Key Points
Erythrocytes suppress neutrophil activation and apoptosis in whole blood.
Sialoglycoproteins on the erythrocyte surface regulate neutrophils through Siglec-9.
Abstract
Healthy blood neutrophils are functionally quiescent in the bloodstream, have a short lifespan, and exit the circulation to carry out innate immune functions, or undergo rapid apoptosis and macrophage-mediated clearance to mitigate host tissue damage. Limitation of unnecessary intravascular neutrophil activation is also important to prevent serious inflammatory pathologies. Because neutrophils become easily activated after purification, we carried out ex vivo comparisons with neutrophils maintained in whole blood. We found a difference in activation state, with purified neutrophils showing signs of increased reactivity: shedding of l-selectin, CD11b upregulation, increased oxidative burst, and faster progression to apoptosis. We discovered that erythrocytes suppressed neutrophil activation ex vivo and in vitro, including reduced l-selectin shedding, oxidative burst, chemotaxis, neutrophil extracellular trap formation, bacterial killing, and induction of apoptosis. Selective and specific modification of sialic acid side chains on erythrocyte surfaces with mild sodium metaperiodate oxidation followed by aldehyde quenching with 4-methyl-3-thiosemicarbazide reduced neutrophil binding to erythrocytes and restored neutrophil activation. By enzyme-linked immunosorbent assay and immunofluorescence, we found that glycophorin A, the most abundant sialoglycoprotein on erythrocytes, engaged neutrophil Siglec-9, a sialic acid–recognizing receptor known to dampen innate immune cell activation. These studies demonstrate a previously unsuspected role for erythrocytes in suppressing neutrophils ex vivo and in vitro and help explain why neutrophils become easily activated after separation from whole blood. We propose that a sialic acid–based “self-associated molecular pattern” on erythrocytes also helps maintain neutrophil quiescence in the bloodstream. Our findings may be relevant to some prior experimental and clinical studies of neutrophils.
Introduction
Erythrocytes comprise almost ∼50% of circulating human blood volume and mediate oxygen and carbon dioxide transport.1 They are abundant, accessible, structurally and functionally simple, and thus one of the best-studied cell types. Erythrocytes are not known to influence inflammation, except when damage releases their inflammatory internal contents.2,3 Notably, erythrocytes outnumber leukocytes in the bloodstream by as much as 1000:1, and the surfaces of these two cell types are in close association. Here, we describe an unexpected and potentially important immune-regulatory interaction of erythrocytes with neutrophils studied in whole blood ex vivo, and in vitro, following standard purification.
Neutrophils are the most abundant circulating leukocyte in humans and first responders against extracellular pathogens.4 In comparison with other leukocytes, neutrophils have a short circulating half-life, even in the absence of infection or inflammation.5,6 Healthy blood neutrophils transmigrate out of the circulation, return to the bone marrow to undergo apoptosis and macrophage-mediated clearance, or both.5,7,8 Neutrophils respond rapidly to injury or infection by chemotaxis. Activated neutrophils have elevated antimicrobial capacity, manifested through enhanced phagocytosis, reactive oxygen species (ROS) production, degranulation, or the formation of neutrophil extracellular traps (NETs) against invading pathogens. These activities limit pathogen access to sterile sites, including the bloodstream.9 Following activation, neutrophils quickly undergo apoptosis and are subsequently phagocytized by macrophages for resolution of inflammation.10 Inappropriate neutrophil activation within the circulation or during clearance after an inflammatory response can cause or aggravate disease states,11 including septic shock,12 adult respiratory distress syndrome,13 and myocardial infarction.13
Neutrophils remain functionally quiescent in healthy circulation, and their activation is assumed to require priming by factors outside the bloodstream.14 Although extravascular stimuli are undoubtedly important, studies of neutrophils ex vivo also suggest functional deterioration and short lifespan after their purification, even when great care is taken to maintain sterility. This phenomenon is typically thought to reflect the normal biology of short-lived neutrophils.6 Here, we propose a mechanism that normally prevents neutrophil activation in circulating blood: loss of a critical inhibitor of activation occurs when neutrophils exit the circulation in response to chemotactic signals or during purification of neutrophils in vitro.
To identify this hypothesized critical inhibitor, we developed a method to directly compare neutrophils maintained in whole blood, with conventionally purified neutrophils from the same samples, and found differences in activation status and apoptosis. We found that neutrophil activation in blood is suppressed by erythrocyte sialoglycoproteins interacting via sialic acids with the inhibitory receptor Siglec-9 on their cell surface. Although amenable only to definitive analysis ex vivo in whole blood, this unexpected anti-inflammatory role for erythrocytes could potentially influence neutrophil inflammatory and innate immune phenotypes in vivo.
Materials and methods
New methods developed for this work are described below. Standard procedures are detailed in the supplemental Methods, available on the Blood Web site.
Cell surface markers on whole blood versus purified neutrophils
Freshly collected heparinized whole blood was probed at 4°C for cell surface activation markers anti-CD66b-allophycocyanin (BD Biosciences catalog no. 555724, Research Resource Identification [RRID]:AB_396067) or isotype control (BD Biosciences catalog no. 555583, RRID:AB_395959); anti-CD11b fluorescein isothiocyanate (FITC) (Tonbo Biosciences catalog no. 70-0112-OWL-A11503, RRID:AB_2621483) or rat immunoglobulin G2b (IgG2b), κ isotype control; or anti-CD62L-FITC (BioLegend catalog no. 304802, RRID:AB_314462) or mouse IgG1, κ isotype control, all at the manufacturers’ recommended final dilutions. After 30 minutes of labeling, samples were fixed in situ by adding 4% weight-to-volume ratio buffered formalin (Fisher Chemical) on ice for 20 minutes. Erythrocytes were lysed using ammonium chloride-potassium lysing buffer (Gibco, Life Sciences) at room temperature (RT) for 5 minutes and resuspended in fluorescence-activated cell sorter (FACS) buffer (1× Gibco Dulbecco's phosphate-buffered saline [DPBS], 1% bovine serum albumin, 10 mM EDTA, and 0.09% sodium azide). Neutrophils from the same samples of blood were also purified using various standardized techniques, washed, labeled as above, and resuspended in FACS buffer. Flow cytometry was performed using BD FACsCalibur or BD FACSCantoII.
Sialic acid side chain modification
Washed erythrocytes were aliquoted at 2 × 109/mL for treatment with DPBS, 4-methyl-3-thiosemicarbazide (MTSC), or NaIO4/MTSC. Sialic acid side chains were oxidized with 2 mM of NaIO4 dissolved in DPBS (Gibco) for 20 minutes on ice in the dark,15,16 and the resulting aldehydes were blocked using either 100 mM FITC-thiosemicarbazide (FTSC)16 or 100 mM of MTSC for 1 hour at 37°C. Modified or sham-treated erythrocytes were washed 3 times or until supernatant was clear, with DPBS and pelleted gently at 1000g with centrifuge deceleration 5.
