Significance
One-third of bipolar disorder (BPD) patients are lithium-responsive (LiR) for unknown reasons. Were lithium’s target to be identified, then BPD’s pathogenesis might be unraveled. We identified and mapped the “lithium-response pathway,” which governs the phosphorylation of CRMP2, a cytoskeleton regulator, particularly for dendritic spines: hence, a neural network modulator. Although “toggling” between inactive (phosphorylated) and active (nonphosphorylated) CRMP2 is physiologic, the “set-point” in LiR BPD is abnormal. Lithium (and other pathway-modulators) normalize that set-point. Hence, BPD is a disorder not of a gene but of the posttranslational regulation of a developmentally critical molecule. Such knowledge should enable better mechanistically based treatments and bioassays. Instructively, lithium was our “molecular can-opener” for “prying” intracellularly to reveal otherwise inscrutable pathophysiology in this complex polygenic disorder.
Keywords: posttranslational modification, proteomics, psychiatric disease modeling, CRMP2, dendrites
Abstract
The molecular pathogenesis of bipolar disorder (BPD) is poorly understood. Using human-induced pluripotent stem cells (hiPSCs) to unravel such mechanisms in polygenic diseases is generally challenging. However, hiPSCs from BPD patients responsive to lithium offered unique opportunities to discern lithium's target and hence gain molecular insight into BPD. By profiling the proteomics of BDP–hiPSC-derived neurons, we found that lithium alters the phosphorylation state of collapsin response mediator protein-2 (CRMP2). Active nonphosphorylated CRMP2, which binds cytoskeleton, is present throughout the neuron; inactive phosphorylated CRMP2, which dissociates from cytoskeleton, exits dendritic spines. CRMP2 elimination yields aberrant dendritogenesis with diminished spine density and lost lithium responsiveness (LiR). The “set-point” for the ratio of pCRMP2:CRMP2 is elevated uniquely in hiPSC-derived neurons from LiR BPD patients, but not with other psychiatric (including lithium-nonresponsive BPD) and neurological disorders. Lithium (and other pathway modulators) lowers pCRMP2, increasing spine area and density. Human BPD brains show similarly elevated ratios and diminished spine densities; lithium therapy normalizes the ratios and spines. Consistent with such “spine-opathies,” human LiR BPD neurons with abnormal ratios evince abnormally steep slopes for calcium flux; lithium normalizes both. Behaviorally, transgenic mice that reproduce lithium's postulated site-of-action in dephosphorylating CRMP2 emulate LiR in BPD. These data suggest that the “lithium response pathway” in BPD governs CRMP2's phosphorylation, which regulates cytoskeletal organization, particularly in spines, modulating neural networks. Aberrations in the posttranslational regulation of this developmentally critical molecule may underlie LiR BPD pathogenesis. Instructively, examining the proteomic profile in hiPSCs of a functional agent—even one whose mechanism-of-action is unknown—might reveal otherwise inscrutable intracellular pathogenic pathways.
Although human induced pluripotent stem cells (hiPSCs) have proven valuable for studying the molecular pathology of monogenic diseases, one of the technique’s greatest challenges has been to offer similar insights into the molecular pathogenesis of polygenic, multifactorial disorders for which the underlying pathophysiology is unknown. The struggle has been to go beyond phenotypic description to discerning underlying molecular mechanisms. Neuropsychiatric illnesses are a prototype for such complex conditions (1–3). They are difficult to model not only because of the likelihood of polygenic influences, but also because of the subjectivity with which these diseases must often be diagnosed, the empirical fashion with which drugs are prescribed, and the heterogeneity of patient response. Of such maladies, bipolar disorder (BPD) type 1, a chronic illness of episodic mania with intervening periods of depression for which the interplay between genetic and environmental factors is poorly understood, is unique in that, for unclear reasons, ∼35% of patients respond to monotherapy with lithium salts (4–7); indeed, lithium responsiveness (LiR) is often regarded as pathognomic of BPD. However, despite our knowledge of lithium’s ubiquitous multisystemic influences and pleiotropic actions (4), the molecular mechanism underlying this drug responsiveness specifically in BPD, as well as BPD’s molecular pathogenesis, are poorly understood. The former, however, could lend insight into the latter. For example, although lithium may suppress hyperexcitability of a subset of neurons in culture (2) [many mechanisms have been proffered (4)], clinical trials have shown that drugs that simply suppress neuronal activity, such as calcium channel blockers, are ineffective in BPD (7). Furthermore, of the one-third of patients who are LiR, many become noncompliant because of frequent adverse side effects (e.g., weight gain, hypothyroidism, tremor, kidney dysfunction, dermatologic reactions, teratogenicity). Such pleiotropic effects underscore our ignorance with regard to lithium’s action specifically for BPD. Additionally, the safety index of lithium is narrow (5, 8). In view of its prevalence (the sixth leading cause of disability worldwide), suboptimal treatment options, and absence of biomarkers for onset and progression, neuropsychiatric disorders in general—and BPD in particular—represent a pressing unmet medical need (1 in 250 sufferers die from complications of BPD). Two obstacles to developing safer, more effective mood stabilizers have been a lack of known clinically relevant molecular drug targets and of drug-screening assays that are rooted in the molecular pathogenesis and pathophysiology of the disorder. Although heritability of BPD is ∼80%, few disease-specific gene associations have been identified with sufficient consistency and statistical significance to guide further studies (9, 10); multiple loci are more likely to contribute to LiR than any single reliable genetic marker, making it challenging for hiPSC disease-modeling technology. The approach presented here might help address these challenges.
Because most of lithium’s actions have been linked to posttranslational regulation rather than to transcription (4), we elected to start with an unbiased differential proteomic approach. Thus, whereas lithium’s action as a modifier of kinase signaling has been described for numerous substrates (4), precisely how phosphorylation plays a role, and what the substrate of that phosphorylation might be that is relevant to BPD, are not understood. Here we describe inroads into probing, mapping, and understanding the regulation of the molecular “lithium-response pathway” in BPD initially using proteomic profiling (by two independent techniques) of patient-derived hiPSCs to identify putative lithium targets, followed by bioinformatic pathway analyses to determine the hierarchy and convergence of these candidates. We validated our conclusions in: (i) biochemical analyses comparing hiPSC-derived neurons from LiR, lithium-nonresponsive (LiNR), and unaffected individuals (as well as those with other psychiatric and neurological conditions); (ii) assays of neuronal function; (iii) neurocytological and behavioral analyses of transgenic mice in which the pathway’s putative central node is eliminated or lithium’s putative site-of-action is reproduced; and (iv) biochemical and histological assessment of primary human patient brain specimens. Extrapolating from the mediators of LiR to conclusions regarding the molecular underpinnings of BPD, our data implicate not a gene defect per se, but rather aberrant posttranslational modification of a developmentally critical molecule: an abnormally high phosphoregulatory set-point for the central cytoskeletal modulator Collapsin Response Mediator Protein-2 (CRMP2) (11–16) which, by determining CRMP2’s active state, in turn influences dendritic form and function and hence, presumably, neural network development and activity.
Results
We generated hiPSCs from cohorts of LiR and LiNR BPD, and unaffected patients, which included two sets of first-degree relatives, each set with an LiR patient and an unaffected family member and one set with a family member with the diagnosis of unipolar/major depression (MD) (SI Appendix, Fig. S1). Additionally, we generated hiPSCs from a patient with Parkinson’s disease (PD) as a neurologically affected nonpsychiatric control (SI Appendix, Fig. S1). hiPSC clones (typically duplicates for each patient sample) (SI Appendix, Fig. S1) were validated to confirm that they: (i) retained a SNP pattern identical to their somatic cell-of-origin; (ii) were immunopositive for OCT4, NANOG, SSEA4, and Tra-1-81 (SI Appendix, Fig. S2A); (iii) showed gene-expression profiles consistent with the pluripotent state (SI Appendix, Fig. S2B); and (iv) were capable of forming embryoid bodies or teratomas containing derivatives of the three primitive germ layers (SI Appendix, Figs. S2 C and D). We observed no differences in neuronal induction efficiency and yield among LiR, LiNR, or unaffected patient-derived hiPSCs, all of which showed similar expression of neuronal markers [Tau, βIII-tubulin (Tuj1), MAP2, vGLUT, GABA] and produced neural progenitor cells (NPCs) and electrophysiologically active (17) glutamatergic (vGLUT+) and GABAergic (GABA+) neurons that initially expressed markers consistent with a dorsal anterior forebrain cortical phenotype (SI Appendix, Figs. S2 E–L and S3 A–J) (17–20). For our in vitro studies, we elected to preserve the distinction made by clinicians between LiR and LiNR patients (5–8) and to probe lithium’s protein targets within LiR BPD neurons (SI Appendix, Figs. S1).
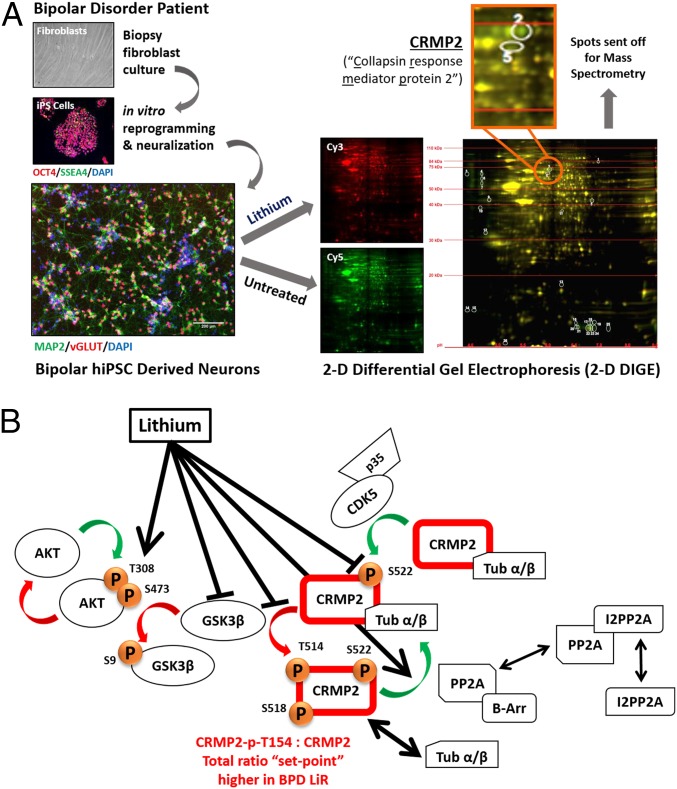
We began unbiased differential proteomic analysis of a single clone of BPD hiPSC-derived neurons by comparing lithium treated to untreated neurons. By 2D differential gel electrophoresis (2D-DIGE), we identified 26 differentially represented protein spots yielding 15 distinct proteins identified by mass spectrometry (Fig. 1A and SI Appendix, Fig. S4A). The genes corresponding to the 15 proteins were queried against a publicly available human gene-expression database constructed from the dorsal lateral prefrontal cortex of 30 BPD patient brains compared with 31 control patient brains (https://www.ncbi.nlm.nih.gov/sites/GDSbrowser?acc=GDS2190): three genes, whose products changed significantly in response to lithium in the 2D-DIGE dataset (and hence were candidate lithium targets), were also differentially expressed in BPD compared with control brains (P < 0.03): CRMP2, mitochondrial ribosomal protein S22 (MRPS22), and cystatin B (CSTB). Interrogation of the Sullivan Lab Evidence Project (SLEP) was performed to indicate proteins whose encoding genes might already be linked to psychiatric disorders by genome-wide association studies or genetic linkages. Interrogation of SLEP (<50 kb for linkage, P < 1 × 10−3 for genome-wide association studies, logarithm of the odds score of 3) showed microsatellite enrichment of 4 of the 15 protein-encoding genes—CRMP2, WD repeat and FYVE domain containing protein-1 (WDFY1), and reticulocalbin-1 (RCN1)—and SNP enrichment of two: nuclear transport factor-2 (NUTF2) and RCN1. Finally, because, 8p21 has been implicated as a susceptibility locus for schizophrenia (21–23), genes linked to that region have also been viewed as risk factors for psychiatric disease more broadly, although the mechanism by which they might predispose to a mental disorder is unknown (21–23). Among the 2D-DIGE candidate proteins, only CRMP2 is encoded within chromosomal region 8p22-21.
