Abstract
Phytochemical analysis of the methanolic and dichloromethane extracts of the aerial parts of Scutellaria pinnatifida led to the isolation of a phenylpropanoid, 1-o-feruloyl-β-D-glucose (1), two known flavonoids including luteolin-7-o-glucoside (2) and apigenin-7-o-glucoside (3), three known phenylethanoid glycosides composed of phlomisethanoside (4), syringalide A (5), and verbascoside (6), and oleic acid (7). Isolation and structural elucidation of compounds were accomplished by HPLC and spectroscopic methods (UV, 1H-NMR, 13C-NMR). The extracts were also evaluated for their radical scavenging activity and insecticidal property by 2,2-diphenyl-1-picryl-hydrazyl (DPPH) assay and contact toxicity method, respectively. Among the extracts, the methanol extract showed the most potent free radical scavenging activity with a RC50 value of 0.044 ± 0.350 mg/mL which could be attributed to the presence of the isolated phenolic compounds. In the case of insecticidal activity, the n-hexane extract displayed the most potent activity and caused 10%, 15%, and 40% mortality to Oryzaephilus mercator at the concentration of 5, 10, and 15 mg/mL after 4 h of exposure.
Keywords: Scutellaria pinnatifida, Phenylpropanoid, Antioxidant activity, Insecticidal activity
INTRODUCTION
Scutellaria pinnatifida (S. pinnatifida) A. Hamilt, commonly known as “skullcaps” is one of the 20 Iranian species of the genus Scutellaria L. (Lamiaceae family) and also has been extensively used in traditional Chinese medicine (1,2). The root of S. baicalensis, is best known candidate of this genus for its anti-inflammatory, antitumor, anticancer, antibacterial, and antioxidant properties, and its flavonoid content (2,3,4). Other species of this genus have also been used by many cultures for the treatment of hypertension, atherosclerosis, inflammatory diseases, hepatitis, cancer, allergy, and have shown sedative, antioxidant, antithrombotic, antimicrobial, neuroprotective, anxiolytic, insecticidal, and antiviral properties (5,6,7). Except for the reports on the chemical composition of the volatile oil from the aerial parts of S. pinnatifida and some biological activities (7,8), to the best of our knowledge, there is no report available to date on phytochemical investigation of this species. From other Scutellaria species, flavonoids especially bioflavonoids (9), iridoids (10), phenylethanoids (11), and diterpenoids (5) have been previously reported. As a part of our continuing studies on the Lamiaceae family and also for having valuable compounds in this genus, we report here the isolation and structure elucidation of a phenylpropanoid, 1-o-feruloyl-ß- D-glucose (1), two known flavonoids including luteolin-7-o-glucoside (2) and apigenin-7-o- glucoside (3), three known phenylethanoid glycosides composed of phlomisethanoside (4), syringalide A (5), and verbascoside (6), and oleic acid (7) as well as biological activities of different extracts of S. pinnatifida.
MATERIALS AND METHODS
Materials
2,2-Diphenyl-1-picrylhydrazyl (DPPH) and Trolox were purchased from Sigma-Aldrich (United Kingdom). All solvents used were procured from Caledon (Canada).
General experimental procedures
Reversed-phase preparative high pressure liquid chromatography (prep-HPLC) analyses were carried out on a HPLC system (Knauer, Germany), equipped with a Knauer PDA detector 2800 (Heraeus, Germany), using a reversed-phase Reprosil 100 C18 column (10 μm particle size, 250 × 20 mm i.d.) (Dr. Maisch, Germany).
The NMR spectroscopic analyses (one- dimensional, 13C spectra) were obtained on a Bruker 200 NMR spectrometer (Bruker, Germany) (200 MHz for 1H, and 50 MHz for 13C). Chemical shifts (δ, ppm) are reported relative to transcranial magnetic stimulation (TMS) as an internal standard. Sephadex LH20 (Amersham Biosciences, Sweden) used for column chromatography and Sep-pak cartridge (10 g, Waters, Ireland) used for fractionating of methanol (MeOH) extract. Thin layer chromatography (TLC) was performed on silica gel GF-254 plates (Merck, Germany) and spots were detected by ultraviolet (UV) illumination.
Plant material
The aerial parts of S. pinnatifida were collected from Payam region near Marand, East Azarbaijan province, Iran. A voucher specimen was identified and preserved (Tbz- Fph-704) in the herbarium of the School of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran.
Extraction and isolation
Air-dried and ground aerial parts of S. pinnatifida (100 g) were Soxhlet-extracted, successively, with n-hexane, dichloromethane (DCM), and MeOH (1.1 L each). All these extracts were separately concentrated using a rotary evaporator at a maximum temperature of 45 °C.
