Abstract
BACKGROUND
Coma after acute head injury is always alarming. Depending on the type of injury, immediate treatment may be life-saving. About a quarter of a million patients are treated for traumatic brain injury in Germany each year. Treatment recommendations must be updated continually in the light of advancing knowledge.
METHODS
This review of treatment recommendations, prognostic factors, and the pathophysiology of coma after acute head injury is based on a 2015 German guideline for the treatment of head injury in adults and on pertinent publications retrieved by a selective search in PubMed for literature on post-traumatic coma.
RESULTS
As soon as the vital functions have been secured, patients with acute head injury should undergo cranial computed tomography, which is the method of choice for identifying intracranial injuries needing immediate treatment. Patients who have an intracranial hematoma with mass effect should be taken to surgery at once. The prognosis is significantly correlated with the patient’s age, the duration of coma, accompanying neurological manifestations, and the site of brain injury. The case fatality rate of patients who have been comatose for 24 hours and who have accompanying lateralizing signs, a unilaterally absent pupillary light reflex, or hemiparesis lies between 30% and 50%. This figure rises to 50-60% in patients with abnormal extensor reflexes and to over 90% in those with bilaterally absent pupillary light reflexes. Current neuropathological and neuroradiological studies indicate that coma after acute head injury is due to reversible or irreversible dysfunction of the brainstem.
CONCLUSION
Brain tissue can tolerate ischemia and elevated pressure only for a very limited time. Comatose head-injured patients must therefore be evaluated urgently to determine whether they can be helped by the surgical removal of a hematoma or by a decompressive hemicraniectomy.
In 2014, 267 186 patients were admitted to German hospitals with an intracranial injury (1). Traumatic brain injury is a common cause of death worldwide at all ages up to young adulthood (2, 3). This article is intended to provide an overview of the diagnosis, treatment, prognosis, and causative mechanisms of post-traumatic coma. It is based on the current guideline on the treatment of head injury in adults that was issued in 2015 by the Association of the Scientific Medical Societies in Germany (Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften [AWMF]) (4), produced under the leadership of the German Neurosurgical Society (Deutsche Gesellschaft für Neurochirurgie) in collaboration with the German societies for anesthesiology and intensive care, neurology, neuroradiology, and trauma surgery. Further information in this article is derived from publications up to 2015 that were retrieved by a selective literature search in PubMed for the key words “unconsciousness,” “coma,” “traumatic brain injury,” and “prognosis.” Most of the recommendations in the guideline are not based on validated scientific evidence; many measures have been assigned a high recommendation level in the light of decades of consistent clinical experience, even though their efficacy cannot be demonstrated in a clinical trial—for example, the recommendation that intracranial hematomas with mass effect in patients with severe neurological deficits should be surgically evacuated as soon as possible.
THE DEFINITION OF UNCONSCIOUSNESS
The terms “unconsciousness” and “coma” are used synonymously by international convention, regardless of the duration of the condition (5). Both refer to a state of absent perception of oneself and one’s environment, from which one cannot be aroused. The Glasgow Coma Scale (GCS) (6), first proposed in 1974, does not contain a precise definition of coma. An international working group of the Neurotraumatology Committee of the World Federation of Neurological Surgeons recommends the following clinical definition of coma: the patient does not follow commands and does not open his or her eyes either spontaneously or in response to a noxious stimulus. Spontaneous movements are compatible with the definition of coma. In consideration of pertinent neurological disturbances, four grades of severity of coma have been proposed (Box 1) (7, 8). Coma, by this definition, corresponds to a Glasgow Coma Score of 7 or less.
BOX 1. Coma Classification of the World Federation of Neurosurgical Societies*.
Grade I Coma without any of the neurological disturbances listed below
Grade II Coma with lateralizing signs, unilateral fixed and dilated pupil, or hemiparesis
Grade III Coma with pathological extensor responses
Grade IV Coma with bilateral fixed and dilated pupils
PRE-HOSPITAL TREATMENT AT THE SCENE OF THE ACCIDENT
First aid for an unconscious trauma victim at the scene of the accident is directed toward securing the vital functions (ABC = airway, breathing, circulation). Manifest bleeding must be controlled. If the neurological examination reveals that the patient is comatose, a consensus of medical opinion holds that the patient should be intubated without delay (4, 9, 10), as clinical experience has shown that swallowing and breathing are often impaired in comatose patients, and their airways are in danger of inhalation of blood, secretions, or vomitus. The neurological examination, as described below, is the basis for further interventions after arrival in the hospital.