Fig. 1.
CRMP2 appears to be the central node in the lithium-response pathway in hiPSC-derived neurons from LiR BPD patients. (A) Differential proteomic analysis by 2D-DIGE of BPD patient hiPSC-derived neurons identified CRMP2 as a target of lithium. Patient-derived dermal fibroblasts were reprogrammed into hiPSCs and differentiated to cortical interneurons (17). (Scale bar: 400 μm, Top; 200 μm, Bottom.) Neurons from a single clone were treated or left untreated with LiCl [red (Cy3) and green (Cy5), respectively], and then subjected to 2D-DIGE, separating proteins based on their molecular weight, charge, and degree of enrichment under each condition. In an overlay of gels, proteins unchanged in response to LiCl fluoresce both green and red, and hence, look yellow; proteins altered by LiCl fluoresce solely red or solely green. Differential protein spots (encircled white) were picked and identified by MS (SI Appendix, Fig. S2), with spots #2 and #5 (enlarged) identified as CRMP2. (B) Proposed model of the lithium-response pathway in BPD, regulating CRMP2’s phosphorylation-state and, hence, its association with cytoskeletal elements. The proposed action of lithium in this context—as mediated by its presumptive direct and indirect targets (black lines radiating from lithium working in concert) (Figs. 2 and 3 and SI Appendix, Figs. S6–S10)—is to promote dephosphorylation of CRMP2 at T514: that is, to reduce the CRMP2-p-T514:CRMP2 ratio, the set-point for which, we propose, is excessively high in LiR BPD (Figs. 2, 3 D and F, and 5 and SI Appendix, Figs. S10 and S12). It does so through both GSK3β-dependent and independent routes. Putative components: phosphorylation of CRMP2 at T514 inactivates CRMP2, such that it dissociates from cytoskeletal molecules (e.g., tubulin heterodimers). That phosphorylation, as well as at S518, T509, and T555 (suggested by IPA analysis to be by GSK3β), must first be “primed” by phosphorylation of S522 (believed to be mediated by CDK5). Phosphorylation of CRMP2 by kinases is balanced by its dephosphorylation by phosphatases (e.g., PP2A, which must be released by i2PP2A and complexed with β-arrestin-2 to function). Lithium can inactivate GSK3β by promoting its phosphorylation at S9 or by increasing p-AKT that can also phospho-inactivate GSK3β. Lithium can also block phosphorylation of S522 or increase phosphatase (PP2A) action. [Not schematized: The influence of PP2A/β-arrestin-2 complexes on AKT in the GSK3β-dependent portion of the schematic (30).]
The 2D-DIGE candidates were then subjected to Ingenuity Pathway Analysis (IPA), as well as modeling with the STRING Network tool (string-db.org) to generate a canonical pathway dendrogram that might indicate the functional relationships between gene products using gene ontology terms (SI Appendix, Fig. S4B). This analysis revealed that lithium treatment of human neurons appears to modulate four pathways significantly: semaphorin (SEMA) signaling in neurons, uracil degradation II (reductive), thymine degradation, and axonal guidance signaling. We noted that CRMP2 was a constituent of each of the four pathways. Additionally, axonal guidance signaling appeared most centralized; its function connected with 75% of the pathways identified in the IPA analysis. Of the 15 candidate protein targets, the one most pivotal to axonal guidance and cytoskeletal dynamics was CRMP2, originally discovered as the mediator of Sema3A’s (initially named “collapsin”) guidance of neurite extension and axonal growth cone development (5–11).
Given the converging proteomic and bioinformatics results, we focused on CRMP2 (a central node in cytoskeletal dynamics). It is known that phosphorylation of CRMP2 at threonine (T) 514 (CRMP2-p-T514) causes its dissociation from cytoskeletal proteins (e.g., tubulin heterodimers) (24–26). However, phosphorylation of CRMP2-T514 (as well as of CRMP2-S518 and CRMP2-T509) must first be primed by phosphorylation at CRMP2-S522. Phosphorylation of CRMP2 by kinases is balanced by its dephosphorylation by phosphatases (24–26) (Fig. 1B). (Note: Although lithium is known to have numerous targets, these 2D-DIGE experiments were designed to highlight ideally only those that differed specifically in BPD in response to lithium; those targets that were not specifically relevant to BPD would cancel each other out.)
IPA of the 2D-DIGE results showed the lithium-response pathway in BPD neurons overlaps significantly with the glycogen synthase kinase 3β (GSK3β) signaling axis, reinforcing presumptions that GSK3β is a major node upstream of CRMP2 (15), and suggesting that phosphorylation of CRMP2 at T514 is significantly influenced, although not necessarily exclusively, by GSK3β, a known substrate of lithium-mediated inhibition (but not the paralog GSK3α) (27). Therefore, we next questioned whether CRMP2 is the primary GSK3β substrate in BPD hiPSC neurons, or if additional substrates downstream of GSK3β may be involved. Surprisingly, bioinformatic analysis revealed that none of the other 15 candidates from our 2D-DIGE analysis contained GSK3β phosphorylation sites, suggesting that CRMP2 may be the major direct downstream effector of GSK3β in the specific context of human NPCs and neurons, particularly from BPD patients.
To further ascertain the extent of the molecular consequences of GSK3β inhibition within neural cells, we used “stable isotope labeling by amino acids in cell culture” (SILAC)-labeled (28) hiPSC-derived neural cells with a highly specific GSK3β inhibitor, CHIR99021, and compared proteolytic digests of treated and untreated cells using high-resolution liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) to identify differentially enriched peptides and proteins (SI Appendix, Fig. S5). As expected, CRMP2-p-T514 peptide was substantially reduced by CHIR99021 (SI Appendix, Fig. S5A). Of putative GSK3β substrates, CRMP2 was among the most robust in terms of overall peptide coverage and dose-dependency. Overall, these data suggested that CRMP2 is a primary mediator of GSK3β-dependent lithium action in human neurons.
We next monitored the time course of CRMP2 and CRMP2-p-T514 levels during differentiation of hiPSCs to neurons. Interestingly, CRMP2-p-T514 is largely restricted to NPCs and neurons, and most abundant in the latter (SI Appendix, Fig. S6A). Note that CRMP2-p-T514 in these neural lineages evolves into three isoforms of slightly different molecular weight, known to result from transcription initiation from alternate start codons, although all transcripts maintain the coding region for the phospho-motif containing T514. [Only isoform 2 shows any detectable phosphorylation in hiPSCs, likely reflecting the microtubule-dependence of the reprogramming process (29).] Isoform 3 (the top, heaviest band in SI Appendix, Fig. S6A) appears most pertinent to neural lineages, does not appear until the NPC stage, increases with maturation, and appears in our hands to be the one most influenced by lithium and CHIR99021 treatment; hence, we focused on the phospho-state of that isoform.
These findings prompted us to study in greater detail the phosphoregulation of CRMP2 in hiPSC-derived neurons, with an eye toward discerning how lithium interposes itself upon that regulation (Fig. 1B). We therefore began our delineation of the lithium-response pathway to and from CRMP2 by applying lithium to neurons derived from LiR BPD hiPSCs compared with LiNR BPD and unaffected patients, as well compared with other psychiatric and nonpsychiatric neurological conditions (e.g., MD and PD, respectively). (The patients used in the specific experiments described are specified in the appropriate figure legends, in SI Appendix, Supplementary Methods and in the table in SI Appendix, Fig. S1).
We found by immunostaining (Fig. 2 and SI Appendix, Fig. S6B) and Western blotting (Fig. 3 and SI Appendix, Fig. S6C) that lithium significantly decreased CRMP-p-T514 in all hiPSC-derived NPCs (Fig. 3B) and neurons, but without significant effect on nonphosphorylated or the total level of CRMP2 protein (Figs. 2A and 3 A and C–E, and SI Appendix, Figs. S3K, S6 B and C, S10, and S11). The effect of LiCl on CRMP-p-T514 was not emulated by other chloride salts: for example, NaCl or MgCl2 (SI Appendix, Fig. S6C). We reasoned that, if lithium lowers CRMP2-p-T514, at least in part, by inhibiting downstream GSK3β, then direct application of a GSK3β inhibitor (e.g., CHIR99021) should similarly lower CRMP2-p-T514 abundance, reducing the CRMP2-p-T514:CRMP2 ratio. This was found to be the case by SILAC (SI Appendix, Fig. S5), immunocytochemical (Fig. 2 and SI Appendix, Fig. S7), and Western blot analysis (Fig. 3), starting as early as the NPC stage (Fig. 3B) and persisting into the more mature neuronal stage (Figs. 2 and 3 A and C–E, and SI Appendix, Figs. S5A and S7). [Suppression of isoform 3 at the developmentally earlier NPC stage (Fig. 3B) appears more complete than in neurons because βIII-tubulin is less abundant in NPCs (Fig. 3A).] LiCl increased the inactive form of GSK3β (GSK3β-p-S9) (30) (within 1 h of exposure) (Fig. 3A), and both LiCl and direct GSK3β inhibition decreased CRMP2-p-T514 in a dose-dependent manner, but not total CRMP2 levels (Figs. 3 A–C and SI Appendix, Figs. S5A and S10C). All inhibitors of GSK3β tested (including CHIR99021, SB216763, and SB415286) lowered CRMP2-p-T514; CHIR99021 had the greatest effect and is the most selective of the three compounds (Fig. 3F). As per the model in Fig. 1B, the reduction of CRMP2-p-T514 (by lithium or GSK3β inhibition) restores the association of CRMP2 with tubulin in BPD hiPSC-derived neurons (without affecting total tubulin) based on coimmunoprecipitation pull-down experiments (Fig. 3C and SI Appendix, Fig. S8).
