Abstract
Small-animal models are needed to test human immunodeficiency virus (HIV) vaccine efficacy following viral challenge. To this end, we examined HIV-1-specific immune responses following immunization of nonobese diabetic-severe combined immunodeficient mice that were repopulated with human peripheral blood lymphocytes (hu-PBL-NOD/SCID mice). Autologous dendritic cells (DC) were transduced ex vivo with replication-defective, helper virus-free, herpes simplex virus type 1 (HSV-1) amplicons that expressed HIV-1 gp120 and were then injected into the hu-PBL-NOD/SCID mice. This resulted in primary HIV-1-specific humoral and cellular immune responses. Serum samples from vaccinated animals contained human immunoglobulin G that reacted with HIV-1 Env proteins by enzyme-linked immunosorbent assay and neutralized the infectivity of HIV-1 LAI and ADA strains. T cells isolated from the mice responded to viral antigens by producing gamma interferon when analyzed by enzyme-linked immunospot assay. Importantly, exposure of the vaccinated animals to infectious HIV-1 demonstrated partial protection against infectious HIV-1 challenge. This was reflected by a reduction in HIV-1ADA and by protection of the engrafted human CD4+ T lymphocytes against HIV-1LAI-induced cytotoxicity. These data demonstrate that transduction of DC by HSV amplicon vectors expressing HIV-1 gp120 induce virus-specific immune responses in hu-PBL-NOD/SCID mice. This mouse model may be a useful tool to evaluate human immune responses and protection against viral infection following vaccination.
The discovery of an effective vaccine for human immunodeficiency virus type 1 (HIV-1) remains the principal objective to help control the AIDS pandemic (2, 9, 12, 16, 25, 34, 49). To date, animal testing of candidate vaccines has focused extensively on nonhuman primates (macaques), since these animals can be readily challenged with infectious, pathogenic, simian immunodeficiency viruses (SIV) and HIV/SIV chimeras (27, 29, 31, 59). This permits direct evaluation of the protective potential of candidate vaccines. On balance, nonhuman primate models have significant disadvantages, which include cost, availability, uncertain value in predicting human immune responses, and the molecular and immunogenetic diversity of SIV, HIV/SIV, and HIV (14, 21, 22, 40, 59). Nonetheless, the limited species tropism of HIV makes finding relevant animal models for vaccine testing difficult. Although small-animal models (mice and rabbits) have been used to test the immunogenicity of HIV-1 antigens (7, 19, 45, 62), they cannot be used to evaluate protection from live-virus challenge. Mice, in particular, may generate strong immune responses to candidate viral vaccines, which subsequently prove to be less immunogenic in nonhuman primates and humans (32).
An ideal animal model system for evaluation of candidate HIV-1 vaccines should incorporate human antigen presenting cells and effector cells, while also permitting direct virus challenge with infectious, pathogenic HIV-1. Within this context, we considered previous experiences using human-mouse chimeras for vaccine testing. Severe combined immunodeficient (SCID) mice reconstituted with human peripheral blood lymphocytes (hu-PBL-SCID mice) develop immune responses to viral antigens (11, 38, 47, 55). Moreover, the model was utilized extensively in our laboratories and by others to study HIV-1 pathogenic mechanisms and for drug testing (33, 37, 39, 43, 44, 55, 56). Nonetheless, a limitation of the hu-PBL-SCID mouse model is the relatively modest level of T-cell engraftment and the dearth of human dendritic cells (DC) in the model system (6, 53). Introduction of exogenous human DC improve the model's value for vaccine testing (28, 61), linked to the potent antigen-presenting and T-cell stimulatory properties of the DC (5, 50, 51).
Due to the limitations of the hu-PBL-SCID mouse model, we elected to use a mouse-human chimeric model system, based on nonobese diabetic (NOD)/SCID mice. These animals allow efficient T-cell engraftment (17, 18, 42). NOD/SCID mice were engrafted with hu-PBL, and the resulting hu-PBL-NOD/SCID mice were immunized with autologous human DC transduced ex vivo with a helper-free herpes simplex virus type 1 (HSV-1) amplicon vector encoding HIV-1 gp120 (HSV gp120 amplicons). Human cellular and humoral immune responses and infectious virus challenge were recorded after vaccination. The studies revealed that HSV gp120 amplicon-transduced DC immunization induced significant envelope-specific human adaptive immune responses. Subsequent experiments revealed that the vaccinated animals were partially protected against infectious virus challenge. These data support the utility of this model as a novel preclinical testing system for HIV-1 vaccines.
MATERIALS AND METHODS
hu-PBL NOD/SCID mice.
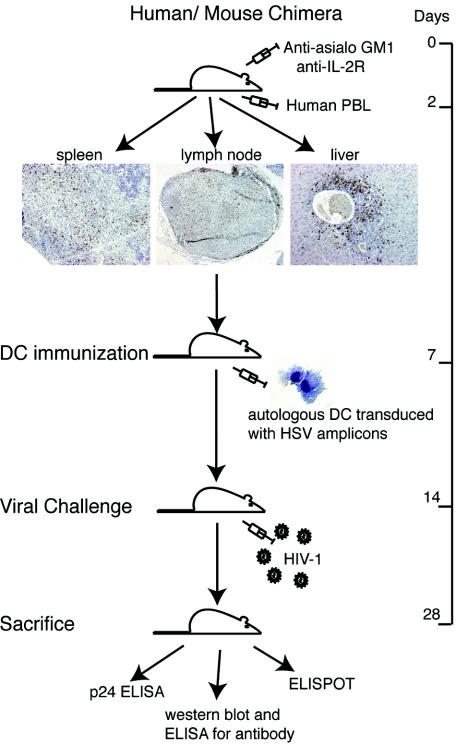
Four-week-old male NOD-C.B-17 SCID mice were purchased from the Jackson Laboratory (Bar Harbor, Maine). Animals were maintained in sterile microisolator cages under pathogen-free conditions in accordance with ethical guidelines for care of laboratory animals at the University of Nebraska Medical Center set forth by the National Institutes of Health (NIH). Animals were injected once intraperitoneally (i.p.) with rat anti-CD122 antibody (Ab) (0.2 mg/mouse) and twice with rabbit asialo-GM1 Ab (0.2 mg/mouse) (both from Wako, Richmond, Va.) at 2 days before and 1 and 3 days after PBL reconstitution, respectively (20 × 106 human PBL were used per mouse). The Abs were used to inhibit mouse NK cell activity and facilitate human PBL engraftment (26, 52). Figure 1 outlines the procedures for reconstitution and subsequent vaccine testing.
FIG. 1.