The Glasgow Coma Score alone is insufficient documentation of the patient’s neurological condition. In an unconscious patient, the pupillary responses must be examined, as well as the motor function of all four limbs (tested separately). The patient’s (lack of) spontaneous movement, response to noxious stimuli, and any pathological flexion or extension responses are to be noted. These findings are crucially important, as they have a bearing on the differential diagnostic considerations in patients with injuries of the brain and spinal cord. Post-traumatic coma is often due to a brain lesion, but it can also be due to an intoxication, endocrine or metabolic disturbances, hypoxia, brain hemorrhage, or other causes (4).
TRANSPORT OF THE COMATOSE PATIENT
The comatose patient, when first seen, should be assumed to have a potentially life-threatening dysfunction of the brain and should therefore be transported immediately to a hospital in which computerized tomography (CT) and neurosurgical treatment are available around the clock. Magnetic resonance imaging (MRI) should also be available, because there may be an accompanying spinal cord injury, and MlRI should be possible for intubated patients. If there is more than one suitable hospital in the area, the shortest transport time (not distance) determines the choice. For the timely and successful removal of life-threatening intracranial hematomas, the interval from the accident to the CT should be less than one hour if possible, as hematomas can expand over time (11, 12). There are scant evidence-based recommendations about drugs to be given during transport. The term “analgosedation” (translation of the common German term Analgosedierung) is misleading and not in general use internationally. The unconscious patient cannot perceive any internal or external stimuli and thus cannot have pain, in the sense of a conscious, unpleasant experience of noxious stimuli. Thus, counteracting pain in coma with analgesic drugs makes little sense. The respiratory-depressive effect of some potent analgesic drugs can be exploited successfully in individual cases of comatose head-injured patients who breathe against the ventilator.
There is no evidence from clinical trials that any further sedation helps (4). In particular, medication to reduce intracranial pressure has not been shown to improve the outcome of treatment. The practical disadvantage of sedation is that it impairs the physician’s ability to assess motor function and thereby detect a hemiparesis or pathological flexion and extension responses, which may indicate acute brainstem dysfunction. If the patient shows signs of transtentorial herniation (increasing impairment of consciousness leading to coma, fixed and dilated pupils, pathological flexion and extension responses), then the administration of osmodiuretics such as mannitol or hyperosmolar saline and/or hyperventilation can acutely lower the intracranial pressure at the scene of the accident, during transport, or on the way to the CT scanner (13– 15). A recommendation to give glucocorticoids at the scene of the accident to patients in post-traumatic coma was issued in 1980 and remained in force for more than two decades; a study published in 2005 (16), however, showed that this practice is contraindicated, as it is associated with significantly higher mortality. 10% of comatose brain-injured patients also have cervical spine injuries. It is thus recommended in many guidelines from around the world (1982 to present) that the cervical spine of all patients found in a coma should be immobilized prophylactically with a hard collar (4).
IN-HOSPITAL TREATMENT OF THE COMATOSE HEAD-INJURED PATIENT
The method of choice for detecting intracranial injuries is head CT. Spiral CT of the entire body is practical, as there is generally no other way to exclude further, extracranial injuries in comatose patients (4). This study takes less time than conventional x-rays while giving more information.
The CT should be obtained as soon as the patient arrives in the hospital, except when the vital signs, circulation, or breathing require immediate life-saving attention. The care of polytraumatized patients should be interdisciplinary. The sequence of treatment of multiple injured organ systems is based on urgency; more than one operation can be performed at the same time if necessary.
The current literature does not indicate that any further sedating or intracranial-pressure-lowering drugs given to the unconscious patient improve the outcome. Barbiturate coma was said to do so (17), but this was not confirmed by a comprehensive analysis (18). Sedation to secure the airway may be needed for practical reasons.
Invasive intracranial pressure (ICP) monitoring can provide a useful early warning of rising ICP; if it is performed via an intraventricular catheter, the ICP can be lowered by drainage of cerebrospinal fluid (CSF). Maintenance of the cerebral perfusion pressure (CPP, defined as the difference between mean arterial blood pressure and intracranial pressure [ABP – ICP]) in the normal range is effected mainly by avoiding arterial hypotension, rather than by lowering the ICP with drugs. In critical situations, drugs often do not suffice to lower the ICP adequately.
There has not yet been any documentation of better outcomes from treatment guided by ICP or CPP measurement, compared to treatment guided by clinical neurological surveillance without any ICP or CPP measurement (19– 21). A consensus holds that intracranial hematomas with mass effect should be surgically evacuated without delay (4). For penetrating injuries, and injuries of the frontal skull base with CSF leakage, it may be advisable not to proceed to surgical repair immediately, as acute post-traumatic brain edema increases the operative risk. The most effective way to lower ICP is decompressive hemicraniectomy (22). Even though the utility of this measure with respect to outcome is debated, and sequelae after reimplantation of the skull flap are not uncommon, there is nonetheless convincing evidence in its favor from observations made in individual cases.