Two gram of the MeOH extract was subjected to solid-phase extraction (SPE) using a C18 Sep-pak cartridge, eluting with a step gradient of MeOH-water mixtures (10:90, 20:80, 40:60, 60:40, 80:20, and 100:0).
The SPE fraction eluted with 20% MeOH was analyzed by prep-HPLC using the mobile phase: 0-24 min, linear gradient of 20-40% MeOH in water; 24-36 min, maintained at 40% MeOH in water to isolate compounds 1 (4.7 mg, tR = 19.25 min), 2 (13.4 mg, tR = 21.63 min), and 3 (12.7 mg, tR = 27.31 min). Similar prep-HPLC analyses of the 40% methanolic SPE fraction (mobile phase: 0-24 min, linear gradient of 40-50% MeOH in water; 24-48 min, maintained in 50% MeOH in water) afforded compounds 4 (7.6 mg, tR = 22.35 min), 5 (4.0 mg, tR = 27.46 min), and 6 (4.7 mg, tR = 36.82 min).
In all above prep-HPLC analyses, the flow rate of the mobile phase was 8.0 mL/min. The structures of all compounds 1-6 were elucidated unequivocally by spectroscopic means and comparing with references. 2 g of the DCM extract was fractionated on a Sephadex LH-20 column chromatography eluted with MeOH with the flow rate of 2.0 mL/min and purified the fractions by TLC on silica gel (mobile phase, CHCl3:MeOH 8:2) yielded compound 7 (4.6 mg).
Free radical scavenging activity
The free radical scavenging activities of the extracts were assessed by the DPPH assay used by Takao, et al (12) with suitable modifications. DPPH (8 mg) was dissolved in MeOH (100 mL) to obtain a concentration of 80 μg/mL.
The methanolic extract of plants was dissolved in MeOH and the n-hexane and DCM extracts were dissolved in chloroform to obtain a concentration of 1 mg/mL. Serial dilutions were prepared to obtain concentrations of, 2.5 × 10-1, 1.25 × 10-1, 6.25 × 10-2, 3.13 × 10-2, 1.56 × 10-2, 7.81× 10-3, 3.91 × 10-3 and 1.95 × 10-3 mg/mL. Diluted solutions (1 mL each) were mixed with DPPH solution (1 mL) and allowed to develop for 30 min. The UV absorbance was measured at 517 nm.
The experiment was performed in triplicate and the average absorption was noted for each concentration. The same procedure was followed for the positive control, quercetin. Percent reduction of the free radical DPPH (R%) was calculated using following equation:
R% = [(AB–AA)/AB] × 100
where, AB is the absorbance of blank and AA is the absorbance of test samples. For calculating the RC50 values (concentration providing 50% reduction), the graph of reduction percentage against extract concentrations was utilized.
Insecticidal assay (contact toxicity method)
Adults of Oryzaephilus mercator (O. mercator) were collected from a laboratory culture. O. mercator was reared on a mixture of whole wheat and maize flour at the ratio 1:1 in glass containers containing 0.5 kg of the mixture.
All insect species were reared at 27 ± 2 °C, 12% moisture content in continuous darkness for about 3 weeks without exposing to insecticides.
Adults (1-3 week old) were used for contact toxicity insecticidal assay. 1, 5, 10, 15 mg of extracts were dissolved in 1 mL volatile organic solvent n-hexane and these solutions were then coated on the inner surface of 20 mL glass vial (four replicates for each concentration). Each glass vial was rotated by hand until the test solution was distributed on the vial inner wall and floor, and organic solvent was mostly evaporated.
When the solvent was completely evaporated, five O. mercator were placed carefully in each vial.
The survival of the insects was assessed after 4, 8, 24, 48 h. Controls consisted of O. mercator in vial, treated only with the carrier solvent (13).
