Abstract
The v-Abl protein tyrosine kinase encoded by Abelson murine leukemia virus (Ab-MLV) induces pre-B-cell transformation. Signals emanating from the SH2 domain of the protein are required for transformation, and several proteins bind this region of v-Abl. One such protein is the adaptor molecule Shc, a protein that complexes with Grb2/Sos and facilitates Ras activation, an event associated with Ab-MLV transformation. To test the role this interaction plays in growth and survival of infected pre-B cells, dominant-negative (DN) Shc proteins were coexpressed with v-Abl and transformation was examined. Expression of DN Shc reduced Ab-MLV pre-B-cell transformation and decreased the ability of v-Abl to stimulate Ras activation and Erk phosphorylation in a Raf-dependent but Rac-independent fashion. Further analysis revealed that Shc is required for v-Abl-mediated Raf tyrosine 340 and 341 phosphorylation, an event associated with Erk phosphorylation. In contrast to effects on proliferation, survival of the cells and activation of Akt were not affected by expression of DN Shc. Together, these data reveal that v-Abl-Shc interactions are a critical part of the growth stimulatory signals delivered during transformation but that they do not affect antiapoptotic pathways. Furthermore, these data highlight a novel role for Shc in signaling from v-Abl to Raf.
Abelson murine leukemia virus (Ab-MLV) arose from recombination between Moloney murine leukemia virus and the cellular proto-oncogene c-abl, an event that resulted in expression of a constitutively active tyrosine kinase called v-Abl. Ab-MLV transforms pre-B cells and NIH 3T3 cells in vitro and induces pre-B-cell lymphoma in vivo (43). Transformation by Ab-MLV requires v-Abl kinase activity and an intact SH2 domain (26, 30, 43). The SH2 domain contains the FLVRES motif that forms a phosphotyrosine-binding pocket involved in protein-protein associations. Disruption of the v-Abl SH2 domain results in defects in cell transformation, suggesting that signaling cascades influenced by the SH2 domain affect multiple pathways (26, 52). Although the v-Abl SH2 domain has been associated with signaling to the Ras, PI3-K, and c-Myc pathways (reviewed in reference 66), the ways that particular SH2 binding proteins transmit signals downstream from this domain have not been elucidated.
The adaptor protein Shc is one molecule that binds to the SH2 domain of v-Abl. Once bound, Shc becomes tyrosine phosphorylated (35, 38) and associates with Grb2/Sos, a complex that can activate Ras. Association with Grb2 requires phosphorylation of Y239, 240, and 313, three tyrosines located in the CH1 domain of Shc (20, 25). These residues become phosphorylated upon activation of several different receptors and are probably targets of v-Abl (reviewed in reference 40). Shc phosphorylation can promote cell growth and survival depending on the cell type and context, and multiple pathways, including those involving Ras/mitogen-activated protein (MAP) kinase and PI3-K/Akt, can be affected (18, 19, 24, 32). In addition to Ab-MLV-mediated transformation, Shc proteins have been implicated in Ret/Ptc2, Trk-T3, ErbB2, and polyomavirus middle-T transformation, and hyperphosphorylation of Shc has been documented for several types of cancer (4, 31, 37, 53).
Shc influences Ras and Myc, two molecules required for Ab-MLV transformation, (16, 47, 48), reinforcing the possible functional link between v-Abl and Shc. In addition, Shc binds P120/R273K at a reduced level (26). This mutant form of v-Abl retains its ability to block apoptosis but is compromised in its ability to stimulate growth of cells (17, 26), suggesting that Shc may be particularly important for v-Abl-induced proliferation. To test this idea directly and to probe the nature of the signals that pass from v-Abl through Shc to downstream effector proteins, we studied transformation of pre-B cells coexpressing Ab-MLV and dominant-negative (DN) mutants of Shc. These experiments revealed that Shc function is important for transformation and influences growth by transmitting signals that integrate downstream with the Ras-Raf-MAP kinase pathway.
MATERIALS AND METHODS
Cells and viruses.
293T and COS7 cells were grown in Dulbecco's modified Eagle's medium (Gibco) supplemented to contain 10% fetal calf serum (Sigma) and 2 mM l-glutamine (Gibco). NIH 3T3 cells were grown in Dulbecco's modified Eagle's medium supplemented to contain 10% calf serum (Sigma) and 2 mM l-glutamine. Ab-MLV-transformed pre-B cells were grown in RPMI 1640 medium (Gibco) supplemented to contain 20% fetal calf serum, 2 mM l-glutamine, and 50 μM 2-mercaptoethanol (Sigma). Viral stocks were prepared by transfection of 293T cells as described elsewhere (26, 63) and titered by infecting NIH 3T3 cells with 0.5 ml of virus containing 8 μg of Polybrene (Sigma)/ml. The cells were harvested 24 h later, and DNA was extracted by incubating the cell pellets in sarcosyl lysis buffer (10 mM Tris [pH 7.6], 10 mM EDTA [pH 8.0], 10 mM NaCl, 0.5% sarcosyl) at 50°C for 16 h. The DNA was recovered by ethanol precipitation, resuspended in water, and amplified with the v-abl primers 5′-GCTTCAACACTCTGGCTGAGTTAGT-3′ and 5′-GATCCATCTCGCTGCGGTAT-3′. PCRs contained 0.2 μM primers and SYBRGreen PCR master mix (Applied Biosystems) and were amplified by using a GeneAmp 5700 sequence detection system (Applied Biosystems). Samples were heated for 10 min at 95°C and amplified for 40 cycles of 95°C for 15 s, 59°C for 1 min, and 81°C for 10 s. The amount of v-abl DNA was determined by using a standard curve generated from v-abl plasmid sequences. The amount of cellular DNA in each sample was normalized by amplifying a portion of the Rag1 locus by use of primers 5′-ATCATCTGTGGTTAGCCGTCTGT-3′ and 5′-ATTATGTATCAGCTCTCACGCCC-3′. Samples were heated for 10 min at 95°C and amplified for 40 cycles of 95°C for 15 s and 60°C for 1 min. Amplified sequences were analyzed using GeneAmp 5700 SDS software. Bone marrow cells were infected with equivalent titers of the different viruses, plated in 35-mm-diameter culture dishes, and monitored for growth. When bone marrow cultures contained 2 × 106 pre-B cells/ml, the dishes were scored as transformed (1, 26, 62).
Plasmids and transfections.
v-abl and Shc sequences were expressed using the pMIA vector, a modified form of the pMIG vector (21, 60) that contains v-abl sequences downstream of the internal ribosome entry site present in pMIG (Fig. 1A). Hemagglutinin (HA)-Shc sequences encoding wild-type Shc and the DN mutants ShcY239F, ShcY313/F, and ShcY239/313F from the LacSwitch pOP13 vector (20) were cloned into the NotI site upstream of the internal ribosome entry site in pMIA. The kinase-inactive v-abl mutant D484N (38) was expressed from the pMIG vector. 293T cells were transfected with plasmids encoding v-Abl, different forms of Shc, pMT3N17Rac-1 (50) encoding DN Rac, pSRalphaMSVtkNeoN17Ras (48) encoding DN Ras, pLNCXRaf-1, pLNCXRaf-1Y340/341F, or pLNCXRaf-1Y340/341D (7) by use of PolyFect transfection reagent (QIAGEN). After 24 h, serum-free medium was added; the cells were harvested after an additional 24 h and processed for protein analysis.
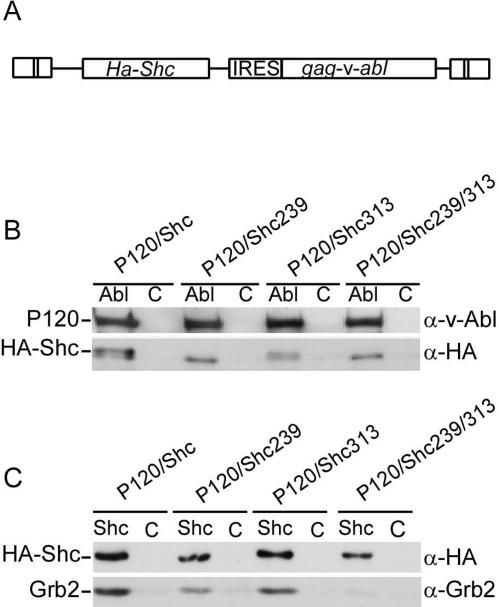
FIG. 1.
DN Shc associates with v-Abl but binds poorly to Grb2. (A) A schematic of the v-Abl/Shc viruses. (B) 293T cells were transfected with 15 μg of DNA expressing P120 and the different forms of Shc and harvested 48 h posttransfection. Following immunoprecipitation of the v-Abl protein by use of α-Gag/v-Abl antibody (Abl) (9) or control antibody (C), v-Abl and HA Shc were visualized by Western blotting using the indicated antibodies. (C) Lysates prepared as described for panel B were immunoprecipitated with α-Shc antibodies (Shc) or control antibody (C) and analyzed by Western blotting using the indicated antibodies.