Surgery for accompanying injuries that are not life-threatening should be deferred until the patient has recovered sufficiently from the head injury to be considered stable (23). Induced hypothermia as a treatment for patients with traumatic brain injury has been studied repeatedly over the past five decades, but no benefit has ever been convincingly shown (24, e1). There is likewise insufficient evidence supporting the utility of hyperbaric oxygen therapy (e2).
THE PROGNOSIS OF PATIENTS WITH POST-TRAUMATIC COMA
In the early phase after traumatic brain injury, the patient’s emergence from coma is just as unpredictable as a sudden worsening of consciousness from fully alert to comatose. As a rule, it is not possible to prognosticate reliably on clinical grounds alone in the first 24 hours after injury. An early clinical classification of traumatic brain injury as mild, moderate, or severe, determined on the basis of the Glasgow Coma Score at the time of the accident or at variable times thereafter (6, 12, or 24 hours), is internationally in widespread use but has not been found to be adequately correlated with outcomes and therefore provides no practical help (25, 26).
In Germany, traumatic brain injury is commonly classified as grade I, II, or III (27) on the basis of the duration of the post-traumatic neurological disturbance: up to 4 days for grade I, up to 3 weeks for grade II, and more than 3 weeks for grade III. This grading system has been found to be prognostically relevant, particularly with respect to the return to work, but it is retrospective and thus not applicable in the acute phase.
The ultimate outcome of patients who are comatose after a traumatic brain injury is significantly correlated with their additional neurological disturbances in the first 24 hours. Mortality is over 90% if both pupils are fixed and dilated, 50–60 % if there are pathological extensor responses, 30–50% if a single pupil is fixed and dilated, and 5–10% if none of these are the case (11). The duration of coma can be determined reliably only if sedating drugs are given in no higher doses than needed for the patient to tolerate mechanical ventilation. Under these circumstances, long-term follow-up studies have shown that a 20-year-old patient who has been unconscious for 18 days has the same low chance of survival (5%) as a 75-year-old patient who has been unconscious for only 5 days. Thus, age (e3) and the duration of coma are of major prognostic significance. An initially comatose patient who regains consciousness once an epidural hematoma has been removed has a much better prognosis than a patient in persistent coma.
Marshall et al. 1991 (e4) proposed a classification of posttraumatic CT findings for use in prognostication (Table), yet no clear correlation has been found between the CT findings of initially comatose patients and the outcome of their treatment (figure 1). There is likewise no clear correlation between the extent of brain contusions seen in CT and the outcome (28, 29).
Table. Classification of traumatic brain injury according to CT findings*.
| Grade | CT findings |
| Diffuse injury, grade I | normal |
