Abstract
Cellular translation is inhibited following infection with most strains of reovirus, but the mechanisms responsible for this phenomenon remain to be elucidated. The extent of host shutoff varies in a strain-dependent manner; infection with the majority of strains leads to strong host shutoff, while infection with strain Dearing results in minimal inhibition of cellular translation. A genetic study with reassortant viruses and subsequent biochemical analyses led to the hypothesis that the interferon-induced, double-stranded RNA-activated protein kinase, PKR, is responsible for reovirus-induced host shutoff. To directly determine whether PKR is responsible for reovirus-induced host shutoff, we used a panel of reovirus strains and mouse embryo fibroblasts derived from knockout mice. This approach revealed that PKR contributes to but is not wholly responsible for reovirus-induced host shutoff. Studies with cells lacking RNase L, the endoribonuclease component of the interferon-regulated 2′,5′-oligoadenylate synthetase-RNase L system, demonstrated that RNase L also down-regulates cellular protein synthesis in reovirus-infected cells. In many viral systems, PKR and RNase L have well-characterized antiviral functions. An analysis of reovirus replication in cells lacking these molecules indicated that, while they contributed to host shutoff, neither PKR nor RNase L exerted an antiviral effect on reovirus growth. In fact, some strains of reovirus replicated more efficiently in the presence of PKR and RNase L than in their absence. Data presented in this report illustrate that the inhibition of cellular translation following reovirus infection is complex and involves multiple interferon-regulated gene products. In addition, our results suggest that reovirus has evolved effective mechanisms to avoid the actions of the interferon-stimulated antiviral pathways that include PKR and RNase L and may even benefit from their expression.
Cellular translation is inhibited following infection with most strains of mammalian reovirus, a phenomenon known as host shutoff (54). However, the extent of reovirus-induced host shutoff varies in a strain-specific manner; infection with strain Dearing has a minimal effect on cellular translation, whereas infection with other strains, such as Jones, clone 8 (c8), clone 87 (c87), and clone 93 (c93), leads to dramatic host shutoff (40, 41). In most situations, even when cellular translation is inhibited, reovirus proteins are efficiently synthesized. Although the mechanisms responsible for reovirus-induced host shutoff remain to be elucidated, they have been defined for other viruses, including poliovirus and rotavirus (14). In poliovirus-infected cells, virus-encoded protease 2Apro leads to cleavage of eukaryotic translation initiation factors eIF4GI and eIF4GII, thus preventing translation of the vast majority of capped cellular mRNAs (10, 17). Uncapped and internal ribosome entry site-containing poliovirus mRNAs, in contrast, require only the C-terminal cleaved portion of eIF4G for translation initiation and thus are efficiently translated under these conditions (32). Although some evidence suggests that reovirus secondary transcripts are uncapped (43), there is no evidence for eIF4G cleavage during reovirus infection (9), nor is there evidence that their short 5′ untranslated regions support internal ribosome entry. Rather than modifying eIF4G, rotavirus encodes a nonstructural protein, NSP3, which effectively competes with the cellular poly(A) binding protein for binding to eIF4G (33). As a viral homolog of poly(A) binding protein, NSP3 leads to the preferential translation of rotavirus transcripts by interacting with eIF4G and a specific sequence in the 3′ end of nonpolyadenylated rotavirus mRNAs (34, 45). Although rotavirus and reovirus both belong to the family Reoviridae, there is no NSP3 homolog in mammalian orthoreoviruses.
The innate immune response to viral infection can also result in translational inhibition. In the presence of double-stranded RNA (dsRNA), whose concentration frequently increases as a consequence of infection, the interferon (IFN)-stimulated protein kinase, PKR, dimerizes and is activated via trans-autophosphorylation. Activated PKR halts translation initiation by phosphorylating serine 51 (S51) in the α subunit of eIF2. This phosphorylation increases the affinity of eIF2 for guanine nucleotide exchange factor eIF2B and thus prevents the recycling of GDP for GTP. Since phosphorylated and GDP-bound eIF2 cannot participate in the formation of the 43S preinitiation complex, translation initiation is halted (5). Multiple lines of evidence have led to the hypothesis that PKR is responsible for reovirus-induced host shutoff. The difference in the host shutoff phenotypes observed after infection with reovirus strains Dearing and Jones was genetically mapped to a single viral gene segment, S4, that encodes viral protein σ3 (41). In addition to its structural role in forming the outer capsid, σ3 is capable of binding dsRNA in a sequence-independent manner (8, 19, 39). σ3 can prevent PKR activation in vitro (20). Furthermore, σ3 functionally substitutes for the PKR-inhibitory molecules VAI RNA and E3L in adenovirus and vaccinia virus deletion mutants, respectively, enabling their replication in cell cultures (3, 26). Finally, the extent of eIF2α phosphorylation was shown to correlate with the extent to which reovirus strains Dearing, Lang, and Jones induce host shutoff (26).
In this study, we set out to directly test the role of the PKR-eIF2α pathway in reovirus-induced host shutoff by examining protein synthesis profiles following infection of cells that lack an intact PKR gene. We found that while PKR contributes to the inhibition of cellular translation during reovirus infection, most notably following strain Jones infection, it is not solely responsible for the strong host shutoff observed after infection with several other reovirus strains. Rather, our results demonstrate that reovirus-induced host shutoff is multifactorial and is mediated by at least two IFN-regulated gene products, PKR and RNase L. In addition, our results indicated that reovirus not only avoids the antiviral effects of PKR and RNase L but actually benefits from their presence because the level of replication of some strains of reovirus is higher in wild-type (wt) mouse embryo fibroblasts (MEFs) than in knockout (KO) cells.