Protein analysis.
Cell pellets were washed twice with phosphate-buffered saline (PBS) and lysed in lysis buffer (10 mM Tris [pH 7.4], 1% sodium dodecyl sulfate [SDS], 1 mM sodium orthovanadate, and 1 mM phenylmethylsulfonyl fluoride). The samples were boiled for 5 min and sheared through a 25-gauge needle, and total cell protein was quantitated using a bicinchoninic acid assay kit (Pierce). In most cases, 50 μg of protein was fractionated through an SDS-polyacrylamide gel and transferred to a polyvinylidene difluoride membrane (U.S. Biochemicals). For immunoprecipitation, cells were lysed in radioimmunoprecipitation assay buffer (10 mM sodium phosphate [pH 7.0], 150 mM sodium chloride, 0.1% SDS, 50 mM NaF, 1% NP-40, 2 mM EDTA, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin/ml) and incubated for 15 min on ice. The lysates were clarified by centrifugation, and protein was quantitated using a bicinchoninic acid assay kit. Equivalent amounts of protein were immunoprecipitated on ice for 1 h. The immune complexes were recovered using protein G-Sepharose beads (Pharmacia), washed with radioimmunoprecipitation assay buffer, eluted by boiling in sample buffer (200 mM Tris [pH 6.8], 4% SDS, 40% glycerol, 0.12% bromophenol blue, 20% 2-mercaptoethanol) for 5 min, fractionated on an SDS polyacrylamide gel, and transferred to a membrane. Membranes were blocked in buffer containing 0.2% I-block (Tropix) and 0.1% Tween 20 in PBS for 1 h, incubated with antibody for 1.5 h, and washed in blocking buffer. The blots were incubated with alkaline phosphatase-conjugated secondary antibodies for 1 h and washed with blocking buffer. Blots were treated with CSPD substrate (Tropix) and exposed to Kodak X-omat AR film. Blots were stripped in stripping buffer (62.5 mM Tris [pH 6.8], 2% SDS, 100 mM 2-mercaptoethanol) and incubated at 55°C for 30 min. Stripped blots were washed in 0.1% Tween 20 in PBS, blocked, and reprobed. The antibodies used included anti-Gag/v-Abl (H548) (9), anti-v-Abl (24-21) (49), anti-Erk (Cell Signaling Technology), anti-phospho-Erk (Cell Signaling Technology), anti-HA (Babco), anti-Akt (Cell Signaling Technology), anti-phospho-Akt serine 473 (Cell Signaling Technology), anti-c-Myc (Oncogene Research Products), anti-Ras (Transduction Laboratories), anti-Myc tag 9B11 (Cell Signaling Technology), anti-phospho-Raf-1 340/341 (Santa Cruz), anti-Raf-1 (Santa Cruz), anti-Grb2 (Transduction Labs or Santa Cruz), anti-Rac (Santa Cruz), antiphosphotyrosine (Upstate), anti-β-actin (Sigma), and alkaline phosphatase-conjugated anti-mouse immunoglobulin G and anti-rabbit antibodies (Promega). Anti-Rb antibody (Calbiochem) was used as an isotype-matched control for v-Abl immunoprecipitations, and rabbit gamma globulin was used as control for Shc immunoprecipitations. Densitometry was used to compare levels of the various proteins and their modified forms and was performed on a minimum of two independent experiments.
Apoptosis and proliferation analysis.
Primary bone marrow transformants were analyzed by using an in situ cell death detection kit (Roche) according to the manufacturer's protocol. Infected bone marrow cells were washed and fixed in a 4% paraformaldehyde solution for 1 h. Fixed cells were washed with 1× PBS and permeabilized in 0.1% Triton X-100 in 0.1% sodium citrate for 2 min. Cells were incubated with terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) reaction mix for 1 h, washed, resuspended in PBS, and analyzed by flow cytometry. Primary bone marrow transformants were analyzed for cell expansion by cell counting using a hemocytometer. In some assays, 5 × 105 fully transformed pre-B cells were treated with a Mek inhibitor, PD98059 (Calbiochem), and counted using a hemocytometer; viability was determined by trypan blue exclusion.
Rac and Ras assays.
Levels of activated Rac were determined by monitoring recovery of active Rac1 via interaction with the Rac1 binding domain of Pak3 fused to glutathione S-transferase protein (GST-PBD) (15). To generate the GST fusion protein, log phase Escherichia coli cells expressing GST-PBD were grown for 2 h in the presence of 50 μg of ampicillin/ml and 0.1 mM isopropyl-β-d-thiogalactopyranoside. The cells were pelleted, lysed in buffer (50 mM Tris [pH 7.5], 20 mM MgCl2, 150 mM NaCl, 0.5% NP-40, 5 mM DTT), and subjected to three freeze-thaw cycles. The lysates were clarified by centrifugation at 11,000 rpm for 30 min, glutathione-Sepharose beads (Pharmacia) were added, and the lysates were incubated for 2 h on a rotating wheel at 4°C. The beads were centrifuged and washed with buffer (50 mM Tris [pH 7.5], 10 mM MgCl2, 200 mM NaCl, 2% NP-40, 10% glycerol, 1 mM dithiothreitol [DTT], 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin/ml). Rac was recovered from COS7 cells transfected with 10 μg of DNA expressing various forms of Shc and v-Abl in the pMIA vector along with 3 μg of pJ3 myc-Rac1 (15) by use of SuperFect reagent (QIAGEN). Cells were harvested 48 h posttransfection and incubated for 10 min in the buffer used to wash the beads. Clarified lysates were incubated with glutathione-Sepharose beads bound to GST-PBD for 30 min on a rotating wheel at 4°C. The samples were washed once in ice-cold buffer (25 mM Tris [pH 7.5], 30 mM MgCl2, 40 mM NaCl, 1% NP-40, 1 mM DTT), twice in ice-cold buffer (25 mM Tris [pH 7.5], 30 mM MgCl2, 40 mM NaCl, 1 mM DTT), boiled in sample buffer, and resolved on a 15% SDS gel. Western blots were probed with anti-Myc tag antibodies to detect the Myc-tagged Rac bound to GST-PBD.
Levels of activated Ras were determined using an EZ-Detect Ras activation kit (Pierce) according to the manufacturer's protocol. Recovery of active Ras occurs via interaction with the Ras binding domain of Raf fused to GST (GST-RBD) (56). 293T cells were transfected via the calcium phosphate method with 15 μg of DNA expressing v-Abl and different forms of Shc. After 24 h, serum-free medium was added. After an additional 24 h, the cells were incubated for 5 min in lysis buffer (25 mM Tris [pH 7.5], 5 mM MgCl2, 150 mM NaCl, 1% NP-40, 5% glycerol, 1 mM DTT, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 1 μg of aprotinin/ml and 1 μg of leupeptin/ml) and centrifuged, and the supernatant was added to a spin column containing a SwellGel immobilized glutathione disk and 80 μg of GST-RBD fusion protein. To some lysates, 0.1 mM GTPγS was added as a positive control. The spin column was incubated for 1 h at 4°C with gentle rocking, and the samples were washed three times with lysis buffer; sample buffer was added to the resin, and the samples were boiled to elute the bound Ras proteins. The eluted proteins were resolved on a 15% SDS gel and transferred to a membrane that was probed with anti-Ras antibodies to detect Ras bound to GST-RBD.
RESULTS
DN Shc binds to v-Abl and reduces Shc/Grb2 complex formation.
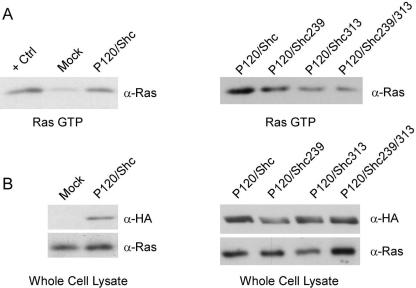
Earlier experiments have implicated signaling through Shc as important for v-Abl transformation and showed that Shc/Grb2 complexes form in the presence of an active v-Abl protein (35, 38); other investigations revealed that expression of ShcY239F, ShcY313F, and ShcY239/313F disrupts Shc/Grb2 association in several cell types (20, 45). To test the effects of these DN mutants in the Ab-MLV system, their ability to bind v-Abl and reduce Shc/Grb2 complex formation was examined by transfecting 293T cells with vectors expressing v-Abl and the various forms of Shc (Fig. 1A). Lysates were prepared 48 h later, immunoprecipitated with antibodies directed against v-Abl, and analyzed by Western blotting with anti-HA antibodies to verify that the DN forms of Shc bind v-Abl (Fig. 1B). HA-Shc was recovered from all samples, indicating that the mutations present in the DN forms do not block interaction with v-Abl. In addition, immunoprecipitation of the lysates with anti-Shc antibodies and Western blot analysis with anti-Grb2 antibodies revealed that three- to fourfold-lower levels of Grb2 were recovered when either ShcY239F or ShcY313F was expressed whereas reductions of 20- to 50-fold were observed with samples expressing ShcY239/313F (Fig. 1C). These data indicate that the DN Shc associates with v-Abl and interferes with the ability of v-Abl to stimulate formation of Shc/Grb2 complexes.
