromal stem cells and cortical epithelial cells, which produce and maintain T cells, and thus, T-cell depletion is a direct result of HCMV replication (23). Both virus yield and histological analyses demonstrated that Toledo, but not AD169, replicated in thymus-liver implants; therefore the SCID-hu system is an excellent model for the evaluation of pathogenesis determinants in the 15-kb region of Toledo that is absent in AD169.
FIG. 1.
Histology of HCMV-infected human thymus-liver implants. The human thymus-liver implants in SCID mice were uninfected (A and B) or inoculated with either AD169 (C and D), Toledo (E and F), or ToledoBAC (G and H). After 13 days, the tissues were collected, processed, and stained with hematoxylin and eosin. Intact T cells in the thymus lobes appeared dark blue (A to D) due to hematoxylin uptake by the nuclei, and necrotic areas appeared red (E to G) from eosin staining of protein. The right panel shows a higher magnification (×98) than the left panel (×19.6) of the area within the outlined blue box of the same section.
ToledoBAC retains its virulence and replicates in SCID-hu mice.
The HCMV BAC technology dramatically simplifies the generation of recombinant HCMV (20, 22, 44). A ToledoBAC strain that promises to facilitate the mutagenesis of the Toledo virus was constructed (26). However, this BAC construct has one potential drawback. A 5-kb fragment encompassing US2 through US8 was deleted from the Toledo genome in order to insert the BAC vector. While this region is dispensable for viral replication in vitro (17), whether it was required for viral growth and pathogenesis in vivo was not known. In addition, a 34-bp loxP site remained in the Toledo genome after the BAC vector was removed by Cre/lox recombination. Thus, it was crucial to test the ability of this mutant Toledo virus to replicate in vivo. We injected 2 × 105 PFU of ToledoBAC into eight thymus-liver xenografts in SCID mice. Thirteen days after inoculation, the infected tissues were harvested and then analyzed for the extent and histological effects of virus replication. Like with the wild-type Toledo virus, ToledoBAC virus growth caused T-cell depletion (Fig. 1G and H), and virus was recovered from all eight implants. Similar to those of its parent strain, ToledoBAC titers were high, ranging from 3 × 103 to 9 × 106 PFU/implant and averaging 3.5 × 106 PFU/implant (Fig. 5). This experiment indicated that ToledoBAC retained its virulence and replicated in vivo as well as the wild-type Toledo strain.
Construction and isolation of ToledoBAC mutants.
By homologous recombination in E. coli, the Toledo genome cloned in the BAC plasmid was manipulated to delete and restore the 15-kb segment, generating the ToledoΔ15kb and ToledoΔ15kbR viruses, respectively. To create ToledoΔ15kb, the 15-kb segment of ToledoBAC was exchanged for a bacterial expression cassette encoding zeocin resistance. In this mutant, ORFs UL137 through UL149 were deleted, UL136 and UL150 were partially deleted (Fig. 2A), and UL133, UL134, UL135, and UL151 were intact. The 15-kb deletion from ToledoΔ15kb was confirmed by PCR analysis, which showed the absence of UL136 and UL149 and the presence of UL133 and UL151 in the flanking regions (Fig. 2B). ToledoΔ15kb BAC DNA was then transfected into HFF cells to generate the ToledoΔ15kb virus. Similarly, Southern blot analysis showed that UL147 was present in both Toledo and ToledoBAC viruses but not in the ToledoΔ15kb virus (Fig. 2C). As a DNA-loading control, UL56 was detected in all three viruses, and a positive control for the zeocin marker showed that it was detected only in ToledoΔ15kb (Fig. 2C). To ensure that deletion of the 15-kb region did not disrupt gene expression in the flanking ORFs, mRNA synthesis from these genes was measured by using an HCMV microarray system, which we developed recently and is similar to the one described in reference 11. The yellow color of the spots for UL133, UL134, UL135, and UL151 indicated that these ORFs were expressed from both ToledoΔ15kb- and ToledoBAC-infected cells (Fig. 2D). Likewise, the green spots for UL136 through UL150 were further confirmation that these ORFs were not present in ToledoΔ15kb.