MATERIALS AND METHODS
Cells and viruses.
Murine L929 cells were maintained as suspension cultures as described previously (21). RNase L KO MEFs (52), MEFs derived from RNase L wt littermates, and MEFs lacking PKR and RNase L in addition to Mx1 (hereafter referred to as double KO) (53) were maintained as monolayer cultures in high-glucose Dulbecco modified Eagle medium (hgDMEM) (Gibco-BRL, Grand Island, N.Y.) supplemented to contain 10% heat-inactivated fetal calf serum (HyClone Laboratories, Logan, Utah), 2 mM glutamine, 50 U of penicillin G/ml, and 50 μg of streptomycin sulfate/ml. PKR KO and PKR wt MEFs were maintained as monolayer cultures in hgDMEM supplemented to contain 15% heat-inactivated fetal calf serum, 2 mM glutamine, 50 U of penicillin G/ml, and 50 μg of streptomycin sulfate/ml (50). Immortalized PKR KO MEFs stably transfected with a bacterial artificial chromosome (BAC) plasmid encoding the human PKR gene (iPKR KO+BAC-huPKR cells) (6) were maintained as monolayer cultures in hgDMEM supplemented to contain 10% heat-inactivated fetal calf serum, 0.2 mg of Zeocin (a glycopeptide antibiotic of the bleomycin family; Invitrogen, Carlsbad, Calif.)/ml, 2 mM glutamine, 50 U of penicillin G/ml, and 50 μg of streptomycin sulfate/ml. Immortalized MEFs expressing wt eIF2α (wt eIF2α MEFs) and S51A eIF2α (S51A eIF2α MEFs) (38) were maintained as monolayer cultures in hgDMEM supplemented to contain 10% fetal calf serum, essential amino acids (Gibco-BRL), 0.1 mM nonessential amino acids (Gibco-BRL), 2 mM glutamine, 50 U of penicillin G/ml, and 50 μg of streptomycin sulfate/ml. Experiments were performed with the medium used to maintain each cell type with two exceptions. Fetal calf serum used to supplement the medium for wt eIF2α MEFs and S51A eIF2α MEFs was heat inactivated, and Zeocin was omitted from the medium for iPKR KO+BAC-huPKR cells.
Reovirus strains Jones, Dearing, and c87/Abney are prototypic laboratory strains. Strains c8 and c93 were originally isolated by Rosen and colleagues (36) and are described elsewhere (21). Third-passage cell lysate stocks were prepared in L929 cells. Purified virions were prepared by CsCl density gradient centrifugation of extracts from cells infected with third-passage stocks (13). Intermediate subvirion particles (ISVPs) were prepared by treating purified virions with chymotrypsin as described elsewhere (29).
Analysis of total protein synthesis.
Cells were plated in duplicate (see Fig. 1) or triplicate (see Fig. 2 to 6) to result in nearly confluent monolayers in 15-mm wells at the time of harvest (Costar, Cambridge, Mass.). The following concentrations were used: 7 × 104 cells/well for PKR wt, RNase L wt, RNase L KO, S51A eIF2α, and wt eIF2α MEFs; 4 × 104 cells/well for PKR KO MEFs; 2 × 105 cells/well for L929 and iPKR KO+BAC-huPKR MEFs; and 1 × 105 cells/well for double KO MEFs. After 4 h of incubation at 37°C, the medium was removed, and cells were infected with purified virions (see Fig. 1A) or ISVPs at various multiplicities of infection (MOIs). Samples were incubated for 2 h at 37°C to allow particles to adsorb, medium was added to a final volume of 1 ml/well, and samples were incubated at 37°C. At various times postinfection (p.i.), cells were preincubated in methionine-free and l-glutamine-free modified Eagle medium (ICN Biomedicals Inc., Aurora, Ohio) for 30 min at 37°C. The medium was removed, and cells were incubated with methionine-free and l-glutamine-free modified Eagle medium supplemented to contain 2 mM glutamine and 50 μCi of [35S]methionine-cysteine (EasyTag; NEN Life Science Products Inc., Boston, Mass.)/ml. After 30 min or 2 h at 37°C, lysates were prepared by resuspending cells in lysis buffer (0.1 M NaCl, 1 mM EDTA, 10 mM Tris [pH 7.4], 0.5% NP-40), and samples were adjusted with concentrated protein sample buffer to achieve final concentrations of 0.3 M sucrose, 0.125 M Tris (pH 8.0), 1% sodium dodecyl sulfate (SDS), 0.01% bromophenol blue, and 50 μl of β-mercaptoethanol/ml. Because our samples were obtained from cells with distinct translational profiles, labeled proteins from equivalent numbers of cells were resolved by electrophoresis on SDS-10% polyacrylamide gels. Labeled proteins were visualized and areas containing cellular proteins were quantified by using a PhosphorImager and ImageQuant software (Molecular Dynamics, Sunnyvale, Calif.).
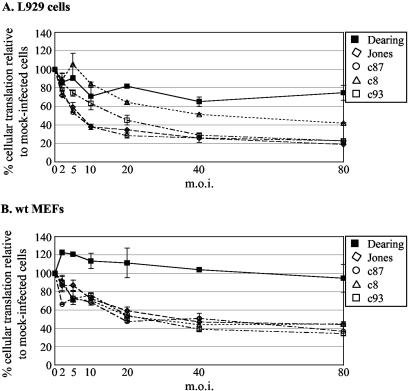
FIG. 1.