DN forms of Shc reduce Ab-MLV-mediated pre-B-cell transformation.
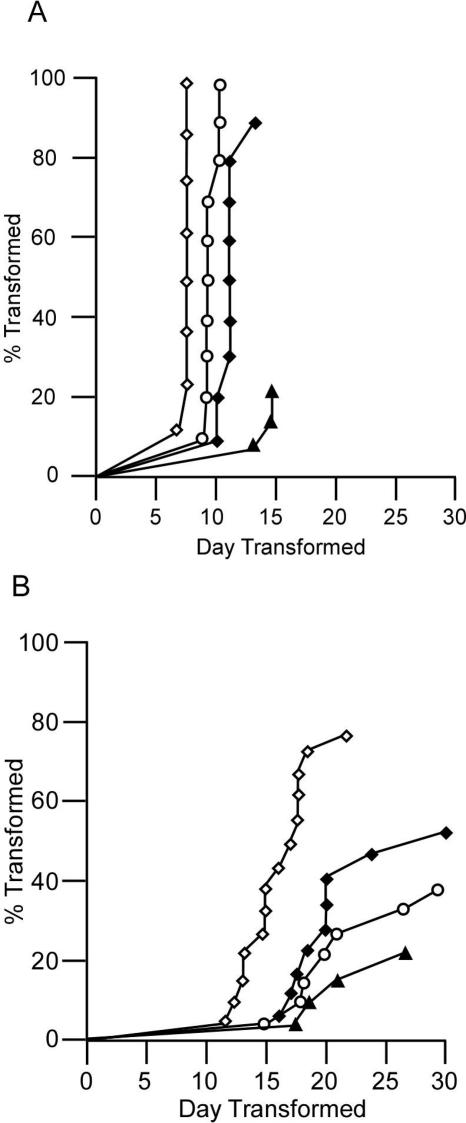
Shc has been postulated to be an important signaling intermediate in Abl-mediated transformation (16, 35, 38). To test this idea directly, bone marrow cells were infected with matched titer stocks expressing v-Abl and DN Shc, plated in liquid cultures, and monitored for pre-B-cell transformation. In this assay, cultures are considered transformed when the density of pre-B cells that can be readily subcultured exceeds 2 × 106 cells/ml (1, 26, 62); the number of dishes that transform and the time required for transformation are directly related to the titer of virus used for infection (Fig. 2A). Analyses of cultures infected with the different Shc viruses revealed that fewer cultures infected with viruses expressing DN Shc underwent transformation and that a longer time period was required before the culture could be scored as transformed (Fig. 2B). Consistent with the results obtained for Shc/Grb2 association, the effect of the ShcY239/313F mutant was greatest with only 4 of 18 cultures undergoing transformation after an extended latent period. These data indicate that functional Shc is required for efficient Ab-MLV-mediated pre-B-cell transformation.
FIG. 2.
Expression of DN Shc reduces Ab-MLV pre-B-cell transformation. (A) Bone marrow cultures were infected with dilutions of Ab-MLV (⋄, undiluted; ○, 1:10; ♦, 1:100; ▴, 1:1,000) and scored as transformed when they contained 2 × 106 pre-B transformants/ml (1, 26, 62). Each point represents an individual culture. The transformation curves were compared using a log-rank test: P120 undiluted versus P120 1:10, P < 0.0001; P120 undiluted versus P120 1:100, P < 0.0001; P120 undiluted versus P120 1:1,000, P < 0.0001. (B) Bone marrow cells were infected with matched titer stocks of P120/Shc or P120/DNShc viruses (⋄, P120/Shc; ♦, P120/ShcY239F; ○, P120/ShcY313F; ▴, P120/ShcY239/313F) and scored as transformed when they contained 2 × 106 cells per ml. Each point represents an individual culture. The transformation curves were compared using a log-rank test: P120/Shc versus P120/ShcY239F, P = 0.0187; P120/Shc versus P120/ShcY313F, P = 0.003; P120/Shc versus P120/ShcY239/313F, P = 0.0002.
DN Shc does not affect pre-B-cell survival, Akt phosphorylation, or c-Myc expression.
Ab-MLV-induced pre-B-cell transformation involves growth stimulation and suppression of apoptosis (8, 26), responses that might require functional Shc. PI3-K and Akt are particularly important for survival of Ab-MLV-transformed cells (55), and Shc can activate this pathway via Gab2, a molecule required for Bcr/Abl mediated-transformation (19, 46). To determine whether Shc facilitates v-Abl-mediated effects on cell survival, bone marrow cells were infected with matched titer stocks and plated in liquid cultures. The cells were collected after uninfected nonadherent cells had disappeared from the cultures, and apoptosis was evaluated by TUNEL and flow cytometry. Apoptosis was low in all cultures, and no differences in apoptosis were associated with expression of DN Shc (Fig. 3A). Consistent with these analyses, cell counting using phase microscopy also revealed the presence of low numbers of dying cells in cultures similar to the results seen with those analyzed by TUNEL. These results suggest that signaling mediated via Shc is not required to maintain the survival of Ab-MLV-infected pre-B cells.
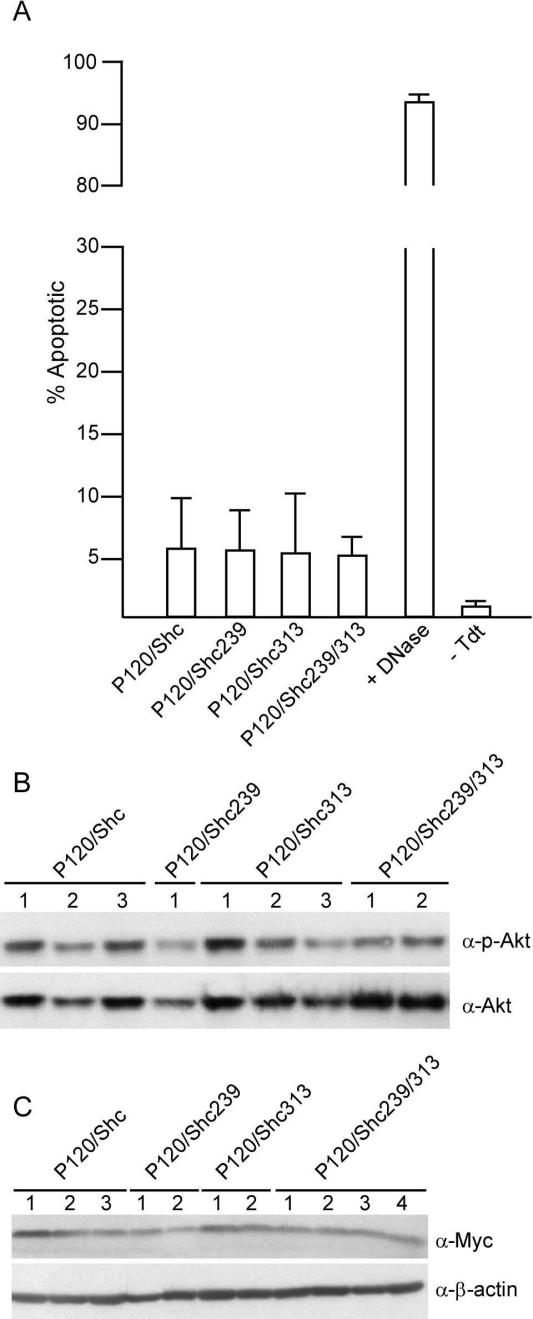
FIG. 3.
Cell survival, Akt phosphorylation, and Myc expression are not affected by DN Shc. (A) Bone marrow cells were infected with matched titer stocks of P120/Shc or P120/DNShc viruses and analyzed by TUNEL and flow cytometry at 17 days postinfection. Values obtained from three independent cultures infected with each virus were averaged; error bars indicate standard deviations. Infected cells treated with 3,000 U of DNase I/ml (+DNase) were used as a positive control, and cells incubated in the absence of TdT (−Tdt) served as a negative control. The data shown are representative of four experiments in which a total of 12 cultures were evaluated for each virus. (B) Bone marrow cultures infected with the different viruses were harvested when they reached a concentration of 2 × 106 pre-B cells/ml and analyzed by Western blotting with the indicated antibodies. Each lane represents an independent culture. (C) Bone marrow cultures as described for panel B were analyzed by Western blotting with the indicated antibodies. Each lane represents an independent culture. Densitometric analysis revealed that levels of p-Akt differed by less than 30% among the various samples. The panel shown is representative of two independent experiments.