RESULTS
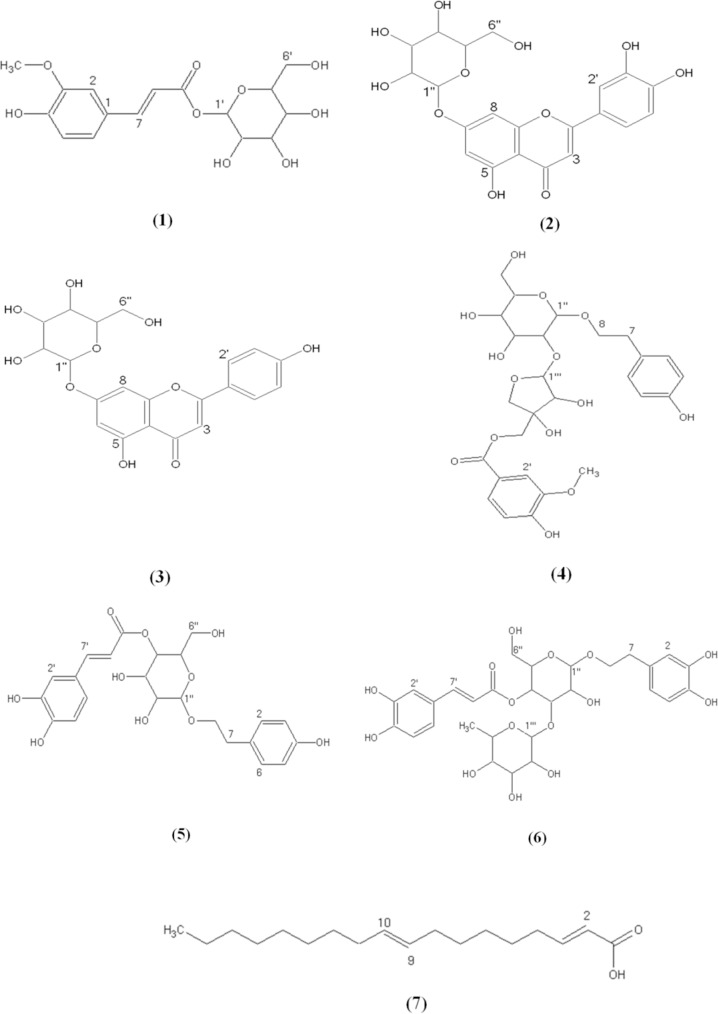
This study was planned to the isolation of 7 compounds from the aerial parts of S. pinnatifida, a phenylpropanoid, 1-o-feruloyl-ß- D-glucose (1), two known flavonoids: luteolin- 7-o-glucoside (2) and apigenin-7-o-glucoside (3), three known phenylethanoid glycosides: phlomisethanoside (4), syringalide A (5), and verbascoside (6), and oleic acid (7) (Fig. 1). The chemical structures of all isolated compounds were elucidated unequivocally through UV and NMR and also all spectroscopic data were in agreement with respective published data (14,15,16,17,18,19,20,21). The data of 1H-NMR and 13C-NMR of the compounds are given as follows:
Fig. 1.
Chemical structures of compounds from S. pinnatifida.
1-O-feruloyl-ß-D-glucose (1): amorphous solid (4.7 mg), 1H-NMR (200 MHz, CD3OD): δH 7.74 (1H, d, J = 15.8, H-7), 7.20 (1H, d, J = 2.0, H-2), 7.09 (1H, dd, J = 8.0, 2.0, H-6), 6.84 (1H, d, J = 8.0, H-5), 6.44 (1H, d, J = 15.8, H- 8), 5.60 (1H, d, J = 8.0, H-’), 3.90 (3H, s, OCH3), 3.30-3.90 (6H, overlapped, glucose protons). These data were in agreement with the published data (14).
Luteolin-7-o-ß-D-glucoside (2): yellow amorphous solid (13.4 mg), UV (MeOH) λmax = 255, 265,351 nm. 1H-NMR (200 MHz, CD3OD): δH 7.46 (1H, dd, J = 8.0, 2.0, H-6’), 7.43 (1H, d, J = 2.0, H-2’), 6.92 (1H, d, J = 8.0, H-5), 6.84 (1H, d, J = 2.0, H-8), 6.70 (1H, s, H-3), 6.51 (1H, d, J = 2.0, H-6), 5.07 (1H, d, J =6.0, H-1’’), 3.20-3.80 (6H, overlapped, glucose protons). 13C-NMR (50 MHz, CD3OD): δC 182.4 (C-4), 165.3 (C-7), 163.4 (C-2), 161.6 (C-5), 157.9 (C-9), 150.2 (C-4’), 145.4 (C-3’), 121.4 (C-’), 120.5 (C- 6’), 116.8 (C-5’), 114.1 (C-2’), 104.1 (C-10), 101.7 (C-1’’), 101.5 (C-3), 96.0 (C-6), 94.6 (C-8), 77.6 (C-5’’), 76.5 (C-3’’), 74.5 (C-2’’), 71.1 (C-4’’), 62.9 (C-6’’). These data are in agreement with the published data (15).
Apigenin-7-o-ß-D-glucoside (3): yellow amorphous solid (12.7 mg), UV (MeOH) λmax = 267, 330, 425 nm. 1H-NMR (200 MHz, CD3OD): δH 7.92 (2H, J = 8.0, H-3’, H-5), 6.95 (2H, d, J = 8.0, H-2’, H-6’), 6.87 (1H, d, J = 2.0, H-8), 6.62 (1H, s, H-3), 6.52 (1H, d, J = 2.0, H-6), 5.01 (1H, d, J = 5.8, H-1’’), 3.203.80 (6H, overlapped, glucose protons). 13C- NMR (50 MHz, CD3OD): δC 180.4 (C-4), 166.6 (C-5), 165.3 (C-7), 164.4 (C-2), 159.9 (C-9), 129.7 (C-2’, C-6’), 123.4 (C-1’), 117.0 (C-3’, C-5’), 109.1 (C-10), 101.5 (C-3), 101.5 (C-1’’), 96.1 (C-6), 94.8 (C-8), 78.6 (C-5’’), 76.6 (C-3’’), 74.5 (C-2’’), 73.5 (C-4’’) 62.6 (C-6’’). These data are in agreement with the published data (16).