Whole blood and buffy coat smear staining, immunofluorescence microscopy, and confocal microscopy
Whole blood and buffy coat smears were made using 5 μL each and were air-dried and fixed in 95% ethanol at −20°C for 15 minutes. Slides were left to air-dry and then washed in phosphate-buffered saline with Tween 20 (PBST). To rehydrate, we incubated slides in 1% bovine serum albumin in PBST overnight at 4°C and then stained them with 10 μg/mL of antiglycophorin A-FITC (eBioscience catalog no. 11-9886-42, RRID:AB_10668830) and 10 μg/mL anti-Siglec-9-bioitinylated (R&D Systems catalog no. BAF1139, RRID:AB_355864) in the dark overnight at 4°C. Smears were washed with PBST and stained with secondary antibody streptavidin Alexafluor594 at 1:500 for 2 hours at RT. Isotype controls were stained with streptavidin-phycoerythrin (PE) and mouse IgG1-FITC. All slides were washed with PBST and stained with 1% Hoechst for 15 minutes in the dark at RT. After a final PBST wash, slides were mounted with Prolong Gold Antifade Reagent with 4′,6-diamidino-2-phenylindole (Molecular Probes) and cover-slipped. Smears were stained in duplicate, and at least 5 images of Siglec-9 positive neutrophils were captured from 3 independent experiments. Digital photomicrographs were taken using a Keyence BZ9000 fluorescence microscope (BIOREVO, BZ-9000, Keyence). Confocal microscopy images were acquired by using a Leica CTR4000 Confocal Microscope with a ×63 objective. Z-stack images were obtained by imaging approximately 6-μm-thick sections of cells in all channels. Red-green-blue graphic profiles were created by analyzing the distribution and intensity of pixels of these colors along a chosen line using ImageJ software.
Results
Neutrophils are activated during purification, shedding l-selectin, and upregulating CD11b integrin
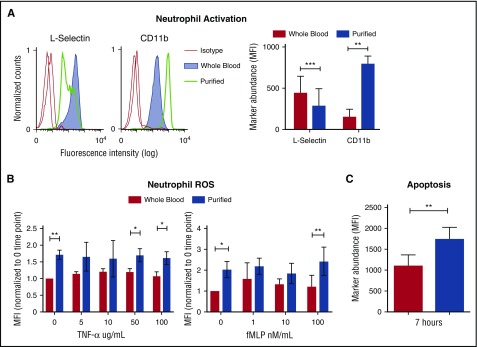
Neutrophils were studied from freshly drawn heparinized human venous whole blood. To avoid accidental activation and unnecessary manipulation, we developed a simple method to directly analyze 3 neutrophil cell surface markers (CD66b, l-selectin, and CD11b).17 Corresponding antibodies were added directly to anticoagulated whole blood, incubated briefly, and fixed immediately in situ, a process that conveniently lysed erythrocytes facilitating flow cytometry analysis. This simple approach allowed us to directly measure the natural state of neutrophils collected ex vivo for comparison with neutrophils isolated in parallel from autologous blood samples using a standard Polymorphprep gradient centrifugation method.18 Neutrophils were gated by forward and side scatter and selected by CD66b marker. Neutrophils in whole blood appeared quiescent, expressing high levels of l-selectin and low levels of CD11b. In contrast, neutrophils purified under sterile conditions showed signs of activation, expressing reduced levels of l-selectin and higher CD11b expression (Figure 1A).
Figure 1.
Purification and separation of human neutrophils from whole blood promotes neutrophil activation and apoptosis. (A) Heparinized human blood or purified neutrophils were evaluated for the expression of l-selectin and CD11b in resting conditions. Expression of cell surface markers was analyzed by flow cytometry and shows mean fluorescence intensity (MFI) gated on CD66b-positive cells. Histograms show the representative MFI from the 5 independent donors (left). Graph shows MFI, ± standard error of the mean (right). **P < .0066; ***P < .0004. (B) MFI of phagosomal ROS was analyzed from neutrophils in blood and purified neutrophils using Fc-OxyBURST Green assay reagent after 15 minutes and with the addition of TNF-α or fMLP; n = 4. *P < .0152 (left); *P < .0133 (right); **P < .0019. (C) Apoptosis of neutrophils was analyzed by TUNEL assay from neutrophils in whole blood and purified neutrophils at 7 hours. All CD66b+ neutrophils (3000 cell events) were analyzed for TUNEL MFI; n = 3. **P < .0020. All statistical data analyzed by Student paired t test versus control values. fMLP, formyl-methionyl-leucyl-phenylalanine; ROS, reactive oxygen species; TNF-α, tumor necrosis factor α; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling.
We also made comparisons with neutrophils in whole blood using 2 other purification methods: Percoll density gradient centrifugation (Amersham Biotech) or a recently described red blood cell depletion kit (EasySep Human Glycophorin A Depletion Kit).19 The latter method uses immunomagnetic particles that bind to glycophorin-expressing erythrocytes, which are then selectively depleted with an EasyStep magnet. Purified neutrophils that used all methods expressed higher CD11b (supplemental Figure 1). However, using the magnetic depletion kit did not cause obvious l-selectin shedding in comparison with whole-blood neutrophils. Overall, regardless of the method, removal of neutrophils from the erythrocytes from whole blood results in varied degrees of activation, with the least activation being associated with the rapid magnetic separation.
Unstimulated purified neutrophils are primed for activation and are apoptotic
To further evaluate the influence of purification on neutrophils, we measured reactive oxygen species (ROS) production by the phagolysosomal oxidative burst9 and neutrophil apoptosis by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) assay. Without stimulation, purified neutrophils produced ROS as monitored over 15 minutes (Figure 1B). In contrast, neutrophils studied in whole blood show absent or minimal increase of baseline ROS production over the same period (Figure 1B). Upon stimulation with low concentrations of tumor necrosis factor α (TNF-α), and formyl-methionyl-leucyl-phenylalanine (fMLP) neutrophils in whole blood also showed minimal-to-no ROS production (Figure 1B). However, purified neutrophils produced greater ROS upon stimulation with fMLP and TNF-α (Figure 1B).
Neutrophils undergo apoptosis to resolve inflammation and prevent damage.9 We compared apoptosis of purified neutrophils with those maintained in their natural blood compartment by using a TUNEL assay for DNA fragmentation in CD66b-positive neutrophils. After 7 hours, neutrophils left in whole blood had significantly reduced apoptosis in comparison with purified neutrophils (Figure 1C). Thus, separation of neutrophil from blood components sensitizes them to activation and triggers apoptosis. Thus, we hypothesized that there are factors in whole blood that normally maintain neutrophil quiescence.