To determine whether expression of DN Shc affects Akt activation, lysates were prepared from cultures infected with the different viruses and the expression and phosphorylation status of Akt was determined. Although the levels of Akt differed from sample to sample, the ratios of phosphorylated Akt to total Akt were similar in all cases (Fig. 3B). These data, coupled with those from the apoptosis assay, indicate that DN Shc does not disrupt signals that activate Akt and is not an important intermediate in the pathway(s) that affects survival of Ab-MLV-infected pre-B cells.
Increased expression of c-Myc is also associated with v-Abl expression (67), and DN Myc blocks Ab-MLV transformation (47). To determine whether DN Shc affects the pathway by which v-Abl stimulates c-Myc expression, lysates were prepared as described for the Akt experiment and analyzed by Western blotting for levels of c-Myc (Fig. 3C). Although the amounts of c-Myc recovered differed for the panels of cells tested, no consistent changes were observed that correlated with the presence of DN Shc. These data indicate that v-Abl signals that affect c-Myc expression do not travel through Shc.
DN Shc disrupts v-Abl-stimulated cell proliferation.
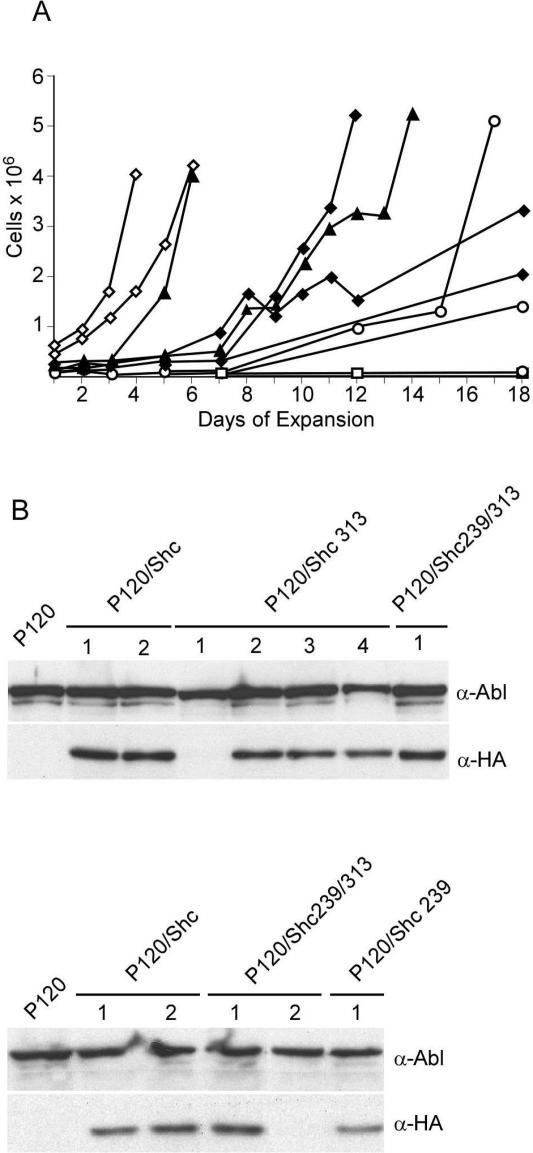
Signals transmitted via Shc can stimulate growth of some cell types through effects on Ras (54), an obligate intermediate in Ab-MLV transformation (48). To determine whether expression of DN Shc affects the ability of Ab-MLV-infected cells to grow, bone marrow cells were infected with matched titer stocks and plated in liquid cultures. Consistent with the transformation experiments, only a fraction of the cultures infected with viruses expressing v-Abl and DN Shc grew during the course of the experiment. Cells in the expanding dishes were counted using a hemocytometer on a regular basis for 3 weeks after control infected cultures had reached the stage of primary transformation. These analyses revealed that most cultures expressing DN Shc, including the representatives shown in Fig. 4A, expand more slowly than cultures expressing P120/Shc. Changes in growth profile correlate with loss of DN Shc expression in some instances (Fig. 4B). Among all cultures examined, 2 of 13 expressing ShcY313F and 3 of 9 expressing ShcY239/313F lost DN Shc expression; 1 of 19 cultures expressing the wild-type form of Shc also lost Shc expression. Other mutations that could compensate for continued expression of DN Shc, such as increased expression of Ras, may occur in other cultures. Taken together, these data indicate that Shc function is important for Ab-MLV-mediated cell proliferation.
FIG. 4.
DN Shc retards expansion of infected bone marrow. (A) Bone marrow cells were infected with matched titer stocks of P120/Shc or P120/DNShc viruses (⋄, P120/Shc; ♦, P120/Shc239; ○, P120/Shc313; ▴, P120/Shc239/313). Beginning 13 days postinfection, the cells were counted using a hemocytometer at regular intervals to assess the growth in the culture. (B) Infected cells were lysed, and Western blots were prepared and probed with the indicated antibodies. Each lane represents an independent culture. The lanes labeled P120 contain control lysates from a cell line infected with wild-type Ab-MLV.
DN Shc alters Ras activation.
Activation of Ras is critical for Ab-MLV transformation (48), and Shc/Grb2 association facilitates Ras activation by bringing the Ras exchange factor Sos into proximity with Ras (13, 44). To determine whether DN Shc interferes with the ability of v-Abl to activate Ras, 293T cells were transfected with DNA expressing v-Abl and the various forms of Shc and lysates were prepared 48 h later. The level of activated Ras was assessed by monitoring the amount of Ras that bound to GST-RBD (22). Expression of v-Abl increased Ras activation fourfold (Fig. 5A). Consistent with the ability of the different DN Shc forms to interfere with Grb2 association, expression of either ShcY239F or ShcY313F reduced Ras activation by about twofold; expression of ShcY239/313F reduced Ras activation threefold (Fig. 5B). Because even ShcY239/313F does not completely abolish Ras activation, these data suggest that a Shc-independent pathway affecting Ras activity probably exists. This idea is consistent with the observation that active Ras is required for expression of v-Abl-stimulated c-Myc (67), a downstream molecule that is not affected by DN Shc. Nonetheless, these results also indicate that Shc is required for efficient Ras activation by v-Abl.
FIG. 5.
Expression of DN Shc reduces v-Abl-mediated Ras activation. 293T cells were transfected with 5 μg of DNA expressing P120 and the various forms of Shc. Cells were serum starved and harvested 48 h posttransfection. (A) Activated Ras was recovered by binding to GST-RBD Raf and analyzed by Western blotting using α-Ras antibodies. Lysate from mock-transfected cells (Mock) was treated with GTPγS as the positive control (+Ctrl). (B) Whole-cell lysates were analyzed by Western blotting to control for the amounts of Ras and HA-Shc present in the samples.
MAP kinase is required for growth and is affected by DN Shc.
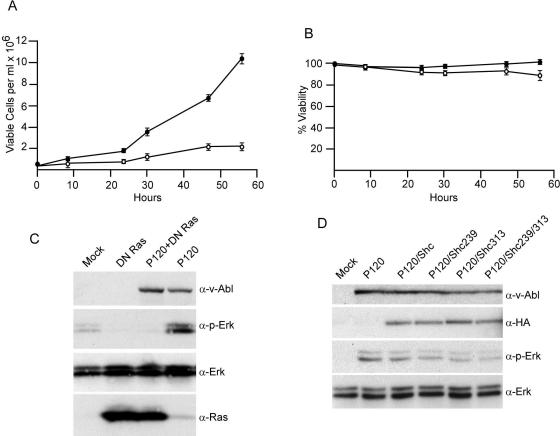
The MAP kinase pathway is one major route by which Ras transmits signals for growth (23). However, earlier studies of Ab-MLV-transformed NIH 3T3 cells (64) suggested that this pathway was not required for cell growth. To examine the role of the MAP kinase pathway in growth of transformed pre-B cells, three fully transformed, clonal cell lines expressing wild-type Ab-MLV were treated with the Mek inhibitor PD98059 (12) and analyzed for growth and survival. In all cases, including that of the representative shown (Fig. 6A), the treated samples grew more slowly than mock-treated controls. Consistent with a role for the MAP kinase pathway in growth, treatment with concentrations of PD98059 that were sufficient to arrest growth did not induce high levels of apoptosis (Fig. 6B).
FIG. 6.