Vaccine scheme and analysis of human immune engraftment in hu-PBL-NOD/SCID mice. NOD/SCID mice were injected with anti-asialo-GM1 Ab and anti-CD122 Ab prior to hu-PBL injection. These Abs were given to inhibit mouse NK cell activity and enhance hu-PBL reconstitution. Immunohistology of mouse spleen, lymph node, and liver obtained 14 days after reconstitution shows the presence of a significant number of human cells. Paraffin sections were stained for human vimentin (brown). Magnification, ×20.
Leukocyte preparation.
Monocytes and PBL were obtained from leukopheresis of HIV-1, HIV-2, and hepatitis B-seronegative (HLA-A3) donors and purified by countercurrent centrifugal elutriation (15). Cell suspensions were >98% monocytes by cell morphology in Wright-stained cytosmears. The PBL fraction was used to reconstitute NOD/SCID mice. Monocytes were differentiated into DC by previously described techniques (23). Briefly, monocytes were cultured in DC proprietary medium, PCGM (GenePrime LLC, Gaithersburg, Md.) at 1 million cells/ml. Culture medium was exchanged every 3 days with fresh medium. The DC obtained were examined by morphology in Wright-stained cytosmears and by flow cytometric analysis for CD1a, CD11c, CD14, HLA-DR, CD80, CD83, and CD86. Appropriate isotype controls were used to set the gates. Allophycocyanin-conjugated Abs and isotype controls were obtained from BD Pharmingen, San Diego, Calif., and cells were analyzed with a FACSCalibur with CellQuest software (BD Immunocytometry Systems, Mountain View, Calif.).
Immunization and HIV-1 challenge.
Monocyte-derived DC were transduced with helper-free HSV amplicon vectors. The vector encoding the beta-galactosidase gene (HSVlac) was used as the control construct in these experiments, while a previously described vector encoding HIV-1 gp120 (HSV gp120MN/LAI) was used as the vaccine candidate (20, 58). The HIV-1 gp120 encoded by the latter construct was a human codon-optimized derivative of HIV-1MN that contained the V3 loop sequences derived from HIV-1LAI, generously supplied by Jurgen Haas (20). For vaccination experiments, human DC were prepared and then transduced ex vivo with HSV amplicon vectors. Transduction was achieved by incubation of DC at a multiplicity of infection (MOI) of 1.0 for 2 h at 37°C. Cells were washed twice with medium and resuspended in phosphate-buffered saline for animal injection. Parallel, replicate cultures were retained for flow cytometric characterization at 48 h after transduction and for analysis of gp120 expression levels with an HIV-1MN gp120-specific monoclonal Ab (clone ID6; NIH AIDS Research and Reference Reagent Program, Bethesda, Md.), in combination with a fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin G (IgG). Control (nontransduced) DC were stained by replicate assays.
Hu-PBL-NOD/SCID mice were injected i.p. twice with amplicon-transduced autologous DC (3 × 106 cells) infused on days 5 and 12 after human PBL reconstitution. Cellular and humoral immune responses were then assessed at days 7, 14, and 21 after the final DC immunization. Four animals per group were sacrificed at each time point. Samples collected at sacrifice included serum (obtained by cardiac puncture) and spleens (used for cell isolation). HIV-1-specific humoral and cellular immune responses were measured by enzyme-linked immunosorbent assay (ELISA), Western blotting, and enzyme-linked immunospot (ELISPOT) assay.
For infectious HIV-1 challenge, hu-PBL-NOD/SCID mice were injected i.p. with 3 × 106 autologous DC that had been transduced ex vivo with HSVlac (control) or HSV gp120MN/LAI (vaccine), on day 5 after PBL reconstitution. Seven days later, the mice were challenged i.p. with infectious HIV-1LAI or HIV-1ADA at a dose of either 105 or 102 50% tissue culture infectious doses (TCID50)/mouse, respectively. The challenge virus doses were selected on the basis of previously published studies which have shown that elevated titers of X4-using viruses are required to establish efficient infection in hu-PBL-SCID mice (13). Each animal group contained five to eight animals. Mice were sacrificed 14 days after challenge and evaluated for humoral and cellular immune responses and viral load. Splenocytes were immunostained with fluorescein isothiocyanate- or phycoerythrin-conjugated Abs specific for human CD3, CD4, CD8, and CD45 (BD Pharmingen) and analyzed by flow cytometry.
Virus strains.
HIV-1LAI was propagated in phytohemagglutinin (PHA)-stimulated peripheral blood mononuclear cells (PHA-blasts), and HIV-1ADA was propagated in monocyte-derived macrophages obtained from HIV-1-seronegative donors. Monocyte-derived macrophages were cultured in macrophage colony-stimulating factor, a generous gift from Genetics Institute, Cambridge, Mass., for 7 days before infection. Viral preparations were screened negative for endotoxin (<10 pg/ml) (Associates of Cape Cod, Woods Hole, Mass.) and mycoplasma (Gen-Probe II; Gen-Probe, San Diego, Calif.). The virus titers were determined on PHA-blasts and were 106 and 103 TCID50/ml for HIV-1LAI and HIV-1ADA, respectively. HIV-1LAI stock was obtained from the NIH AIDS Research and Reference Reagent Program, and HIV-1ADA was isolated from virus isolated from the peripheral blood mononuclear cells of an AIDS patient with Kaposi's sarcoma (15).
ELISA and Western blotting for HIV-1-specific Abs.
Sera collected from DC-immunized animals were analyzed for HIV-1-specific human IgG by ELISA with HIV-1MN gp160 (20) and by Western blotting with HIV-1 nitrocellulose strips from Calypte, Alameda, Calif. The strips were incubated overnight in 1:5 diluted serum samples, with shaking at 4°C; horseradish peroxidase conjugated anti-human IgG was used as a secondary Ab. The strips were developed with a chemiluminescent substrate (Pierce Biotechnology, Inc., Rockford, Ill.) and were exposed to X-ray film. HIV-1-seropositive and -negative human sera were used as positive and negative controls at 1:1,000 dilutions, respectively.
Viral neutralization tests.
Mouse sera were analyzed for their virus-neutralizing Abs in vitro, with PHA-blasts as virus-susceptible target cells. Briefly, sera were serially diluted in RPMI-1640 medium with 10% fetal calf serum, and 10-μl aliquots of diluted serum samples were then placed into a 96-well plate in triplicate. To this, 40 μl of either HIV-1LAI or HIV-1ADA (100 TCID50) was added and incubated at 37°C for 1 h. PHA-blasts were then added to each well (105 cells in 150 μl of medium, containing 50 U of interleukin-2/ml). After 3 days, cells were washed, and fresh medium with interleukin-2 was added. Supernatants were collected on day seven and virus production was measured by reverse transcriptase (RT) assay. RT was determined by incubating 10 μl of supernatant with a reaction mixture consisting of 0.05% NP-40 (Sigma) and [3H]dTTP (2 Ci/mmol; Amersham Corp., Arlington Heights, Ill.) in Tris-HCl buffer (pH 7.9) for 24 h at 37°C. Radiolabeled nucleotides were precipitated on paper filters in an automatic cell harvester (Skatron, Sterling, Va.) by using cold 10% trichloroacetate and 95% ethanol. Incorporated activity was measured by liquid scintillation spectroscopy (24).