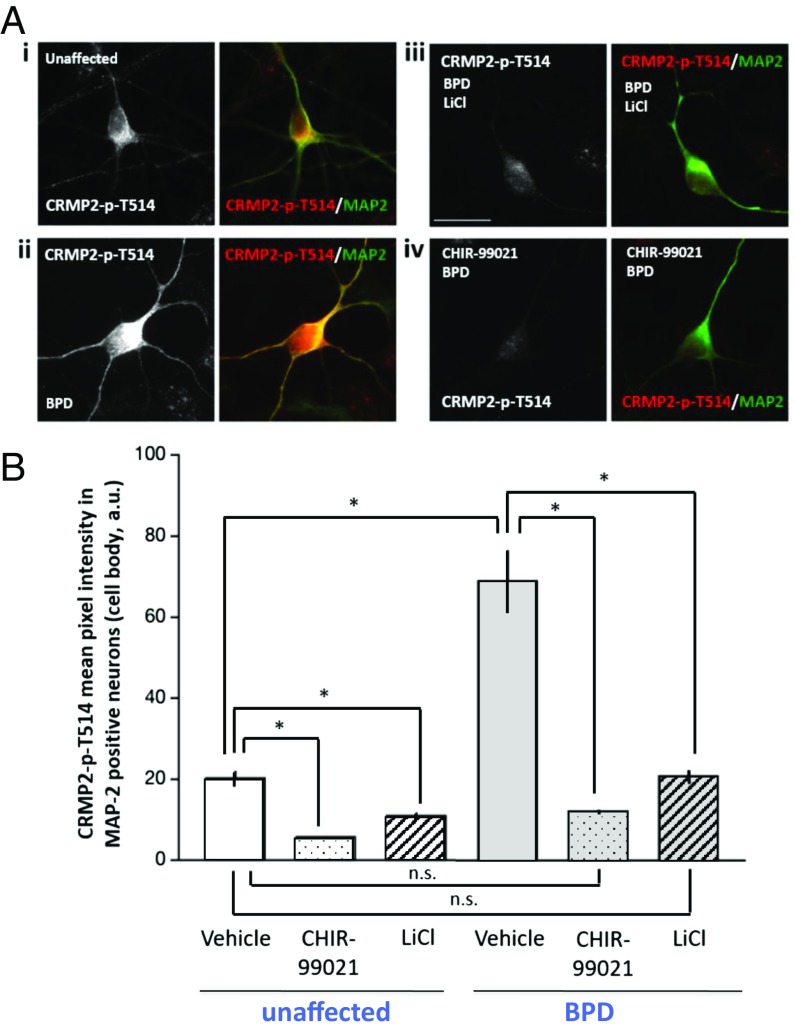
Fig. 2.
Immunocytochemical analysis showing that the baseline intracellular level of CRMP2-p-T514 is higher in LiR BPD than in unaffected neurons but is reduced to normal levels (i.e., those in untreated unaffected neurons) by LiCl or GSK3β inhibition. (A) Image captures of hiPSC-derived MAP2+ (green) neurons from an unaffected individual (i) compared with those from an untreated BPD patient (ii), both immunostained for CRMP2-p-T514 (red). The BPD neurons were then treated with LiCl (iii) or with the GSK3β inhibitor CHIR99021 (iv). With either treatment, the high initial CRMP2-p-T514 immunofluorescence in the BPD neurons was returned to the level of the unaffected neurons (i). (Scale bar: 20 μm.) (B) Quantification of images in A: mean ± SEM of CRMP2-p-T514 pixel intensity in the cell body of hiPSC-derived MAP2+ neurons in each of three conditions: untreated, LiCl-treated, CHIR99021-treated. Two-tailed t test confirmed that CRMP2-p-T514 is significantly more abundant at baseline in BPD neurons than in unaffected neurons. One-way ANOVA revealed a significant effect of CHIR99021 and lithium on lowering CRMP2-p-T514 levels in BPD (F2, 63 = 44.59, *P < 0.0001) as well as in unaffected (F2, 114 = 44.59, *P < 0.0001) neurons (Tukey’s HSD post hoc test). Shown are Pt-UC-6 (clone 1) and Pt-LiR-7 (clone 1). Methods specific for this figure are in SI Appendix.
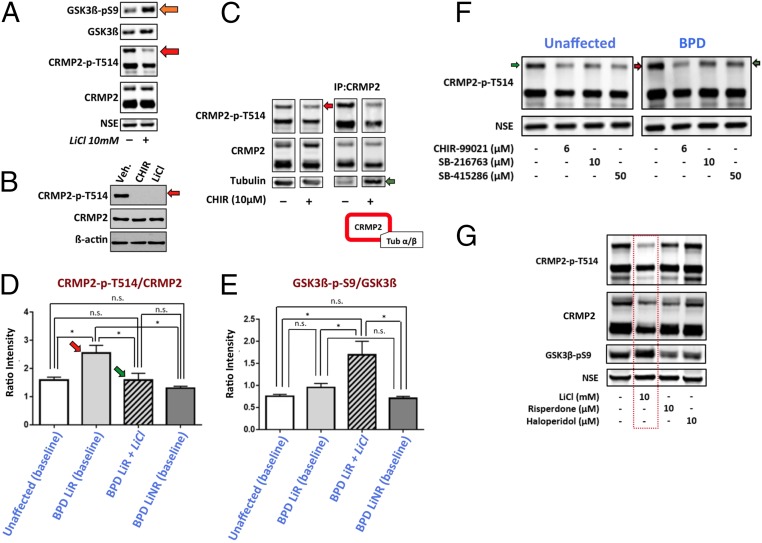
Fig. 3.
Western analyses show that the baseline CRMP2-p-T514:CRMP2 ratio is higher in LiR BPD than in unaffected or LiNR BPD neurons (although GSK3β-p-S9:GSK3β is not reduced), and is normalized by LiCl (an action replicated by direct GSK3β inhibition but not by other psychotropic drugs) and, in so doing, promotes reassociation of CRMP2 with cytoskeletal elements. (A) LiCl reduces CRMP2-p-T514 (but not CRMP2) in mature hiPSC-derived BPD neurons in part by inhibiting (phosphorylating) GSK3β-S9 (orange arrow). Decreased T514-CRMP2 phosphorylation is most prominent in isoform 3 (Top band; red arrow), the isoform most pertinent to neural lineages. (Data are shown for patient Pt-LiR-1, although similar results were observed for all clones from at least eight patients examined in this manner: Pt-LiR-2, Pt-LiR-3, Pt-LiR4, Pt-UC-1, Pt-UC-2, Pt-UC-3, Pt-UC-4; six biological replicates, each with two technical replicates. See SI Appendix, Fig. S1 for patient information.) Neuronal protein was normalized to neuron-specific enolase (NSE; which also served as the loading control) to eliminate confounding protein contaminants. (B) LiCl’s suppression of CRMP2 phosphorylation starts as early as the NPC stage shown here, an action matched by direct chemical inhibition of GSK3β with CHIR99021. Only isoform 3 is shown, suppression of which, at this younger stage, is more complete because βIII-tubulin is less abundant than in neurons. (C) Reduction of CRMP2-p-T514 (red arrow) increases CRMP2’s association with tubulin in BPD hiPSC-derived neurons, as illustrated here by increased coimmunoprecipitation of CRMP2 and tubulin (green arrow) in a manner not seen under high CRMP2-p-T514 conditions. (Performed on all clones from Pt-LiR-1, Pt-LiR-3, Pt-UC-2, Pt-UC-1, Pt-UC-6; two technical replicates each.) See also SI Appendix, Fig. S8. (D) Across multiple patients, the baseline CRMP2-p-T514:CRMP2 ratio is higher in LiR BPD neurons (red arrow) than in neurons from unaffected or LiNR BPD patients (the latter approximating each other). Lithium reduced CRMP2-p-T514 in all patient groups (see SI Appendix, Figs. S10 and S11 for expanded data), and hence, by shifting the balance away from phosphorylated CRMP2, established a lower CRMP2-p-T514:CRMP2 ratio in LiR BPD neurons now comparable to the baseline of untreated unaffected control neurons (green arrow). (Western blot in F is representative of the primary data from which pixel intensity was measured, normalized to NSE, and ratios calculated. Western blots in F are representative of the primary data from which pixel intensity was measured, normalized to NSE, and ratios calculated. Statistics based on Pt-UC-2, Pt-UC-3, Pt-UC-4, Pt-LiR-2, Pt-LiR-3, Pt-LiR-4, Pt-LiR-5, Pt-LiNR-1, Pt-LiNR-2, Pt-LiNR-3, each with three technical replicates; t test; *P < 0.0001; ns, not significant in D and E.) (E) Inhibitory S9 phosphorylation of GSK3β was increased in all neurons treated with LiCl. However, surprisingly, across the same patients as in D, baseline GSK3β-p-S9:GSK3β ratios did not differ. Given that CRMP2 is a GSK3β substrate, one explanation for the abnormally high baseline inactive CRMP2-p-T514 seen in D might have been that baseline GSK3β-p-S9 is too low, allowing GSK3β levels to rise, and hence elevating CRMP2-p-T514 in LiR BPD neurons: that is, a manifestation solely of GSK3β dysregulation. However, in the absence of decreased baseline GSK3β-p-S9 in those neurons, their abnormal regulation of CRMP2 must be attributable to upstream pathways independent of GSK3β regulation (Fig. 1B; see also SI Appendix, Fig. S10). (F) Representative Western blot on which the statistical analyses in D were performed, suggesting that the baseline CRMP2-p-T514 level in LiR BPD patients is higher than normal (red arrow), but that lithium exposure or GSK3β inhibition (shown here) reduces it to a level as low (or lower than) that in untreated unaffected patients (green arrows). (The patients shown here—Pt-LiR-1 and Pt-UC-1—are first-degree relatives.) High baseline CRMP2-p-T514 is not seen in LiNR BPD (D), in other psychiatric disorders (e.g., MD; another first-degree relative with MD is shown in SI Appendix, Fig. S11), or in other neurologic disorder [e.g., PD (SI Appendix, Fig. S11)], which have levels no higher than in unaffected patients, suggesting that elevated ratios have disease-specificity. All GSK3β inhibitors tested (CHIR99021, SB216763, SB415286) lowered CRMP2-p-T514; CHIR99021, the most specific, had the greatest impact. (G) Western analyses of hiPSC-derived neurons suggest that CRMP2-p-T514 reduction (without altering CRMP2) and GSK3β inhibition by increased S9 phosphorylation are drug-specific actions of lithium (red box), but not of other mood-stabilizing agents often prescribed for LiNR BPD (risperidone and haloperidol shown). (Shown are representative patients Pt-UC-1 and Pt-LiR-1, each with two technical replicates, but applies also to all clones from Pt-LiR-2, Pt-LiR-3, Pt-LiR4, Pt-UC-2, Pt-UC-3, Pt-UC-4. See SI Appendix, Fig. S1 for patient information.)
Finally, further fleshing out lithium’s putative interposition upon the phosphoregulation of CRMP2 in hiPSC neural derivatives, we observed that LiCl increased the active AKT phospho-form, AKT-p-T308 (SI Appendix, Fig. S9A), for which GSK3β-S9 is a known substrate, hence indirectly decreasing GSK3β’s activity because pf elevation of GSK3β-p-S9 levels.