FIG. 2.
Construction of the ToledoΔ15kb clone. (A) The 15-kb fragment containing ORFs UL136 through UL150 (in the dashed-line box) was deleted from ToledoBAC and replaced by a zeocin resistance gene to generate ToledoΔ15kb BAC. (B) The parental ToledoBAC (B lanes) and ToledoΔ15kb (Δ lanes) clones were confirmed by PCR analysis. The specific ORFs (indicated above the lane designations) in the 15-kb region were amplified, and the PCR products were observed on an agarose gel. (C) The ToledoBAC (lane B), ToledoΔ15kb (lane Δ), and Toledo (lane T) strains were digested with EcoRI and run on an agarose gel. Southern blots were performed using 32P-labeled probes for UL56, UL147, and the zeocin resistance gene (Zeocin). (D) HFF cells were infected with the ToledoBAC and ToledoΔ15kb viruses. Total RNAs were isolated from the infected cells and used to synthesize cDNAs. ToledoBAC-infected cDNA was labeled with Cy3 (green), and ToledoΔ15kb-infected cDNA was labeled with Cy5 (red). Both cDNAs were mixed and used for hybridization in an HCMV microarray. A partial microarray image shows the levels of expression of the ORFs in the 15-kb region from both viruses. Yellow signals indicate that the RNAs are detected from both pools of virally infected cells, and green signals indicate that the RNAs were detected from ToledoBAC transcripts and not from ToledoΔ15kb.
To demonstrate that any growth defect of ToledoΔ15kb was a direct result of the 15-kb deletion and not spurious changes that occurred during recombinant virus construction, a rescue virus, ToledoΔ15kb, was generated by inserting the 15-kb segment back into the ToledoΔ15kb virus. This was accomplished in two steps, as shown in Fig. 3A and B. The first step was to insert a selectable marker between UL130 and UL149 into the 15-kb segment of ToledoBAC (Fig. 3A). This new plasmid, ToledoOK, was analyzed by PCR analysis, and the presence of the oriV/Kanr cassette was confirmed in ToledoOK but not in ToledoBAC (Fig. 3C, lane 1). The correct position of the cassette was verified when two primers outside the cassette amplified a 2.1-kb fragment from ToledoOK and only a 100-bp fragment from ToledoBAC (Fig. 3C, lane 2). This location was further verified by analyses of the left and right junctions by using PCR analysis. The left junction was confirmed by using a primer upstream of insertion and a primer within the insertion, which gave a 700-bp PCR product in ToledoOK (Fig. 3C, lane 3). With a similar assay, the right junction yielded a 600-bp fragment in ToledoOK (Fig. 3C, lane 4). Neither of these junction fragments was produced from ToledoBAC (Fig. 3C, lanes 3 and 4). PCR analyses also indicated that both adjacent ORFs, UL130 and UL149, were intact in ToledoOK (Fig. 3C, lanes 5 and 6).
The second step in constructing the rescue virus was to insert the 15-kb sequence from ToledoOK, now identifiable by kanamycin resistance and increased to 17 kb, back into ToledoΔ15kb. The 17-kb fragment containing the 15-kb sequence from wild-type Toledo and the oriV/Kanr cassette was purified and electroporated into the E. coli DY380 strain harboring ToledoΔ15kb, and the correct recombinant clones were selected and confirmed by PCR and Southern blot analyses. These assays showed that UL137, UL144, UL149, and UL150 (within the 15-kb segment) were deleted from ToledoΔ15kb but present in both ToledoBAC and ToledoΔ15kbR. Furthermore, UL136 (partially deleted in ToledoΔ15kb) and UL151 were present in all three viruses (Fig. 3D and E). The recombinant ToledoΔ15kbR DNA was transfected into HFF cells to produce the recombinant virus. A Cre-expressing plasmid was cotransfected with the BAC DNA so that the BAC vector and the oriV/Kanr marker in ToledoΔ15kbR were removed from the recombinant virus and the loxP sites were left in the dispensable regions of the virus. PCR and Southern analyses were used to verify that the vector and the marker were indeed removed from the viruses (data not shown). Deleting these extra sequences from ToledoΔ15kb and ToledoΔ15kbR genomes was important because they could make the virus grow more slowly.