Phlomisethanoside (4): pale yellow amorphous solid (7.6 mg), UV (MeOH) λmax = 220, 261, 287, 325 nm. 1H-NMR (200 MHz, CD3OD): δH 7.64 (1H, dd, J = 8.0,2.0, H-6), 7.56 (1H, d, J = 2.0, H-2), 6.92 (1H, d, J = 8.0, H-5), 6.86 (2H, d, J = 8.0, H-2, H-6), 6.64 (2H, d, J = 8.0, H-3, H-5), 5.38 (1H, s, H- 1’’’), 4.35, 4.42 (2H, d, J = 11.2, 2H-5’), 4.28 (1H, d, J = 7.8, H-1’’), 4.05 (1H, s, H- 2’’’), 3.78, 4.16 (2H, d, J = 9.9, 2H-4’’’), 3.85 (3H, s, OCH3), 3.61, 3.94 (2H, m, H-8), 3.20-3.90 (6H, overlapped, glucose protons), 2.80 (2H, t, J = 7.6, H-7), 13C-NMR (50 MHz, CD3OD): δC 166.6 (C-7), 155.1 (C-3’), 154.2 (C-4), 150.2 (C-4’), 129.4 (C-2, C-6), 129.2 (C-1), 124.0 (C-6’), 121.1(C-1’), 114.6 (C-3, C-5), 114.6 (C-5’), 112.4 (C-2’), 108.8 (C- 1’’’), 104.1 (C-1’’), 79.3 (C-3’’’), 78.7 (C- 3’’), 77.9 (C-5’’), 77.8 (C-2’’), 77.5 (C-2’’’), 76.4 (C-4’’’), 70.3 (C-8), 70.3 (C-4’’), 67.1 (C-5’’’), 61.3 (C-6’’), 35.0 (C-7). These data are consistant with the published data (17).
Syringalide A (5): yellow amorphous solid (4.0 mg), UV (MeOH) λmax = 210, 230, 265, 325 nm. 1H-NMR (200 MHz, CD3OD): δH 7.70 (1H, d, J = 15.9, H-7’), 6.95 (1H, d, J = 2.0, H-2’), 6.83 (1H, d, J = 8.0, H6’), 6.79 (2H, d, J = 8.0, H-2, H-6), 6.62 (1H, dd, J = 8.0, 2.0, H-5’), 6.33(1H, d, , J = 15.9, H-8’), 6.33 (2H, d, J = 8.0, H-3, H-5), 4.40 (1H, d, J = 7.2, H-1’’), 3.31, 3.71 (2H, m, H-8), 3.203.90 (6H, overlapped, glucose protons), 2.82 (2H, t, J = 7.5, H-7), 13C-NMR (50 MHz, CD3OD): δC 162.8 (C-9’), 153.8 (C-4), 148.7 (C-3’), 148.1 (C-4’), 147.7 (C-7’), 130.94 (C- 2, C-6), 130.4 (C-1), 121.2 (C-6’), 121.1(C- 1’), 116.5 (C-5’), 116.3 (C-3, C-5), 115.1 (C- 8’), 110.9 (C-2’), 103.1 (C-1’’), 77.8 (C-3’’), 76.2 (C-5’’), 75.4 (C-2’’), 72.3 (C-8), 71.7 (C- 4’’), 62.7 (C-6’’), 36.5 (C-7), These data are in agreement with the published data (18,19).
Verbascoside (6): yellow amorphous solid (4.7 mg), UV (MeOH) λmax = 215, 245, 285, 330 nm. 1H-NMR (200 MHz, CD3OD): δH 7.44 (1H, d, J = 16.1, H-7’), 7.06 (1H, d, J = 2.0, H-2’), 6.98 (1H, dd, J = 8.0, 2.0, H-6’), 6.80 (1H, d, J = 8.0, H-5’), 6.70 (1H, d, J = 2.0, H-2), 6.66 (1H, d, J = 8.0, H-5), 6.59 (1H, dd, J = 8.0, 2.0, H-6), 5.19 (1H, bs, H-1’’’), 4.41 (1H, d, J = 8.0, H-1’’), 4.05, 3.86 (2H, m, J = 9.0, H-8), 3.20-4.95 (6H, overlapped, glucose protons), 3.20-3.90 (4H, overlapped, rhamnose protons), 2.80 (2H, t, J = 8.3, H-7), 1.11 (3H, d, J = 6.1, H-6’’’). These data are in line with the published data (20).