DN Shc decreases Erk activation, an event associated with reduced cell growth. Pre-B cells fully transformed with wild-type Ab-MLV were plated at 5 × 105 cells/ml and treated with 70 μM PD98059 (○) or dimethyl sulfoxide (•); triplicate cultures were stained with trypan blue and counted using a hemocytometer on a regular basis. The numbers of viable cells (A) and the percentages of viability (B) were averaged for each time point; error bars represent standard deviations. These data are representative of experiments conducted with three independent cell lines that were each analyzed two to three times. (C) 293T cells were transfected with 5 μg of DNA expressing P120 and 2 μg of DNA expressing DN Ras, the cells were serum starved, and lysates prepared 48 h later were analyzed by Western blotting with the indicated antibodies. (D) 293T cells were transfected with 5 μg of DNA expressing P120 and the various forms of Shc and serum starved, and lysates prepared 48 h later were analyzed by Western blotting with the indicated antibodies.
To determine whether DN Shc interferes with the ability of v-Abl to activate Erk, 293T cells were transfected with DNA expressing v-Abl and the various forms of Shc, and the phosphorylation status of Erk was examined by Western blotting with anti-phospho-Erk antibodies. As expected (39, 64), expression of v-Abl increased Erk phosphorylation and DN Ras blocked v-Abl-dependent Erk phosphorylation, confirming that Ras is required for v-Abl signaling to Erk (Fig. 6C). Expression of Shc Y239F or Shc Y313F reduced the amount of v-Abl-induced Erk phosphorylation by about twofold, while expression of Shc Y239/313F reduced Erk phosphorylation by approximately threefold (Fig. 6D). Thus, DN Shc reduces but does not fully block v-Abl-mediated Erk phosphorylation, a feature that is consistent with the ability of the mutants to reduce but not fully block Ras activation and transformation.
DN Shc does not alter Rac activation.
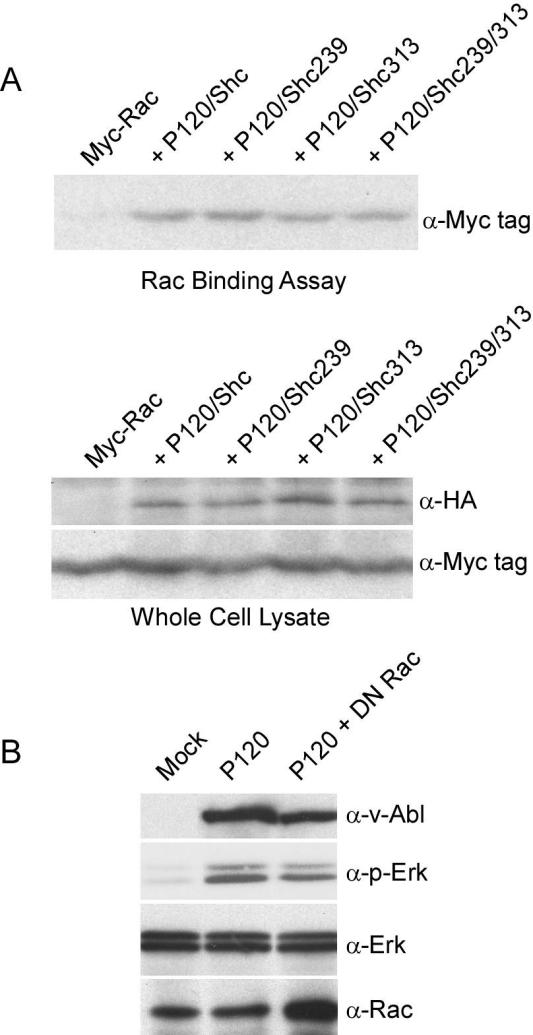
DN Shc does not block Erk activation completely, indicating that v-Abl can signal to Ras and the MAP kinase pathway in multiple ways. Indeed, earlier results indicated that the GTPase Rac is an important mediator of this process in Ab-MLV-transformed cells (42). To determine whether DN Shc interferes with the ability of v-Abl to activate Rac, COS7 cells were transfected with DNA expressing v-Abl, Myc-tagged Rac, and the various forms of Shc and lysates were prepared 48 h later. The level of activated Rac was determined using GST-PBD (3). Although these analyses confirmed that v-Abl activates Rac, they also demonstrate that DN Shc does not affect Rac activity (Fig. 7A). In contrast, expression of Myc-tagged DN Rac reduced Erk phosphorylation by about twofold (Fig. 7B), a reduction similar to that observed in another study (42). These data, coupled with those demonstrating the effect of DN Shc on Erk phosphorylation, indicate that v-Abl activates Erk via at least two pathways, one involving Rac and another involving signals transmitted through Shc. This redundancy likely contributes to the incomplete ability of DN Shc to block Ab-MLV transformation.
FIG. 7.
Expression of DN Shc does not alter v-Abl-mediated Rac activation. (A) COS7 cells were transfected with the 10 μg of DNA encoding P120 and the various forms of Shc and 3 μg of pJ3 myc-Rac1. Lysates prepared 48 h posttransfection were incubated with GST-PBD, and Rac was recovered and analyzed by Western blotting with the indicated antibodies. (B) 293T cells were transfected with 5 μg of DNA expressing P120 and 1 μg of DNA expressing DN Rac and serum starved, and lysates prepared 48 h later were analyzed by Western blotting with the indicated antibodies. The autoradiogram shown is representative of three independent experiments in which densitometry revealed that levels of p-Erk were decreased by approximately twofold in the presence of DN Rac.
DN Shc affects tyrosine phosphorylation of Raf.
The Rac-MAP kinase pathway is not affected by DN Shc. However, Raf connects Ras to the MAP kinase pathway via effects on Mek, an event that is dependent on phosphorylation of Raf at tyrosines 340 and 341 (14). Although the specific residues have not been identified, v-Abl has been shown to promote Raf phosphorylation (64), suggesting that signals transmitted via Raf might be involved in the circuit connecting v-Abl and Shc to the MAP kinase pathway. To determine whether v-Abl stimulates phosphorylation of Raf on tyrosines 340 and 341, Ab-MLV-transformed pre-B cells were treated for 4 h with the v-Abl kinase inhibitor STI-571 (33) and lysates were analyzed by Western blotting with antibodies specific for Raf that is tyrosine phosphorylated on residues 340 and 341. The treated samples contained about 10-fold less Raf modified by Y340/341 phosphorylation (Fig. 8A). Thus, an active v-Abl influences Raf activity by stimulating modifications that influence MAP kinase activity.
FIG. 8.
Raf tyrosine 340/341 phosphorylation affects v-Abl signals to Erk. (A) Transformed pre-B cells were treated with various concentrations of STI-571 for 4 h, lysed, and analyzed by Western blotting with the indicated antibodies. (B) 293T cells were transfected with 5 μg of DNA expressing P120 and 1 μg of DNA expressing the different Raf plasmids and serum starved, and lysates prepared 48 h later were analyzed by Western blotting with the indicated antibodies. The autoradiogram shown is representative of three independent experiments in which densitometry revealed that levels of p-Erk were decreased by approximately threefold in the presence of Raf Y340/341F. (C) 293T cells were transfected with 5 μg of DNA expressing P120 and the different forms of Shc and serum starved, and lysates prepared 48 h later were analyzed by Western blotting with the indicated antibodies.
Consistent with a role for Raf in the pathway from v-Abl to Erk, 293T cells transfected with v-Abl and Raf Y340/341F (57), a variant of Raf that cannot be phosphorylated on residues 340 and 341, displayed threefold-lower levels of Erk phosphorylation in comparison to expression of v-Abl and wild-type Raf (Fig. 8B). Furthermore, expression of Raf Y340/341D (57), a mutant that mimics constitutive phosphorylation, supplied the necessary signals to phosphorylate Erk in the presence of the kinase-deficient v-Abl mutant, P120D484N (38), indicating that phosphorylation of Raf on Y340/341 is important for v-Abl-mediated Erk phosphorylation.
To determine whether DN Shc is an intermediate in this pathway, Raf Y340/341 phosphorylation was examined in 293T cells that had been cotransfected with P120 and the various forms of Shc. As expected, expression of P120/Shc increased Raf Y340/341 phosphorylation. In contrast, expression of P120 and the different DN Shc forms reduced Raf Y340/341 phosphorylation. Reductions of about twofold were observed when Shc Y239F and Shc Y313F were present, and expression of Shc Y239/313F reduced Raf Y340/341 phosphorylation by approximately fivefold (Fig. 8C). These data, coupled with the results presented earlier, suggest that Shc affects v-Abl-mediated transformation by reducing signals transmitted to Ras and downstream to the MAP kinase pathway via the Raf protein.