The 15-kb segment is not required for Toledo growth in HFF cells.
To confirm that the process of constructing recombinant viruses did not impair their ability to propagate in cultured cells, the Toledo strains were purified and tested in HFF cells, and the titers for the strains were determined. The growth properties of ToledoΔ15kb, ToledoΔ15kbR, and the parental virus ToledoBAC were compared in single-step (MOI = 5) and multiple-step (MOI = 0.1) growth curve analyses. Both the growth kinetics and peak titers of ToledoBAC and ToledoΔ15kbR were similar (Fig. 4). In contrast, the results showed that at a high MOI, ToledoΔ15kb produced infectious virus 1 day later than the parental or rescued viruses, although their growth rates were similar (Fig. 4A). This defect was more pronounced when the viruses were inoculated at a low MOI. ToledoBAC and ToledoΔ15kbR entered exponential growth by 3 days after infection, whereas ToledoΔ15kb was still growing very slowly after 5 days. The yield of ToledoΔ15kb was also consistently 10-fold less than those of ToledoBAC and ToledoΔ15kbR, even after 17 days (Fig. 4B). These experiments indicated that although the 15-kb fragment containing ORFs UL137 through UL150 was not essential for Toledo replication in HFF cells, genes in this region promoted viral replication, because the deletion virus grew more slowly and had a lower yield than the parental virus. Importantly, this growth defect was probably not caused by mutations outside the 15-kb region, because the phenotype was completely reversed in the rescue virus.
The 15-kb segment is essential for Toledo replication in T-cell xenografts in SCID-hu mice.
Whether the genes located in the 15-kb segment are required for viral replication in vivo was examined by using the SCID-hu model. Purified ToledoΔ15kb and ToledoΔ15kbR viruses were inoculated into thymus-liver implants in SCID-hu mice. Thirteen days after inoculation, the infected tissues were collected, and the titers of the viruses in these tissues were determined. The results showed that none of the implants inoculated with ToledoΔ15kb virus yielded virus, while the rescue virus, ToledoΔ15kbR, replicated to high titers, averaging 106 PFU/implant (Fig. 5). This experiment demonstrates that the 15-kb fragment contains crucial genes for viral replication in vivo. Furthermore, it suggests that possible mutations (if any) outside of this 15-kb region were not responsible for the growth defect phenotype of ToledoΔ15kb.
DISCUSSION
In this study, we combined HCMV BAC technology, a highly efficient recombination system, and the SCID-hu virus replication model, and we demonstrated that the 15-kb region absent from AD169 is indeed a determinant of pathogenesis. By use of SCID-hu mice to study HCMV growth in vivo, the virulence of the parental ToledoBAC virus was confirmed for the first time. It was then possible to test the hypothesis that the 15-kb region found only in wild-type clinical isolates conferred the ability to replicate in vivo. After precise deletion and then restoration of the 15-kb region, the phenotypes of the mutant viruses were revealed in cultured cells and in the SCID-hu model. This combined approach was effective for evaluating the importance of HCMV genes in replication in human tissues.
The challenges of studying HCMV pathogenesis are highlighted by the different phenotypes of the ToledoΔ15kb virus in HFF cells and in the SCID-hu model. The deletion mutant virus showed only a slight growth defect in cultured cells, primarily at a low MOI, yet it was unable to replicate in thymus-liver implants in vivo. This work and that of others show how valuable the SCID-hu model is for testing HCMV virulence determinants, despite the complexity and limitations of the system (9, 19, 23). Possible reasons why HCMV replication is more restricted in thymus-liver implants than in HFF monolayers are that (i) there are many cell types and the permissive cells may not be in close contact, (ii) the cellular microenvironment is unique to the thymus-like organ, (iii) the cells are differentiated, and (iv) innate antiviral defenses may be active. For these reasons and more, HCMV requires a broader spectrum of its ORFs to replicate under the cellular and structural conditions found in thymus-liver implants.