ELISPOT assay.
Single-cell suspensions of splenocytes collected from control and vaccinated animals were stimulated with autologous DC loaded with HIV-1 antigens (41). Briefly, DC were pulsed with a 10-mer peptide (RGPGRAFVTI;Alpha Diagnostics, San Antonio, Tex.) for 1 h at 37°C. This peptide has previously been shown to have the ability to bind to multiple major histocompatibility complex class I molecules, including HLA-A2 and HLA-A3 (1, 4). These cells were used as stimulators to activate HIV-1-specific human T cells present within the mouse splenocyte population. Stimulator and responder cells were plated in human gamma interferon (IFN-γ) Ab-coated, nitrocellulose membrane-lined 96-well microtiter plates at a ratio of 1:10. The ELISPOT assay was performed after 24 to 36 h with reagents obtained from BD Pharmingen. DC loaded with heat-inactivated HIV-1 particles (corresponding to HIV-1LAI or HIV-1ADA, incubated at 65°C for 2 h) were also used for stimulation; PHA at a concentration of 1 μg/ml was used as a positive control. Unloaded DC were used as nonspecific stimulators (negative control), and ELISPOT values from these cultures were subtracted from those for all other experimental groups to calculate numbers of antigen-specific IFN-γ-positive spots. Assay validation was performed with cells from an HIV-1-infected patient.
Detection of HIV-1 infection.
HIV-1 infection in hu-PBL NOD/SCID mice was measured by determining HIV-1 p24 antigens and RNA copies in the sera from control and vaccinated animals challenged with virus. HIV-1 p24 was measured with an ELISA kit from Becton Dickinson. HIV-1 RNA copies were detected in the serum with the Cobas Amplicor HIV-1 monitor test kit (Roche Diagnostics, Basel, Switzerland), following the manufacturer's instructions.
Statistical analysis.
Data were analyzed using Excel with Student's t test and analysis of variance for comparisons. A P value of <0.05 was considered statistically significant. All results are presented as means ± standard errors of the mean (SEM). The viral loads in nonvaccinated and vaccinated animals were compared by a nonparametric Wilcoxon test.
RESULTS
HSV gp120MN/LAI amplicon transduction of human DC.
Helper-free HSV-1 amplicons effectively transduced human DC (60). DC were infected with HSV gp120MN/LAI or HSVlac amplicons at an MOI of 1.0 for 2 h and analyzed 48 h later for expression of major histocompatibility complex class II (HLA-DR), costimulatory molecules (CD80 and CD86), and the maturation antigen (CD83). Figure 2 shows the flow cytometric analysis of DC with and without HSV amplicons. The results show that HSV amplicon-treated DC undergo partial maturation when compared to untreated DC. An upregulation of HLA-DR, CD80, CD86, and CD83 expression was observed. The expression of the surface markers was comparable to that of lipopolysaccharide-treated DC, a potent inducer of DC maturation. In addition, 50 to 60% of HLA-DR-positive DC transduced with the HSV gp120MN/LAI amplicon expressed HIV-1 gp120 on their surface—indicating that amplicon-mediated gene transfer was highly efficient.
FIG. 2.
Flow cytometric analysis of DC after amplicon transduction. DC transduced in vitro with HSV gp120MN/LAI amplicons (MOI, 1) showed abundant surface expression of gp120 as detected by flow cytometry with gp120-specific Ab. Sixty percent of HLA-DR-positive cells expressed HIV-1 gp120. Increased expression of HLA-DR, costimulatory molecules (CD80 and CD86), and the maturation marker (CD83) was detected after HSV transduction compared to that of nontransduced DC. Surface expression of these antigens was analyzed 48 h posttransduction. Appropriate isotype controls were used to set the gates. The expression of surface markers upon HSV amplicon transduction was analyzed in at least four individual experiments using cells from different donors. The results shown are from a representative experiment.
Transduced DC were analyzed for their ability to present antigen to T cells by the mixed lymphocyte assay (data not shown). DC could efficiently stimulate T-cell proliferation, consistent with data obtained from previous studies utilizing HSV amplicons (60).
HSV gp120MN/LAI amplicons generate HIV-1-specific humoral immune responses.
Mice immunized with HSV gp120MN/LAI-transduced DC produced HIV-1 gp120-specific human IgG in sera at significant levels as detected by Western blot (Fig. 3A) and ELISA tests (Fig. 3B). The highest titers of HIV-1 gp120-specific human IgG were observed at day 7 after DC immunization, with a titer of 1:160. The second DC immunization did not affect the titer, and the endpoint HIV-1-specific IgG decreased to 1:25 by 21 days after immunization. In contrast, sera from mice injected with HSVlac-transduced DC showed no HIV-1-specific Abs.
FIG. 3.
Humoral immune responses in immunized hu-PBL-NOD/SCID mice. (A) Western blot analysis for HIV-1 gp120 Abs. The nitrocellulose membrane was probed with the following sera: (i) HIV-1-infected patient serum (1:1,000), (ii) pooled serum samples from mice that received DC transduced with HSV gp120MN/LAI (1:5 dilution), and (iii) pooled serum samples from mice that received DC transduced with a control amplicon vector (HSVlac). Sera were collected and pooled 7 days after DC immunization. Blots were incubated with the secondary Ab, a horseradish peroxidase-conjugated anti-human IgG, and developed with a chemiluminescence substrate. (B) IgG ELISA for HIV-1 envelope-reactive Abs in vaccinated mice. Mice were immunized with HSV-transduced DC; 7 days later, the animals were either challenged with infectious HIV-1 (strain HIV-1ADA or HIV-1LAI) or mock challenged (uninfected mice). Serum samples were then collected 14 days after challenge and analyzed by ELISA with an anti-human IgG Ab; the results are shown for pooled serum samples from six to eight mice. Sera from healthy, noninfected mice and HIV-infected patient sera were included in the assay as negative and positive controls, respectively (results not shown). (C) Neutralization assay results with sera from immunized mice. Sera from immunized mice were collected at day 7, following infusion of amplicon-transduced DC (HSV gp120MN/LAI or HSVlac in the immunized and control populations, respectively). Sera from a total of four mice per group were then pooled and tested for the ability to inhibit HIV infection of PHA-blasts at the indicated dilutions. Neutralization assays were performed with both LAI and ADA viral strains, with an initial MOI of 0.001 (102 TCID50 of cell-free virus was added to 105 PHA-blasts). On day 7 after viral infection, virus replication was measured by RT activity in cell-free culture supernatants.