Erythrocytes inhibit neutrophil activation
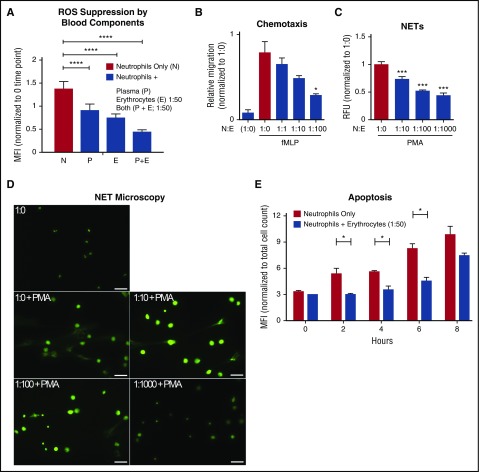
The major components of blood are plasma and erythrocytes; platelets and leukocytes comprise small components by mass. To identify blood factors that suppress neutrophil activation, we added back each blood component to freshly purified neutrophils and measured ROS produced as an indicator of spontaneous activation. Coincubation of autologous erythrocytes, plasma, or both significantly inhibited neutrophil ROS production (Figure 2A). From this point onward, we studied only the immunoregulatory effects of erythrocytes on neutrophil quiescence.
Figure 2.
Autologous erythrocytes inhibit neutrophil oxidative burst, chemotaxis, extracellular trap formation and apoptosis. (A) Purified human neutrophils were coincubated in whole-blood components: plasma, erythrocytes, or both plasma and erythrocytes. Coincubation was done with whole plasma, and the erythrocyte concentration was at a 1:50 neutrophil:erythrocyte ratio. MFI of phagosomal ROS production was analyzed using Fc-OxyBURST Green assay reagent after 15 minutes; n = 3 Statistics were analyzed by ordinary 1-way analysis of variance (ANOVA). ****P < .0001. (B) The effect of neutrophil chemotaxis in combination with erythrocytes (neutrophils:erythrocytes; upper well) in the presence of 100 nM of fMLP (lower well) was determined using a transwell system. Statistics were analyzed by ordinary 1-way ANOVA; n = 4. *P < .03 versus control values considered statistically significant. (C) NET formation was quantified to determine the effect of erythrocytes incubated with neutrophils; n = 3. Statistics were analyzed by ordinary 1-way ANOVA. ***P < .0001 versus control values considered statistically significant. (D) NET formation microscopy analysis with increasing concentrations of erythrocytes plus phorbol myristate acetate (25 nM) where indicated. Scale bar, 50 μm. (E) Detection of apoptotic neutrophils was analyzed by TUNEL assay with and without incubation with erythrocytes up to 8 hours. Neutrophils were gated using forward and side scatter by flow cytometry and by TUNEL MFI of 10 000 cell counts. Results were analyzed by Student paired t test; n = 2. *P < .05 versus control values considered statistically significant. N:E, neutrophil:erythrocyte; PMA, phorbol myristate acetate; RFU, relative fluorescence units.
In vivo, neutrophils exit through capillaries and postcapillary venules.7 In these narrow vessels, cells are squeezed into single file, facilitating translocation and chemotaxis and effectively separating erythrocytes from neutrophils. To determine whether separation from erythrocytes is required for effective translocation and chemotaxis, we tested neutrophil migration across a Transwell filter in response to a bacterial chemotactic stimulus, fMLP, in the absence or presence of differing numbers of erythrocytes. Neutrophil transmigration was proportionately inhibited by increasing ratios of erythrocytes (1:1, 1:10, and 1:100; Figure 2B). Notably, we did observe some erythrocytes falling through the membrane filter (data not shown), indicating that the membranes were not clogging nor interfering with neutrophil migration.
Another important neutrophil function is forming DNA-based NETs. This is a specialized mechanism for entrapping bacteria to prevent the spread of infection.20,21 NETs are increasingly recognized as a potential contributor to pathological inflammatory conditions21; therefore, their release must be critically protected. As quantified by extracellular DNA release, increasing concentrations of erythrocytes significantly suppressed NET formation in response to the potent agonist phorbol myristate acetate (PMA) (Figure 2C-D).
Ex vivo, purified neutrophils die by apoptosis over a period of 8-36 hours.22,23 A 1:50 ratio of neutrophil:erythrocyte was sufficient to significantly suppress the onset of neutrophil apoptosis in vitro (Figure 2E). Neutrophils maintained in whole blood had a longer lifespan, measured by propidium iodide staining. Lifespan was further increased by decreasing temperature from 37°C to RT to 4°C, with neutrophil quiescence maintained for up 24 hours in whole blood at 4°C (supplemental Figure 2).
Selective modification of erythrocyte surface sialic acids reveals their role in neutrophil quiescence
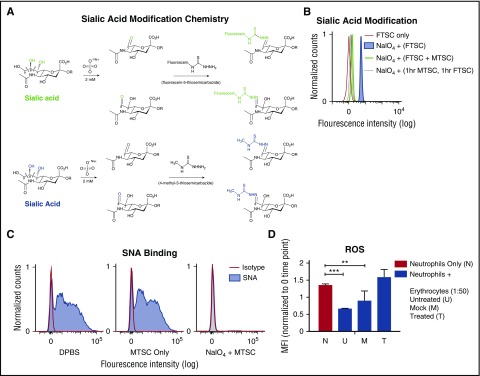
Erythrocyte cell surfaces are covered in a high density of sialic acids attached to the termini of sialoglycoprotein glycans.24 To determine whether sialic acids inhibit neutrophil activation, we could potentially remove them with a sialidase. However, sialidase treatment induces gross biophysical changes, including loss of cell hydrophilicity and negative charge, and also exposes underlying galactose residues that could engage receptors such as galectins.25,26 Therefore, we selectively modified sialic acids on the erythrocyte surface using mild sodium periodate oxidation (NaIO4), a well-known classic method that specifically modifies the exocyclic side chain of terminal sialic acids on sialoglycoproteins.15 Although this mild treatment has previously been shown to be highly selective for sialic acids on erythrocytes,15,27 it leaves behind reactive aldehyde groups on the side chain, which could affect further functional studies. Quenching the aldehydes with sodium borohydride15 would damage erythrocytes because of alkaline conditions and generation of borates. We therefore made use of a recent observation that FTSC can selectively react with periodate-generated aldehydes under gentle conditions.16 We used 4-methyl-3-thiosemicarbazide (MTSC), instead of the bulky FTSC, to leave behind an inert, but slightly modified, sialic acid side chain (Figure 3A). Flow cytometry demonstrated that FTSC integration on the erythrocyte surface depends on sodium periodate pretreatment (Figure 3B). When equal concentrations of FTSC and MTSC were incubated with erythrocytes after mild periodate oxidation, FTSC labeling was reduced in half (Figure 3B). Finally, when erythrocytes were mildly treated with periodate, followed by incubation of MTSC for 1 hour and FTSC for an additional 1 hour, FTCS labeling was almost eliminated (Figure 3B). Thus, the MTSC reaction went to completion; all aldehydes available for reaction with FTSC had reacted with MTSC.
Figure 3.