Providing a challenging environment for HCMV replication is an advantage of the SCID-hu model and also exposes its limitations. Replication in thymus-liver implants does not mimic the pattern of HCMV disease in humans, a systemic infection that reaches every organ in the body (8). Thus, the complete range of tissue tropism, such as endothelial cells, smooth muscle cells, and macrophages, cannot be fully studied in the SCID-hu model, nor can latency and reactivation. Similarly, there is no acquired immune response to HCMV in the SCID-hu model, so postvaccine challenge is not feasible. These limitations with the model are shared with, and tolerated by, others who work on human-restricted viruses such as human immunodeficiency virus type 1, measles virus, and varicella-zoster virus (1, 6, 16, 24, 25, 40) because the SCID-hu mouse model is still the preferred system for determining their molecular basis of pathogenesis.
Cloning an entire CMV genome as a BAC offers significant advantages over previously used methods for creating recombinant viruses. This method allows for the rapid generation of different types of recombinant viruses and their rescue viruses (7, 20, 22, 44). In recent studies, BAC technology was applied to systematically mutate every ORF of HCMV and study its role in viral replication (13, 43). Approximately 40% of the ORFs are required for HCMV replication in HFF cells, and 60% are dispensable. The nonessential ORFs in HFF cells might be important for viral growth in other cell types in vivo and pathogenesis. The SCID-hu model provides a valuable system for testing many of these HCMV mutants and strains and for studying their functions.
Both the AD169 and Towne strains have been extensively cultured in fibroblasts, and many genetic alternations are expected to have occurred during the course of these passages. Any of these changes may contribute to the attenuation of these strains. Recently, the sequencing analyses of AD169, Towne, Toledo, and three other clinical isolates indicated there is substantial amino acid variation between the conserved ORFs of these strains (26). Therefore, it is difficult to predict which changes in AD169 and Towne contribute to attenuation. However, the 15-kb deletions from the AD169 and Towne strains are the most notable genetic difference between attenuated and virulent strains and are likely responsible for their attenuation. This assertion is supported by the results presented here, although other changes outside the 15-kb region may also contribute to attenuation.
Although the genes located in the 15-kb segment are not required for viral replication in HFF cells, the 15-kb deletion virus showed slowed growth kinetics at both high and low MOIs and produced about 10 times less virus at a low MOI. The defect is caused by the 15-kb deletion, because this mild defect is completely reversed when the 15-kb segment is inserted back into the deletion virus (Fig. 4). This result suggests that even though the genes in this region are not essential for viral replication in HFF cells, they play roles in enhancing viral growth in this system. On the other hand, deleting the 15-kb segment from Toledo made its genomic structure much more similar to those of AD169 and Towne, but Toledo grew slower than those strains. This result suggests that mutations outside the 15-kb segment, which arose during repeated passage in human fibroblasts, contribute to the fast-growth phenotypes for AD169 and Towne, while low-passage Toledo may not contain these mutations.
It is clear that although the genes located in the 15-kb region are crucial for viral replication in vivo, their precise functions are unknown. Several of these ORFs encode putative glycoproteins (10), which suggests a role in viral adsorption, tissue tropism, or viral dissemination. This region also encodes several other interesting proteins, including two α (CXC) chemokines (UL146 and UL147) and a member of the tumor necrosis factor receptor family (UL144) (33). UL146 encodes a 117-amino-acid glycoprotein that is secreted from infected cells and is a potent IL-8 homologue. Even when expressed in E. coli, the UL146 protein is a functional chemokine (28). This viral chemokine may allow HCMV to escape immune clearance in vivo, achieving more-robust infection and dissemination. Recently, three potential new ORFs, C-ORF24, C-ORF25, and C-ORF26, were also identified in this region (26). Some of these 18 ORFs likely play significant roles in the pathogenesis of the virus. We plan to use the same approach presented here to individually test these genes for their importance in HCMV replication in thymus-liver implants, with the ultimate goal of learning their functions in HCMV disease.