Serum from DC-immunized but unchallenged mice contained Ab levels similar to those present in DC-immunized and virus-challenged animals (Fig. 3B). Ab levels were slightly reduced in HIV-1LAI-challenged mice compared to HIV-1ADA-challenged mice. However, no Abs were generated against other HIV proteins after virus challenge (data not shown), and no Env-specific Abs were detected in animals that were not immunized with HSV gp120MN/LAI. This was true even after infectious virus challenge (Fig. 3B, vaccinated versus control mice for the HIV-1 LAI and ADA groups).
Analysis of virus-neutralizing Abs is shown in Fig. 3C. Sera from immunized animals (i.e., animals which received HSV gp120MN/LAI-transduced DC) showed neutralizing activity against both LAI and ADA viral strains that was effective at a 1:4 dilution, whereas sera from control animals (animals which received HSVlac-transduced DC) did not show neutralizing activity.
HSV gp120MN/LAI amplicons generate HIV-1-specific cellular immune responses.
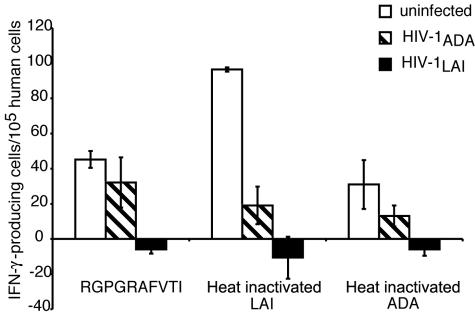
Generation of cellular immune responses against HIV-1 is critical in vaccine candidate evaluation. To this end, we monitored virus-specific T-cell responses induced in immunized animals by a human IFN-γ ELISPOT assay. A significant antigen-specific T-cell response was detected against the immunodominant cytotoxic T lymphocyte epitope peptide (RGPGRAFVTI) and also against heat-inactivated HIV-1 antigen prepared from different HIV-1 strains (LAI > ADA). HIV-1-specific primary cellular immune responses peaked at day 7 after the first immunization with 270 ± 50 IFN-γ-secreting cells/105 human cells. This declined by day 21 after immunization to 55 ± 24 IFN-γ-secreting cells/105 human cells, possibly reflecting the gradual decline of primary immune response. After viral challenge, HIV-1-specific cellular immune responses were reduced in ADA-challenged mice but were lost in LAI-challenged animals (Fig. 4). This may have reflected virus-induced human immune cell depletion in the mice (see Fig. 6).
FIG. 4.
Cellular immune responses in hu-PBL-NOD/SCID mice. Mice were immunized with HSV-transduced DC; 7 days later, the animals were either challenged with infectious HIV-1 (strain HIV-1ADA or HIV-1LAI) or mock challenged (uninfected mice). Splenocytes were then harvested 14 days after challenge and analyzed by IFN-γ ELISPOT. Briefly, autologous DC were pulsed with either an HLA-A2-restricted peptide from the HIV-1LAI V3 loop (RGPGRAFVTI) or heat-inactivated whole-virus preparations (HIV-1 strains LAI and ADA) and mixed with splenocytes at a 1:10 ratio. Unpulsed DC were used as nonspecific stimulators. The number of IFN-γ-positive spots per 105 human T cells was calculated from the percentage of CD3+ cells in spleen as detected by flow cytometry. Each group contained six to eight animals, and the results shown represent mean values (± SEM) for six to eight mice per group. The open bar, cross-hatched bar, and solid bar represent the number of HIV-specific cytotoxic lymphocytes in uninfected, HIV-1LAI challenged, and HIV-1ADA challenged animals, respectively. Note that the reduction in IFN-γ-positive spots in samples from the challenged animals (compared to samples from uninfected mice) may reflect in part the loss of CD4+ T cells, due to HIV-1 infection (see Fig. 6).
FIG. 6.
Protection of human CD4+ T cells following HIV-1 challenge. Mice were immunized with HSV-transduced DC (HSV gp120MN/LAI or HSVlac in the vaccinated and control groups, respectively). Seven days later, the animals were challenged with infectious HIV-1LAI. Splenocytes were harvested 14 days after challenge and subjected to immunophenotypic analysis, using monoclonal Abs specific for human CD4 and CD8. (A) Flow cytometric analysis. Pooled splenocytes from six to eight mice per group were stained for CD4+ and CD8+ T cells. (B) Bar graph representing the mean ± SEM values for CD4+ and CD8+ human T cells, obtained from the control and vaccinated mice challenged with LAI (six to eight animals per group). *, P < 0.05 by Student's t test.
HSV gp120MN/LAI amplicons generate partial protection following HIV-1 challenge.
Mice immunized with HSV gp120MN/LAI-transduced DC and HSVlac-transduced DC were challenged with HIV-1 to test whether the immune responses could attenuate or prevent viral infection. In challenge experiments, mice were given only one DC immunization, since peak immune responses were detected at day 7. The HIV-1 challenge was then performed at the peak level of immune responses with an X4-using lymphotropic strain (LAI; 105 TCID50) and an R5-using macrophage tropic strain (ADA; 102 TCID50). A high-titer X4-using lymphotropic strain is necessary to achieve efficient infection of hu-PBL-NOD/SCID mice (13, 37). The amount of HIV-1 in sera of the mice was then measured following virus challenge by both HIV-1 p24 ELISA and quantitative RT-PCR (to determine the viral RNA copy number). The levels of normalized HIV-1 p24 protein in ADA-infected mice reached 225.7 ± 97.1 pg/ml in control mice inoculated with HSVlac transduced DC versus 69.0 ± 32.9 pg/ml in vaccinated mice inoculated with HSV gp120MN/LAI-transduced DC (P < 0.08). In LAI-infected mice, no differences between control and vaccinated mice were detected (67.1 ± 46.5 versus 64.7 ± 47.1; P < 0.97). Analysis of viral RNA copy number in these serum samples yielded very similar results. The number of viral copies in sera of control animals infected with ADA was 4.3 ± 0.2 versus 3.9 ± 0.2 log per ml of plasma in vaccinated (P < 0.24) mice. In control and vaccinated mice infected with LAI, viral load was similar (3.6 ± 0.2 versus 3.5 ± 0.2 log; P < 0.82). Since viral load was greatly dependent on the number of engrafted human cells, results from both assays were normalized for the percentage of human CD45+ cells in the spleen of each animal (Fig. 5). Normalization showed statistically significant downregulation of HIV-1ADA replication in vaccinated animals (P < 0.05). Collectively, these results demonstrate partial protection in the immunized animals following challenge with infectious HIV-1ADA.
FIG. 5.