Oleic acid (7): pale yellow oily liquid (4.6 mg). 1H-NMR (200 MHz, CD3OD): δH 5.38 (2H, m, Olefinic protons), 2.31 (2H, t, J = 8.0, H-2), 2.04 (4H, m, H-8, H-11), 1.64 (2H, m, H-3), 1.20-1.40 (32H, m, H-4, H-5, H-6, H-7, H-12, H-13, H-14, H-15, H-16, H-17), 0.87 (3H, t, H-18). 13C-NMR (50 MHz, CD3OD): δC 178.0 (C-1), 129.8 (C-10), 129.1 (C-9), 34.9 (C-2), 26.0-31.0 (C-4, C-5, C-6, C-7, C-8, C-11, C-12, C-13, C-14, C-15, C-16), 24.9 (C- 3), 22.3 (C-17), 0.86 (C-18). These data are in line with the published data (21).
Antioxidant activity of S. pinnatifida extracts
The results of inhibiting free radicals obtained from extracts of S. pinnatifida are given in Table 1.
Table 1.
Free radical scavenging activity of the extracts of S. pinnatifida determined by DPPH assay.
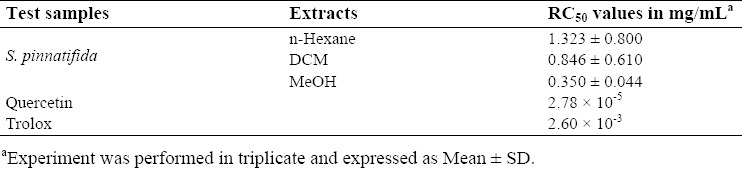
RC50 values of MeOH, DCM, and n-hexane extracts were 0.044 ± 0.350, 0.846 ± 0.610, and 1.323 ± 0.800 mg/mL, respectively in comparison with the RC50 values of quercetin as a positive control which was 2.78 × 10-5 mg/mL.
Insecticidal activity of aerial parts of S. pinnatifida extracts
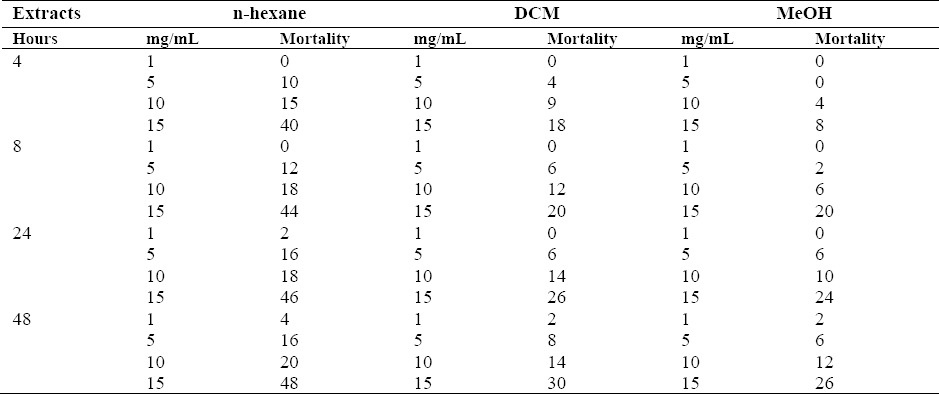
The results are shown in Table 2; amongst the extracts, the n-hexane extract displayed the most potent activity and caused 10%, 15%, and 40% mortality to O. mercator at the dose of 5, 10, 15 mg/mL after 4 h of exposure.
Table 2.
Percent mortality of Oryzeaphilus mercator exposed to 1 mL of extracts of S. pinnatifida at concentrations of 1, 5, 10, 15 mg/mL after 4, 8, 24 and 48 h.