Acknowledgments
We thank T. Shenk for the ToledoBAC clone, pCMV-pp71, and advice, Neal Copeland and Craig Stranthdee for the DY380 strain, and L. Enquist for pGS403. P. Soteropoulos and S. Ghanny assisted with the HCMV microarray. Rebecca Greenblatt and Heidi Zapata assisted in the SCID-hu mouse experiments. C. Patterson critically read the manuscript.
This work was supported by NIH grants AI050709-01 (H.Z.) and AI052168-01 (J.F.M.), New Jersey Commission on Cancer Research grant 702021 (H.Z.), and UMDNJ Foundation grant 104727 (H.Z.).
REFERENCES
- 1.Auwaerter, P. G., H. Kaneshima, J. M. McCune, G. Wiegand, and D. E. Griffin. 1996. Measles virus infection of thymic epithelium in the SCID-hu mouse leads to thymocyte apoptosis. J. Virol. 70:3734-3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldick, C. J., Jr., A. Marchini, C. E. Patterson, and T. Shenk. 1997. Human cytomegalovirus tegument protein pp71 (ppUL82) enhances the infectivity of viral DNA and accelerates the infectious cycle. J. Virol. 71:4400-4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balfour, H. H., Jr., G. W. Sachs, P. Welo, R. C. Gehrz, R. L. Simmons, and J. S. Najarian. 1984. Cytomegalovirus vaccine in renal transplant candidates: progress report of a randomized, placebo-controlled, double-blind trial. Birth Defects Orig. Artic. Ser. 20:289-304. [PubMed] [Google Scholar]
- 4.Bidanset, D. J., R. J. Rybak, C. B. Hartline, and E. R. Kern. 2001. Replication of human cytomegalovirus in severe combined immunodeficient mice implanted with human retinal tissue. J. Infect. Dis. 184:192-195. [DOI] [PubMed] [Google Scholar]
- 5.Boehncke, W. H. 1999. The SCID-hu xenogeneic transplantation model: complex but telling. Arch. Dermatol. Res. 291:367-373. [DOI] [PubMed] [Google Scholar]
- 6.Bonyhadi, M. L., and H. Kaneshima. 1997. The SCID-hu mouse: an in vivo model for HIV-1 infection in humans. Mol. Med. Today 3:246-253. [DOI] [PubMed] [Google Scholar]
- 7.Borst, E.-M., G. Hahn, U. H. Koszinowski, and M. Messerle. 1999. Cloning of the human cytomegalovirus (HCMV) genome as an infectious bacterial artificial chromosome in Escherichia coli: a new approach for construction of HCMV mutants. J. Virol. 73:8320-8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Britt, W. J., and C. A. Alford. 1996. Cytomegalovirus, p. 2493-2523. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 9.Brown, J. M., H. Kaneshima, and E. S. Mocarski. 1995. Dramatic interstrain differences in the replication of human cytomegalovirus in SCID-hu mice. J. Infect. Dis. 171:1599-1603. [DOI] [PubMed] [Google Scholar]
- 10.Cha, T.-A., E. Tom, G. W. Kemble, G. M. Duke, E. S. Mocarski, and R. R. Spaete. 1996. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J. Virol. 70:78-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers, J., A. Angulo, D. Amaratunga, H. Guo, Y. Jiang, J. S. Wan, A. Bittner, K. Frueh, M. R. Jackson, P. A. Peterson, M. G. Erlander, and P. Ghazal. 1999. DNA microarrays of the complex human cytomegalovirus genome: profiling kinetic class with drug sensitivity of viral gene expression. J. Virol. 73:5757-5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiLoreto, D., Jr., L. G. Epstein, E. S. Lazar, W. J. Britt, and M. del Cerro. 1994. Cytomegalovirus infection of human retinal tissue: an in vivo model. Lab. Investig. 71:141-148. [PubMed] [Google Scholar]
- 13.Dunn, W., C. Chou, H. Li, R. Hai, D. Patterson, V. Stolc, H. Zhu, and F. Liu. 2003. Functional profiling of a human cytomegalovirus genome. Proc. Natl. Acad. Sci. USA 100:14223-14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elek, S. D., and H. Stern. 1974. Development of a vaccine against mental retardation caused by cytomegalovirus infection in utero. Lancet i:1-5. [DOI] [PubMed]