Protection against HIV-1 infection in vaccinated hu-PBL-NOD/SCID mice. Mice were immunized with HSV-transduced DC (HSV gp120MN/LAI or HSVlacZ in the vaccinated and control groups, respectively). Seven days later, the animals were challenged with infectious HIV-1 (strain HIV-1ADA or HIV-1LAI). Sera were then collected 14 days after challenge and analyzed by HIV-1 p24 ELISA or RT-PCR (to quantitate HIV RNA). Results are expressed as the ratio of viral load or nanograms per milliliter of HIV-1 p24 to the number of human CD45+ cells, as determined by flow cytometry. Open bars, data from individual animals; shaded bars, mean ± SEM of data from the two groups of mice. Top and bottom panels are from HIV-1LAI- and HIV-1ADA-infected animals, respectively. The experiment was set up with six to eight animals per group; samples from animals that did not reconstitute with human PBL were excluded from analysis. *, P < 0.05, calculated with Student's t test.
HSV gp120MN/LAI DC immunization protects CD4+ T cells.
HIV-1LAI challenge led to a significant depletion of CD4+ T lymphocytes (Fig. 6), which was largely prevented in mice inoculated with HSV gp120MN/LAI-transduced DC. Thus, even though the virus load was not reduced in these animals (Fig. 5), the engrafted human CD4+ T lymphocytes were significantly protected from virus-induced cytolysis following X4-using lymphotropic virus strain (LAI) infection. In contrast, HIV-1ADA challenge had no effect on human CD4+ T lymphocyte counts in the hu-PBL-NOD/SCID mice; CD4+ T cell levels, therefore, remained equivalent in both the vaccinated and control animal groups in this case (6.05 ± 2.30 versus 6.16 ± 3.00). This was expected, as HIV-1ADA does not affect bystander death of CD4+ T cells.
DISCUSSION
Our results demonstrate that hu-PBL-NOD/SCID mice can evaluate human humoral and cellular immune responses to candidate HIV-1 vaccines along with protective efficacy of such responses in an infectious challenge model. This has clear implications for preclinical evaluation of HIV-1 vaccines. Furthermore, that one can successfully study immune responses elicited following inoculation of hu-PBL-engrafted mice with autologous human DC suggests that the hu-PBL-NOD/SCID mouse model system can also be used to study a range of vaccine candidates. This may have utility for both the preclinical evaluation of prophylactic vaccines for HIV/AIDS and prophylactic or therapeutic vaccines for cancer. This study, to the best of our knowledge, is the first to use viral vector-transduced DC expressing HIV-1 antigens demonstrating potent immune responses in a mouse model for human disease.
HSV-1 has broad cellular tropism that includes DC (35, 60). The virus also infects mucosal surfaces and has the ability to establish latent infection in the host, which is characterized by the prolonged expression of a small subset of viral genes. The helper-free HSV-1 amplicon system has several advantages for vaccine delivery, including delivery of multiple copies of the packaged DNA to the target cell. Importantly, the packaged amplicon DNA does not express HSV-1 proteins (such as ICP47 or other viral immune modulatory molecules). Furthermore, transduction of human DC with helper-free HSV-1 amplicons is highly efficient and results in phenotypic maturation of the DC (as reflected by rises in cell surface expression of HLA-DR, CD80, CD83, and CD86), without suppressing their immunostimulatory function (as assessed by multilinear regression) (60). These factors may contribute to the robust immune responses detected in the hu-PBL-NOD/SCID mice that received the HSV gp120MN/LAI-transduced DC, as DC maturation was shown to be a key factor in generating immune responses in hu-PBL-SCID mice (26, 61).
The same amplicon vector used in the present study (HSV gp120MN/LAI) was previously shown to induce potent HIV-1 specific cellular and humoral responses in BALB/c mice (20, 58). In the present report, we have shown that human DC transduced with this vector elicit robust human humoral and cellular immune responses in hu-PBL-NOD/SCID mice. Such immune responses were comparable to previous reports with HIV-1-pulsed DC-based vaccines (28, 61). Taken together, these findings support the notion that the broad host range of HSV-1, coupled with its ability to infect DC, makes the HSV-1 amplicon vector an excellent vehicle for antigen delivery and induction of virus-specific immunity (20, 35, 48, 57).
In the present study, two different virus strains were employed in our challenge experiments. HIV-1LAI was chosen in part because the LAI V3 loop sequence was incorporated within the HIV-1 gp120 gene contained in the vaccine construct; however, the remainder was derived from HIV-1MN (20). HIV-1ADA was also used for challenge experiments because hu-PBL-SCID mice have been shown to be more susceptible to R5 viruses than X4 viruses (13, 37). Macrophage tropic viruses are the major transmittable viruses for HIV infection (36, 46). Partial protection from HIV-1ADA and none against HIV-1LAI was observed. This result is less impressive than that previously reported by Yoshida and colleagues (61), who observed complete protection from infectious virus challenge in hu-PBL-SCID mice immunized with autologous HIV-1 pulsed DC. However, there are several differences between the studies. First, we immunized animals once i.p. and examined virus load at 2 weeks after challenge. In contrast, Yoshida and colleagues coinjected the human PBL and antigen-pulsed DC by the intrasplenic route, then followed with a booster DC immunization 5 days later, and measured viral load at 1 week rather than 2 weeks after challenge, as in our study. Second, we used a large amount of viral inoculum of HIV-1 strain LAI (105 TCID50), whereas Yoshida et al. (61) and Lapenta et al. (28) used an autologous macrophage tropic viral strain for challenges. Finally, and perhaps most importantly, our experiments involved the transduction of the immunizing DC with a vector that expressed only HIV-1 gp120 with limited neutralizing viral responses. For these reasons, direct comparisons between the studies cannot be made. Rather, we would conclude that these results provide proof of principle for the use of the hu-PBL-NOD/SCID model to assess the induction of primary human immune responses to candidate HIV-1 vaccines and further exploit the model to examine immunization-induced protection from virus challenge. Significant sparing of the engrafted human CD4+ T-cell population following HIV-1LAI infection in hu-PBL-NOD/SCID mice injected with HSV gp120MN/LAI-transduced DC suggests that partial protective immune responses were achieved in the animals. Future efforts will focus on the evaluation of immune responses elicited by amplicon vectors, which express more optimal HIV-1 Env constructs, alone and in combination with other viral antigens (such as Gag, Pol, Nef, and Tat), as well as appropriate molecular adjuvants (3, 10, 54).