MOI dependence of reovirus-induced host shutoff. L929 cells (A) or wt MEFs (B) were mock infected or infected in duplicate at a variety of MOIs with the indicated strains of reovirus. At 20 h p.i., cells were pulse-labeled with [35S]methionine-cysteine for 30 min, extracts were prepared, and proteins were separated by SDS-PAGE. Cellular translation was quantified as described in the legend to Fig. 2. The graphs depict the average percent cellular translation relative to that in mock-infected cells; error bars indicate deviations from the average.
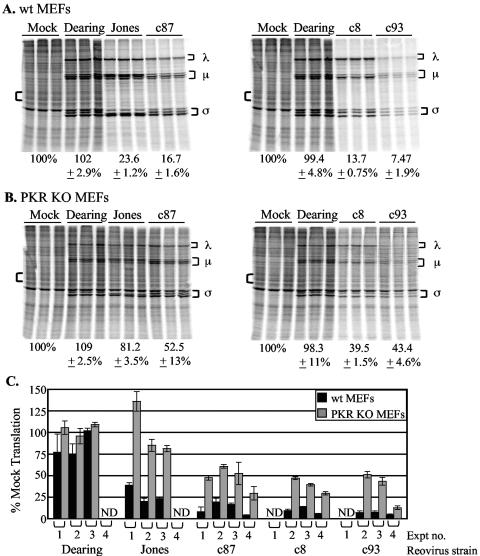
FIG. 2.
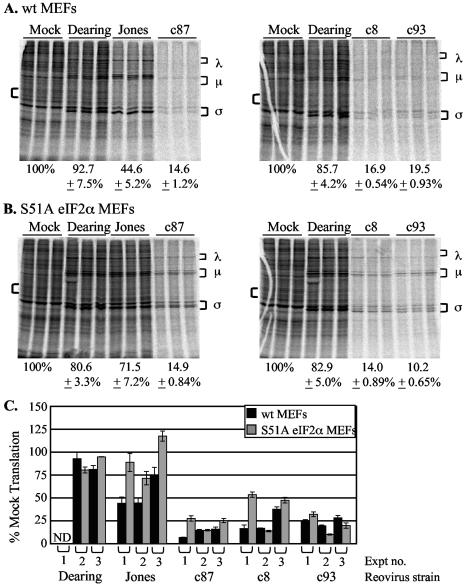
Impact of PKR expression on reovirus-induced host shutoff. (A and B) wt (A) or PKR KO (B) MEFs were mock infected or infected in triplicate with the indicated reovirus strains at an MOI of 80 PFU/cell. Cells were metabolically labeled at 20 h p.i. with [35S]methionine-cysteine for 30 min, and proteins were separated by SDS-PAGE. Reovirus proteins are indicated by brackets to the right of each gel image. Percentages below the gel images represent the percent cellular translation quantified from the area indicated by the left bracket relative to the level of cellular translation in uninfected cells ± the standard deviation. (C) Graph depicting results from four separate experiments (Expt). The black bars represent the extent of cellular translation in wt MEFs; the grey bars represent that in PKR KO MEFs. ND, not determined.
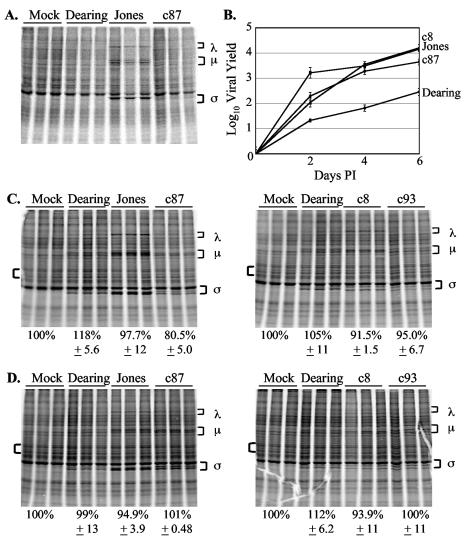
FIG. 6.
Impact of PKR and RNase L deletions on reovirus replication and reovirus-induced host shutoff. (A) Double KO MEFs were mock infected or infected in triplicate with the indicated reovirus strains at an MOI of 80 PFU/cell. Levels of cellular translation were analyzed at 20 h p.i. as described in the legend to Fig. 2. (B) Double KO MEFs were infected with the indicated strains of reovirus at an MOI of 2 PFU/cell. Infectious virus present at 0, 2, 4, or 6 days p.i. was measured by plaque assay. Each time point represents the mean ± standard deviation derived from three independent samples. (C and D) Double KO MEFs were mock infected or infected in triplicate with the indicated reovirus strains at an MOI of 80 PFU/cell. Cells were metabolically labeled at 20 (C) or 44 (D) h p.i. with [35S]methionine-cysteine for 2 h. Levels of cellular translation were determined as described in the legend to Fig. 2.
Analysis of PKR expression.