DISCUSSION
These experiments have directly demonstrated that Shc is functionally important for Ab-MLV pre-B-cell transformation and reveal that disruption of the v-Abl-dependent Shc/Grb2/Sos complex by expression of DN Shc reduces the ability of infected pre-B cells to grow but not their ability to survive. Decreased growth potential correlates with decreases in activation of a subset of proteins that are stimulated in cells expressing v-Abl. Ras activity, Raf phosphorylation, and MAP kinase activation, events dependent on v-Abl-mediated stimulation, are all decreased in the presence of DN Shc. In contrast, expression of c-Myc and activation of Akt, two other important downstream consequences of v-Abl expression (47, 55), are not altered in the presence of DN Shc. These data illustrate how v-Abl interaction with one protein can affect only a subset of downstream intermediates, stimulate specific pathways, and trigger a portion of the events required for transformation.
Expression of DN Shc interferes with v-Abl-stimulated growth and correlates with decreased activation of the MAP kinase pathway, indicating that the primary function of this pathway in Ab-MLV-transformed pre-B cells is to promote cell growth. This idea is consistent with the observation that alteration of the FLVRES motif in the v-Abl SH2 domain reduces interaction with SH2 binding partners, including Shc, decreases Erk phosphorylation, and interferes with growth but not survival of transformed pre-B cells (17, 26). The ability of the Mek inhibitor PD98059 to block growth without disrupting cell survival also supports a critical role for the MAP kinase cascade in this aspect of Ab-MLV transformation. This paradigm appears to extend to the related Bcr/Abl oncoprotein, which also stimulates growth and survival via separate pathways (10, 11) that correlate with activation of the PI3-K/Akt and MAP kinase pathways (27, 34). However, in this instance, additional signaling cascades affect these circuits because Gab2, an adaptor molecule that can interact with Grb2 in an Shc-independent fashion, has been reported to activate both the PI3-K and MAP kinase pathways (46). In at least some cytokine-mediated signaling, interactions involving Gab2 appear to be involved in growth stimulation and not survival (19), even though both growth and survival appear affected by Gab2 in Bcr/Abl-expressing cells (46). Additional information on the possible role of Gab2 in v-Abl-mediated transformation is needed to determine how this protein may affect the process. Perhaps functionally similar pathways exist in Ab-MLV-transformed cells, but v-Abl does not contain a residue analogous to Y177, the presumptive Bcr/Abl docking site for the Grb2-Gab2 complex.
Despite the ability of all the DN Shc forms to decrease v-Abl-induced Shc/Grb2 association and downstream MAP kinase signaling, DN Shc did not completely abolish transformation. One factor that may contribute to this result is the inability of even Shc Y239/313F to completely block Shc/Grb2 association, a feature that has been noted by other investigators (20). The level of DN Shc expressed in the pre-B cells is similar to the levels of endogenous Shc. Because Shc and v-Abl association involves interaction with the amino-terminal portion of Shc (38), a region not altered in the DN alleles, DN Shc does not block association of Shc and v-Abl (our unpublished data). Thus, cells expressing the DN alleles contain some v-Abl proteins that are bound to endogenous Shc, complexes which may still transmit signals. Secondary mutations affecting the v-Abl-Shc pathway may be responsible in some instances. Several transformants no longer expressed DN Shc, and some others expressed higher levels of Ras than transformants that did not express DN Shc (our unpublished data). In addition, Shc-independent pathways to transformation may exist. v-Abl can activate Ras via p62Dok/RasGAP, another molecule that binds to the v-Abl SH2 domain (6, 26, 65), via the adapter protein Nck, which binds to the v-Abl COOH terminus (41), or via effects on the PI3-K pathway (52). Thus, these pathways might be sufficiently active in a subset of infected cells to allow transformation to occur.
The Shc Y239F and Y313F mutants have a reduced effect on transformation and signaling compared to the ShcY239/313F mutant. The increased effect of the double mutant is consistent with the ability of this form to markedly reduce association with Grb2 and with other studies (59, 61) which indicate that both Y239 and Y313 are important for association with Grb2. The observation that fewer transformants were derived from cultures expressing v-Abl and ShcY313F may suggest that this residue is more important than Y239, as originally suggested (36). However, very little difference in signal disruption levels was seen when this mutant was compared with the Y239F mutant. Nonetheless, information concerning the magnitude of the signal that is required to activate downstream pathways and stimulate cell growth is scant, and it is possible that effects in some cells are more pronounced than in others, leading to an overall decrease in transformation following bone marrow cell infection.
A functional Ras protein is required for v-Abl-mediated stimulation of Erk phosphorylation, but there are several ways in which Ras can be activated in Ab-MLV-infected cells. Several investigators have suggested that v-Abl promotes Erk activation via a Rac-dependent pathway (42, 64). Consistent with these experiments, DN Rac expression reduced Erk phosphorylation, but to a more modest degree than expression of DN Ras, suggesting that v-Abl also activates Erk via a Rac-independent pathway. In addition, DN Shc did not affect the ability of Rac to stimulate Erk phosphorylation, placing Shc on a Rac-independent pathway to Erk. A recent study indicating that v-Abl activates Rac via recruitment of molecules at the carboxy terminus (51) also supports a separation of the pathways that lead to Rac from those involving the SH2-interacting Shc protein.
Raf proteins are known to function downstream of Ras and activate the MAP kinase pathway in a number of systems (reviewed in reference 5), and earlier work (58) implicated Raf in v-Abl-stimulated Erk phosphorylation. The ability of v-Abl to stimulate Raf phosphorylation at Y340/341, a modification associated with colocalization of Raf with Ras at the plasma membrane (2, 28, 29), lends support to this idea. More importantly, the ability of DN Shc to interfere with this phosphorylation places Shc on the pathway by which v-Abl leads to Raf and to the MAP kinase pathway.
The ability of DN Shc to affect Raf phosphorylation and the effects of DN Shc on growth of Ab-MLV-infected pre-B cells identify Raf as an important intermediate on the growth pathways required for v-Abl transformation. Raf has previously been associated with both v-Abl- and Bcr/Abl-mediated apoptotic suppression (27, 34, 64), an event involving translocation of Raf to the mitochondria that does not influence Erk phosphorylation. Phosphorylation of Raf at serine 338 via Pak has recently been associated with a mitochondrion-dependent role for Raf in endothelial cell survival (2) and may be important in Abl-mediated responses as well. Indeed, v-Abl also promotes phosphorylation of Raf at serine 338 (our unpublished observations), suggesting that differential modification of Raf mediates different subcellular localizations with distinct biological consequences. The analyses of Raf modification in the presence of DN Shc suggest that v-Abl affects Raf in two distinct ways by stimulating phosphorylation of either serine 338 or tyrosines 340 and 341. Depending on the modification, Raf functions at distinct cellular locations to mediate different biological responses. The Ab-MLV transformation system provides a useful tool to understand the way v-Abl and other oncoproteins impose specificity on such downstream signals, leading to different aspects of the transformation process.
Acknowledgments
We thank Larry Feig, Brent Cochran, Jeffrey Frost, and Anthony De Franco for the gifts of plasmids used in these experiments and Caleb Lee for helpful discussions.
This work was supported by CA 24420 from the National Cancer Institute.