Overall, our results support prior studies demonstrating that DC-based HIV vaccines effectively induce humoral and cellular immune responses in hu-PBL-SCID mice (28, 61). HSV-1 amplicons effectively transduce DC and elicit high-level viral antigen expression. This facilitates their use in eliciting strong immune responses to encoded HIV-1 antigens, which may persist over time (20). DC-based vaccine approaches may further extend the utility of this vector system and could (for example) allow one to avoid any deleterious effects associated with preexisting anti-vector immunity (by allowing the performance of vector transduction ex vivo, in the absence of serum Abs). DC vaccine strategies may also have significant utility in therapeutic settings, including not only HIV/AIDS but also cancer (8, 29, 30). These results further support the use of the hu-PBL-NOD/SCID mouse model for preclinical screening of potential vaccine candidates. Human immune responses and protection against viral challenge can be tested. This may prove to be particularly useful for vaccine candidates that are difficult to test in macaques. Overall, the chimeric mouse model is significant in its ability to evaluate immune responses that regulate HIV infection. Such responses induced by the types of immunization approaches developed in this report are necessary elements for developing an effective HIV vaccine.
Acknowledgments
We thank Robin Taylor for review of the manuscript and administrative assistance, Linda Wilke for FACS analysis, and Laura and Brian Lauer for generous donations for vaccine research in HIV/AIDS.
NIH research grants supporting this work included P01 MH57556, P01NS31492, R01NS34239, R37NS36136 (H.E.G.), NCRR P20RR15635 (L.P. and H.E.G), F31AI054330 (K.S.), P01 AI056356 (S.D., W.J.B., and H.J.F.), and NS31492-06 (H.E.G. and S.G.).
REFERENCES
- 1.Achour, A., O. Picard, J. P. Mbika, A. Willer, R. Snart, B. Bizzini, C. Carelli, A. Burny, and D. Zagury. 1993. Envelope protein and p18(IIIB) peptide recognized by cytotoxic T lymphocytes from humans immunized with human immunodeficiency virus envelope. Vaccine 11:609-701. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad, K. 2003. High hopes for HIV vaccine. Lancet Infect. Dis. 3:62. [DOI] [PubMed] [Google Scholar]
- 3.Ahonen, C. L., S. J. Gibson, R. M. Smith, L. K. Pederson, J. M. Lindh, M. A. Tomai, and J. P. Vasilakos. 1999. Dendritic cell maturation and subsequent enhanced T-cell stimulation induced with the novel synthetic immune response modifier R-848. Cell Immunol. 197:62-72. [DOI] [PubMed] [Google Scholar]
- 4.Alexander-Miller, M. A., K. C. Parker, T. Tsukui, C. D. Pendleton, J. E. Coligan, and J. A. Berzofsky. 1996. Molecular analysis of presentation by HLA-A2.1 of a promiscuously binding V3 loop peptide from the HIV-envelope protein to human cytotoxic T lymphocytes. Int. Immunol. 8:641-649. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau, J., J. Fay, V. Pascual, and A. K. Palucka. 2003. Dendritic cells: controllers of the immune system and a new promise for immunotherapy. Novartis Found. Symp. 252:226-235. [PubMed] [Google Scholar]
- 6.Bombil, F., J. P. Kints, J. M. Scheiff, H. Bazin, and D. Latinne. 1996. A promising model of primary human immunization in human-scid mouse. Immunobiology 195:360-375. [DOI] [PubMed] [Google Scholar]
- 7.Bower, J. F., X. Yang, J. Sodroski, and T. M. Ross. 2004. Elicitation of neutralizing antibodies with DNA vaccines expressing soluble stabilized human immunodeficiency virus type 1 envelope glycoprotein trimers conjugated to C3d. J. Virol. 78:4710-4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown, K., W. Gao, S. Alber, A. Trichel, M. Murphey-Corb, S. C. Watkins, A. Gambotto, and S. M. Barratt-Boyes. 2003. Adenovirus-transduced dendritic cells injected into skin or lymph node prime potent simian immunodeficiency virus-specific T cell immunity in monkeys. J. Immunol. 171:6875-6882. [DOI] [PubMed] [Google Scholar]
- 9.Calarota, S. A., and D. B. Weiner. 2003. Present status of human HIV vaccine development. Aids 17(Suppl. 4):S73-S84. [DOI] [PubMed] [Google Scholar]
- 10.Chakrabarti, B. K., W. P. Kong, B. Y. Wu, Z. Y. Yang, J. Friborg, X. Ling, S. R. King, D. C. Montefiori, and G. J. Nabel. 2002. Modifications of the human immunodeficiency virus envelope glycoprotein enhance immunogenicity for genetic immunization. J. Virol. 76:5357-5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chargui, J., D. Dye, J. Blomberg, C. Desgranges, and J. L. Touraine. 1995. The humanized severe combined immunodeficient mouse as a model for primary human humoral response against HIV1 peptides. J. Immunol. Methods 181:91-100. [DOI] [PubMed] [Google Scholar]
- 12.Emini, E. A., and W. C. Koff. 2004. AIDS/HIV. Developing an AIDS vaccine: need, uncertainty, hope. Science 304:1913-1914. [DOI] [PubMed] [Google Scholar]
- 13.Fais, S., C. Lapenta, S. M. Santini, M. Spada, S. Parlato, M. Logozzi, P. Rizza, and F. Belardelli. 1999. Human immunodeficiency virus type 1 strains R5 and X4 induce different pathogenic effects in hu-PBL-SCID mice, depending on the state of activation/differentiation of human target cells at the time of primary infection. J. Virol. 73:6453-6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardner, M. B., and P. A. Luciw. 1989. Animal models of AIDS. FASEB J. 3:2593-2606. [DOI] [PubMed] [Google Scholar]
- 15.Gendelman, H. E., J. M. Orenstein, M. A. Martin, C. Ferrua, R. Mitra, T. Phipps, L. A. Wahl, H. C. Lane, A. S. Fauci, D. S. Burke, et al. 1988. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J. Exp. Med. 167:1428-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilliam, B. L., and R. R. Redfield. 2003. Therapeutic HIV vaccines. Curr. Top. Med. Chem. 3:1536-1553. [DOI] [PubMed] [Google Scholar]
