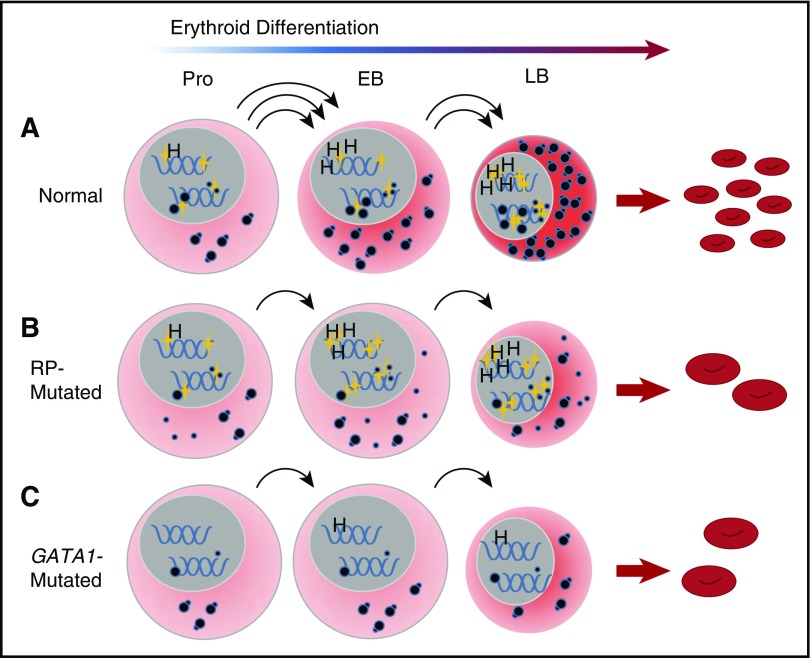
Figure 7.
A convergent model for the effect of RP and GATA1 mutations on erythroid differentiation in DBA. (A) Under conditions of normal hematopoiesis, rising GATA1L levels (gold stars, nuclear) in CD41−/CD44+/CD235− cells promote the erythroid program by stimulating the expression of canonical GATA1 targets, such as heme synthesis genes, as well as enhancing expression of components of the translational apparatus, including ribosomal RNA, snoRNA, and ribosomal proteins. The net effect of these activities is the accumulation of sufficient translational competence (cytoplasmic, illustrated as mature 80S ribosomes) to support normal differentiation and proliferation from proerythroblasts into early and late basophilic erythroblasts. (B) In RP-mutated erythropoiesis, GATA1L is present to promote heme synthesis as well as upregulation of the translational apparatus; however, abortive ribosomal assembly (illustrated as a large-subunit defect with accumulation of free cytoplasmic 40S ribosomes) limits accretion of translational competence, with reduced proliferation of erythroid progenitors, delays in the maturational program, and anemia. (C) In GATA1-mediated DBA, the absence of GATA1L limits upregulation of the translational apparatus without abortive ribosome assembly, but with similar consequences to proliferation and differentiation of erythroid progenitors. This model places GATA1 as a master regulator in DBA that operates upstream from RP gene alterations. EB, early basophilic erythroblasts; H, heme synthesis genes; LB, late basophilic erythroblasts; Pro, proerythroblasts.