Modification of side chains of terminal sialic acids on erythrocyte surface by mild periodate and MTSC. (A) Mild oxidation using sodium periodate (NaIO4) generates aldehydes on sialic acid–containing glycoproteins, followed by direct labeling of aldehydes with a fluorescent tag, FTSC (top, green). A smaller compound, MTSC, replaced FTSC, which would generate the same sialic acid modification without the fluorescein molecule (bottom, blue). (B) By flow cytometry, modification of sialic acid by both FTSC and MTSC was tested for reactivity and competition on the erythrocytes surface. Erythrocytes were treated with sodium periodate (NaIO4) for 20 minutes on ice, followed by the addition of FTSC or MTSC, where noted, for 1 hour at 37°C; n = 2. (C) Sialic acid modification of treated erythrocytes (NaIO4 + MTSC, MTSC only) and untreated erythrocytes (DPBS) were stained with biotinylated SNA lectin, which preferentially binds to sialic acids attached to terminal galactose in α2-6-linkage and was measured by flow cytometry. Isotype control (streptavidin-PE); n = 4. (D) Purified neutrophils were incubated with erythrocytes (DPBS, MTSC only, and NaIO4 + MTSC). Erythrocyte concentration was at 1:50 neutrophil:erythrocyte ratio. MFI of phagosomal ROS production was analyzed using Fc-OxyBURST Green assay reagent at 15 minutes. Statistics were analyzed by ordinary 1-way ANOVA; n = 2. **P < .0072 versus control values considered statistically significant; ***P < .0004.
This kind of mild sodium periodate oxidation on the erythrocyte surface is well known to be sialic acid specific and to not affect other molecules.15 As further evidence for erythrocyte sialic acid modification by periodate and MTSC, we measured loss of Sambucus nigra (SNA) lectin binding, which targets α2-6-linked cell surface sialic acids with intact side chains.28,29 Treated erythrocytes lost SNA binding (Figure 3C) in comparison with nontreated and mock-treated erythrocytes. Flow cytometry showed only a small decrease in expression of erythrocyte glycophorin A (GPA) after NaIO4/MTSC (supplemental Figure 3), showing that the antibody epitope is largely intact after erythrocyte treatment and that it is independent of sialylation.
After successful erythrocyte sialic acid modification, we asked whether the suppression of neutrophil activation was lost. We purified neutrophils with untreated (DPBS), mock (MTSC only), and treated erythrocytes (NaIO4/MTSC), measuring production of ROS after 15 minutes with no stimulus. This subtle modification of cell surface sialic acids was indeed sufficient to markedly reduce the inhibitory effect of erythrocytes on neutrophil activation (Figure 3D).
Surface-expressed sialylated glycophorin A on erythrocytes engage neutrophils via Siglec-9
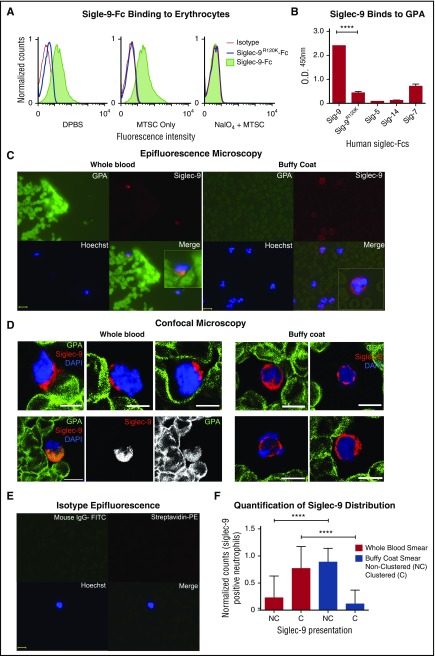
Sialic acid–binding Ig-like lectins (Siglecs) are inhibitory receptors that recognize sialic acids as self-associated molecular patterns (SAMPs)30 and suppress leukocyte activation.31-34 Siglec-9 is the most abundant Siglec constitutively expressed on neutrophils,35,36 and a candidate for the erythrocyte sialic acid–mediated neutrophil suppression. We thus tested loss of Siglec-9 binding on erythrocytes after NAIO4/MTSC treatment. As is shown in Figure 4A, recombinant soluble human Siglec-9-Fc binds to human erythrocytes, and binding was abrogated by a single amino acid mutation known to eliminate sialic acid recognition (Siglec-9R120K-Fc).35 Consistent with this finding, NaIO4/MTSC-treated erythrocytes with sialic acid side chain modification also lost Siglec-9-Fc binding. Attempts to further analyze the interaction using blocking and nonblocking anti-Siglec-9 antibodies were confounded by the fact that these reagents trigger immediate signaling, followed by rapid clearance by endocytosis,37 a process we found to be prominent in neutrophils.
Figure 4.
GPA, the major surface sialoglycoprotein on erythrocytes, engages Siglec-9 via sialic acids. (A) Treated (NaIO4/MTSC), mock treated (MTSC only), and untreated (DPBS) erythrocytes were evaluated for the binding of Siglec-9-Fc and analyzed by flow cytometry. Isotype control (goat anti-human IgG); n = 4. (B) Five human recombinant Siglec-Fcs were measured for their ability to bind immobilized GPA by enzyme-linked immunosorbent assay; n = 3. Statistics were analyzed by ordinary 1-way ANOVA. ****P < .0001 versus control values considered statistically significant. (C) Smears prepared immediately after drawing whole blood (left) or those prepared from buffy coat (right), fixed and costained for the erythrocyte GPA (green), Siglec-9 on neutrophils (red), and nucleus (Hoechst). Clustering (Merge) of GPA with neutrophils Siglec-9 upon contact (1 representative image of each shown). Scale bar, 10 μm. (D) Smears were stained similarly as in panel C and analyzed by confocal microscopy. Whole-blood smears (left) confirm polarized clustering of Siglec-9 on neutrophils toward GPA-covered red blood cells (4 representative patterns are shown). One staining pattern in which GPA colocalizes with Siglec-9 (lower left); individual GPA and Siglec-9 channels are in black and white. Scale bars, 5 µm. (E) Isotype controls were stained with streptavidin-PE, mouse IgG1-FITC, and Hoechst stain. Scale bar, 10 μm. (F) Siglec-9 clustering and nonclustering in neutrophils from epifluorescent blood smears were counted and normalized to the total number of Siglec-9 positive neutrophils. Representative images from panel C or pooled data percentage (normalized to Siglec-9 positive cells) are shown (+/− standard error of the mean). Statistical analysis was performed using ordinary 1-way ANOVA with Holm–Sidak's multiple comparison test. ****P < .0001. DAPI, 4′,6-diamidino-2-phenylindole; O.D., optical densitiy; Sig, Siglec.
GPA is the most abundant erythrocyte surface sialoglycoprotein, with approximately 1 × 106 copies on each cell,1,38 and accounts for a large fraction of the total sialic acid on erythrocyte glycoproteins. Among a variety of Siglec-Fc molecules (Siglec-9, −9R120K, −5, −14, and −7-Fc)39 tested for interaction with immobilized GPA, Siglec-9-Fc was the only one that showed prominent binding, and binding was lost in the mutant Siglec-9R120K-Fc (Figure 4B).