- 17.Greiner, D. L., L. D. Shultz, J. Yates, M. C. Appel, G. Perdrizet, R. M. Hesselton, I. Schweitzer, W. G. Beamer, K. L. Shultz, S. C. Pelsue, and et al. 1995. Improved engraftment of human spleen cells in NOD/LtSz-scid/scid mice as compared with C.B-17-scid/scid mice. Am. J. Pathol. 146:888-902. [PMC free article] [PubMed] [Google Scholar]
- 18.Hesselton, R. M., R. A. Koup, M. A. Cromwell, B. S. Graham, M. Johns, and J. L. Sullivan. 1993. Human peripheral blood xenografts in the SCID mouse: characterization of immunologic reconstitution. J. Infect. Dis. 168:630-640. [DOI] [PubMed] [Google Scholar]
- 19.Hewer, R., and D. Meyer. 2003. Peptide immunogens based on the envelope region of HIV-1 are recognized by HIV/AIDS patient polyclonal antibodies and induce strong humoral immune responses in mice and rabbits. Mol. Immunol. 40:327-335. [DOI] [PubMed] [Google Scholar]
- 20.Hocknell, P. K., R. D. Wiley, X. Wang, T. G. Evans, W. J. Bowers, T. Hanke, H. J. Federoff, and S. Dewhurst. 2002. Expression of human immunodeficiency virus type 1 gp120 from herpes simplex virus type 1-derived amplicons results in potent, specific, and durable cellular and humoral immune responses. J. Virol. 76:5565-5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joag, S. V. 2000. Primate models of AIDS. Microbes Infect. 2:223-229. [DOI] [PubMed] [Google Scholar]
- 22.Johnston, M. I. 2000. The role of nonhuman primate models in AIDS vaccine development. Mol. Med. Today 6:267-270. [DOI] [PubMed] [Google Scholar]
- 23.Joshi, S. S., U. E. Vu, T. R. Lovgren, M. Lorkovic, W. Patel, G. L. Todd, C. Kuszynski, B. J. Joshi, and H. P. Dave. 2002. Comparison of phenotypic and functional dendritic cells derived from human umbilical cord blood and peripheral blood mononuclear cells. J. Hematother. Stem Cell Res. 11:337-347. [DOI] [PubMed] [Google Scholar]
- 24.Kalter, D. C., H. E. Gendelman, and M. S. Meltzer. 1991. Inhibition of human immunodeficiency virus infection in monocytes by monoclonal antibodies against leukocyte adhesion molecules. Immunol. Lett. 30:219-227. [DOI] [PubMed] [Google Scholar]
- 25.Kaur, A., and R. P. Johnson. 2003. HIV pathogenesis and vaccine development. Top. HIV Med. 11:76-85. [PubMed] [Google Scholar]
- 26.Koyanagi, Y., Y. Tanaka, J. Kira, M. Ito, K. Hioki, N. Misawa, Y. Kawano, K. Yamasaki, R. Tanaka, Y. Suzuki, Y. Ueyama, E. Terada, T. Tanaka, M. Miyasaka, T. Kobayashi, Y. Kumazawa, and N. Yamamoto. 1997. Primary human immunodeficiency virus type 1 viremia and central nervous system invasion in a novel hu-PBL-immunodeficient mouse strain. J. Virol. 71:2417-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LaFranco-Scheuch, L., K. Abel, N. Makori, K. Rothaeusler, and C. J. Miller. 2004. High beta-chemokine expression levels in lymphoid tissues of simian/human immunodeficiency virus 89.6-vaccinated rhesus macaques are associated with uncontrolled replication of simian immunodeficiency virus challenge inoculum. J. Virol. 78:6399-6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lapenta, C., S. M. Santini, M. Logozzi, M. Spada, M. Andreotti, T. Di Pucchio, S. Parlato, and F. Belardelli. 2003. Potent immune response against HIV-1 and protection from virus challenge in hu-PBL-SCID mice immunized with inactivated virus-pulsed dendritic cells generated in the presence of IFN-alpha. J. Exp. Med. 198:361-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu, W., X. Wu, Y. Lu, W. Guo, and J. M. Andrieu. 2003. Therapeutic dendritic-cell vaccine for simian AIDS. Nat. Med. 9:27-32. [DOI] [PubMed] [Google Scholar]
- 30.Marovich, M. A., J. R. Mascola, M. A. Eller, M. K. Louder, P. A. Caudrelier, R. El-Habib, S. Ratto-Kim, J. H. Cox, J. R. Currier, B. L. Levine, C. H. June, W. B. Bernstein, M. L. Robb, B. Schuler-Thurner, R. M. Steinman, D. L. Birx, and S. Schlesinger-Frankel. 2002. Preparation of clinical-grade recombinant canarypox-human immunodeficiency virus vaccine-loaded human dendritic cells. J. Infect. Dis. 186:1242-1252. [DOI] [PubMed] [Google Scholar]
- 31.Matano, T., M. Kobayashi, H. Igarashi, A. Takeda, H. Nakamura, M. Kano, C. Sugimoto, K. Mori, A. Iida, T. Hirata, M. Hasegawa, T. Yuasa, M. Miyazawa, Y. Takahashi, M. Yasunami, A. Kimura, D. H. O'Connor, D. I. Watkins, and Y. Nagai. 2004. Cytotoxic T lymphocyte-based control of simian immunodeficiency virus replication in a preclinical AIDS vaccine trial. J. Exp. Med. 199:1709-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCluskie, M. J., C. L. Brazolot Millan, R. A. Gramzinski, H. L. Robinson, J. C. Santoro, J. T. Fuller, G. Widera, J. R. Haynes, R. H. Purcell, and H. L. Davis. 1999. Route and method of delivery of DNA vaccine influence immune responses in mice and non-human primates. Mol. Med. 5:287-300. [PMC free article] [PubMed] [Google Scholar]
- 33.McKinney, D. M., D. A. Lewinsohn, S. R. Riddell, P. D. Greenberg, and D. E. Mosier. 1999. The antiviral activity of HIV-specific CD8+ CTL clones is limited by elimination due to encounter with HIV-infected targets. J. Immunol. 163:861-867. [PubMed] [Google Scholar]
- 34.McMichael, A. J., and T. Hanke. 2003. HIV vaccines 1983-2003. Nat. Med. 9:874-880. [DOI] [PubMed] [Google Scholar]
- 35.Mikloska, Z., L. Bosnjak, and A. L. Cunningham. 2001. Immature monocyte-derived dendritic cells are productively infected with herpes simplex virus type 1. J. Virol. 75:5958-5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosier, D., and H. Sieburg. 1994. Macrophage-tropic HIV: critical for AIDS pathogenesis? Immunol. Today 15:332-339. [DOI] [PubMed] [Google Scholar]
- 37.Mosier, D. E., R. J. Gulizia, S. M. Baird, D. B. Wilson, D. H. Spector, and S. A. Spector. 1991. Human immunodeficiency virus infection of human-PBL-SCID mice. Science 251:791-794. [DOI] [PubMed] [Google Scholar]