Cells were harvested in phosphate-buffered saline, collected by centrifugation, and lysed in Tris lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 50 mM NaF, 10 mM β-glycerophosphate, 0.1 mM EDTA, 10% glycerol, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 2 μg of leupeptin/ml, 2 μg of pepstatin/ml, 2 μg of aprotinin/ml). After 20 min on ice, samples were pelleted by centrifugation at 10,000 × g for 20 min. Cell lysates were normalized for protein content by using a protein assay kit (DC protein assay; Bio-Rad Laboratories, Hercules, Calif.) and were solubilized in protein sample buffer. Proteins were resolved by electrophoresis on SDS-10% polyacrylamide gels and transferred to nitrocellulose membranes (Bio-Rad) by electroblotting for 1.75 h at 100 V in transfer buffer (25 mM Tris, 192 mM glycine, 20% methanol). Nitrocellulose membranes were blocked overnight in Tris-buffered saline (10 mM Tris [pH 8.0], 150 mM NaCl) with 0.4% Tween 20 (TBST) and 10% nonfat dry milk and were washed with TBST prior to incubation with the primary antibody. PKR was detected by using a PKR-specific monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.) diluted in TBST. Membranes were washed with TBST and incubated with horseradish peroxidase-conjugated anti-mouse immunoglobulin G (Pierce Chemical Company, Rockford, Ill.). Bound antibodies were detected by enhanced chemiluminescence (ECL detection reagents; Amersham, Arlington Heights, Ill.) and exposure to X-ray film (Full Speed Blue; Henry Schein, Melville, N.Y.).
Analysis of viral growth.
Cells were infected at various MOIs, and adsorption was allowed to proceed for 1 h on ice at 4°C. After adsorption, cells were concentrated by low-speed centrifugation and resuspended in fresh medium. ISVPs and cells then were added to dram vials containing 1 ml of cold medium at cell densities to result in near confluence (4 × 104 PKR KO MEFs, 8 × 104 PKR wt MEFs, and 1 × 105 each RNase L wt MEFs, RNase L KO MEFs, and double KO MEFs/vial). Triplicate samples were prepared for each time point. One set of samples (time zero) was frozen immediately at −20°C. The remaining samples were incubated at 37°C until the desired time point was reached. Harvested samples were subjected to three cycles of freezing and thawing and titrated by plaque assays as described elsewhere (46). Viral yields were calculated with the formula log10(PFU/ml)t = xd − log10(PFU/ml)t = 0, where t is time and xd is day postinfection.
RESULTS
Reovirus-induced host shutoff is MOI dependent.
Sharpe and Fields characterized the effects of reovirus infection on translation in L929 cells (41). Their analysis revealed that strain Jones-induced host shutoff is highly MOI dependent and does not occur at less than 80 PFU/cell. At this high MOI, strain Dearing had minimal effects on cellular protein synthesis. To determine whether the host shutoff induced by other strains of reovirus was similarly MOI dependent, we compared the capacities of five different reovirus strains to induce host shutoff in L929 cells or wt MEFs when infection was initiated at various MOIs. For these studies, we infected MEFs with ISVPs because there is a block to reovirus uncoating in some MEFs that is overcome by pretreatment of virions with chymotrypsin (16). At 20 h p.i., cells were metabolically labeled with [35S]methionine, and total protein synthesis was analyzed by SDS-polyacrylamide gel electrophoresis (PAGE). As expected, infection with strain Dearing had a minimal effect on cellular translation, even at an MOI of 80, the highest MOI examined. Like strain Jones (41), other strains induced host shutoff in an MOI-dependent manner. This characteristic was not cell type specific, as similar results were obtained with L929 cells (Fig. 1A) and wt MEFs (Fig. 1B). We chose an MOI of 80 PFU/cell for the rest of our protein labeling experiments because this MOI was historically used for examining cellular translation in reovirus-infected cells (26, 40, 41) and maximized strain-specific phenotypes.
PKR is not solely responsible for the inhibition of cellular translation during reovirus infection.
To directly test the hypothesis that PKR is responsible for reovirus-induced host shutoff, we compared the capacities of several reovirus strains to induce host shutoff in MEFs from PKR KO mice and MEFs from wt littermate control mice (50). Previous work with L929 and HeLa cells indicated that reovirus-induced host shutoff is first detected at ∼12 h p.i. and that its extent increases over the course of infection (11, 41). We assessed host shutoff at 20 h p.i. by metabolically labeling cells with [35S]methionine and analyzing total protein synthesis by SDS-PAGE. Synthesized proteins were visualized by PhosphorImager analysis, and levels of translation were quantified. We chose this time point because viral yields are significant in both MEFs and L929 cells by 24 h p.i. and because cell viability, as measured by trypan blue exclusion, remains at ≥60% (data not shown). A representative experiment is shown in Fig. 2A and B. Whereas infection of wt MEFs with strain Jones, c87, c8, or c93 resulted in host shutoff (Fig. 2A), there was a less dramatic inhibition of cellular translation in PKR KO MEFs (Fig. 2B). The role of PKR was most apparent in Jones-infected cells; the host shutoff phenotype characteristic of this strain was largely abolished in PKR KO cells. Infection with other strains, particularly c8 and c93, clearly resulted in host shutoff in PKR KO MEFs, although not to the same extent as in wt MEFs. Figure 2C demonstrates the consistency of host shutoff phenotypes in independent replicate experiments, each with infections performed and characterized in triplicate. These results revealed that PKR contributes to reovirus-induced host shutoff but is not solely responsible for the strong inhibition of cellular translation observed following infection with c87, c8, or c93.
Strain Jones-induced host shutoff depends on PKR expression.