To further document interactions between erythrocyte GPA and neutrophil Siglec-9 in blood ex vivo, we compared blood smears made from freshly collected whole blood or from erythrocyte-poor, neutrophil-rich buffy coat preparations made from the same sample. Figure 4C shows interactions between erythrocyte GPA (green) and neutrophil Siglec-9 (red) when undiluted samples were smeared immediately after collection. Polarized clustering of Siglec-9 toward GPA on erythrocytes was frequently observed on smears prepared from whole blood (Figure 4C). By contrast, the pattern of Siglec-9 staining appeared much more uniform in smears made from buffy coat preparations (Figure 4C). To obtain a clearer image of the differences between whole-blood and buffy coat smears, we also performed confocal microscopy (Figure 4D). These images not only confirm the polarized clustering of Siglec-9 in peripheral smears we observed by epifluorescence in Figure 4C but also showed the proximity of such polarized clusters of Siglec-9 on neutrophils to GPA on erythrocytes. In many instances, colocalization between GPA and Siglec-9 was observed (Figure 4D, lower left), consistent with our finding that Siglec-9-Fc binds erythrocytes. Taken together, these findings illustrate how the brief physical separation involved in the preparation of the buffy coat is sufficient to markedly reduce the Siglec-9 clustering toward GPA on erythrocytes (Figure 4C-D, right), which was predominantly observed in fresh blood smears (Figure 4C-D, left). Figure 4E shows an isotype control. Quantifying multiple fields (see the Methods section), we found that >75% of neutrophil Siglec-9 was clustered in erythrocyte-rich whole-blood smears, whereas >85% of neutrophil Siglec-9 was nonclustered in erythrocyte-poor buffy coat smears (Figure 4F).
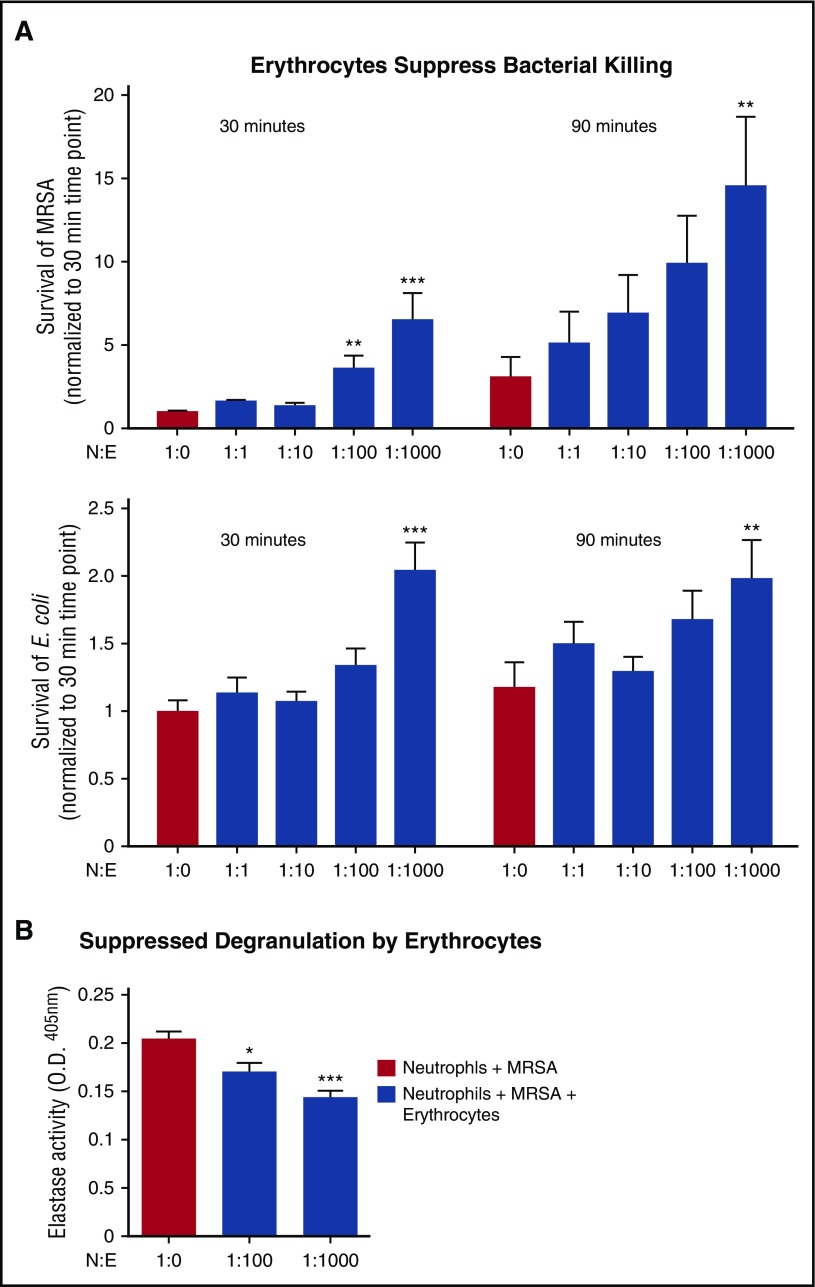
Erythrocyte’s suppression of neutrophils in whole blood does not completely suppress bacterial killing
Neutrophils are the first responders to bacterial infections in tissues; however, bacterial killing assays indicate that neutrophils can kill bacteria in whole blood, that is, they are not completely inert in the presence of erythrocytes. Bacterial killing was evaluated across several neutrophil:erythrocyte ratios, with the leading blood-borne pathogen being methicillin-resistant Staphylococcus aureus (MRSA) and a nonpathogenic Escherichia coli (E coli) strain at a multiplicity of infection (MOI) of 1 bacterium per neutrophil (MOI = 1). Addition of erythrocytes did not completely suppress neutrophil activity; however, clearance of both species was less efficient in the presence of 1:100 and 1:1000 neutrophil:erythrocyte ratios (Figure 5A). Likewise, neutrophil degranulation to release antimicrobial effectors was partially suppressed by erythrocytes (Figure 5B).
Figure 5.
Erythrocytes suppress neutrophils in whole blood, but do not completely suppress bacterial killing. (A) Neutrophil killing of MRSA or E coli K12 was evaluated with increasing concentrations of erythrocytes at 30 and 90 minutes. Neutrophil killing of MRSA (top). Positive control is neutrophils plus MRSA. Statistics were analyzed by repeated measures one-way ANOVA and post hoc Holm–Sidak's multiple comparison test; n = 3. **P < .0057; ***P < .0001 versus control values considered statistically significant. Neutrophil killing of E coli (bottom). Positive control is neutrophils plus E coli. Statistics were analyzed by repeated measures 1-way ANOVA and post hoc Holm–Sidak's multiple comparison test; n = 3. **P < .05 versus control values considered statistically significant; ***P < .0003. (B) Neutrophils were incubated with MRSA for 90 minutes, and degranulation was measured by elastase release on fluorescent plate reader at ex. 488/emm.530; n = 3. Statistics were analyzed by ordinary 1-way ANOVA. ***P < .0001 versus control values considered statistically significant. N:E, neutrophil:erythrocyte.