DISCUSSION
A combination of SPE and reversed-phase prep-HPLC analyses of the methanolic extract and also using Sephadex LH-20 column chromatography of the aerial parts of S. pinnatifida led to the characterization of a phenylpropanoid: 1-o-feruloyl-ß-D-glucose (1) (14), two known flavonoids: luteolin-7-o- glucoside (2) (15) and apigenin-7-o-glucoside (3) (16), three known phenylethanoid glycosides: phlomisethanoside (4) (17), syringalide A (5) (18,19), and verbascoside (6) (20), and oleic acid (7) (21), respectively. The 1H-NMR spectrum of compound (1) displayed signals for three aromatic methine protons at δH 7.20 (1H, d, J = 2.0, H-2), 6.84 (1H, d, J = 8.0, H-5), 7.09 (1H, dd, J = 8.0, 2.0, H-6) suggesting the presence of a 1, 3, 4 tri substituted phenyl moiety, two trans-olefinic protons at δH 7.74 (1H, d, J = 15.8, H-7), 6.44 (1H, d, J = 15.8, H-8) indicating the presence of an α, ß-unsaturated carbonyl functionality, a singlet proton resonance at 3.90 (3H, s) exhibiting the presence of a methoxy group and a doublet proton resonance at 5.60 (1H, d, J = 8.0, H-1’) was readily assigned to the anomeric proton of a glucose moiety, indicating the monoglycosidic structure in (1). As a result, the structure of (1) was identified as a phenylpropanoid, 1-o-feruloyl-ß-D- glucose which was previously isolated as an antioxidant agent from the fruits of Luffa cylindrica (L.) (14). But to the best of our knowledge, this is the first report on the isolation of this compound from genus of Scutellaria. In the case of compounds (2) and (3), UV spectra were identical with flavone monoglucoside (22), which was supported by 1H-NMR and 13C-NMR spectrums, showing characteristic signals appeared at δH 6.70 (1H, s, H-3), 6.62 (1H, s, H-3), indicating the chromophore are flavones, luteolin, and apigenin, respectively. A literature survey indicated that luteolin-7-o-ß-D-glucoside (2) and apigenin-7-o-ß-D-glucoside (3) have previously been isolated from Scutellaria genus (23,24). The UV spectroscopic data of compound (4) revealed its phenolic nature. The 1H-NMR data of (4) showed proton resonances ascribed to the acyl group characterized as three signals appearing at δH 7.56 (1H, d, J = 2.0, H-2’), 6.92 (1H, d, J = 8.0, H-5’), and 7.64 (1H, dd, J = 8.0,2.0, H-6’) that suggested the presence of a trisubstituted phenyl moiety in the structure of (4). Additionally, the presence of a methoxyl moiety 3.85, s) and related carbon signals indicated a vanilloyl (4’-hydroxy-3’-methoxy- benzoic acid) moiety as a part of structure. Furthermore, the 1H-NMR of (4) suggested the presence of an aromatic A2X2 system with two doublets with ortho coupling constants (J = 8.0 Hz) at δH 6.86 (2H, d, J = 8.0, H-2, H-6) and 6.64 (2H, d, J = 8.0, H-3, H-5). According to the chemical shifts and splitting patterns of the sugar protons 5.38, s; δH 4.28, d, J = 7.8), the two sugar moieties were indicated as apiose and glucose, respectively. Based on the above evidences and literature survey (17), compound (4) was established to be a vanillic acid ester phenylethanoid glycoside, named phlomisethanoside and the spectrum of 13C- NMR also confirmed this estimated structure. This compound was previously reported from the Phlomis species (17), however, it was the first time to be isolated from the genus Scutellaria. In the case of compound (5), the 1H-NMR spectrum indicated the presence of a trisubstituted phenyl moiety that characterized by three signals appearing at δH 6.95 (1H, d, J = 2.0, H-2’), 6.83 (1H, dd, J = 8.0, 2.0, H- 6’), 6.62 (1H, d, J = 8.0, H-5’), a p-hydroxyphenethyl alcohol moiety with proton resonances at δH 6.79 (2H, d, J = 8.0, H-2, H- 6), 6.33 (2H, d, J = 8.0, H-3, H-5), 2.82 (2H, t, J = 7.5, H-7), 3.31, 3.71 (2H, m, H-8) and two olefinic protons (δH 7.70, d, J = 15.9; δH 6.33, d, J = 15.9, AX system) ascribable to H-7’ and H-8’ of the caffeic acid derivative. Moreover, one anomeric proton signal was observed at δH 4.40 (1H, d, J = 7.2, H-1’’) which was consistent with the β- glucopyranose unit. The related anomeric carbon resonances was at δC 103.1. Based on these results, the structure of (5) was identified as a phenylethanoid, syringalide A, whose spectral data were consistent with the literature (18,19). Syringalide A was previously isolated from the leaves of Syringa species (Oleaceae family) but, to the best of our knowledge, this is the first report on the occurrence of this compound in the genus Scutellaria. Compound (6) was identified on the basis of its 1H-NMR and 13C- NMR data to be a well-known phenylethanoid, verbascoside (acteoside). A comparison of the spectroscopic data with previous published data (20) confirmed the estimated structure. This compound was previously reported from Scutellaria genus (25,26), however, it is the first time to be obtained from this species. The structure of compound (7) was identified by NMR comparison with published data (21). As a wide-spread fatty acid, oleic acid was previously reported from Scutellaria species (27). Furthermore, free radical scavenging activity of the corresponding extracts was evaluated in vitro by the DPPH assay. The RC50 values of all extracts are presented in Table 1. The DPPH-scavenging capacity of the extracts was compared with known antioxidants, quercetin, and trolox as positive controls. Among the extracts, the methanolic extract showed the most potent free-radical- scavenging activity with a RC50 value of 0.044 ± 0.350 mg/mL which could be attributed to the presence of the isolated phenolics. Phenylpropanoids, phenylethanoids, and flavones exhibited potent antioxidant activities in various studies (5,28). Both DCM and n- hexane extracts showed low potency in this assay which may be explained by deficiency of hydrogen donating components. The insecticidal property of the extracts of S. pinnatifida has been evaluated by the assay described by Freedman B (1982) (13). Among the extracts, n-hexane extract displayed the most potent activity and caused 10%, 15%, 40% mortality to O. mercator at the dose of 5, 10, 15 mg/mL after 4 h of exposure. Over 48% mortality at 2 days after treatment was achieved at the dose of 15 mg/mL of n-hexane extract. Responses varied according to the concentration of extracts and exposure time and it was concentration dependent. Previous researches demonstrated that most of the lipophilic compounds can penetrate into insects’ membranes rapidly and disturb their vital physiological functions (29). Therefore, the possibility of the higher insecticidal activity exhibited by n-hexane extract reported here would be due to the presence of these types of lipophilic compounds.