Although inhibition of GSK3β is a prominent node in the lithium-response pathway’s reduction of CRMP2-p-T514, we observed LiCl treatment also resulted in a reduction of phosphorylation at CRMP2-S522 (and subsequent decrease in CRMP2-p-T514) (SI Appendix, Fig. S9B), implicating other parallel (or additive) GSK3β-independent lithium interactions (Fig. 1B). Interestingly, inhibitors of CDK5 (cyclin-dependent kinase-5), the presumed S522 kinase that primes other CRMP2 sites for subsequent GSK3β phosphorylation (25), did not decrease CRMP2-p-T514, suggesting that lithium decreases CRMP2-p-S522 independently of CDK5 in human neurons (SI Appendix, Fig. S9C).
Protein phosphatase 2A (PP2A) (26, 27) dephosphorylates CRMP2 as a counterpoise to the kinases (Fig. 1B), Among the 15 putative lithium candidates revealed by 2D-DIGE, i2PP2A, an inhibitor of PP2A, was decreased 1.5-fold in response to lithium (SI Appendix, Fig. S4A), suggesting that lithium relieves inhibition of PP2A, thereby decreasing CRMP2-p-T514. Following its dissociation from i2PP2A, PP2A requires binding to β-arrestin-2 for it to be active (26, 27); lithium increased β-arrestin-2 levels (SI Appendix, Fig. S9D). LiCl does not alter the phosphorylation of CRMP2-p-T555, the kinase for which is Rho-associated protein kinase. Finally, we also assessed the possibility that lithium may affect other upstream interactors of CRMP2. However, neither lithium nor CHIR99021 altered protein levels of SEMA3A (CRMP2’s most prominent upstream partner) (SI Appendix, Fig. S9E) nor the tyrosine kinase YES1.
We next determined whether these actions on CRMP2 regulation (i.e., decreasing CRMP2-p-T514 in hiPSC-derived neurons) was specific to lithium among mood-stabilizing medications. In contrast to lithium, other psychotropic agents, including those routinely used clinically in LiNR BPD patients and other psychiatric disorders (e.g., haloperidol, risperidone, clozapine, valproic acid), did not similarly reduce CRMP2-p-T514 (Fig. 3G). Furthermore, of these drugs, only lithium appeared to increase phosphorylation of GSK3β-S9, thereby inhibiting GSK3β action (Fig. 3G).
“Toggling” between active and inactive CRMP2 is a normal physiologic process speculated to facilitate corticogenic lamination during development and to inhibit abnormal sprouting following acute CNS trauma (12, 13, 16, 24). Accordingly, it was not unexpected that lithium reduced CRMP2-p-T514 (and hence the CRMP2-p-T514:CRMP2 ratio) in all hiPSC-derived NPCs and neurons regardless of the donor patient’s diagnosis, including unaffected individuals with no psychiatric or neurologic disease (SI Appendix, Fig. S1). Strikingly, the baseline level of CRMP2-p-T514 was significantly higher in hiPSC-derived neurons from LiR BPD patients compared with those from other individuals, including those with LiNR BPD. Even at the single human neuron level, quantitative analysis of the intracellular immunofluorescence signal demonstrated a significantly higher baseline level of CRMP2-p-T514 immunoreactivity within BPD compared with unaffected neurons (Fig. 2). Intriguingly, lithium treatment, as well as GSK3β inhibition, of BPD neurons reduced elevated CRMP2-p-T514 levels to that of normal neurons (Fig. 2B).
To test the generalizability of this observation in hiPSC-derived neurons across many patient samples, we extended our study to semiquantitative Western blot analysis of the CRMP2-p-T514:CRMP2 ratios across a spectrum of conditions and patients (Fig. 3 and SI Appendix, Figs. S1, S10, and S11): again, CRMP2-p-T514 was abnormally high in LiR BPD neurons in contrast to neurons from unaffected patients, patients with other neuropsychiatric disorders (e.g., MD, including first-degree relatives of LiR BPD patients), other neurologic diseases (e.g., PD), and strikingly, even LiNR BPD. Again, although lithium (and GSK3β inhibition) decreased CRMP2-p-T514 in hiPSC-derived neurons from all patients (without altering CRMP2), abnormally high CRMP2-p-T514 levels in LiR BPD were reduced to a level approximating the baseline level of unaffected individuals, which, in turn, was not significantly different from the baseline levels in patients with LiNR BPD, MD, and Parkinsonism. Hence, an abnormally high CRMP2-p-T514:CRMP2 ratio appeared to be disease-specific, prompting the hypothesis that the set-point for the CRMP2-p-T514:CRMP2 ratio may be abnormally high in LiR BPD and, at least with respect to hiPSC-based analysis, a molecular hallmark of LiR BPD (Fig. 3D). Interestingly, we did not observe decreased baseline levels of GSK3β-p-S9 in LiR BPD cells. Given that CRMP2 is a GSK3β substrate, one explanation for an abnormally high inactive CRMP2-p-T514 might be that the inactive inhibitory form of GSK3β (i.e., GSK3β-p-S9) is simply too low, allowing GSK3β levels to rise, and hence rendering the observation of an elevated CRMP2-p-T514 as just the manifestation of a GSK3β problem. However, in not finding decreased baseline levels of GSK3β-p-S9 in LiR BPD cells, the abnormal regulation of CRMP2 (a central actor in its own right) must be attributable to additional converging upstream pathways independent of GSK3β regulation (Fig. 3E).
Given these observations from hiPSCs in vitro, we next needed to (i) validate their relevance to actual primary patient specimens (Fig. 4), as well as (ii) determine what physiological relevance CRMP2 might have to BPD (Figs. 5–7). These investigations were pursued in parallel, the latter providing guidance as to what metrics should be assessed in the former.
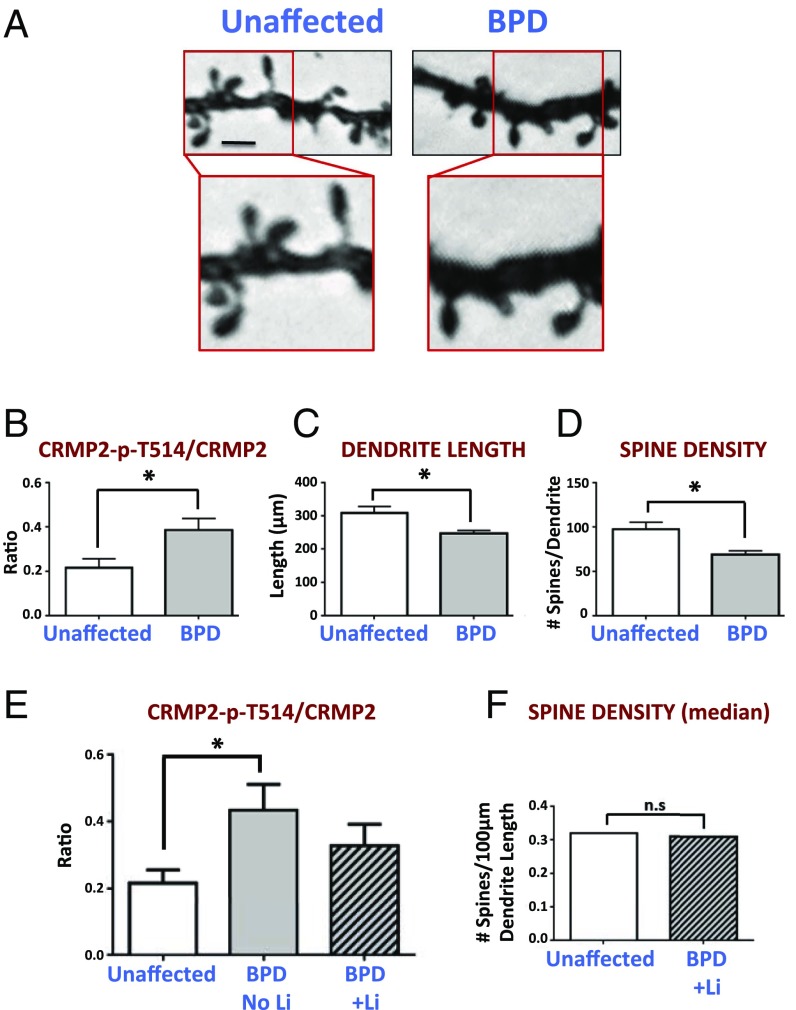
Fig. 4.
Excessively elevated CRMP2-p-T514:CRMP2 ratios are associated with diminished dendritic spine morphology in primary human postmortem specimens, both of which are improved by lithium. Shown are data from the dorsolateral prefrontal cortex of BPD patients (n = 9 patients) in which CRMP2-p-T514 ratios (by Western blot) were observed to correlate with dendritic spine morphologies (based on Golgi stain analysis). (A) Golgi-stained dorsolateral prefrontal cortex from a representative BPD and unaffected patient showing diminished spine density in the former. (Compare with similar data from the CRMP2-KO mouse in Fig. 6 E and F.) (Scale bar: 0.3 µm, Upper and 0.15 µm, Lower.) The average CRMP2-p-T514:CRMP2 ratio was significantly (*) increased (P = 0.01) (B) and the dendritic length (P = 0.011) (C) and spine density (P = 0.003) (D) were significantly (*) reduced in BPD patients not treated with lithium compared with unaffected individuals (n = 5 and n = 16, respectively). Diminished dendritic spine density had a moderately strong coefficient-of-correlation of 0.61 with the elevated ratio. Treatment of BPD patients with lithium (n = 4 patients) provided a normalizing effect for both decreasing the ratio (E) and increasing spine density (F), such that they were no longer significantly different from unaffected patients (“ns”). Note that spine density was confirmed by two separate measurements which were in agreement: “number of spines per dendrite” and “number of spines per 100-µm dendrite length.” (See also biochemical data in SI Appendix, Fig. S12.)
Fig. 5.