Discussion
Erythrocytes are one of the best-studied cell types and known for their pivotal function of oxygen and carbon dioxide transport. Despite these past studies and their numerical dominance in flowing blood, erythrocytes are assumed to have no major involvement in regulation of inflammation. Here, we identify an unexpected function of erythrocytes of likely biological importance and their interaction with neutrophils. Our data show that sialic acids present SAMPs30 on the erythrocyte surface to suppress neutrophil reactivity and maintain quiescence ex vivo and in vitro. Suppression of neutrophil activation involves a cluster of sialic acids on the most abundant erythrocyte sialoglycoprotein, GPA, which engages neutrophil Siglec-9, a receptor previously known to dampen innate immune cell activation.35,36
The new finding that erythrocyte surface sialic acids suppress neutrophil activation ex vivo and in vitro may help explain some common laboratory observations. We clearly demonstrate how separation of erythrocytes from neutrophils was sufficient to activate neutrophils and initiate apoptosis. Our results suggest that neutrophils do not require priming by factors outside the bloodstream to become activated. Rather, neutrophil activation can be initiated by removing them from whole blood, and all of its components, initiating an extravascular program of increased activation potential and, ultimately, apoptosis.
Plasma glycoproteins and platelets, whose surfaces are also sialic acid rich, might supplement the erythrocyte contribution to neutrophil quiescence in the bloodstream and extend the SAMP hypothesis.30 Indeed, our study showed that autologous plasma also has a suppressive effect on neutrophil activation (Figure 2A), a finding meriting further investigation. A prior study showed that incubation with viable and fixed unstimulated platelets, or fixed activated platelets, resulted in inhibition of neutrophil apoptosis.40 The mechanism responsible was not defined in the study, but a similar engagement of abundant platelet sialoglycoproteins with neutrophil Siglec-9 can be considered in future studies.
We found 2 prior studies that evaluated the importance of erythrocytes in preventing neutrophil cell death. Aoshiba et al described effects of erythrocytes inhibiting apoptosis of purified human neutrophils in vitro.41 This study used exposure to exogenous H2O2 to promote induction of apoptosis and found that having erythrocytes present served as a “sink” to scavenge oxygen radicals and prolong the lifespan of neutrophils. An older study by Van Asbeck et al described how insufflation of intact erythrocytes into the tracheobronchial tree of hyperoxic rats increased their survival due to the ROS-scavenging effects of erythrocytes.42 Molecular mechanisms were not explored in either study, but our SAMP model was likely operant during these experimental setups. Comparing our results, we suggest that a ratio of 1000:1 (erythrocytes:neutrophils) not only protects against apoptosis but also suppresses neutrophil activation in the bloodstream. Antioxidant properties of erythrocytes may further mitigate one of the inflammatory consequences of neutrophil activation (ie, ROS); however, we did not examine this aspect in the current study because modifying erythrocyte sialic acids was sufficient by itself to eliminate the neutrophil suppression effect.
Our observation that whole-blood smears, but not buffy coat smears show high percentages of neutrophil Siglec-9 clustering at points of contact with erythrocytes was initially surprising (Figure 4). However, centrifugation of whole blood involves separation of erythrocytes from leukocytes, followed by formation of the buffy coat. This finding suggests that neutrophil Siglec-9 may be in frequent contact with erythrocyte sialic acids in vivo. However, separation would occur when individual cells pass single file through capillaries7; thus, complete separation of erythrocytes from neutrophils might facilitate translocation and chemotaxis upon reaching postcapillary venules. Furthermore, our chemotaxis assays show that decreasing numbers of erythrocytes allow for more transmigration of neutrophils, modeling the in vivo circumstance in which neutrophils leave the circulation to subsequently exhibit activation phenotypes such as ROS generation and NET production in infected tissues.
Our findings are relevant to in vitro experiments involving neutrophils and may explain variable results of some prior studies of isolated neutrophils. Because loss of contact with erythrocytes favors rapid neutrophil activation and apoptosis, the exact timing at which neutrophils are used in in vitro studies (eg, microbial killing) after purification could be important. To avoid accidental activation, we took care to maintain sterility of neutrophil isolation in this study, and experiments were completed within <2 hours. Polymorphprep solution was the primary method used to isolate a pure granulocyte fraction from human blood by centrifugation.43 This method has been used by many investigators because of its efficacy for enriching neutrophils. An earlier study compared several different methods for isolating neutrophils.43 The investigators assessed purity, function, and expression of cell surface markers of enriched granulocytes obtained by Ficoll density centrifugation, anti-CD15 antibody conjugated magnetic microbeads (positive selection), or density centrifugation using Polymorphprep. Depending on the method used, all 3 strategies showed no major differences in enriched granulocyte purity when selected with CD66b, the same marker used in this study to gate on neutrophils. No significant cell death was observed during the isolation procedure. Spontaneous ROS production levels did not differ in unstimulated granulocytes. No differences were observed in phagocytosis of E coli, and the expression of l-selectin was similar in all groups. However, a control to show how neutrophil activation and function behaved in whole blood was not performed.
For future studies using density centrifugation methods, adding back autologous erythrocytes may augment neutrophil lifespan for studies of longer duration. For studies requiring minimal activation,17 the magnetic removal of erythrocytes described here may be useful. If rapid isolation of pure neutrophils is desired, a method such as the MACSxpress Neutrophil Isolation Kit might be beneficial. This approach uses negative selection to remove all blood components except neutrophils. Preliminary analysis comparing this method to whole blood shows slight activation (higher expression of CD11b) but no difference in l-selectin, supporting our hypothesis of neutrophil activation when separated from blood components. This is likely due to rapid purification, minimal manipulation, and immediate use. We also noted that neutrophils maintained in whole blood had a much longer lifespan, especially at 4°C, at which temperature they underwent minimal activation, and is of potential practical relevance when storing or shipping samples for research studies. In the future, it would be interesting to see whether similar issues affect isolation procedures for other leukocytes expressing Siglecs, such as monocytes.
As with any unexpected observation, our findings raise questions for future investigation, including the full relevance of erythrocyte-mediated neutrophil suppression in vivo. Although we studied the phenomenon in freshly collected whole blood, we are unable to think of a logical and ethical experiment that could definitively prove the importance of this mechanism in vivo. One cannot rapidly deplete erythrocytes from the circulation without also causing major oxygenation and hemodynamic changes. On the other hand, simple evaluation of the heightened activation state of neutrophils migrating from the circulation into the tissues in response to chemotactic stimulus would not definitively prove that their escape from erythrocyte suppression was the key factor.
There are, however, some prior observations that support the current hypothesis and may be of practical relevance to our discovery. Experimental neutrophil half-lives reported in human subjects vary between ∼8 hours5 and 3+ days.6,44,45 Notably, the shorter times were found in studies using purified and retransfused neutrophils. In this regard, our findings are possibly relevant to granulocyte transfusions in neutropenic patients, a theoretically useful approach with mixed results to date,46 complicated by neutrophil sequestration in lung vasculature.47-50 Given our findings, adding back some autologous erythrocytes to granulocytes prepared for transfusion could possibly improve stability and efficacy and reduce adverse effects.