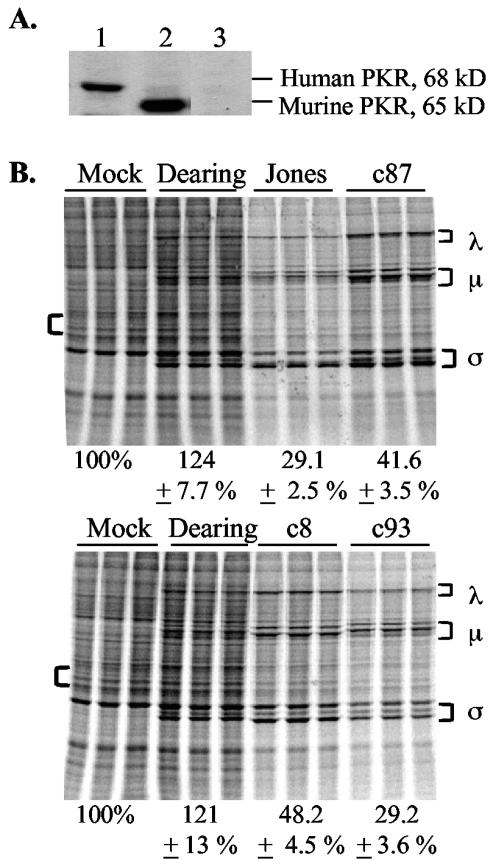
To determine whether the decreased host shutoff in PKR KO MEFs was due to the disruption of the PKR gene rather than a secondary effect in the embryos from which these cells were derived, we examined levels of cellular translation in infected PKR KO MEFs stably transfected with a BAC plasmid encoding the human PKR gene (Fig. 3A) (6). Cells were infected with ISVPs and labeled at 20 h p.i. Whereas the host shutoff characteristic of Jones was not apparent in PKR KO MEFs (Fig. 2B), Jones infection induced a dramatic decrease in cellular translation in iPKR KO+BAC-huPKR MEFs (Fig. 3B). These results argue that the diminished host shutoff in Jones-infected PKR KO MEFs is due to the absence of the PKR protein. In contrast, the expression of the human PKR gene in PKR KO MEFs did not have as dramatic an effect on the host shutoff induced by reovirus strains c87, c8, and c93, consistent with the conclusion that another molecule contributes to host shutoff in cells infected with these strains.
FIG. 3.
Reovirus-induced host shutoff in PKR KO MEFs transfected with human PKR. (A) PKR expression in cell extracts from iPKR KO+BAC-huPKR cells, PKR wt MEFs, and PKR KO MEFs (lanes 1 to 3, respectively) was examined by immunoblot analysis. (B) iPKR KO+BAC-huPKR cells were mock infected or infected in triplicate with the indicated reovirus strains at an MOI of 80 PFU/cell. Levels of cellular translation were analyzed at 20 h p.i. as described in the legend to Fig. 2.
PKR regulates cellular translation in strain Jones-infected cells through its capacity to phosphorylate eIF2α.
PKR activation is most commonly associated with its ability to phosphorylate eIF2α and inhibit translation initiation (5). However, PKR can also affect translation through its effects on other signaling pathways (48). To determine whether PKR functions through its ability to phosphorylate and inactivate eIF2α in reovirus-infected cells, we used MEFs in which S51 of eIF2α has been mutated by homologous recombination to nonphosphorylatable A51 (38). This S51A substitution results in constitutively active eIF2α. At 20 h p.i., wt eIF2α MEFs infected with Jones, c87, c8, or c93 displayed dramatic host shutoff, whereas infection with strain Dearing had a minimal effect on cellular translation (Fig. 4A). When parallel infections were performed with mutant S51A eIF2α MEFs, infection with Jones no longer led to the strong host shutoff that is characteristic of this strain (Fig. 4B). In contrast, host shutoff was still apparent in S51A eIF2α MEFs following infection with c87, c8, or c93. Figure 4C demonstrates the consistency of the host shutoff phenotypes in multiple independent experiments. For reasons that are not yet clear, c87, c8, and c93 viral protein synthesis appeared compromised in this set of MEFs when infection led to strong host shutoff. Interestingly, despite the apparently poor viral protein synthesis visualized by metabolic labeling, all strains analyzed reached final yields comparable to those achieved in other MEFs (J. Smith, R. Kaufman, and L. Schiff, unpublished data).
FIG. 4.
Effect of nonphosphorylatable, constitutively active eIF2α on reovirus-induced host shutoff. (A and B) wt eIF2α (A) or S51A eIF2α (B) MEFs were mock infected or infected in triplicate with the indicated reovirus strains at an MOI of 80 PFU/cell. Cells were metabolically labeled at 20 h p.i. with [35S]methionine-cysteine for 2 h. Levels of cellular translation were determined as described in the legend to Fig. 2. (C) Graph depicting results from three separate experiments (Expt). The black bars represent the level of cellular translation in wt eIF2α MEFs; the grey bars represent protein synthesis in S51A eIF2α MEFs. ND, not determined.
These data support a model in which host shutoff induced by infection with reovirus strain Jones is largely due to the capacity of PKR to phosphorylate and inactivate eIF2α. Host shutoff by this strain is severely decreased in the absence of PKR (Fig. 2B) and when eIF2α is constitutively active (Fig. 4B). However, these data indicate that other mechanisms contribute to the strong host shutoff induced by reovirus strains c87, c8, and c93 because host shutoff is diminished but not absent in cells lacking PKR (Fig. 2B) and cellular translation is inhibited in MEFs that contain constitutively active eIF2α (Fig. 4B).
RNase L contributes to reovirus-induced host shutoff.