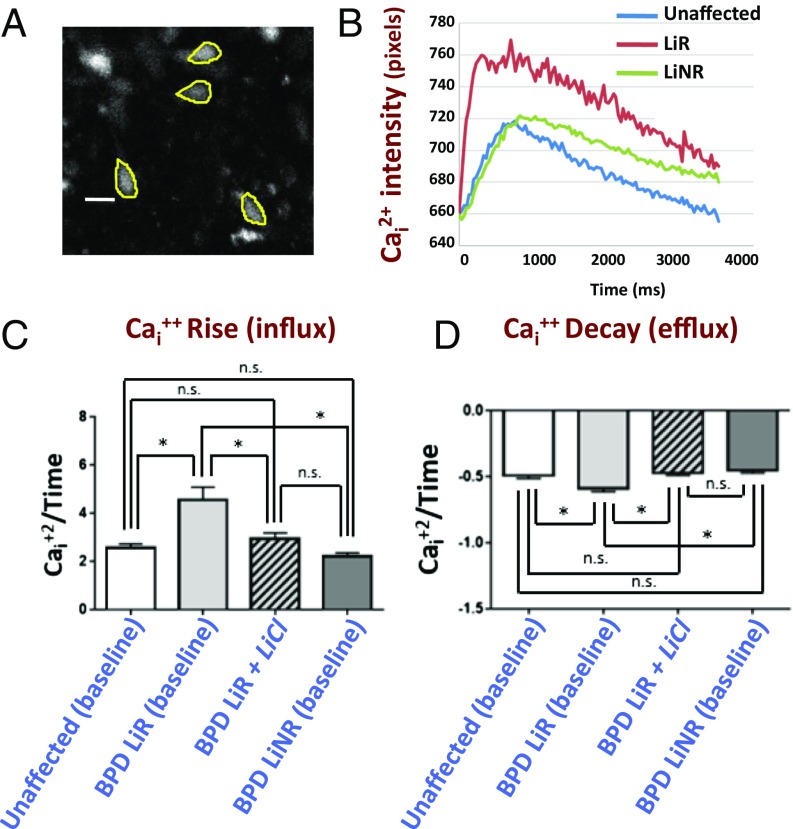
Neuronal function (as assayed by single neuron Cai+2 transients) is altered in LiR BPD hiPSC-derived neurons in a manner predicted by diminished dendritic spine density (34, 35) (excessively steep Cai2+ infux/efflux slopes), but is improved by lithium. (A) hiPSC-derived neurons, which exhibit spontaneous Cai2+ currents (examples of which are traced here), were studied individually from unaffected (n = 125 neurons from Pt-UC-3 and Pt-UC-4), LiR BPD (n = 188 neurons from Pt-LiR-2 and Pt-LiR-4), and LiNR BPD (n = 115 from Pt-LiNR-1 and Pt-LiNR-2) patients; two clones per patient. All neurons showed spontaneous Cai2+ currents, consistent with their being electrophysiologically active. (Scale bar: 25 µm.) Movie S1 is a real-time recording of Ca2+ flux in a representative field of neurons from Pt-LiR-4. (B) A representative intensity recording of kinetic image cytometry of Cai2+ flux in spontaneously firing hiPSC-derived neurons showing the influx and efflux slopes from LiR BPD neurons (red) to be steeper compared with those from LiNR BPD (green) and unaffected neurons (blue), which approximate each other. The average of each type of slope—rise/influx (C) and decay/efflux (D)—is calculated for neurons that fire once and resolve within 10 s. Each trace represents 303 images captured over 10 s. The slopes of each spontaneously active neuron were calculated and are presented graphically in C and D: the influx and efflux slopes of LiR BPD neurons are significantly higher (*P < 0.01) than those in LiNR and unaffected neurons, which are not significantly different (“ns”) from each other. LiCl treatment makes the influx and efflux slopes for LiR BPD patient neurons decrease to match those of the unaffected and LiNR neurons, eliminating any statistical differences between disease categories (“ns”). Additional data presented in SI Appendix, Fig. S13.
Fig. 7.
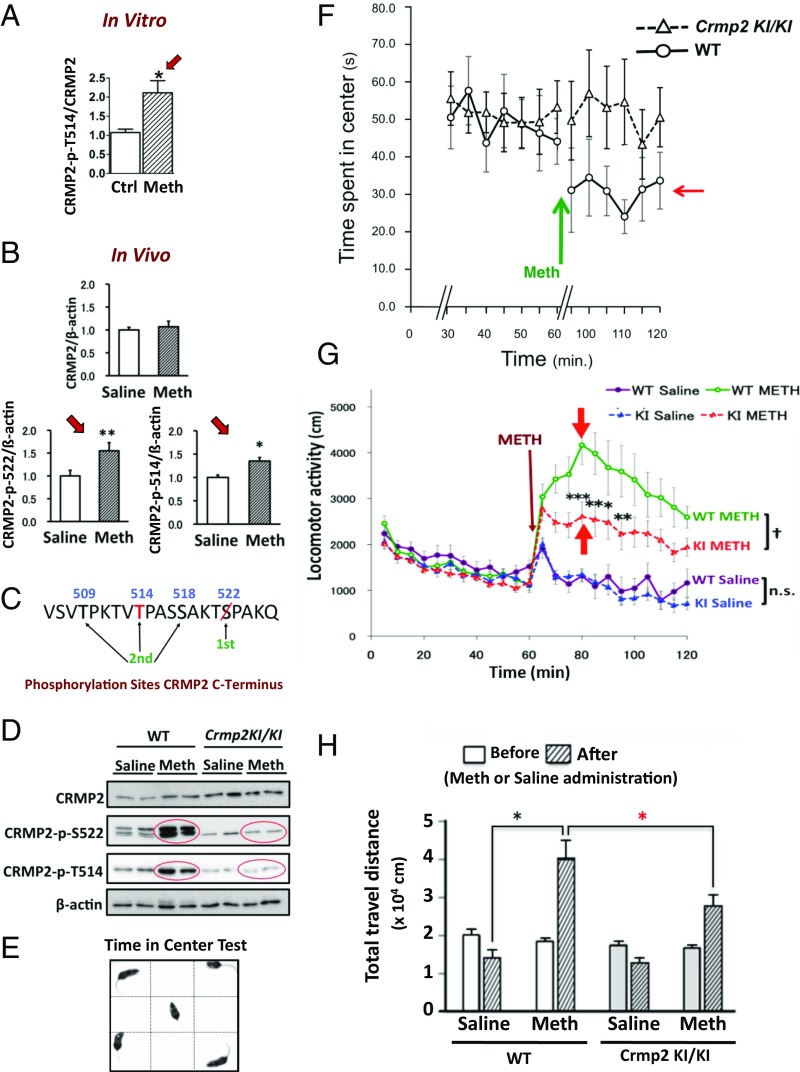
Blocking phosphorylation of CRMP2 promotes behavioral improvement in animal models of LiR BPD. (A and B) Methamphetamine (“Meth”; 200 µM), an accepted agent for experimentally-inducing LiR BPD-like behavior (e.g., mania) in mice, increases CRMP2-p-S522 and CRMP2-p-T514 levels (although not total CRMP2) in mouse hippocampal neurons both (A) in vitro [mean ± SEM; unpaired t test *P < 0.005, number of fields: control (“ctrl”) = 41, Meth = 43] and (B) in vivo (9-wk-old C57BL/6J mice, 60 min postinjection of 2 mg/kg Meth i.p., relative to β-actin; compared with saline, *P < 0.05, **P < 0.001; data expressed as means ± SEM; n = 3 mice-per-condition). Striatum is presented here but all CRMP2-expressing regions yield similar findings (Dunnett test). (C) In CRMP2ki/ki mice, the S522 priming phospho-site is eliminated preventing phosphorylation of T509, T514, and S518. We postulate that T514 and S522 are lithium’s site-of-action, suppressing CRMP2 phosphorylation; the mutation mimics lithium’s posited action. (D) Western blot confirming that Meth (2 mg/kg, i.p.) increases (within 60 min) phosphorylation of CRMP2 at S522 and T514 in WT but not CRMP2ki/ki mouse brains. (Residual bands at S522 reflect minor cross-reactivity of the antibody with CRMP1-p-S522.) (E) Open-field test for quantifying nonmanic behavior (time spent exploring the unprotected center) vs. manic behavior (little time in the center, more time “manically” circling the periphery). (F–H) Comparisons of CRMP2ki/ki and WT mouse behavior after Meth administration confirms that the mutation preventing CRMP2 phosphorylation (emulating lithium’s posited site-of-action) decreases BPD-like behaviors. (F) WT littermates (7- to 12-wk-old, n = 10) (○) injected with Meth (2 mg/kg, i.p.; green arrow) become “manic” and hyperlocomotor, spending less time in the center (red arrow), whereas the nonphosphorylatable CRMP2ki/ki mice (n = 17) (△), similarly treated with Meth, spend no less time in the center, (i.e., no change from their untreated baseline; means ± SEM), not displaying this BPD-like behavior. (In order not to contaminate this assay of anxiety/mania, the two vertical hash marks on the x axis represent a 20- to 30-min recording gap to allow the mice to reacclimate to the cage after handling and receiving an injection and for Meth to reach a steady-state brain level.) (G) Meth-treated CRMP2ki/ki mice (“KI”, red dots) display less locomotor activity compared with Meth-treated WT littermates (green dots) (means ± SEM by repeated measures ANOVA: †P < 0.05; by Bonferroni posttest: **P < 0.01, ***P < 0.001; n.s., not significant; WT+Meth, n = 10; KI+Meth, n = 17; WT+saline, n = 7; KI+saline, n = 9). (H) Hyperlocomotor WT mice travel a greater distance following Meth administration than Meth-treated but nonmanic CRMP2ki/ki mice (means ± SEM by Bonferroni, *P < 0.05; n as per G). In short, the nonphosphorylatable CRMP2ki/ki mouse remains nonmanic and minimally affected by Meth as if on chronic lithium.