- 15.Fleisher, G. R., S. E. Starr, H. M. Friedman, and S. A. Plotkin. 1982. Vaccination of pediatric nurses with live attenuated cytomegalovirus. Am. J. Dis. Child. 136:294-296. [DOI] [PubMed] [Google Scholar]
- 16.Jamieson, B. D., G. M. Aldrovandi, and J. A. Zack. 1996. The SCID-hu mouse: an in-vivo model for HIV-1 pathogenesis and stem cell gene therapy for AIDS. Semin. Immunol. 8:215-221. [DOI] [PubMed] [Google Scholar]
- 17.Jones, T. R., and V. P. Muzithras. 1992. A cluster of dispensable genes within the human cytomegalovirus genome short component: IRS1, US1 through US5, and the US6 family. J. Virol. 66:2541-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Just, M., A. Buergin-Wolff, G. Emoedi, and R. Hernandez. 1975. Immunisation trials with live attenuated cytomegalovirus TOWNE 125. Infection 3:111-114. [DOI] [PubMed] [Google Scholar]
- 19.Kern, E. R., R. J. Rybak, C. B. Hartline, and D. J. Bidanset. 2001. Predictive efficacy of SCID-hu mouse models for treatment of human cytomegalovirus infections. Antivir. Chem. Chemother. 12:149-156. [PubMed] [Google Scholar]
- 20.Marchini, A., H. Liu, and H. Zhu. 2001. Human cytomegalovirus with IE-2 (UL122) deleted fails to express early lytic genes. J. Virol. 75:1870-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCune, J. M., R. Namikawa, H. Kaneshima, L. D. Shultz, M. Lieberman, and I. L. Weissman. 1988. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science 241:1632-1639. [DOI] [PubMed] [Google Scholar]
- 22.Messerle, M., I. Crnkovic, W. Hammerschmidt, H. Ziegler, and U. H. Koszinowski. 1997. Cloning and mutagenesis of a herpesvirus genome as an infectious bacterial artificial chromosome. Proc. Natl. Acad. Sci. USA 94:14759-14763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mocarski, E. S., M. Bonyhadi, S. Salimi, J. M. McCune, and H. Kaneshima. 1993. Human cytomegalovirus in a SCID-hu mouse: thymic epithelial cells are prominent targets of viral replication. Proc. Natl. Acad. Sci. USA 90:104-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moffat, J. F., M. D. Stein, H. Kaneshima, and A. M. Arvin. 1995. Tropism of varicella-zoster virus for human CD4+ and CD8+ T lymphocytes and epidermal cells in SCID-hu mice. J. Virol. 69:5236-5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moffat, J. F., L. Zerboni, P. R. Kinchington, C. Grose, H. Kaneshima, and A. M. Arvin. 1998. Attenuation of the vaccine Oka strain of varicella-zoster virus and role of glycoprotein C in alphaherpesvirus virulence demonstrated in the SCID-hu mouse. J. Virol. 72:965-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy, E., D. Yu, J. Grimwood, J. Schmutz, M. Dickson, M. A. Jarvis, G. Hahn, J. A. Nelson, R. M. Myers, and T. E. Shenk. 2003. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc. Natl. Acad. Sci. USA 100:14976-14981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pass, R. F. 2001. Cytomegalovirus, p. 2675-2705. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 28.Penfold, M. E., D. J. Dairaghi, G. M. Duke, N. Saederup, E. S. Mocarski, G. W. Kemble, and T. J. Schall. 1999. Cytomegalovirus encodes a potent alpha chemokine. Proc. Natl. Acad. Sci. USA 96:9839-9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plotkin, S. A., J. Farquhar, and E. Horberger. 1976. Clinical trials of immunization with the Towne 125 strain of human cytomegalovirus. J. Infect. Dis. 134:470-475. [DOI] [PubMed] [Google Scholar]