CONCLUSION
In summary, the results of the present study revealed the isolation of six compounds from the methanolic extract of S. pinnatifida including 1-o-feruloyl-ß-D- glucose, phlomisethanoside, syringalide A and verbascoside, luteolin-7-o-glucoside and apigenin-7-o-glucoside of which 1-o- feruloyl-ß-D-glucose, phlomisethanoside and syringalide A have not been reported previously in Scutellaria genus. Free radical scavenging activity of methanolic extract is attributed to presence of phenolic compound identified in this study and indicates good medicinal potentials of this which requires further biological and pharmacological studies. Furthermore, the pure compounds of the n- hexane extract which exhibited high insecticidal activity could be isolated and evaluated for their biological activities.
ACKNOWLEDGMENTS
The authors would like to acknowledge Atefeh Ebrahimi for identification of plant materials.
REFERENCES
- 1.Mozaffarian V. Dictionary of Iranian plant names: Latin-English-Persian. Farhang Mo’aser. 4th ed. Tehran: 1996. p. 498. [Google Scholar]
- 2.Rechinger Kh. Flora Iranica. 7th ed. Graze, Austria: verlagsanstalt; 1982. p. 75. [Google Scholar]
- 3.Zhang Y, Wang X, Wang X, Xu Z, Liu Z, Ni Q, et al. Protective effect of flavonoids from Scutellaria baicalensis Georgi on cerebral ischemia injury. J. Ethnopharmacol. 2006;108(3):355–360. doi: 10.1016/j.jep.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 4.Du Z, Wang K, Tao Y, Chen L, Qiu F. Purification of baicalin and wogonoside from Scutellaria baicalensis extracts by macroporous resin adsorption chromatography. J. Chromatogr B Analyt Technol Biomed Life Sci. 2012;908:143–149. doi: 10.1016/j.jchromb.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 5.Nie XP, Qu GW, Yue XD, Li GS, Dai SJ. Scutelinquanines A–C, three new cytotoxic neo- clerodane diterpenoid from Scutellaria barbata. Phytochem Lett. 2010;3(4):190–193. [Google Scholar]
- 6.Shang X, He X, He X, Li M, Zhang R, Fan P, et al. The genus Scutellaria an ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2010;128(2):279–313. doi: 10.1016/j.jep.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Sauvage S, Granger M, Samson E, Majumdar A, Nigam P, Nahar L, et al. Assessment of free-radical- scavenging and antibacterial activities, and brine shrimp toxicity of Scutellaria pinnatifida (Lamiaceae) Orient Pharm Exp Med. 2010;10:304–309. [Google Scholar]
- 8.Ghannadi A, Mehregan I. Essential oil of one of the Iranian skullcaps. Z Naturforsch. 2003;58(5-6):316–318. doi: 10.1515/znc-2003-5-604. [DOI] [PubMed] [Google Scholar]
- 9.Liu G, Rajesh N, Wang X, Zhang M, Wu Q, Li S, et al. Identification of flavonoids in the stems and leaves of Scutellaria baicalensis Georgi. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(13-14):1023–1028. doi: 10.1016/j.jchromb.2011.02.050. [DOI] [PubMed] [Google Scholar]
- 10.Gousiadou C, Karioti A, Heilmann J, Skaltsa H. Iridoids from Scutellaria albida ssp. Phytochemistry. 2007;68(13):1799–1804. doi: 10.1016/j.phytochem.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Ersoz T, Tasdemir D, Calis I, Ireland CM. Phenylethanoid glycosides from Scutellaria galericulata. Turk J Chem. 2002;26(4):465–471. [Google Scholar]
- 12.Takao T, Kitatani F, Watanabe N, Yagi A, Sakata K. A simple screening method for antioxidants and isolation of several method for antioxidants and isolation of several antioxidants produced by marine from fish and shellfish. Biosci Biotechnol Biochem. 1994;58(10):1780–1783. [Google Scholar]