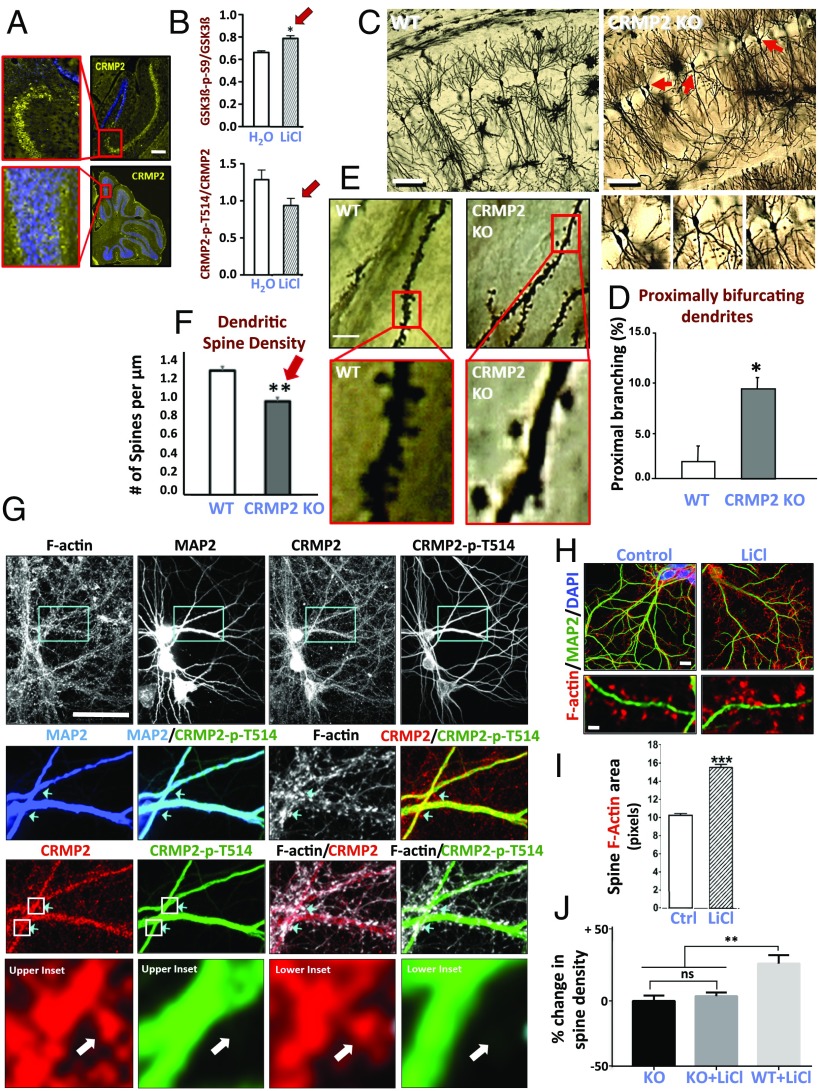
Because determining CRMP2’s role in neurons in situ entails experimental manipulations in living systems not feasible in humans or human postmortem material and beyond the state-of-the-art for hiPSCs (e.g., high-resolution dendrite and dendritic spine morphometrics), we turned to rodents for a series of studies to provide guidance. We performed an immunohistochemical survey of the intact adult mouse brain and determined that CRMP2 is highly expressed in neurons of the hippocampus (e.g., CA1 pyramidal neurons) (Fig. 6A), cerebral cortex, olfactory bulb, and cerebellum (Purkinje neurons), regions postulated to be involved in BPD (31). We verified that, as was seen in BPD hiPSC-derived neurons in vitro, lithium administration to mice increases the inactive form of GSK3β (GSK3β-p-S9) and lowers CRMP2-p-T514 in vivo (Fig. 6B). We next eliminated CRMP2 function systemically by generating a constitutive CRMP2-KO mouse strain (recognizing that CRMP2 is also expressed in nonneural systems and that lithium is a systemically administered drug). In contrast to a brain-specific CRMP2-deleted mouse generated contemporaneously (32), these animals had grossly normal bodies and brains (as per actual BPD patients), except for a unique dendrite aberrancy: The brains of adult CRMP2-KO mice were characterized by a fivefold increase in bifurcation of apical dendrites proximally (creating increased dendritic branching points at the expense of main trunk dendrites) (33) (Fig. 6 C and D). Prominent as well was the loss of dendritic spine density (25% fewer spines in CRMP2-KO mice compared with WT) (Fig. 6 E and F). These perturbations in dendritogenesis prompted us to examine the subcellular localization of CRMP2 in neurons, particularly in relation to its phosphorylation state as modified by lithium. (Examining the dendritic arbor of primary hippocampal neurons in situ across the stratum radiatum is regarded as the most valid and reproducible system for rigorously evaluating anatomical dendritic spine parameters and cannot yet be reproduced in cell culture.) An antibody that detects CRMP2 independent of phosphorylation state showed that CRMP2 was expressed throughout the neuron, including the dendritic shaft, branches, and spines (Fig. 6G). In contrast, inactive CRMP2-p-T514, which dissociates from tubulin, was not detectable in dendritic spines (Fig. 6G), suggesting that, when CRMP2 becomes phosphorylated (i.e., inactivated), it exits or is excluded from the spines. LiCl administration, however, which, as noted above, decreases the proportion of phosphorylated inactive CRMP2 in vitro and in vivo (hence increasing the amount of active CRMP2), induced a 60% increase in dendritic spine area (Figs. 6 H and I) and a 36% increase in spine density. This lithium response is lost in CRMP2-null neurons (an informative loss-of-function observation) (Fig. 6J). (Interestingly, CRMP2-KO mice are hyperactive and overanxious in stressful environments and show briefer social interactions compared with WT littermates).
Fig. 6.
CRMP2 function is pivotal for proper dendritic branching and spine organization in vivo. (A) Representative photomicrographs from an immunohistochemical survey of the adult mouse brain determining the regions and cell types expressing phosphorylated CRMP2 in situ. Shown are sections through the CA1 region of the hippocampus (Upper) and the cerebellum (Lower), costained with an antibody against CRMP2-p-T514 (yellow) and DAPI (blue). Regions in the red boxes are magnified (Left) to visualize the pyramidal and Purkinje cell layers, respectively. Other regions in which neurons showed expression were cerebral cortex, olfactory bulb, and striatum. (Scale bar: 250 µm, Right, and 100 µm, Left.) (B) LiCl administration to mice increases levels of inactive (phosphorylated) GSK3β (red arrow) and lowers levels of inactive CRMP2-p-T514 (red arrow) compared with water (H2O) (based on quantification of Western blot analysis of hippocampal protein). (n = 7, H2O-treated; n = 7, LiCl-treated, *P = 0.05.) (C–F) Constitutive Crmp2-KO mice have grossly normal bodies and brains but are characterized at adulthood by defects in dendritic morphology (compared with WT) in the regions where CRMP2 is expressed [e.g., in CA1 of the hippocampus, as shown here in Golgi stains examined along the stratum radiatum (33), but also seen in striatum and cortex]. (C and D) CRMP2-KO mice show a fivefold increase in bifurcation of apical dendrites proximally (creating increased dendritic branching points at the expense of main trunk dendrites). The representative pyramidal neurons indicated by red arrows in C are each magnified in the respective Insets below the overview and are quantified in D. (Scale bar, 100 µm.) (Data shown are mean ± SEM from 49 to 76 neurons from three mice of each genotype; Student t test: *P < 0.001 compared with WT.) (E and F) The dendrites themselves are characterized by a decreased density of spines (i.e., average number of spines per micrometer). The red blocked areas in E are magnified in the Insets and the data are quantified in F. (Scale bar: 10 µm; 2.5 µm in Insets.) (Data shown are mean ± SEM from >20 dendrites from each of three WT and three CRMP2-KO mice; **P = 0.0006 compared with WT.) This diminished dendritic spine density and length in the CRMP2-KO mouse is strikingly similar to that seen in the primary human postmortem brain specimens from LiR BPD patients (Fig. 4 A, C, and D) and would be consistent with the functional consequences seen in LiR BPD neurons (Fig. 5). (G) Differential localization of nonphosphorylated and phosphorylated CRMP2, in and out of spines, respectively. A representative field of neurons examined in situ along the stratum radiatum in the rat hippocampus. Cyan boxes (first row) indicate the dendritic regions magnified in the second row and stained, respectively, for filamentous actin (F-actin, white), the neuronal marker MAP2 (blue), CRMP2 (red), and CRMP2-p-T514 (green); these same regions, in their respective columns in the third row, are costained with combinations of the other markers and the images merged. Arrowheads indicate phalloidin-stained dendritic spines; they contain nonphosphorylated CRMP2 (red) but do not stain for CRMP2-p-T514 (green). Magnified images from the white boxed areas in the third row are magnified in the fourth row and again show that nonphosphorylated CRMP2 (red) is expressed throughout the neuron, including the dendritic shaft, branches, and spines; CRMP2-p-T514 (green) is not expressed in the spines, suggesting that, when CRMP2 becomes phosphorylated, it leaves or is excluded from the spines. In the fourth row, a white arrow points to the same representative dendritic spines in both Left and Right panels nicely showing that, although nonphosphorylated CRMP2 fills the spines, phosphorylated CRMP2 is absent. (Scale bar: 90 μm, first row; 40 μm, second and third rows; 15 μm, fourth row.) (H–J) LiCl increases dendritic spine volume and density, an action abrogated by KO of CRMP2. Rat hippocampal neurons (MAP2+, green) show an increase in F-actin staining (red) after 7 d of incubation with LiCl (3 mM) (H), presented quantitatively in I. (Scale bar: 15 μm, Top; 7 μm, Bottom.) F-actin area correlates with dendritic spine volume (44, 45). LiCl induced a 60% increase in the area of F-actin puncta associated with dendritic spines and increased spine density by 36% [11 spines/10 µm (untreated) vs. 15 spines/10 µm (LiCl)]. (Data expressed as mean ± SEM; ***P < 0.001 unpaired Student's t test; n = 23 neurons per group; 3,255 actin spines measured in control neurons, 3,812 spines in LiCl-treated neurons.) (J) In contrast, hippocampal neurons from the CRMP2-KO mouse evince no increase in spine density from their baseline when similarly treated with LiCl, in contrast to WT littermates. (KO untreated, n = 13; KO+LiCl, n = 11; WT+LiCl, n = 9. One-way ANOVA, **P = 0.0013.)
Given that subcellular localization of CRMP2 (based on its lithium-modifiable phosphorylation state) might influence dendritic spine regulation, we next sought validation in actual human BPD patients (Fig. 4 and SI Appendix, Fig. S12). Our prediction was that, in BPD patients, levels of inactive CRMP2-p-T514 would be abnormally high and that, accordingly (as in the CRMP-KO mouse), dendritic spine densities would be diminished (compared with unaffected patients). First we performed Western blot analysis on protein preparations from primary postmortem brains of BPD patients. Indeed, CRMP2-p-T514:CRMP2 ratios were elevated in samples from unmedicated BPD compared with unaffected patients, suggesting that CRMP2 might be aberrantly regulated (Fig. 4B and SI Appendix, Fig. S12). Next we observed cytoarchitecturally that BPD patients, compared with unaffected patients, had diminished dendritic spine densities (Fig. 4A), a picture reminiscent of that seen in the CRMP-KO mouse (Fig. 6 E and F). As presented in Fig. 4 B–F, we next determined that there was a moderately strong coefficient-of-correlation (0.61) between abnormally elevated CRMP2-p-T514:CRMP2 ratios in BPD patient brains (compared with brains of unaffected patients with lower ratios) and diminished dendritic spine density and decreased dendritic length [a metric of excess proximal branching, as per the CRMP2-KO mice (Fig. 6 C and D)] in those brains. As further shown in Fig. 4 E and F, LiR BPD patients placed on lithium had a decrease in their levels of CRMP2-p-T514 toward normal ratios, as well as an improvement in dendritic spine density such that it was no longer significantly different from unaffected individuals, again with a moderately strong correlation, suggesting (within the limitations of assessing archived postmortem material) that lithium treatment might have a normalizing influence in LiR BPD patients on both CRMP2 ratios and dendritic spine abnormalities that appear linked. At a minimum, these data support the hypothesis that the set-point for CRMP2-p-T514 is higher than normal in LiR BPD patients.
Whereas actual primary patient specimens provide the ultimate histopathologic and biochemical validation, because one cannot glean functionality from postmortem material, we turned again to living human neurons derived from BPD patient hiPSCs. Diminished dendritic spine density, as seen in primary human neurons in situ in BPD patient pathological specimens, predicts that there should be altered regulation of intracellular Cai2+ transients within individual neurons (i.e., more rapid calcium flux) (34, 35). Altered calcium dynamics has long been suspected in BPD (2, 36), although calcium channel blockers have proved clinically ineffective as a monotherapy for this condition (13), speaking against hyperexcitability as the primary target of LiR (2). Although previous studies looked at the total number of firings of populations of neurons over a given period (2), we sought to analyze Cai2+ flux in individual LiR BPD neurons (Fig. 5A) with abnormally high CRMP2-p-T514 levels (as per Fig. 2). Cai2+ imaging of LiR BPD hiPSC-derived neurons (Fig. 5 B–D) showed a more rapid ingress and egress of Ca2+, reflected as steeper slopes (35, 36), compared with neurons from LiNR and unaffected patients (the latter two approximating each other) (Fig. 5 B–D and Movie S1), as would be expected under conditions of diminished spine density. In other words, LiR BPD neurons flux Cai2+ at a greater rate; they not only fire and resolve at a quicker rate, as shown by graphs of their rise/influx and decay/efflux slopes, but also have a more intense firing as shown by their amplitudes (SI Appendix, Fig. S13). Lithium administration to the LiR BPD neurons shifted their slopes to approximate those of the other two categories of patient-derived neurons, unaffected and LiNR BPD (Fig. 5 C and D). In other words, the LiR BPD neurons’ Cai2+ influx, efflux, and amplitude now showed a significant reduction toward normal.