| Diffuse injury, grade II | cisterns preserved, midline shift <6 mm, contusions <25 cm 2 |
| Diffuse injury, grade III | cisterns narrowed |
| Diffuse injury, grade IV | midline shift >5 mm, surgically evacuated hematoma, or hematoma >25 cm2 |
* modified from (15); CT, computerized tomography
Figure 1.
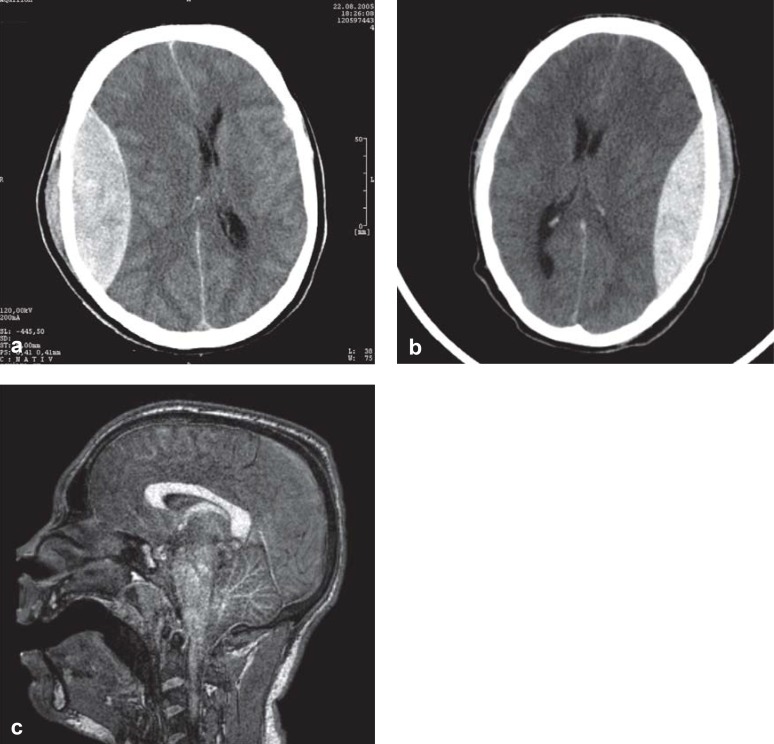
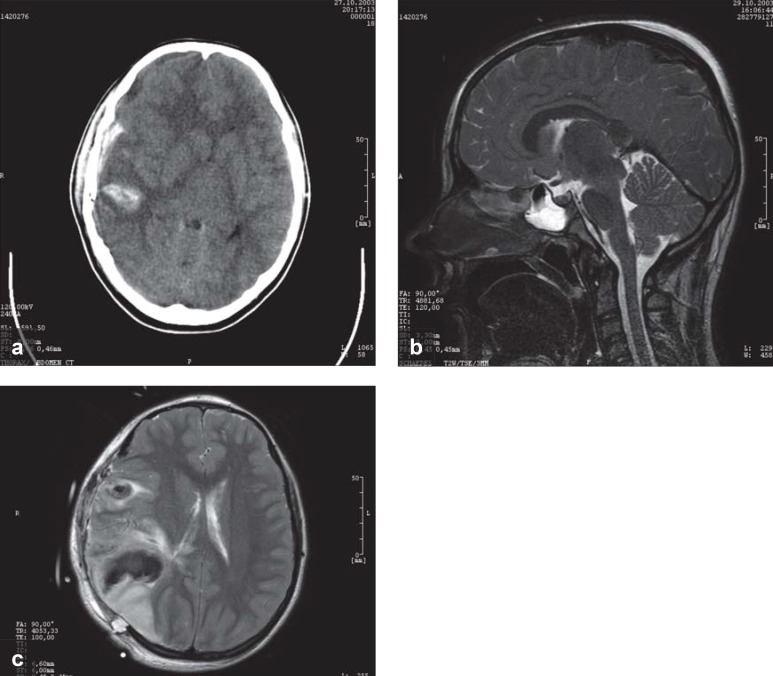
Head CT and MRI in patients with traumatic brain injury.
This 42-year-old woman (physician) was comatose 3 hours after an accident, with acute pathological extensor responses. The CT shows an epidural hematoma. She awakened promptly after surgical removal of the hematoma.
This 20-year-old man fell, remained mentally lucid, and went home; a few hours later, he was found unconscious in bed with fixed and dilated pupils. The CT shows an epidural hematoma, which was removed immediately, like the one in (a).
After surgery, he remained comatose and his pupils remained fixed and dilated. The MRI shows increased signal intensity throughout the brainstem, evidently reflecting a pathological abnormality that arose secondarily, after the lucid interval, as a result of sustained pressure on the brainstem arising from the epidural hematoma. The superiority of MRI to CT in this case is clear, as the lethal brainstem injury cannot be seen in the CT in (b), just as the CT in (a) cannot show the absence of a brainstem lesion.
Somatosensory evoked potentials (SSEPs) are prognostically significant: if the SSEPs are bilaterally absent, the mortality is 80–90% (e5– e7).
Pioneer studies performed decades ago already suggested that the ICP was of lesser prognostic significance than the patient’s clinical condition (30). Multiple studies performed since then have not modified this conclusion. No clear correlation has been found between mildly or moderately elevated ICP values and the ultimate morbidity or mortality of brain-injured patients. It is true, however, that persistently and markedly elevated, life-threatening ICP that cannot be pharmacologically lowered is correlated with increased mortality (31).
MRI reveals traumatic lesions in the brain (particularly the brainstem) in much greater detail than CT (Figure 1) and is thus more useful for prognostication (32). It is more time-consuming, however, and it is no better than CT at showing the bony and intracranial traumatic lesions that require surgical treatment (4).
It has only become clear since the advent of MRI that brainstem lesions are of major prognostic significance with respect to mortality, as well as morbidity in surviving patients (32) (Box 2). The MRI findings can be used for a four-level classification of the severity of traumatic brain injury:
BOX 2. Classification of traumatic brain injury according to MRI findings*.
Grade I Exclusively supratentorial injury without any brainstem injury.
Grade II Unilateral brainstem injury at any level, with or without an additional grade I injury.
Grade III Bilateral midbrain injury with or without an additional grade II injury.
Grade IV Bilateral pontine injury with or without an additional grade III injury.