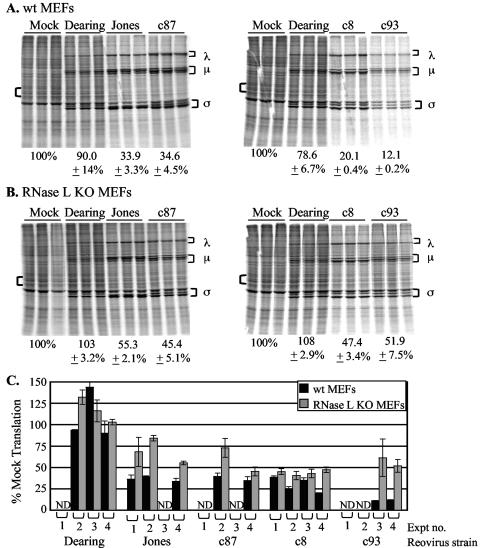
Since we found that several strains of reovirus inhibit cellular translation in the absence of PKR, we examined whether another well-characterized IFN-regulated gene product, RNase L, plays a role in reovirus-induced host shutoff. RNase L is the endoribonuclease component of the antiviral 2-5A system. In the presence of dsRNA, oligoadenylate synthetases are activated and synthesized from ATP short 2′,5′-oligoadenylates. The binding of these molecules to RNase L enables its dimerization and activation of its nuclease domain. Activated RNase L cleaves single-stranded RNA after UU and UA sequences and interferes with translation by degrading mRNA and/or rRNA (42). To determine whether RNase L activity contributes to reovirus-induced host shutoff, we analyzed cellular translation in RNase L KO MEFs (52). As expected, infection of wt littermate control MEFs with strain Jones, c87, c8, or c93 resulted in strong host shutoff, whereas infection with strain Dearing had a minimal effect on the level of cellular translation (Fig. 5A). In RNase L KO MEFs, host translation was inhibited following infection with Jones, c87, c8, or c93, although the extent was diminished (to various degrees) relative to what was observed in wt cells (Fig. 5B and C). These results reveal that, like PKR, RNase L plays a role in reovirus-induced host shutoff.
FIG. 5.
Consequences of RNase L expression for reovirus-induced host shutoff. (A and B) wt (A) or RNase L KO (B) MEFs were mock infected or infected in triplicate with the indicated reovirus strains at an MOI of 80 PFU/cell. Levels of cellular translation were analyzed at 20 h p.i. as described in the legend to Fig. 2. (C) Graph depicting results from four separate experiments (Expt). The black bars represent the extent of cellular translation in wt MEFs; the grey bars represent that in RNase L KO MEFs. ND, not determined.
Reovirus-induced host shutoff does not occur in the absence of both PKR and RNase L.
We hypothesized that the combined activities of PKR and RNase L might be responsible for the dramatic inhibition of cellular translation that we observed following infection with c87, c8, and c93, since these reovirus strains induced host shutoff in the absence of either molecule alone. To test this hypothesis, we analyzed host shutoff phenotypes in double KO MEFs lacking both PKR and RNase L (53). When we used our standard metabolic labeling protocol, which involves a 30-min pulse with [35S]methionine at 20 h p.i., we could not readily detect viral protein synthesis, except in strain Jones-infected cells (Fig. 6A). To determine the extent to which reovirus was capable of replicating in double KO MEFs, we performed single-cycle growth analyses with strains Dearing, Jones, c8, and c87. The results of this experiment indicated that all four reovirus strains replicated in double KO MEFs, with maximal titers reached by 6 d p.i. (Fig. 6B). Hypothesizing that the efficiency of viral protein synthesis might be altered in double KO MEFs, we extended metabolic labeling times from 30 min to 2 h. At 20 h p.i., cellular translation was not inhibited and minimal viral protein synthesis was visualized by metabolic labeling (Fig. 6C). Since some aspect of viral replication is necessary for Jones-induced host shutoff (41) and the kinetics of replication were somewhat slower in double KO cells than in either of the single KO cells (compare Fig. 6B and Fig. 7), we also analyzed total protein synthesis in double KO MEFs at 44 h p.i. (Fig. 6D). Consistent with our observations at 20 h p.i., we found that even at this later time (when Jones, c8, and c87 were replicating to high yields), reovirus infection did not lead to inhibition of cellular translation in MEFs lacking both PKR and RNase L. These results are consistent with a model in which the combined effects of PKR and RNase L lead to the dramatic host shutoff observed in cells infected with c87, c8, and c93.
FIG. 7.
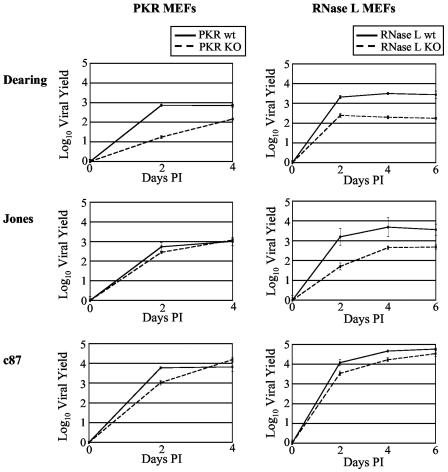
Effect of PKR and RNase L on reovirus replication. wt and KO PKR and RNase L MEFs were infected with the indicated reovirus strains at an MOI of 2 PFU/cell. Infectious virus present at 0, 2, 4 or 6 days p.i. was determined as described in the legend to Fig. 6.
Reovirus replication is not inhibited by PKR or RNase L.