- 30.Plotkin, S. A., T. Furukawa, N. Zygraich, and C. Huygelen. 1975. Candidate cytomegalovirus strain for human vaccination. Infect. Immun. 12:521-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plotkin, S. A., and E. S. Huang. 1985. Cytomegalovirus vaccine virus (Towne strain) does not induce latency. J. Infect. Dis. 152:395-397. [DOI] [PubMed] [Google Scholar]
- 32.Plotkin, S. A., S. E. Starr, H. M. Friedman, E. Gonczol, and R. E. Weibel. 1989. Protective effects of Towne cytomegalovirus vaccine against low-passage cytomegalovirus administered as a challenge. J. Infect. Dis. 159:860-865. [DOI] [PubMed] [Google Scholar]
- 33.Prichard, M. N., M. E. Penfold, G. M. Duke, R. R. Spaete, and G. W. Kemble. 2001. A review of genetic differences between limited and extensively passaged human cytomegalovirus strains. Rev. Med. Virol. 11:191-200. [DOI] [PubMed] [Google Scholar]
- 34.Quinnan, G. V., Jr., M. Delery, A. H. Rook, W. R. Frederick, J. S. Epstein, J. F. Manischewitz, L. Jackson, K. M. Ramsey, K. Mittal, S. A. Plotkin, et al. 1984. Comparative virulence and immunogenicity of the Towne strain and a nonattenuated strain of cytomegalovirus. Ann. Intern. Med. 101:478-483. [DOI] [PubMed] [Google Scholar]
- 35.Soderberg-Naucler, C., K. N. Fish, and J. A. Nelson. 1997. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell 91:119-126. [DOI] [PubMed] [Google Scholar]
- 36.Soderberg-Naucler, C., and J. Y. Nelson. 1999. Human cytomegalovirus latency and reactivation—a delicate balance between the virus and its host's immune system. Intervirology 42:314-321. [DOI] [PubMed] [Google Scholar]
- 37.Staczek, J. 1990. Animal cytomegaloviruses. Microbiol. Rev. 54:247-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swaminathan, S., H. M. Ellis, L. S. Waters, D. Yu, E. C. Lee, D. L. Court, and S. K. Sharan. 2001. Rapid engineering of bacterial artificial chromosomes using oligonucleotides. Genesis 29:14-21. [DOI] [PubMed] [Google Scholar]
- 39.Ulger, C., G. A. Toruner, M. Alkan, M. Mohammed, S. Damani, J. Kang, A. Galante, H. Aviv, P. Soteropoulos, P. P. Tolias, M. N. Schwalb, and J. J. Dermody. 2003. Comprehensive genome-wide comparison of DNA and RNA level scan using microarray technology for identification of candidate cancer-related genes in the HL-60 cell line. Cancer Genet. Cytogenet. 147:28-35. [DOI] [PubMed] [Google Scholar]
- 40.Valsamakis, A., P. G. Auwaerter, B. K. Rima, H. Kaneshima, and D. E. Griffin. 1999. Altered virulence of vaccine strains of measles virus after prolonged replication in human tissue. J. Virol. 73:8791-8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang, W., C. E. Patterson, S. Yang, and H. Zhu. 2004. Coupling generation of cytomegalovirus deletion mutants and amplification of viral BAC clones. J. Virol. Methods 121:137-143. [DOI] [PubMed] [Google Scholar]
- 42.Yu, D., H. M. Ellis, E. C. Lee, N. A. Jenkins, N. G. Copeland, and D. L. Court. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 97:5978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu, D., M. C. Silva, and T. Shenk. 2003. Functional map of human cytomegalovirus AD169 defined by global mutational analysis. Proc. Natl. Acad. Sci. USA 100:12396-12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu, D., G. A. Smith, L. W. Enquist, and T. Shenk. 2002. Construction of a self-excisable bacterial artificial chromosome containing the human cytomegalovirus genome and mutagenesis of the diploid TRL/IRL13 gene. J. Virol. 76:2316-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]