REFERENCES
- 1.Afar, D. E. H., A. Goga, J. McLaughlin, O. N. Witte, and C. L. Sawyers. 1994. Differential complementation of Bcr-Abl point mutants with c-Myc. Science 264:424-426. [DOI] [PubMed] [Google Scholar]
- 2.Alavi, A., J. D. Hood, R. Frausto, D. G. Stupack, and D. A. Cheresh. 2003. Role of Raf in vascular protection from distinct apoptotic stimuli. Science 301:94-96. [DOI] [PubMed] [Google Scholar]
- 3.Bagrodia, S., S. J. Taylor, C. L. Creasy, J. Chernoff, and R. A. Cerione. 1995. Identification of a mouse p21Cdc42/Rac activated kinase. J. Biol. Chem. 270:22731-22737. [DOI] [PubMed] [Google Scholar]
- 4.Campell, K. S., E. Orgis, B. Burke, W. Su, K. R. Auger, B. J. Druker, B. S. Schaffhausen, T. M. Roberts, and D. C. Pallas. 1994. Polyoma middle tumor antigen interacts with Shc protein via the NPTY (Asn-Pro-Thr-Tyr) motif in middle tumor antigen. Proc. Natl. Acad. Sci. USA 91:6344-6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campell, S. L., R. Khosravi-Far, K. L. Rossman, G. J. Clark, and C. J. Der. 1998. Increasing complexity of Ras signaling. Oncogene 17:1395-1413. [DOI] [PubMed] [Google Scholar]
- 6.Carpino, N., D. Wisniewski, A. Strife, D. Marshak, R. Kobayashi, B. Stillman, and B. Clarkson. 1997. p62(dok): a constitutively tyrosine-phosphorylated, GAP-associated protein in chronic myelogenous leukemia progenitor cells. Cell 88:197-204. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhary, A., W. G. King, M. D. Mattalian, J. A. Frost, B. Diaz, D. K. Morrison, M. H. Cobb, M. S. Marshall, and J. S. Brugge. 2000. Phosphatidylinositol 3-kinase regulates Raf1 through Pak phosphorylation of serine 338. Curr. Biol. 10:551-554. [DOI] [PubMed] [Google Scholar]
- 8.Chen, Y. Y., and N. Rosenberg. 1992. Lymphoid cells transformed by Abelson virus require the v-abl protein tyrosine kinase only during early G1. Proc. Natl. Acad. Sci. USA 89:6683-6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chesebro, B., K. Wehrly, M. Cloyd, W. Britt, J. Portis, J. Collins, and J. Nishio. 1981. Characterization of mouse monoclonal antibodies specific for Friend murine leukemia virus-induced erythroleukemia cells: friend-specific and FMR-specific antigens. Virology 112:131-144. [DOI] [PubMed] [Google Scholar]
- 10.Cortez, D., L. Kadlec, and A. M. Pendergast. 1995. Structural and signaling requirements for BCR-ABL-mediated transformation and inhibition of apoptosis. Mol. Cell. Biol. 15:5531-5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortez, D., G. Reuther, and A. M. Pendergast. 1997. The Bcr-Abl tyrosine kinase activates mitogenic signaling pathways and stimulates G1-to-S phase transition in hematopoietic cells. Oncogene 15:2333-2342. [DOI] [PubMed] [Google Scholar]
- 12.Dudley, D. T., L. Pang, S. J. Decker, A. J. Bridges, and A. R. Saltiel. 1995. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. USA 92:7686-7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egan, S. E., B. W. Giddings, M. W. Brooks, L. Buday, A. M. Sizeland, and R. A. Weinberg. 1993. Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature (London) 363:45-51. [DOI] [PubMed] [Google Scholar]
- 14.Fabian, J. R., I. O. Daar, and D. K. Morrison. 1993. Critical tyrosine residues regulate the enzymatic and biological activity of Raf-1 kinase. Mol. Cell. Biol. 13:7170-7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glaven, J. A., I. Whitehead, S. Bagrodia, R. Kay, and R. A. Cerione. 1999. The Dbl-related protein, Lfc, localizes to microtubules and mediates the activation of Rac signaling pathways in cells. J. Biol. Chem. 274:2279-2285. [DOI] [PubMed] [Google Scholar]
- 16.Goga, A., J. McLaughlin, D. E. H. Afar, D. C. Saffran, and O. N. Witte. 1995. Alternative signals to Ras for hematopoietic transformation by the Bcr-Abl oncogene. Cell 82:981-988. [DOI] [PubMed] [Google Scholar]
- 17.Gong, L., I. Unnikrishnan, A. Raghavan, K. Parmar, and N. Rosenberg. 2004. Active Akt and functional p53 modulate apoptosis in Abelson virus-transformed pre-B cells. J. Virol. 78:1636-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gotoh, N., A. Tojo, and M. Shibuya. 1996. A novel pathway from phosphorylation of tyrosine residues 239/240 of Shc, contributing to suppress apoptosis by IL-3. EMBO J. 15:6197-6204. [PMC free article] [PubMed] [Google Scholar]
- 19.Gu, H., H. Maeda, J. J. Moon, J. D. Lord, M. Yoakim, B. H. Nelson, and B. G. Neel. 2000. New role for Shc in activation of the phosphatidylinositol 3-kinase/Akt pathway. Mol. Cell. Biol. 20:7109-7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harmer, S. L., and A. L. DeFranco. 1997. Shc contains two Grb2 binding sites needed for efficient formation of complexes with SOS in lymphocytes. Mol. Cell. Biochem. 17:4087-4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hawley, R. G., F. H. Lieu, A. Z. Fong, and T. S. Hawley. 1994. Versatile retroviral vectors for potential use in gene therapy. Gene Ther. 1:136-138. [PubMed] [Google Scholar]
- 22.Hermann, C., G. A. Martin, and A. Wittinghofer. 1995. Quantitative analysis of the complex between p21ras and the Ras-binding domain of the human Raf-1 protein kinase. J. Biol. Chem. 270:2901-2905. [DOI] [PubMed] [Google Scholar]
- 23.Khosravi-Far, R., S. Campbell, K. L. Rossman, and C. J. Der. 1998. Increasing complexity of Ras signal transduction: involvement of Rho family proteins. Adv. Cancer Res. 72:57-107. [DOI] [PubMed] [Google Scholar]
- 24.Lord, J. D., B. C. McIntosh, P. D. Greenberg, and B. H. Nelson. 1998. The IL-2 receptor promotes proliferation, bcl-2 and bcl-x induction, but not cell viability through the adapter molecule Shc. J. Immunol. 161:4627-4633. [PubMed] [Google Scholar]
- 25.Lowenstein, E. J., R. J. Daly, A. G. Batzer, W. Li, B. Margolis, R. Lammers, A. Ullrich, E. Y. Skolink, D. Bar-Sagi, and J. Schlessinger. 1992. The SH2 and SH3 domain-containing protein Grb2 links receptor tyrosine kinases to ras signaling. Cell 70:431-442. [DOI] [PubMed] [Google Scholar]
- 26.Mainville, C., K. Parmar, I. Unnikrishnan, G. D. Raffel, L. Gong, and N. Rosenberg. 2001. Temperature-sensitive transformation by an Abelson virus mutant encoding an altered SH2 domain. J. Virol. 75:1816-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Majewski, M., M. Nieborowska-Skorska, P. Salomoni, A. Slupianek, K. Reiss, R. Trotta, B. Calabretta, and T. Skorski. 1999. Activation of mitochondrial Raf-1 is involved in the antiapoptotic effects of Akt. Cancer Res. 59:2815-2819. [PubMed] [Google Scholar]
- 28.Marais, R., Y. Light, H. F. Paterson, and C. J. Marshall. 1995. Ras recruits Raf-1 to the plasma membrane for activation by tyrosine phosphorylation. EMBO J. 14:3136-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mason, C. S., C. J. Springer, R. G. Cooper, G. Superti-Furga, C. J. Marshall, and R. Marais. 1999. Serine and tyrosine phosphorylations cooperate in Raf-1, but not B-Raf activation. EMBO J. 18:2137-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayer, B. J., P. K. Jackson, R. A. Van Etten, and D. Baltimore. 1992. Point mutations in the abl SH2 domain coordinately impair phosphotyrosine binding in vitro and transforming activity in vivo. Mol. Cell. Biol. 12:609-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mercalli, E., S. Ghizzoni, E. Arighi, L. Alberti, S. R., M. T. Radice, M. L. Gishizky, M. A. Pierotti, and M. G. Borrello. 2001. Key role of Shc signaling in the transforming pathway triggered by Ret/ptc2 oncoprotein. Oncogene 20:3475-3485. [DOI] [PubMed] [Google Scholar]
- 32.Mettouchi, A., S. Klein, W. Gou, M. Lopez-Lago, E. Lemichez, J. K. Westwick, and F. G. Giancotti. 2001. Integrin-specific activation of Rac controls progression through the G1 phase of the cell cycle. Mol. Cell 8:115-127. [DOI] [PubMed] [Google Scholar]
- 33.Muljo, S. A., and M. S. Schlissel. 2003. A small molecular Abl kinase inhibitor induces differentiation of Abelson-virus-transformed pre-B cell lines. Nat. Immunol. 4:31-37. [DOI] [PubMed] [Google Scholar]