* from Firsching et al. (32); MRI, magnetic resonance imaging
-
grade I (no brainstem lesion)
seen in 39% of comatose patients
mortality, 4.5%
-
grade II (unilateral brainstem lesion)
seen in 22% of comatose patients
mortality, 15.9%
-
grade III (bilateral midbrain lesions)
seen in 19 % of patients
mortality, 23.5%
-
grade IV (bilateral pontine lesions)
seen in 20% of comatose patients
mortality, 97.3%.
69% of patients with a grade I injury survived without functional impairment, while only 25% of patients with a grade II injury did. Not one patient with a grade III or grade IV injury survived without functional impairment.
THE PATHOPHYSIOLOGY OF COMA
Experts disagree little with regard to the diagnostic evaluation, treatment, and prognosis of comatose head-injured patients, yet much controversy surrounds the question, “Which brain structures are responsible for coma?” The longstanding observation that an epidural hematoma can be followed by a lucid interval and then by acute coma that resolves when the hematoma is removed (33) has been explained as reflecting reversible brainstem dysfunction due to compression (34). Another concept of the organic cause of coma arose from the histopathological observation of massive neuronal injury, with axonal lesions extending far into the white matter of the cerebral hemispheres.
From 1982 onward, diffuse axonal injury was considered to be the cause of post-traumatic coma, when the CT showed no hematoma exerting a mass effect, and the patient remained comatose for 6 hours or more after the trauma. It was concluded from multiple histopathological case reports that brainstem injury was rare and not the cause of coma (35, 36). Coma was attributed to damage of neural pathways that ascend from the brainstem in the setting of diffuse axonal injury to the hemispheric white matter. With this concept maintaining the upper hand, the contrary notion that coma, pupillary areflexia, and pathological extensor responses are signs of brainstem damage was vehemently disputed as recently as 2002 (the “brainstem damage saga”) (37).
Other teams of researchers, however, reported neuropathological findings that conflicted with this interpretation (38), including findings from patients who had remained comatose from the time of the accident until death: all, without exception, had brainstem injuries (39). This histological evidence was further supported by a prospective study involving serial MRI scans in comatose patients (32). A statistically significant correlation was found between coma and brainstem lesions in the first 8 days. All patients who did not emerge from coma within 8 days had structural brainstem damage on MRI. An association of bilateral pontine injury with especially high mortality (over 90%) was described for the first time. Moreover, pupillary areflexia and pathological flexor and extensor responses (traditionally considered clinical signs of brainstem dysfunction) were indeed significantly correlated with structural brainstem lesions seen on MRI.
Thus, current evidence suggests that post-traumatic coma is due to brainstem dysfunction rather than hemispheric axonal disruption (40). This conclusion is of practical significance: there is no way to reconnect torn axons, but brainstem compression can be counteracted by lowering the ICP (Figure 2). If needed, a far more effective way to lower ICP than any drug treatment is the surgical evacuation of a hematoma that is exerting a mass effect, and/or an extensive decompressive hemicraniectomy. These options should be available to all patients who are comatose after an acute traumatic brain injury.
Figure 2.
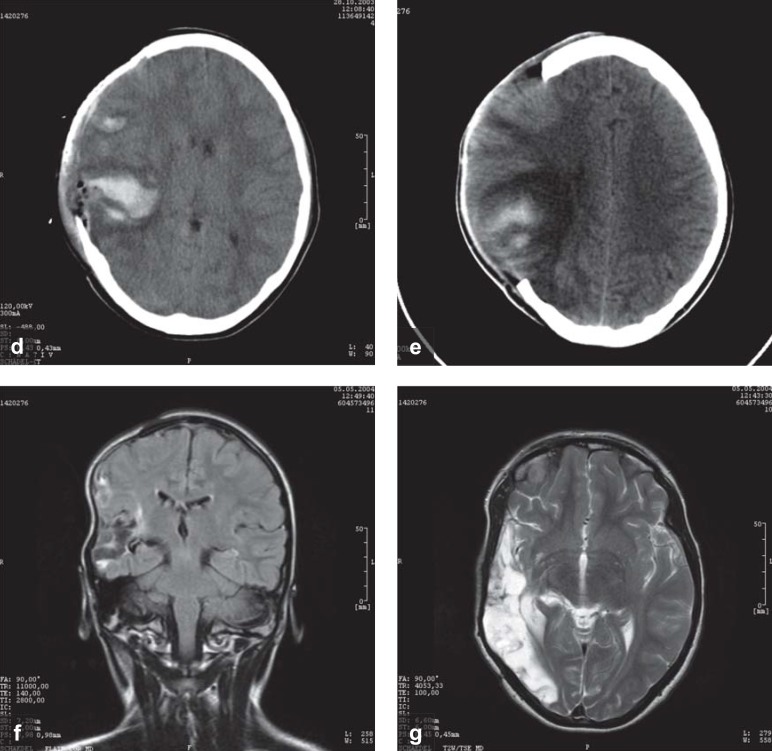
Head CT and MRI scans of a 12-year-old girl who was struck by an automobile while crossing the street and was immediately rendered unconscious, with a fixed and dilated right pupil.