We were struck by the fact that, in our metabolic labeling experiments, viral protein synthesis did not appear to increase in the single KO MEFs, as would be expected if either PKR or RNase L inhibited reovirus growth. To directly examine the capacity of these two IFN-induced molecules to inhibit reovirus replication, we compared single-cycle growth kinetics and final yields in wt and KO MEFs (Fig. 7). We found that the replication kinetics of all three strains of reovirus were similar in wt and KO MEFs, although final yields varied in a strain-dependent manner. Final yields in wt cells were equivalent to or higher than those in KO cells, indicating that neither PKR nor RNase L has an inhibitory effect on reovirus replication in fibroblasts. These results contrast with published reports suggesting that RNase L inhibits reovirus growth (7, 31).
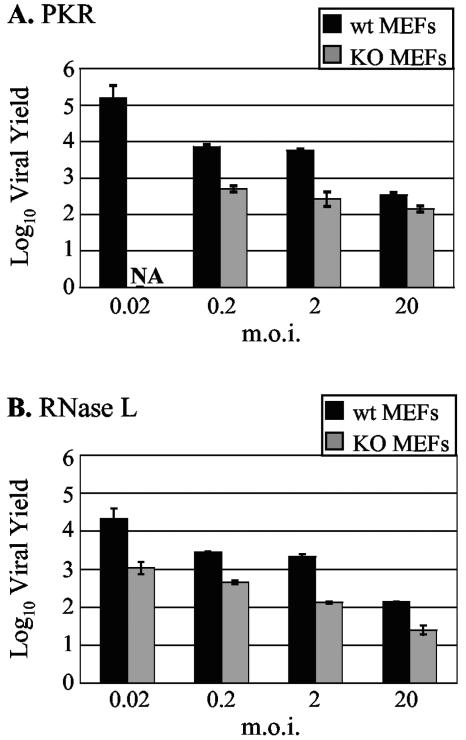
Given the surprising finding that reovirus strain Dearing replicated to higher yields in the presence of PKR and RNase L than in the absence of either molecule, we examined whether enhanced reovirus replication in the presence of these antiviral molecules depends on the MOI used to initiate infection. There is precedent for an MOI-dependent antiviral effect of PKR against vesicular stomatitis virus and encephalomyocarditis virus, with increased PKR-dependent antiviral activity at lower MOIs (22). As shown in Fig. 8, Dearing replicated to higher final yields in wt MEFs than in KO MEFs at all MOIs tested.
FIG. 8.
MOI dependence of reovirus strain Dearing replication in PKR and RNase L MEFs. wt and KO PKR (A) and RNase L (B) MEFs were infected with strain Dearing at the indicated MOIs. Infectious yields at 5 days p.i. were determined as described in the legend to Fig. 6. NA, not available.
DISCUSSION
A variety of biochemical and genetic data have led to the hypothesis that PKR is responsible for the inhibition of cellular translation following reovirus infection (3, 20, 26, 41). We directly addressed this hypothesis by analyzing infection in MEFs deficient in this IFN-induced, dsRNA-activated kinase. Our results demonstrate conclusively that PKR is involved in reovirus-induced host shutoff because the extent of host shutoff decreases following infection of cells that lack the full-length PKR gene. PKR plays a crucial role in the inhibition of cellular translation following infection with strain Jones because host shutoff is largely abolished in PKR KO MEFs after infection with this strain of reovirus. In contrast, PKR is only partially responsible for the host shutoff observed following infection with reovirus strains c8, c87, and c93.
Our results reveal that the importance of PKR and eIF2α phosphorylation in reovirus-induced host shutoff is strain dependent. The inability of strain Jones to induce host shutoff in MEFs containing constitutively active eIF2α (S51A) indicates that it is the capacity of PKR to phosphorylate eIF2α and inhibit translation initiation that is responsible for host shutoff following infection with this reovirus strain. Although PKR is clearly involved in host shutoff after infection with strains c87, c8, and c93, their capacity to induce host shutoff is unaffected by the ability of eIF2α to be phosphorylated. These data argue that the contribution of PKR to host shutoff following infection with these strains of reovirus involves a pathway other than that which leads to eIF2α phosphorylation. PKR has been linked to the activation pathways for multiple signaling molecules, including nuclear factor κB, signal transducer and activator of transcription 1 and 3, mitogen-activated protein kinase kinases 4/7 and 3/6, and protein phosphatase 2A (PP2A) (48). Of these molecules, PP2A is known to directly affect the efficiency of translation initiation. The B56α subunit of PP2A dephosphorylates eIF4E, the eukaryotic translation initiation factor responsible for binding to 5′ caps during recruitment of the eIF4F cap binding complex to mRNA (49). Since phosphorylated eIF4E has a higher affinity for capped mRNA than dephosphorylated eIF4E (15), the ability of PKR to activate PP2A could contribute to the host shutoff observed following infection with reovirus strains c87, c8, and c93. Future studies will explore this hypothesis.
The ability of strains c87, c8, and c93 to induce host shutoff in PKR KO MEFs reflects the involvement of a PKR-independent molecule in reovirus-induced host shutoff. Our data indicate that the endoribonuclease RNase L is one such molecule. At this point it is unclear if RNase L is contributing to host shutoff through its capacity to nonspecifically degrade mRNA and rRNA (42). We do not believe nonspecific RNA degradation is responsible for the host shutoff that is observed following infection with strains c87, c8, and c93 because rRNA cleavage is minimal in infected L929 cells and strain differences in the extent of cleavage do not correlate with host shutoff phenotypes (data not shown). RNase L could impact cellular translation without affecting global RNA levels. It has recently been demonstrated that RNase L is capable of targeting specific mRNAs for degradation (23, 25). This activity of RNase L has been suggested to play an important role in ensuring the transient nature of the IFN response. Thus, RNase L-dependent changes in the half-lives of specific mRNAs could contribute to the inhibition of cellular translation that is observed as a consequence of reovirus infection.