Given its regulation by lithium, we next questioned whether CRMP2 may be required for lithium-mediated behavioral changes in widely accepted animal models of LiR BPD. One such model is methamphetamine-induced hyperlocomotion/mania, which is known to be responsive to lithium in many mouse strains (37). Interestingly, although no molecular mechanisms have been offered, methamphetamine has also been suspected of modulating GSK3β signaling in the nucleus accumbens (38), impinging on dendritic spine formation (39) and effecting Ca2+ channel expression (40). We therefore questioned whether methamphetamine may also influence CRMP2 ratios (Fig. 7 A–D). Indeed, we observed that treatment of primary rat hippocampal neurons in vitro with methamphetamine (hence independent of dopamine handling) (Fig. 7A), as well as administration of methamphetamine to mice in vivo, significantly increased the proportion of phosphorylated CRMP2 (both CRMP2-p-S522 and CRMP2-p-T415) without influencing total CRMP2, hence increasing the inactive:active CRMP2 ratio (Fig. 7 B–D). Methamphetamine administration to normal mice provokes characteristic LiR BPD behavior, including manic exploring of the periphery of an open field with little time spent in the unprotected center (37). We predicted that, were the lithium-response pathway in BPD to be one that converges on inhibiting CRMP2-p-T514, then the behavior of a mouse in which CRMP2 is incapable of being phosphorylated at that motif (CRMP2ki/ki)—lithium’s postulated site-of-action—would emulate chronic lithium treatment (Fig. 7C). Indeed, CRMP2ki/ki mice failed to experience methamphetamine-mediated phosphorylation of CRMP2 (Fig. 7D) and were resistant to functional methamphetamine provocation, abrogating LiR BPD-like behaviors (peripheral circling, mania, hyperlocomotion) in contrast to methamphetamine-exposed WT mice (Fig. 7 E–H) (which would require lithium treatment). This observation (both a critical loss-of-function and reproduction-of-function experiment) suggested that CRMP2 phosphorylation is required, at least in part, for methamphetamine-induced BPD-like mania, lending further support to our model of BPD pathogenesis based on aberrant CRMP2 phosphoregulation and lithium’s therapeutic modification of it at that motif (Fig. 1B).
Discussion
In summary, through a combination of unbiased, differential proteomic and bioinformatic pathway analyses of hiPSC-derived NPCs and neurons from LiR BPD patients (and control patients with other disorders, including LiNR BPD and other psychiatric and neurological conditions), followed by node-by-node mapping, animal modeling, functional validation in vitro and in vivo, and corroboration in human BPD postmortem brains, our results suggest that the molecular lithium-response pathway in BPD acts via CRMP2 to alter neuronal cytoskeletal dynamics, most particularly dendrite and dendritic spine formation, and presumably function: hence, neural network development and activity. By “mapping” the upstream and downstream interactors of CRMP2, we observed that lithium does not impact its direct upstream activator, collapsin (SEMA3A), but does regulate GSK3β and AKT kinases, the arrestin–PP2A complex, and hence, the phospho-sites (e.g., T514, S522) that govern CRMP2’s central role in cytoskeleton regulation. The phosphorylation state of CRMP2 (influenced by both GSK3β-dependent and -independent pathways) determines its association with cytoskeletal elements: nonphosphorylated active CRMP2 binds them, phosphorylated inactive CRMP2 dissociates from them. Our observations in hiPSCs and then in human postmortem brain specimens suggests that the inactive CRMP2-p-T514:active CRMP2 ratio set-point is uniquely elevated in LiR BPD patients. Lithium lowers this ratio to a level observed in unaffected patients. Nullifying CRMP2 function entirely by KO elicits dendrite and spine pathology. It also eliminates lithium’s increase of spine density. Abrogating phosphorylation of CRMP2 at lithium’s postulated site of action, hence emulating lithium’s proposed action, also reproduces lithium’s therapeutic action in accepted behavioral models of LiR BPD. Data from primary BPD patient brains further confirm the predicted link between abnormally elevated CRMP2-p-T514 and dendritic spine abnormalities, as well as evidence that lithium treatment of patients acts to normalize both CRMP2 ratios and dendritic spine density and length.
We emphasize that these actions of lithium in BPD do not rule out potential roles for that cation’s multiple other actions (4–7), which may function additively or synergistically in this condition. We now simply identify a promising regulatable molecular pathway upon which to focus etiologically and pharmacologically.
Elevated baseline CRMP2-p-T514 might be associated with LiR as a clinical classification of BPD (potentially a biomarker). (Prospective studies in large cohorts of living patients will inevitably be required to help define what the critical clinical threshold for a CRMP2-p-T514:CRMP2 ratio should be.) Our data cannot yet determine whether the CRMP2-p-T514:CRMP2 set-point is chronically high in LiR BPD patients or rather that the response of LiR BPD patients to stimuli that increase CRMP2-p-T514 is more pronounced, prolonged, or of earlier onset than in unaffected patients, or a combination of these. Nevertheless, these results may provide impetus for biomarker assay development, for example measuring CRMP2-p-T514:CRMP2 ratios in reprogrammed patient-derived cells (including those obtained from the peripheral blood, like some of ours) as a diagnostic aid to predict drug responsiveness. A qualitative, not just quantitative, distinction between LiR and LiNR BPD based on an abnormally high set-point for an otherwise physiologic posttranslational modification of a cytoskeletal regulator (uniquely in LiR BPD) invites speculation that LiNR BPD is actually a separate disease that “pheno-copies” BPD but is unrelated pathophysiologically to the lithium-response pathway. (See SI Appendix, Figs. S9 and S12 for further discussion of this possibility based on biochemical and functional data, respectively.) Although CRMP2 may play a role in other neuropsychiatric diseases—for example, total CRMP2 levels may be abnormal in postmortem brains of schizophrenics—aberrantly elevated inactive:active CRMP2 ratios with excessively high CRMP2-p-T514 seems specific to LiR BPD.
The lithium-response pathway impinging on CRMP2 likely has additional modes of input that go beyond those illustrated in Fig. 1B. For example, brain-derived neurotrophic factor (BDNF) also reduced CRMP2-p-T514 in a manner similar to lithium (SI Appendix, Fig. S13A), suggesting that systems downstream of tyrosine receptor kinase B (TRKB, the BDNF receptor) might be integrated into the pathway (SI Appendix, Fig. S13B). Moreover, CRMP2 has been associated with tauopathies via its interactions with microtubule-associated protein tau and presenillin 1. Indeed, CRMP2 interacts with amyloid precursor protein, and has been noted to be a component of neurofibrillary tangles in Alzheimer’s disease (41). Hence, not only might this axis be implicated in diseases other than BPD (cytoskeletal dynamics coming to be recognized as central to a growing number of neuropathological processes), but also drugs that lower CRMP2-p-T514 may potentially be more widely therapeutic (particularly in disorders characterized by deposition of cytoskeletal elements such as tau either as a cause or a biomarker).
If our premise is correct that identifying a therapeutic pathway also implicates that pathway as central to that disease’s pathogenesis, then certain broader ideas warrant mentioning. First, whereas BPD has heritable features and developmental underpinnings, it would appear to be a disorder not of a defective gene per se but rather of dysregulated posttranslational modulation of a normally produced gene product that, although developmentally critical, plays a physiologic role in all individuals throughout life. Second, attributing pathophysiology to aberrant cytoskeletal dynamics (dendritic disorganization representing one consequence) would appear to implicate not merely defective neurons but also dysregulated interneuronal networks. Whether these observations are particular to LiR BPD or are applicable more widely to other neuropsychiatric disorders warrants study.
With regard to disease modeling in general, this study suggests a strategy for merging hiPSC technology with proteomics to discern underlying pathophysiological mechanisms in complex, polygenic, multifactorial diseases in which causative genes, cells, proteins, and pathways are not well-understood. If there exists an agent that is known to be functionally impactful even if its molecular mechanism-of-action is uncertain (like lithium in BPD), such an agent may allow an investigator to probe otherwise inscrutable intracellular signaling by identifying its target and then reconstructing the regulatory molecular routes upstream and downstream of that node with an eye toward mapping underlying pathogenic pathways and identifying more specific drug targets for the development of safer, cheaper, or more effective pharmacotherapeutics. In this way, hiPSCs may be used in the most challenging diseases not only to reflect phenomenology and a phenotype, but also to identify underlying molecular mechanisms.
Materials and Methods
Human fibroblasts or lymphoblasts from multiple well-sourced patients (SI Appendix, Fig. S1) were reprogrammed to hiPSCs via nonintegrating episomal-mediated (42), lentivirus-mediated (1), or retrovirus-mediated (19) gene transfer, characterized (43), and differentiated to NPCs and cortical interneurons, as per our routine and as previously described (17–20). Protein isolation, 2D-DIGE and SILAC, Western blotting, and coimmunoprecipitation were performed as described previously (17, 28). Immunofluorescence was quantified by pixel number captured via image analysis software using unbiased stereology. All MS data are publicly accessible; for SILAC data, the mass spectra may be downloaded from MassIVE (massive.ucsd.edu) using accession no. MSV000080975; the data are directly accessible via ftp://massive.ucsd.edu (MSV000080975). Creation and analysis of CRMP2 knockout and knockin mice used standard transgenic techniques. Analysis of primary cultures of rodent hippocampal neurons also followed standard techniques (44). Cai2+ transients and flux were measured via kinetic imaging analysis (34, 35). Animal modeling of BPD behavior (to determine the response to lithium-response pathway manipulation) was as described previously (37–39). All animal use was conducted in accordance with the NIH guidelines and approved by the Yokohama City University Institutional Animal Care and Use Committee. Human postmortem material was obtained from the University of Pittsburgh and from the McLean Hospital. Patient samples were obtained following informed consent; all patient identification was removed and the material was processed according to IRB approvals including from Nova Scotia Health Authority Research Ethics Board, National Institutes of Mental Health, and the Sanford-Burnham Prebys Medical Discovery Institute. Dendrite and dendritic spine morphology were assessed as per routine procedures with Golgi stains and image-assisted quantification (44, 45). Bioinformatic analysis was performed as per the Sullivan Lab Evidence Project, ProtKIN, and IPA. See SI Appendix, Supplemental Methods for details.