This CT scan was obtained within 2 hours of the injury: at this time, both pupils were fixed and dilated. There is a right frontotemporal subdural hematoma of the same width as the calvaria, as well as a small right temporal hemorrhagic contusion and very marked right-to-left shift of the midline structures, mainly due to swelling of the right hemisphere. These findings would be classified as grade IV in the scheme of Marshall et al. 1991 (e4). A short time after this image was taken, an extensive right hemicraniectomy was performed, along with removal of the acute subdural hematoma.
and c) MRI on day 2 shows a large right temporal contusion, but no brainstem injury.
Continuation of Figure 2:
and e) Two weeks later, follow-up CTs show persistent brain swelling, with incipient resorption of the hemorrhagic contusion.
and g) Six months later, before the skull flap was surgically reinserted, follow-up MRI shows a large right temporo-occipital tissue defect. At this time, the patient has a moderately severe left visual field defect, but no other neurological deficits. She is back at school in her Gymnasium (academically-oriented German secondary school): she is reportedly doing poorly in mathematics and has difficulty with spatial processing, but her language ability is above average. Coma and pupillary areflexia at the time of the injury are best explained as reflecting acute, but reversible brainstem dysfunction, due to the pressure that was exerted mainly by the swelling of the brain contusion and, to a lesser extent, by the subdural hematoma. The mass effect was rapidly and successfully reduced by hemicraniectomy, preventing irreversible structural damage to the brainstem.
Key Messages.
Traumatic brain injury is a common cause of death worldwide at all ages up to young adulthood.
Comatose patients may have life-threatening brain dysfunction and should therefore be taken immediately to a hospital where computerized tomography (CT) and neurosurgical treatment are available around the clock.
In the early phase after traumatic brain injury, the patient’s emergence from coma is just as unpredictable as a sudden worsening of consciousness that may put him or her back in coma.
The most effective way to lower elevated intracranial pressure is by decompressive hemicraniectomy.
Current evidence suggests that post-traumatic coma is due to brainstem dysfunction.
Acknowledgments
Acknowledgement
I am very grateful to Professor Skalej, chief of neuroradiology, for generously supplying the impressive radiological images reproduced here and for his scientific assistance in our clinical work.
Dedication
This article is dedicated to Professor Frowein, emeritus chief of neurosurgery at the University of Cologne, in honor of his ninety-third birthday.
Footnotes
Conflict of interest statement
The author states that he has no conflict of interest.
Translated from the original German by Ethan Taub, M.D.
References
- 1.Statistisches Bundesamt. Statistisches Jahrbuch 2015. www.destatis.de/DE/Publikationen/StatistischesJahrbuch/StatistischesJahrbuch2015pdf?__blob=publicationFile" (last accessed on 13 April 2017) [Google Scholar]
- 2.Steudel WI, Cottbus F, Schwerdtfeger K. Acta Neurochir. Vol. 147. (Wien): 2005. Epidemiology and prevention of fatal head injuries in Germany—trends and the impact of the reunification; pp. 231–242. [DOI] [PubMed] [Google Scholar]
- 3.Bullock MR, Hovda DA. Introduction to traumatic brain injury Youmans neurologiscal surgery. In: Winn HR, editor. Elsevier Saunders. 6. 4, Chapter 322. Philadelphia: 2011. 3267 pp. [Google Scholar]
- 4.Firsching R, Rickels E, Mauer UM, et al. AWMF: „Leitlinie Schädel-Hirn-Trauma im Erwachsenenalter“. 2015 [Google Scholar]
- 5.Anonymos. Acta Neurochir. Vol. 25. (Wien): 1979. Glossary of neurotraumatology; pp. 1–63. [PubMed] [Google Scholar]
- 6.Teasdale G, Jennett B. Assessment of coma and impaired consciousness A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 7.Frowein R. Classification of coma. Acta Neurochir (Wien) 1976;34:5–10. doi: 10.1007/BF01405858. [DOI] [PubMed] [Google Scholar]