- 34.Neshat, M. S., A. B. Raitano, H. G. Wang, J. C. Reed, and C. L. Sawyers. 2000. The survival function of the Bcr-Abl oncogene is mediated by Bad-dependent and -independent pathways: roles for phosphatidylinositol 3-kinase and Raf. Mol. Cell. Biol. 20:1179-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Owen-Lynch, P. J., A. K. Wong, and A. D. Whetton. 1995. v-Abl-mediated apoptotic suppression is associated with SHC phosphorylation without concomitant mitogen-activated protein kinase activation. J. Biol. Chem. 270:5956-5962. [DOI] [PubMed] [Google Scholar]
- 36.Pelicci, G., L. Lanfrancone, F. Grignani, J. McGlade, F. Cavallo, G. Forni, I. Nicoletti, F. Grignani, T. Pawson, and P. G. Pelicci. 1992. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell 70:93-104. [DOI] [PubMed] [Google Scholar]
- 37.Pelicci, G., L. Lanfrancone, A. E. Salcini, A. Romano, G. Mele, M. Grazia Borrello, O. Segatto, P. P. Di Fiore, and P. G. Pelicci. 1995. Constitutive phosphorylation of Shc proteins in human tumors. Oncogene 11:899-907. [PubMed] [Google Scholar]
- 38.Raffel, G. D., K. Parmar, and N. Rosenberg. 1996. In vivo association of v-Abl with Shc mediated by a non-phosphotyrosine-dependent SH2 interaction. J. Biol. Chem. 271:4640-4645. [DOI] [PubMed] [Google Scholar]
- 39.Raitano, A. B., J. R. Halpern, T. M. Hambuch, and C. L. Sawyers. 1995. The Bcr-Abl leukemia oncogene activates Jun kinase and requires Jun for transformation. Proc. Natl. Acad. Sci. USA 92:11746-11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ravichandran, K. S. 2001. Signaling via Shc family adapter proteins. Oncogene 20:6322-6330. [DOI] [PubMed] [Google Scholar]
- 41.Ren, R., Z.-S. Ye, and D. Baltimore. 1994. Abl protein-tyrosine kinase selects the crk adapter as a substrate using SH3-binding sites. Genes Dev. 8:783-795. [DOI] [PubMed] [Google Scholar]
- 42.Renshaw, M. W., E. Lea-Chou, and J. Y. J. Wang. 1996. Rac is required for v-Abl tyrosine kinase to activate mitogenesis. Curr. Biol. 6:76-83. [DOI] [PubMed] [Google Scholar]
- 43.Rosenberg, N., and O. N. Witte. 1988. The viral and cellular forms of the Abelson (abl) oncogene. Adv. Virus Res. 35:39-81. [DOI] [PubMed] [Google Scholar]
- 44.Rozakis-Adcock, M., J. McGlade, G. Mbamulu, G. Pelicci, R. J. Daly, W. Li, A. G. Batzer, S. Thomas, J. Brugge, P. G. Pelicci, J. Schlessinger, and T. Pawson. 1993. Association of the Shc and Grb2/Sem5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinases. Nature (London) 260:689-692. [DOI] [PubMed] [Google Scholar]
- 45.Salcini, A. E., J. McGlade, G. Pelicci, I. Nicoletti, T. Pawson, and P. G. Pelicci. 1994. Formation of Shc-Grb2 complexes is necessary to induce neoplastic transformation by overexpression of Shc proteins. Oncogene 9:2827-2836. [PubMed] [Google Scholar]
- 46.Sattler, M., M. G. Mohi, Y. B. Pride, L. R. Quinnan, N. A. Malouf, K. Podar, F. Gesbert, H. Iwasaki, S. Li, R. A. Van Etten, H. Gu, J. D. Griffin, and B. G. Neel. 2002. Critical role for Gab2 in transformation by BCR/ABL. Cancer Cell 1:479-492. [DOI] [PubMed] [Google Scholar]
- 47.Sawyers, C. L., W. Callahan, and O. N. Witte. 1992. Dominant negative myc blocks transformation by abl oncogenes. Cell 70:901-910. [DOI] [PubMed] [Google Scholar]
- 48.Sawyers, C. L., J. McLaughlin, and O. N. Witte. 1995. Genetic requirement for ras in the transformation of fibroblasts and hematopoietic cells by the bcr-abl oncogene. J. Exp. Med. 181:307-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schiff-Maker, L., M. C. Burns, J. B. Konopka, S. Clark, O. N. Witte, and N. Rosenberg. 1986. Monoclonal antibodies specific for v-abl- and c-abl-encoded molecules. J. Virol. 57:1182-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simon, A. R., H. G. Vikis, S. Stewart, B. L. Fanburg, B. H. Cochran, and K. L. Guan. 2000. Regulation of STAT3 by direct binding to the Rac1 GTPase. Science 290:144-147. [DOI] [PubMed] [Google Scholar]
- 51.Sini, P., A. Cannas, A. J. Koleske, P. P. Di Fiore, and G. Scita. 2004. Abl-dependent tyrosine phosphorylation of Sos-1 mediates growth-factor-induced Rac activation. Nat. Cell Biol. 6:268-274. [DOI] [PubMed] [Google Scholar]
- 52.Skorski, T., A. Bellacosa, M. Nieborowska-Skorska, M. Majewski, R. Martinez, J. K. Choi, R. Trotta, P. Wlodarski, D. Perrotti, T. O. Chan, M. A. Wasik, P. N. Tsichlis, and B. Calabretta. 1997. Transformation of hematopoietic cells by BCR/ABL requires activation of a PI-3K/Akt-dependent pathway. EMBO J. 16:6151-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stevenson, L. E., and A. R. Frackelton. 1998. Constitutively tyrosine phosphorylated p52 Shc in breast cancer cells: correlation with ErbB2 and p66 Shc expression. Breast Cancer Res. Treat. 49:119-128. [DOI] [PubMed] [Google Scholar]
- 54.Stevenson, L. E., K. S. Ravichandran, and R. A. Frackelton. 1999. Shc dominant negative disrupts cell cycle progression in both G0-G1 and G2-M of ErbB2-positive breast cancer cells. Cell Growth Diff. 10:61-71. [PubMed] [Google Scholar]
- 55.Tang, X., C. P. Downes, A. D. Whetton, and P. J. Owen-Lych. 2000. Role of phosphatidylinositol 3-kinase and specific protein kinase B isoforms in the suppression of apoptosis mediated by the Abelson protein-tyrosine kinase. J. Biol. Chem. 275:13142-13148. [DOI] [PubMed] [Google Scholar]
- 56.Taylor, S. J., and D. Shalloway. 1996. Cell cycle-dependent activation of Ras. Curr. Biol. 6:1621-1627. [DOI] [PubMed] [Google Scholar]
- 57.Tran, N. H., and J. A. Frost. 2003. Phosphorylation of Raf-1 by p21-activated kinase 1 and Src regulates Raf-1 autoinhibition. J. Biol. Chem. 278:11221-11226. [DOI] [PubMed] [Google Scholar]
- 58.Troppmair, J., J. T. Bruder, H. Munoz, P. A. Lloyd, J. Kyriakis, P. Banerjee, J. Avruch, and U. R. Rapp. 1994. Mitogen-activated protein kinase/extracellular signal-regulated protein kinase activation by oncogenes, serum and 12-O-tetradecanoylphorbol-13-acetate requires Raf and is necessary for transformation. J. Biol. Chem. 269:7030-7035. [PubMed] [Google Scholar]
- 59.van der Geer, P., S. Wiley, G. D. Gish, and T. Pawson. 1996. The Shc adaptor protein is highly phosphorylated at conserved, twin tyrosine residues (Y239/240) that mediate protein-protein interactions. Curr. Biol. 6:1435-1444. [DOI] [PubMed] [Google Scholar]
- 60.Van Parijs, L., Y. Refaeli, J. D. Lord, B. H. Nelson, A. K. Abbas, and D. Baltimore. 1999. Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation induced cell death. Immunity 11:281. [DOI] [PubMed] [Google Scholar]
- 61.Walk, S. F., M. E. March, and K. S. Ravichandran. 1998. Roles of Lck, Syk and Zap-70 tyrosine kinases in TCR-mediated phosphorylation of the adapter protein Shc. Eur. J. Immunol. 8:2265-2275. [DOI] [PubMed] [Google Scholar]
- 62.Warren, D., D. Griffin, C. A. Mainville, and N. Rosenberg. 2003. The extreme carboxyl terminus of v-Abl protein is required for lymphoid transformation by Abelson virus. J. Virol. 77:4617-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Warren, D., A. J. Heilpern, K. Berg, and N. Rosenberg. 2000. The carboxyl terminus of v-Abl protein can augment SH2 domain function. J. Virol. 74:4495-4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weissinger, E. M., G. Eissner, C. Grammer, A. Fackler, B. Haefner, L. S. Yoon, K. S. Lu, A. Bazarov, J. M. Sedivy, H. Mischak, and W. Kolch. 1997. Inhibition of the Raf-1 kinase by cyclin AMP agonists causes apoptosis of v-abl-transformed cells. Mol. Cell. Biol. 17:3229-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamanashi, Y., and D. Baltimore. 1997. Identification of the Abl- and rasGAP-associated 62 kDa protein as a docking protein, Dok. Cell 88:205-211. [DOI] [PubMed] [Google Scholar]
- 66.Zou, X., and K. Calame. 1999. Signaling pathways activated by oncogenic forms of Abl tyrosine kinase. J. Biol. Chem. 274:18141-18144. [DOI] [PubMed] [Google Scholar]
- 67.Zou, X., S. Rudchenko, K.-K. Wong, and K. Calame. 1997. Induction of c-myc transcription by the v-Abl tyrosine kinase requires Ras, Raf1, and cyclin-dependent kinases. Genes Dev. 11:654-662. [DOI] [PubMed] [Google Scholar]