Supplementary Material
Acknowledgments
We thank M. Niepel, N. Moerke, C. Shamu, and P. Sorger for kinase perturbagen screens; J. Wong and V. Chen for help with Cai2+ imaging; S. Ghose and the Harvard Brain Tissue Resource Center for access to postmortem brains. This study was supported by Grant RC2MH090011 (to E.Y.S.); NIH’s Library of Integrated Network-based Cellular Signatures Program (E.Y.S.); the Viterbi Foundation Neuroscience Initiative (E.Y.S.); Stanley Medical Research Institute Grants R21MH093958, R33MH087896, and R01MH095088 (to S.J.H.); the Tau Consortium (S.J.H.); NIH Grant R01MH087823 (to S.H.); California Institute of Regenerative Medicine training grants (to B.T.D.T., L.D., and C.D.); a University of California, San Diego T32 training grant in psychiatry (to B.T.D.T.); the California Bipolar Foundation; the International Bipolar Foundation; and Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program in the Project for Developing Innovation Systems from the Ministry of Education, Science, Sports and Culture in Japan (Grant 42890001) (Y.G.). Dedicated to the memory of Dr. Jeffrey Nye and his contributions to neuropsychopharmacology.
Footnotes
Conflict of interest statement: R.C.B.B., L.M.B., G.C., J.S.N., H.M., and J.H.P. are employees of private companies. Their role in the study was solely as researchers with no financial or proprietary involvement.
Data deposition: All MS data are publicly accessible; for SILAC data, the mass spectra may be downloaded from MassIVE, massive.ucsd.edu (accession no. MSV000080975); the data are directly accessible via ftp://massive.ucsd.edu/MSV000080975.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1700111114/-/DCSupplemental.
References
- 1.Tobe BT, Snyder EY, Nye JS. Modeling complex neuropsychiatric disorders with human induced pluripotent stem cells. Curr Opin Pharmacol. 2011;11:521–527. doi: 10.1016/j.coph.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mertens J, et al. Pharmacogenomics of Bipolar Disorder Study Differential responses to lithium in hyperexcitable neurons from patients with bipolar disorder. Nature. 2015;527:95–99. doi: 10.1038/nature15526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brennand KJ, et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–225. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malhi GS, Tanious M, Das P, Coulston CM, Berk M. Potential mechanisms of action of lithium in bipolar disorder. Current understanding. CNS Drugs. 2013;27:135–153. doi: 10.1007/s40263-013-0039-0. [DOI] [PubMed] [Google Scholar]
- 5.Gershon S, Chengappa KN, Malhi GS. Lithium specificity in bipolar illness: A classic agent for the classic disorder. Bipolar Disord. 2009;11:34–44. doi: 10.1111/j.1399-5618.2009.00709.x. [DOI] [PubMed] [Google Scholar]
- 6.Miller C, Bauer MS. Excess mortality in bipolar disorders. Curr Psychiatry Rep. 2014;16:499. doi: 10.1007/s11920-014-0499-z. [DOI] [PubMed] [Google Scholar]
- 7.Levy NA, Janicak PG. Calcium channel antagonists for the treatment of bipolar disorder. Bipolar Disord. 2000;2:108–119. doi: 10.1034/j.1399-5618.2000.020204.x. [DOI] [PubMed] [Google Scholar]
- 8.Baldessarini RJ, et al. Decreased risk of suicides and attempts during long-term lithium treatment: A meta-analytic review. Bipolar Disord. 2006;8:625–639. doi: 10.1111/j.1399-5618.2006.00344.x. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan PF, Daly MJ, O'Donovan M. Genetic architectures of psychiatric disorders: The emerging picture and its implications. Nat Rev Genet. 2012;13:537–551. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charney AW, et al. Evidence for genetic heterogeneity between clinical subtypes of bipolar disorder. Transl Psychiatry. 2017;7:e993. doi: 10.1038/tp.2016.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goshima Y, Nakamura F, Strittmatter P, Strittmatter SM. Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature. 1995;376:509–514. doi: 10.1038/376509a0. [DOI] [PubMed] [Google Scholar]
- 12.Ip JP, et al. α2-chimaerin controls neuronal migration and functioning of the cerebral cortex through CRMP-2. Nat Neurosci. 2011;15:39–47. doi: 10.1038/nn.2972. [DOI] [PubMed] [Google Scholar]
- 13.Marques JM, et al. CRMP2 tethers kainate receptor activity to cytoskeleton dynamics during neuronal maturation. J Neurosci. 2013;33:18298–18310. doi: 10.1523/JNEUROSCI.3136-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uchida Y, et al. Semaphorin3A signalling is mediated via sequential Cdk5 and GSK3beta phosphorylation of CRMP2: Implication of common phosphorylating mechanism underlying axon guidance and Alzheimer’s disease. Genes Cells. 2005;10:165–179. doi: 10.1111/j.1365-2443.2005.00827.x. [DOI] [PubMed] [Google Scholar]
- 15.Kaneko S, et al. A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat Med. 2006;12:1380–1389. doi: 10.1038/nm1505. [DOI] [PubMed] [Google Scholar]
- 16.Wilson SM, et al. Prevention of posttraumatic axon sprouting by blocking collapsin response mediator protein 2-mediated neurite outgrowth and tubulin polymerization. Neuroscience. 2012;210:451–466. doi: 10.1016/j.neuroscience.2012.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singec I, et al. Quantitative analysis of human pluripotency & neural specification by in-depth (phospho)proteomic profiling. Stem Cell Rep. 2016;7:527–542. doi: 10.1016/j.stemcr.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li W, et al. Rapid induction and long-term self-renewal of primitive neural precursors from human embryonic stem cells by small molecule inhibitors. Proc Natl Acad Sci USA. 2011;108:8299–8304. doi: 10.1073/pnas.1014041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madison JM, et al. Characterization of bipolar disorder patient-specific induced pluripotent stem cells from a family reveals neurodevelopmental and mRNA expression abnormalities. Mol Psychiatry. 2015;20:703–717. doi: 10.1038/mp.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maroof AM, et al. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell. 2013;12:559–572. doi: 10.1016/j.stem.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blouin JL, et al. Schizophrenia susceptibility loci on chromosomes 13q32 and 8p21. Nat Genet. 1998;20:70–73. doi: 10.1038/1734. [DOI] [PubMed] [Google Scholar]
- 22.Fallin MD, et al. Bipolar I disorder and schizophrenia: A 440-single-nucleotide polymorphism screen of 64 candidate genes among Ashkenazi Jewish case-parent trios. Am J Hum Genet. 2005;77:918–936. doi: 10.1086/497703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fallin MD, et al. Linkage and association on 8p21.2-p21.1 in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2011;156:188–197. doi: 10.1002/ajmg.b.31154. [DOI] [PubMed] [Google Scholar]
- 24.Yoshimura T, et al. GSK-3β regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120:137–149. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Cole AR, et al. Distinct priming kinases contribute to differential regulation of collapsin response mediator proteins by glycogen synthase kinase-3 in vivo. J Biol Chem. 2006;281:16591–16598. doi: 10.1074/jbc.M513344200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu LQ, et al. Protein phosphatase 2A facilitates axonogenesis by dephosphorylating CRMP2. J Neurosci. 2010;30:3839–3848. doi: 10.1523/JNEUROSCI.5174-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soutar MP, et al. Evidence that glycogen synthase kinase-3 isoforms have distinct substrate preference in the brain. J Neurochem. 2010;115:974–983. doi: 10.1111/j.1471-4159.2010.06988.x. [DOI] [PubMed] [Google Scholar]
- 28.Hoedt E, Zhang G, Neubert TA. Stable isotope labeling by amino acids in cell culture (SILAC) for quantitative proteomics. Adv Exp Med Biol. 2014;806:93–106. doi: 10.1007/978-3-319-06068-2_5. [DOI] [PubMed] [Google Scholar]
- 29.Sakurai K, et al. Kinome-wide functional analysis highlights the role of cytoskeletal remodeling in somatic cell reprogramming. Cell Stem Cell. 2014;14:523–534. doi: 10.1016/j.stem.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beaulieu JM, et al. A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell. 2008;132:125–136. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- 31.Strakowski SM, et al. The functional neuroanatomy of bipolar disorder: A consensus model. Bipolar Disord. 2012;14:313–325. doi: 10.1111/j.1399-5618.2012.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, et al. Brain-specific Crmp2 deletion leads to neuronal development deficits and behavioural impairments in mice. Nat Commun. 2016;7:11773. doi: 10.1038/ncomms11773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura F, et al. Increased proximal bifurcation of CA1 pyramidal apical dendrites in sema3A mutant mice. J Comp Neurol. 2009;516:360–375. doi: 10.1002/cne.22125. [DOI] [PubMed] [Google Scholar]
- 34.Sabatini BL, Oertner TG, Svoboda K. The life cycle of Ca(2+) ions in dendritic spines. Neuron. 2002;33:439–452. doi: 10.1016/s0896-6273(02)00573-1. [DOI] [PubMed] [Google Scholar]
- 35.Merriam EB, et al. Synaptic regulation of microtubule dynamics in dendritic spines by calcium, F-actin, and drebrin. J Neurosci. 2013;33:16471–16482. doi: 10.1523/JNEUROSCI.0661-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen HM, et al. Transcripts involved in calcium signaling and telencephalic neuronal fate are altered in induced pluripotent stem cells from bipolar disorder patients. Transl Psychiatry. 2014;4:e375. doi: 10.1038/tp.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gould TD, O’Donnell KC, Picchini AM, Manji HK. Strain differences in lithium attenuation of d-amphetamine-induced hyperlocomotion: A mouse model for the genetics of clinical response to lithium. Neuropsychopharmacology. 2007;32:1321–1333. doi: 10.1038/sj.npp.1301254. [DOI] [PubMed] [Google Scholar]
- 38.Xu CM, et al. Glycogen synthase kinase 3β in the nucleus accumbens core is critical for methamphetamine-induced behavioral sensitization. J Neurochem. 2011;118:126–139. doi: 10.1111/j.1471-4159.2011.07281.x. [DOI] [PubMed] [Google Scholar]
- 39.Young EJ, et al. Selective, retrieval-independent disruption of methamphetamine-associated memory by actin depolymerization. Biol Psychiatry. 2014;75:96–104. doi: 10.1016/j.biopsych.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shibasaki M, Kurokawa K, Ohkuma S. Upregulation of L-type Ca(v)1 channels in the development of psychological dependence. Synapse. 2010;64:440–444. doi: 10.1002/syn.20745. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida H, Watanabe A, Ihara Y. Collapsin response mediator protein-2 is associated with neurofibrillary tangles in Alzheimer’s disease. J Biol Chem. 1998;273:9761–9768. doi: 10.1074/jbc.273.16.9761. [DOI] [PubMed] [Google Scholar]
- 42.Okita K, et al. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011;8:409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- 43.Müller FJ, et al. A bioinformatic assay for pluripotency in human cells. Nat Methods. 2011;8:315–317. doi: 10.1038/nmeth.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calabrese B, Halpain S. Essential role for the PKC target MARCKS in maintaining dendritic spine morphology. Neuron. 2005;48:77–90. doi: 10.1016/j.neuron.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 45.Hotulainen P, Hoogenraad CC. Actin in dendritic spines: Connecting dynamics to function. J Cell Biol. 2010;189:619–629. doi: 10.1083/jcb.201003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.