Circulating erythrocyte levels could also be relevant to neutrophil activation in circulation, a process involved in several disease states,11 including leukocytoclastic vasculitis,51 organ reperfusion injury,52 adult respiratory distress syndrome,13 and septic shock.12 It may also be interesting to ask whether patients with genetically inherited erythrocyte membrane abnormalities have altered ligand levels for Siglec-9.
Our study also introduces a simple technique for flow cytometry analysis of multiple neutrophil markers directly detected in intact whole blood as well as a refinement of the classic method of mild metaperiodate oxidation of sialic acid side chains. By aldehyde quenching with MTSC, we generated intact erythrocytes that can no longer bind Siglec-9. This subtle modification of cell surface sialic acids was sufficient to reduce the inhibitory effect of erythrocytes on neutrophil activation, emphasizing the specificity of this interaction.
Many of the experimental and clinical issues discussed here remain speculative. However, taken together with our ex vivo findings, they suggest that this unexpected discovery of erythrocyte suppression of neutrophils may have diverse implications for situations involving neutrophil biology. It also remains to be seen whether erythrocyte sialic acids interact with inhibitory Siglecs on other leukocytes within the bloodstream.
Acknowledgments
The authors thank Stevan Springer and Nissi Varki (both from the University of California, San Diego) for their critical review of the manuscript.
This work was supported by National Institutes of Health (NIH) National Heart, Lung, and Blood Institute grant P01HL107150 (A.V. and V.N.). C.R. was supported by the NIH National Cancer Institute award T32CA121938. A.L. is a University of California, San Diego, Institutional Research and Academic Career Development fellow and supported by NIH National Institute of General Medical Sciences award K12GM068524.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.L. conducted or contributed to the experiments shown in Figures 1, 2A, 3C-D, 4, 5A, and supplemental Figures 1-3; I.S. contributed to the establishment of the study, and conducted or contributed to the experiments shown in Figures 2E and 4A; S.D. conducted or contributed to the experiments shown in Figures 2C-D and 5; R.C. conducted or contributed to the experiment shown in Figure 2B; C.R. and P.G. contributed whole blood and buffy coat confocal microscopy images shown in Figure 4D; S.D. contributed to sialic acid quantification on erythrocytes by high-performance liquid chromatography (not shown); L.D. conducted or contributed to the experiments shown in Figures 3A-B and 4A; V.N. and A.V. contributed to the design, interpretation, and supervision of the study; and A.L., V.N., and A.V. wrote the paper with input and comments from all of the authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for I.S. is Instituto de Biotecnología, Universidad Nacional Autonóma de México, Cuernavaca, Mexico.
The current affiliation for L.D. is GlycoMimetics, Inc., Rockville, MD.
Correspondence: Ajit Varki, Department of Cellular and Molecular Medicine, University of California, San Diego, 9500 Gilman Dr, MC 0687, BRF2, Room 4126, La Jolla, CA 92093-0687; e-mail: a1varki@ucsd.edu; or Victor Nizet, Department of Medicine and Pediatrics, University of California, San Diego, 9500 Gilman Dr, MC 0687, BRF2, Room 4126, La Jolla, CA 92093-0687; e-mail: vnizet@ucsd.edu.
References
- 1.Mohandas N. Structure and composition of the erythrocyte. In: Kaushansky EA, ed. Williams Hematology. New York, NY: McGraw-Hill; 2016:461-478. [Google Scholar]
- 2.Belcher JD, Chen C, Nguyen J, et al. . Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood. 2014;123(3):377-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen G, Zhang D, Fuchs TA, Manwani D, Wagner DD, Frenette PS. Heme-induced neutrophil extracellular traps contribute to the pathogenesis of sickle cell disease. Blood. 2014;123(24):3818-3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silvestre-Roig C, Hidalgo A, Soehnlein O. Neutrophil heterogeneity: implications for homeostasis and pathogenesis. Blood. 2016;127(18):2173-2181. [DOI] [PubMed] [Google Scholar]
- 5.Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31(8):318-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tak T, Tesselaar K, Pillay J, Borghans JA, Koenderman L. What’s your age again? Determination of human neutrophil half-lives revisited. J Leukoc Biol. 2013;94(4):595-601. [DOI] [PubMed] [Google Scholar]
- 7.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159-175. [DOI] [PubMed] [Google Scholar]
- 8.Luo HR, Loison F. Constitutive neutrophil apoptosis: mechanisms and regulation. Am J Hematol. 2008;83(4):288-295. [DOI] [PubMed] [Google Scholar]
- 9.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459-489. [DOI] [PubMed] [Google Scholar]
- 10.Mócsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med. 2013;210(7):1283-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11(8):519-531. [DOI] [PubMed] [Google Scholar]
- 12.Brown KA, Brain SD, Pearson JD, Edgeworth JD, Lewis SM, Treacher DF. Neutrophils in development of multiple organ failure in sepsis. Lancet. 2006;368(9530):157-169. [DOI] [PubMed] [Google Scholar]
- 13.Segel GB, Halterman MW, Lichtman MA. The paradox of the neutrophil’s role in tissue injury. J Leukoc Biol. 2011;89(3):359-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheiermann C, Frenette PS, Hidalgo A. Regulation of leucocyte homeostasis in the circulation. Cardiovasc Res. 2015;107(3):340-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gahmberg CG, Andersson LC. Selective radioactive labeling of cell surface sialoglycoproteins by periodate-tritiated borohydride. J Biol Chem. 1977;252(16):5888-5894. [PubMed] [Google Scholar]
- 16.Zhang Y, Yuan J, Song J, Wang Z, Huang L. An efficient method for selectively imaging and quantifying in situ the expression of sialylated glycoproteins on living cells. Glycobiology. 2013;23(6):643-653. [DOI] [PubMed] [Google Scholar]
- 17.Lewkowicz N, Mycko MP, Przygodzka P, et al. . Induction of human IL-10-producing neutrophils by LPS-stimulated Treg cells and IL-10. Mucosal Immunol. 2016;9(2):364-378. [DOI] [PubMed] [Google Scholar]
- 18.Quinn MT, DeLeo FR, Bokoch GM. Neutrophil methods and protocols. Preface. Methods Mol Biol. 2007;412:vii-viii. [DOI] [PubMed] [Google Scholar]
- 19.Lee SK, Goh SY, Wong YQ, Ding JL. Response of neutrophils to extracellular haemoglobin and LTA in human blood system. EBioMedicine. 2015;2(3):225-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brinkmann V, Reichard U, Goosmann C, et al. . Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532-1535. [DOI] [PubMed] [Google Scholar]