- 13.Freedman B, Mikolajczak KL, Smith CR, Jr, Kwolek WF, Burkholder WE. Olfactory and aggregation responses of Oryzaephilus surinamensis (L.) to extracts from oats. J Stored Prod Res. 1982;18(2):75–82. [Google Scholar]
- 14.Du Q, Wang K. Preparative separation of phenolic constituents in the fruits of Luffa cylindrica (L.) roem using slow rotary countercurrent chromatography. J Liq Chromatogr Relat Technol. 2007;30(13):1915–1922. [Google Scholar]
- 15.Chiruvella KK, Mohammad A, Dampuri G, Ghanta RG, Raghavan SC. Phytochemical and antimicrobial studies of methyl angolensate and luteolin-7-O- glucoside isolated from callus cultures of Soymida febrífuga. Intl J Biomed Sci. 2007;3(4):269–278. [PMC free article] [PubMed] [Google Scholar]
- 16.Norbak R, Nielsen JK, Kondo T. Flavonoids from flowers of two Crocus chrysanthus-biflorus cultivars: “Eye-catcher” and “Spring Pearl” (Iridaceae) Phytochem. 1999;51(8):1139–1146. [Google Scholar]
- 17.Takeda Y, kinugawa M, Masuda T, Honda G, Otsuka H, Sezik E, et al. Phlomisethanoside, a phenylethanoid glycoside from Phlomis grandiflora var. grandiflora. Phytochem. 1999;51(2):323–325. [Google Scholar]
- 18.Kikuchi M, Yamauchi Y, Tanabe F. Studies on the constituents of Syringa species. III. Isolation and structures of acylated glycosides from the leaves of Syringa reticulata (BLUME) HARA. Yakugaku Zasshi. 1987;107(5):350–354. [Google Scholar]
- 19.Li Q, Li SC, Li H, Cai MS, Li ZJ. Total synthesis of syringalide B, a phenylpropanoid glycoside. Carbohydr Res. 2005;340(9):1601–1604. doi: 10.1016/j.carres.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Tsao R, Liu Z, Liu S, Yang R, Young JC, et al. Isolation and purification of acteoside and isoacteoside from Plantago psyllium L. by highspeed counter-current chromatography. J Chromatogr A. 2005;1063(1-2):161–169. doi: 10.1016/j.chroma.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 21.SDBS 2014. Spectral Database for Organic Compounds, National Institute of Advanced Industrial Science and Technology (AIST) Available from: URL: http://sdbs.db.aist.go.jp/sdbs/cgibin/direct_frame_top.cgi .
- 22.Mabry T, Markham KR, Thomas MB. The systematic identification of flavonids. New York: Springer; 1970. p. 82. Heidelberg. Berlin. [Google Scholar]
- 23.Eshbakova K, Toshmatov ZO, Yili A, Aisa HA, Abdullaev ND. Flavonoid galacturonides and glucoronide from the aerial part of Scutellaria schachristanica. Chem Nat Comp. 2013;49(1):103–105. [Google Scholar]
- 24.Nurul Islam M, Downey F, Y.Ng CK. Comprehensive profiling of flavonoids in Scutellaria incana L. using LC-Q-TOF-MS. Acta Chromatogr. 2013;25(3):1–24. [Google Scholar]
- 25.Calis I, Saracoglu I, Basaran AA, Sticher O. Two phenethyl alcohol glycosides from Scutellaria orientalis subsp. Pinnatifida. Phytochemistry. 2002;26(4):581–588. doi: 10.1016/0031-9422(93)85194-v. [DOI] [PubMed] [Google Scholar]
- 26.Ersoz T, Harput US, Saracoglu I, Calis I, Ogihara Y. Phenolic compounds from Scutellaria pontica. Turk J Chem. 2002;26(4):581–588. [Google Scholar]
- 27.Mamadalieva N, Vinciguerra V, Ovidi E, Tiezzi A. Identification and isolation of non-polar compounds from the chloroform extract of Scutellaria ramosissima. Nat Prod Res. 2013;27(21):2059–2062. doi: 10.1080/14786419.2013.819508. [DOI] [PubMed] [Google Scholar]
- 28.Choudhary MI, Begum A, Abbaskhan A, Musharraf SG, Ejaz A, Atta-ur-Rahman Two new antioxidant phenylpropanoids from Lindelofia stylosa. Chem Biodivers. 2008;5(12):2676–2683. doi: 10.1002/cbdv.200890221. [DOI] [PubMed] [Google Scholar]
- 29.Lee S, Peterson CJ, Coats JR. Fumigation toxicity of monoterpenoids to several stored product insects. J Stored Prod Res. 2002;39(1):77–85. [Google Scholar]