- 8.Brihaye J, Frowein RA, Lindgren S, Loew F, Stroobandt G. Acta Neurochir. Vol. 40. (Wien): 1978. Report on the meeting of the WFNS Neuro-Traumatology Committee Brussels. I. Comascaling; pp. 181–186. [Google Scholar]
- 9.Bernard SA, Nguyen V, Cameron P, et al. Prehospital rapid sequence intubation improves functional outcome for patients with severe traumatic brain injury: a randomized controlled trial. Ann Surg. 2010;252:959–965. doi: 10.1097/SLA.0b013e3181efc15f. [DOI] [PubMed] [Google Scholar]
- 10.Maryglothling J, Duane T, Gibbs M, et al. Emergency tracheal intubation immediately following traumatic injury. J Trauma. 2012;73:333–340. doi: 10.1097/TA.0b013e31827018a5. [DOI] [PubMed] [Google Scholar]
- 11.Frowein R, Firsching R. Classification of head injury. In: Vinken PJ, Bruyn GW, editors. Handbook of clinical neurology. Vol. 13. Amsterdam Elsevier: 1990. pp. 101–122. [Google Scholar]
- 12.Firsching R, Heimann M, Frowein RA. Early dynamcis of extradural and subdural hematomas. Neurol Res. 1997;19:257–260. doi: 10.1080/01616412.1997.11740810. [DOI] [PubMed] [Google Scholar]
- 13.Bulger EM, May S, Brasel KJ, et al. Out-of-hospital hypertonic resuscitation following severe traumatic brain injury: a randomized controlled trial. JAMA. 2010;304 1:455–464. doi: 10.1001/jama.2010.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cottenceau V, Masson F, Mahamid E, et al. Comparison of effects of equiosmolar doses of mannitol and hypertonic saline on cerebral blood flow and metabolism in traumatic brain injury. J Neurotrauma. 2011;28:2003–2012. doi: 10.1089/neu.2011.1929. [DOI] [PubMed] [Google Scholar]
- 15.Wakai A, McCabe A, Roberts I, Schierhout G. Mannitol for acute traumatic brain injury. Cochrane Database Syst Rev. 2013;8 doi: 10.1002/14651858.CD001049.pub5. CD001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alderson P, Roberts I. Corticosteroids for acute traumatic brain injury. Cochrane Database Syst Rev. 2005;1 doi: 10.1002/14651858.CD000196.pub2. CD000196.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brain Trauma Foundation. Guidelines for the management of severe head injury. www.braintrauma.org/uploads/11/14/Guidelines_Management_2007w_bookmarks_2.pdf (last accessed on 27 March 2017) [Google Scholar]
- 18.Roberts I, Sydenham E. Barbiturates for acute traumatic brain injury. Cochrane Database Syst Rev. 2012;12 doi: 10.1002/14651858.CD000033.pub2. CD000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chesnut RM, Temkin N, Carney N, et al. Trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2012;367:2471–2481. doi: 10.1056/NEJMoa1207363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forsyth RJ, Wolny S, Rodrigues B. Routine intracranial pressure monitoring in acute coma. Cochrane Database Syst Rev. 2010;2 doi: 10.1002/14651858.CD002043.pub2. CD002043. [DOI] [PubMed] [Google Scholar]
- 21.Shafi S, Diaz-Arrastia R, Madden C, Gentilello L. Intracranial pressure monitoring in brain-injured patients is associated with worsening of survival. J Trauma. 2008;64:335–340. doi: 10.1097/TA.0b013e31815dd017. [DOI] [PubMed] [Google Scholar]
- 22.Cooper DJ, Rosenfeld JV, Murray L, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011;364:1493–1502. doi: 10.1056/NEJMoa1102077. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez R, Firsching R, Lobato R, et al. Guidelines for treatment of head injury in adults. Zentralbl Neurochir. 1997;58:72–74. [PubMed] [Google Scholar]
- 24.Clifton GL, Miller ER, Choi SC, et al. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344:556–563. doi: 10.1056/NEJM200102223440803. [DOI] [PubMed] [Google Scholar]
- 25.Balestreri M, Czosnyka M, Chatfield DA, et al. Predictive value of glasgow coma scale after brain trauma: change in trend over the past ten years. J Neurol Neurosurg Psychiatry. 2004;75:161–162. [PMC free article] [PubMed] [Google Scholar]
- 26.Moskopp D, Stähle C, Wassmann H. Problems of the glasgow coma scale with early intubated haematoma in head injurd adults. Neurosurg Rev. 1995;18:253–257. doi: 10.1007/BF00383876. [DOI] [PubMed] [Google Scholar]