- 21.Sørensen OE, Borregaard N. Neutrophil extracellular traps—the dark side of neutrophils. J Clin Invest. 2016;126(5):1612-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee A, Whyte MK, Haslett C. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J Leukoc Biol. 1993;54(4):283-288. [PubMed] [Google Scholar]
- 23.Kobayashi SD, Voyich JM, Whitney AR, DeLeo FR. Spontaneous neutrophil apoptosis and regulation of cell survival by granulocyte macrophage-colony stimulating factor. J Leukoc Biol. 2005;78(6):1408-1418. [DOI] [PubMed] [Google Scholar]
- 24.Cook GM. Glycobiology of the cell surface: its debt to cell electrophoresis 1940-65. Electrophoresis. 2016;37:(11)1399-1406. [DOI] [PubMed] [Google Scholar]
- 25.Baum LG, Garner OB, Schaefer K, Lee B. Microbe-host interactions are positively and negatively regulated by galectin-glycan interactions. Front Immunol. 2014;5:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rabinovich GA, Croci DO. Regulatory circuits mediated by lectin-glycan interactions in autoimmunity and cancer. Immunity. 2012;36(3):322-335. [DOI] [PubMed] [Google Scholar]
- 27.Van Lenten L, Ashwell G. Studies on the chemical and enzymatic modification of glycoproteins. A general method for the tritiation of sialic acid-containing glycoproteins. J Biol Chem. 1971;246(6):1889-1894. [PubMed] [Google Scholar]
- 28.Shibuya N, Goldstein IJ, Broekaert WF, Nsimba-Lubaki M, Peeters B, Peumans WJ. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(alpha 2-6)Gal/GalNAc sequence. J Biol Chem. 1987;262(4):1596-1601. [PubMed] [Google Scholar]
- 29.Brinkman-Van der Linden ECM, Sonnenburg JL, Varki A. Effects of sialic acid substitutions on recognition by Sambucus nigra agglutinin and Maackia amurensis hemagglutinin. Anal Biochem. 2002;303(1):98-104. [DOI] [PubMed] [Google Scholar]
- 30.Varki A. Since there are PAMPs and DAMPs, there must be SAMPs? Glycan “self-associated molecular patterns” dampen innate immunity, but pathogens can mimic them. Glycobiology. 2011;21(9):1121-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crocker PR, Redelinghuys P. Siglecs as positive and negative regulators of the immune system. Biochem Soc Trans. 2008;36(6):1467-1471. [DOI] [PubMed] [Google Scholar]
- 32.Macauley MS, Crocker PR, Paulson JC. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol. 2014;14(10):653-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bochner BS, Zimmermann N. Role of siglecs and related glycan-binding proteins in immune responses and immunoregulation. J Allergy Clin Immunol. 2015;135(3):598-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angata T, Nycholat CM, Macauley MS. Therapeutic targeting of Siglecs using antibody- and glycan-based approaches. Trends Pharmacol Sci. 2015;36(10):645-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Angata T, Varki A. Cloning, characterization, and phylogenetic analysis of siglec-9, a new member of the CD33-related group of siglecs. Evidence for co-evolution with sialic acid synthesis pathways. J Biol Chem. 2000;275(29):22127-22135. [DOI] [PubMed] [Google Scholar]
- 36.Zhang JQ, Nicoll G, Jones C, Crocker PR. Siglec-9, a novel sialic acid binding member of the immunoglobulin superfamily expressed broadly on human blood leukocytes. J Biol Chem. 2000;275(29):22121-22126. [DOI] [PubMed] [Google Scholar]
- 37.O’Reilly MK, Paulson JC. Siglecs as targets for therapy in immune-cell-mediated disease. Trends Pharmacol Sci. 2009;30(5):240-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baum J, Ward RH, Conway DJ. Natural selection on the erythrocyte surface. Mol Biol Evol. 2002;19(3):223-229. [DOI] [PubMed] [Google Scholar]
- 39.Padler-Karavani V, Hurtado-Ziola N, Chang YC, et al. . Rapid evolution of binding specificities and expression patterns of inhibitory CD33-related Siglecs in primates. FASEB J. 2014;28(3):1280-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andonegui G, Trevani AS, López DH, Raiden S, Giordano M, Geffner JR. Inhibition of human neutrophil apoptosis by platelets. J Immunol. 1997;158(7):3372-3377. [PubMed] [Google Scholar]
- 41.Aoshiba K, Nakajima Y, Yasui S, Tamaoki J, Nagai A. Red blood cells inhibit apoptosis of human neutrophils. Blood. 1999;93(11):4006-4010. [PubMed] [Google Scholar]
- 42.van Asbeck BS, Hoidal J, Vercellotti GM, Schwartz BA, Moldow CF, Jacob HS. Protection against lethal hyperoxia by tracheal insufflation of erythrocytes: role of red cell glutathione. Science. 1985;227(4688):756-759. [DOI] [PubMed] [Google Scholar]
- 43.Zhou L, Somasundaram R, Nederhof RF, et al. . Impact of human granulocyte and monocyte isolation procedures on functional studies. Clin Vaccine Immunol. 2012;19(7):1065-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pillay J, den Braber I, Vrisekoop N, et al. . In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood. 2010;116(4):625-627. [DOI] [PubMed] [Google Scholar]
- 45.Lahoz-Beneytez J, Elemans M, Zhang Y, et al. . Human neutrophil kinetics: modeling of stable isotope labeling data supports short blood neutrophil half-lives. Blood. 2016;127(26):3431-3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dale DC, Liles WC, Llewellyn C, Rodger E, Price TH. Neutrophil transfusions: kinetics and functions of neutrophils mobilized with granulocyte-colony-stimulating factor and dexamethasone. Transfusion. 1998;38(8):713-721. [DOI] [PubMed] [Google Scholar]
- 47.Strauss RG, Connett JE, Gale RP, et al. . A controlled trial of prophylactic granulocyte transfusions during initial induction chemotherapy for acute myelogenous leukemia. N Engl J Med. 1981;305(11):597-603. [DOI] [PubMed] [Google Scholar]
- 48.Chanock SJ, Gorlin JB. Granulocyte transfusions. Time for a second look. Infect Dis Clin North Am. 1996;10(2):327-343. [DOI] [PubMed] [Google Scholar]
- 49.Lee JJ, Chung IJ, Park MR, et al. . Clinical efficacy of granulocyte transfusion therapy in patients with neutropenia-related infections. Leukemia. 2001;15(2):203-207. [DOI] [PubMed] [Google Scholar]
- 50.Cugno C, Deola S, Filippini P, Stroncek DF, Rutella S. Granulocyte transfusions in children and adults with hematological malignancies: benefits and controversies. J Transl Med. 2015;13:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Boysson H, Martin Silva N, de Moreuil C, et al. ; French Vasculitis Study Group, French Internal Medicine Society. Neutrophilic dermatoses in antineutrophil cytoplasmic antibody-associated vasculitis: a French multicenter study of 17 cases and literature review. Medicine (Baltimore). 2016;95(11):e2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heusch G. The coronary circulation as a target of cardioprotection. Circ Res. 2016;118(10):1643-1658. [DOI] [PubMed] [Google Scholar]