- 38.Mosier, D. E., R. J. Gulizia, P. MacIsaac, B. J. Mathieson, G. Smith, S. L. Hu, L. Corey, and P. Greenberg. 1992. Evaluation of gp160 vaccinees in the hu-PBL-SCID mouse model. AIDS Res. Hum. Retrovir. 8:1387. [DOI] [PubMed] [Google Scholar]
- 39.Mosier, D. E., R. J. Gulizia, P. D. MacIsaac, B. E. Torbett, and J. A. Levy. 1993. Rapid loss of CD4+ T cells in human-PBL-SCID mice by noncytopathic HIV isolates. Science 260:689-692. [DOI] [PubMed] [Google Scholar]
- 40.Nath, B. M., K. E. Schumann, and J. D. Boyer. 2000. The chimpanzee and other non-human-primate models in HIV-1 vaccine research. Trends Microbiol. 8:426-431. [DOI] [PubMed] [Google Scholar]
- 41.Nehete, P. N., R. Gambhira, B. P. Nehete, and K. J. Sastry. 2003. Dendritic cells enhance detection of antigen-specific cellular immune responses by lymphocytes from rhesus macaques immunized with an HIV envelope peptide cocktail vaccine. J. Med. Primatol. 32:67-73. [DOI] [PubMed] [Google Scholar]
- 42.Pollock, P. L., D. R. Germolec, C. E. Comment, G. J. Rosenthal, and M. I. Luster. 1994. Development of human lymphocyte-engrafted SCID mice as a model for immunotoxicity assessment. Fundam. Appl. Toxicol. 22:130-138. [DOI] [PubMed] [Google Scholar]
- 43.Poluektova, L., S. Gorantla, J. Faraci, K. Birusingh, H. Dou, and H. E. Gendelman. 2004. Neuroregulatory events follow adaptive immune-mediated elimination of HIV-1-infected macrophages: studies in a murine model of viral encephalitis. J. Immunol. 172:7610-7617. [DOI] [PubMed] [Google Scholar]
- 44.Poluektova, L. Y., D. H. Munn, Y. Persidsky, and H. E. Gendelman. 2002. Generation of cytotoxic T cells against virus-infected human brain macrophages in a murine model of HIV-1 encephalitis. J. Immunol. 168:3941-3949. [DOI] [PubMed] [Google Scholar]
- 45.Rollman, E., J. Hinkula, J. Arteaga, B. Zuber, A. Kjerrstrom, M. Liu, B. Wahren, and K. Ljungberg. 2004. Multi-subtype gp160 DNA immunization induces broadly neutralizing anti-HIV antibodies. Gene Ther. 11:1146-1154. [DOI] [PubMed] [Google Scholar]
- 46.Schuitemaker, H. 1994. Macrophage-tropic HIV-1 variants: initiators of infection and AIDS pathogenesis? J. Leukoc. Biol. 56:218-224. [DOI] [PubMed] [Google Scholar]
- 47.Segall, H., I. Lubin, H. Marcus, A. Canaan, and Y. Reisner. 1996. Generation of primary antigen-specific human cytotoxic T lymphocytes in human/mouse radiation chimera. Blood 88:721-730. [PubMed] [Google Scholar]
- 48.Sena-Esteves, M., Y. Saeki, C. Fraefel, and X. O. Breakefield. 2000. HSV-1 amplicon vectors—simplicity and versatility. Mol. Ther. 2:9-15. [DOI] [PubMed] [Google Scholar]
- 49.Spearman, P. 2003. HIV vaccine development: lessons from the past and promise for the future. Curr. HIV Res. 1:101-120. [DOI] [PubMed] [Google Scholar]
- 50.Steinman, R. M. 2000. The dendritic cell advantage: New focus for immune-based therapies. Drug News Perspect. 13:581-586. [DOI] [PubMed] [Google Scholar]
- 51.Steinman, R. M. 1996. Dendritic cells and immune-based therapies. Exp. Hematol. 24:859-862. [PubMed] [Google Scholar]
- 52.Tanaka, T., M. Tsudo, H. Karasuyama, F. Kitamura, T. Kono, M. Hatakeyama, T. Taniguchi, and M. Miyasaka. 1991. A novel monoclonal antibody against murine IL-2 receptor beta-chain. Characterization of receptor expression in normal lymphoid cells and EL-4 cells. J. Immunol. 147:2222-2228. [PubMed] [Google Scholar]
- 53.Tary-Lehmann, M., A. Saxon, and P. V. Lehmann. 1995. The human immune system in hu-PBL-SCID mice. Immunol. Today 16:529-533. [DOI] [PubMed] [Google Scholar]
- 54.Thomsen, L. L., P. Topley, M. G. Daly, S. J. Brett, and J. P. Tite. 2004. Imiquimod and resiquimod in a mouse model: adjuvants for DNA vaccination by particle-mediated immunotherapeutic delivery. Vaccine 22:1799-1809. [DOI] [PubMed] [Google Scholar]
- 55.Torbett, B. E., G. Picchio, and D. E. Mosier. 1991. hu-PBL-SCID mice: a model for human immune function, AIDS, and lymphomagenesis. Immunol. Rev. 124:139-164. [DOI] [PubMed] [Google Scholar]
- 56.van Kuyk, R., B. E. Torbett, R. J. Gulizia, S. Leath, D. E. Mosier, and S. Koenig. 1994. Cloned human CD8+ cytotoxic T lymphocytes protect human peripheral blood leukocyte-severe combined immunodeficient mice from HIV-1 infection by an HLA-unrestricted mechanism. J. Immunol. 153:4826-4833. [PubMed] [Google Scholar]
- 57.Wang, S., C. Fraefel, and X. Breakefield. 2002. HSV-1 amplicon vectors. Methods Enzymol. 346:593-603. [DOI] [PubMed] [Google Scholar]
- 58.Wang, X., R. D. Wiley, T. G. Evans, W. J. Bowers, H. J. Federoff, and S. Dewhurst. 2003. Cellular immune responses to helper-free HSV-1 amplicon particles encoding HIV-1 gp120 are enhanced by DNA priming. Vaccine 21:2288-2297. [DOI] [PubMed] [Google Scholar]
- 59.Warren, J. 2002. Preclinical AIDS vaccine research: survey of SIV, SHIV, and HIV challenge studies in vaccinated nonhuman primates. J. Med. Primatol. 31:237-256. [DOI] [PubMed] [Google Scholar]
- 60.Willis, R. A., W. J. Bowers, M. J. Turner, T. L. Fisher, C. S. Abdul-Alim, D. F. Howard, H. J. Federoff, E. M. Lord, and J. G. Frelinger. 2001. Dendritic cells transduced with HSV-1 amplicons expressing prostate-specific antigen generate antitumor immunity in mice. Hum. Gene Ther. 12:1867-1879. [DOI] [PubMed] [Google Scholar]
- 61.Yoshida, A., R. Tanaka, T. Murakami, Y. Takahashi, Y. Koyanagi, M. Nakamura, M. Ito, N. Yamamoto, and Y. Tanaka. 2003. Induction of protective immune responses against R5 human immunodeficiency virus type 1 (HIV-1) infection in hu-PBL-SCID mice by intrasplenic immunization with HIV-1-pulsed dendritic cells: possible involvement of a novel factor of human CD4+ T-cell origin. J. Virol. 77:8719-8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhan, X., K. S. Slobod, S. Surman, S. A. Brown, C. Coleclough, and J. L. Hurwitz. 2004. Minor components of a multienvelope HIV vaccine are recognized by type-specific T-helper cells. Vaccine 22:1206-1213. [DOI] [PubMed] [Google Scholar]