- 27.Tönnis W, Loew F. Einteilung der gedeckten Hirnschädigungen. Ärztliche Praxis. 1953;5:13–14. [Google Scholar]
- 28.Stammler U, Frowein RA. Repeated early CT examinations of closed head injury. Neurosurg Rev. 1989;12:159–168. doi: 10.1007/BF01790640. [DOI] [PubMed] [Google Scholar]
- 29.Yamaura A, Ono J, Watanabe Y, Saeki N. CT findings and outcome in head injuries—effects of aging. Neurosurg Rev. 1989;12:178–183. doi: 10.1007/BF01790643. [DOI] [PubMed] [Google Scholar]
- 30.Miller JD, Becker DP, Ward JD, Sullivan HG, Adams WE, Rosner MJ. Significance of intracranial hypertension in severe head injury. J Neurosurg. 1977;47:503–516. doi: 10.3171/jns.1977.47.4.0503. [DOI] [PubMed] [Google Scholar]
- 31.Miller JD. Prediction of outcome after head injury A critical review Extracerebral collections. Advances in Neurotraumatology. In: Vigouroux RP, editor. Springer. Wien-New York: 1986. [Google Scholar]
- 32.Firsching R, Woischneck D, Reissberg S, Döhring W, Peters B. Prognostische Bedeutung der MRT bei Bewusstlosigkeit nach Schädel-Hirn-Verletzung. Dtsch Arztebl. 2003;27 A-1868-74. [Google Scholar]
- 33.von Bergmann E. Deutsche Chirurgie Ferdinand Enke Verlag. Stuttgart: 1889. Die Lehre von den Kopfverletzungen; 298 pp. [Google Scholar]
- 34.Breslauer F. Zur Frage des Hirndrucks. Arch Klin Chir. 1914 103:478–496. [Google Scholar]
- 35.Adams J, Graham D, Murray L, Scott G. Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann Neurol. 1982;12:557–563. doi: 10.1002/ana.410120610. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell DE, Adams J. Primary focal impact damage to the brain stem in blunt head injuries Does it exist? Lancet. 1973;2:215–218. doi: 10.1016/s0140-6736(73)93128-0. [DOI] [PubMed] [Google Scholar]
- 37.Sahuquillo J, Poca M. Diffuse axonal injury after head trauma A review. Adv Tech Stand Neurosurg. 2002;27:23–86. doi: 10.1007/978-3-7091-6174-6_2. [DOI] [PubMed] [Google Scholar]
- 38.Blumbergs P, Scott G, Manavis J, et al. Topography of axonal injury as defined by amyloid precursor protein and the sector scoring method in mild and severe closed head injury. J Neurotrauma. 1995;12:565–572. doi: 10.1089/neu.1995.12.565. [DOI] [PubMed] [Google Scholar]
- 39.Rosenblum WI, Greenberg RP, Seelig JM, et al. Midbrain lesions: frequent and significant prognostic feature in closed head injury. Neurosurgery. 1981;9:613–620. doi: 10.1227/00006123-198112000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Rosenblum W. Immediate, irreversible, posttraumatic coma: a review indicating that bilateral brainstem injury rather than widespread hemispheric damage is essential for its production. J Neuropathol Exp Neurol. 2015;74:198–202. doi: 10.1097/NEN.0000000000000170. [DOI] [PubMed] [Google Scholar]
- E1.Harris OA, Colford JM Jr, Good MC, Matz PG. The role of hypothermia in the management of severe brain injury: a meta-analysis. Arch Neurol. 2002;59:1077–1083. doi: 10.1001/archneur.59.7.1077. [DOI] [PubMed] [Google Scholar]
- E2.Bennett M, Heard R. Hyperbaric oxygen therapy for multiple sclerosis. Cochrane Database Syst Rev. 2004;1 doi: 10.1002/14651858.CD003057.pub2. CD003057.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E3.Kulesza B, Nogalski A, Kulesza T, Prystupa A. Prognostic factors in traumatic brain injury and their association with outcome. J Pre-clinical and Clinical Res. 2015;9:163–166. [Google Scholar]
- E4.Marshall LF, Marshall SB, Klauber MR. A new classification of head injury based on CT. J Neurosurg. 1991;75:14–20. [Google Scholar]
- E5.Greenberg R, Newlon P, Hyatt M, Narayan R, Becker DP. Prognostic implications of early multimodality evoked potentials in severely head imjured patients. J Neurosurg. 1981;55:227–236. doi: 10.3171/jns.1981.55.2.0227. [DOI] [PubMed] [Google Scholar]
- E6.Firsching R, Frowein R. Multimodality evoked potentials and early prognosis in comatose patients. Neurosurg Rev. 1990;13:141–146. doi: 10.1007/BF00383655. [DOI] [PubMed] [Google Scholar]
- E7.Guérit JM. Evoked potentials in severe brain injury. Prog Brain Res. 2005;150:415–426. doi: 10.1016/S0079-6123(05)50029-3. [DOI] [PubMed] [Google Scholar]