PKR and RNase L contribute to, but are not individually responsible for, c87-, c8-, and c93-induced host shutoff. Since we did not detect host shutoff after infection of double KO MEFs, these data are consistent with a model in which the combined activities of PKR and RNase L lead to host shutoff after infection with these strains. A caveat to this conclusion is that viral protein synthesis, as measured by metabolic labeling, appears compromised in the double KO MEFs. Because it has been reported that reovirus strain Jones-induced host shutoff requires viral replication (41), it is possible that host shutoff depends on the accumulation of a critical level of viral proteins. It is also noteworthy that c93 viral protein synthesis, as measured by metabolic labeling in several MEFs, appears to be low relative to that in the other strains used in this study. Based on light microscopy examination of cells, this strain of reovirus induces the most cytopathology. It is possible that such damaging effects to the cell come at a cost to viral fitness.
Although PKR and RNase L have well-documented antiviral activities (2, 7, 24, 27, 31, 51, 53) and are activated as a consequence of reovirus infection (1, 18, 26, 30, 37), our data indicate that, at least in fibroblasts, these molecules do not inhibit reovirus replication. In fact, it appears that they facilitate the replication of some reovirus strains. Our findings that reovirus protein synthesis and viral yields are not enhanced in PKR KO cells are not consistent with the model put forth by Strong and colleagues that reovirus preferentially replicates in Ras-transformed cells due to a block in PKR activation (44). Our results indicate that inhibition of cellular translation by PKR and RNase L is not detrimental to reovirus replication, at least in cell culture, because strains Jones and c87 replicated as well as or better than non-shutoff-inducing strain Dearing in all of the fibroblast cell lines examined.
How might PKR and RNase L enhance reovirus replication? One possibility is that by decreasing cellular protein synthesis, the activity of these IFN-regulated gene products creates an environment that favors the translation of reovirus transcripts, thus increasing progeny virion production. Supporting evidence in the literature proposes that competition exists between cellular mRNA and reovirus mRNA for limited translational machinery (4, 28, 35, 47). Work by Schmechel et al. suggests that specific areas within reovirus-infected cells may be spared from translational inhibition as a result of the localization of σ3 (40). σ3 could locally inhibit PKR and RNase L in and around perinuclear viral factories, while active PKR and RNase L could inhibit cellular translation elsewhere in the cytoplasm. This scenario would shift the translational machinery toward the synthesis of viral proteins. A similar hypothesis was put forth by Francois and colleagues to explain how hepatitis C virus translation occurs in the presence of activated PKR and 2′,5′-oligoadenylate synthetases (12).
In summary, the data presented in this report indicate that reovirus-induced host shutoff is more complex than initially anticipated. As hypothesized, the IFN-regulated dsRNA-activated kinase PKR is involved in reovirus-induced host shutoff, but our results indicate that it may function through pathways in addition to that which leads to eIF2α phosphorylation. Other evidence reveals that RNase L contributes to the inhibition of cellular translation that is characteristic of most reovirus infections. The abilities of several reovirus strains to replicate more efficiently in the presence rather than in the absence of PKR and RNase L clearly indicate that these two molecules do not exert an antiviral effect against reovirus. Rather, our results suggest that the same cellular molecules that contribute to reovirus-induced host shutoff facilitate replication through as-yet-uncharacterized mechanisms.
Acknowledgments
We thank Donalyn Scheuner and Randall Kaufman for wt eIF2α MEFs and S51A eIF2α MEFs. We are grateful to Jayashree Parahjape for assistance in determining 18S rRNA cleavage in reovirus-infected cells. We thank Stephen Rice, Susanna Winston, and Joseph Golden for critical reviews of preliminary versions of the manuscript.
This work was supported by NIH grants CA44059 to R.H.S, AI34039 to B.R.G.W., and AI45990 to L.A.S.
REFERENCES
- 1.Baglioni, C., A. De Benedetti, and G. J. Williams. 1984. Cleavage of nascent reovirus mRNA by localized activation of the 2′-5′-oligoadenylate-dependent endoribonuclease. J. Virol. 52:865-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balachandran, S., P. C. Roberts, L. E. Brown, H. Truong, A. K. Pattnaik, D. R. Archer, and G. N. Barber. 2000. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13:129-141. [DOI] [PubMed] [Google Scholar]
- 3.Beattie, E., K. L. Denzler, J. Tartaglia, M. E. Perkus, E. Paoletti, and B. L. Jacobs. 1994. Reversal of the interferon-sensitive phenotype of a vaccinia virus lacking E3L by expression of the reovirus S4 gene. J. Virol. 69:499-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brendler, T., T. Godefroy-Colburn, S. Yu, and R. E. Thach. 1981. The role of mRNA competition in regulating translation. III. Comparison of in vitro and in vivo results. J. Biol. Chem. 256:11755-11761. [PubMed] [Google Scholar]
- 5.Clemens, M. J. 1996. Protein kinases that phosphorylate eIF2 and eIF2B, and their role in eukaryotic cell translational control, p. 139-172. In J. Hershey, M. Mathews, and N. Sonenberg (ed.), Translational control. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
- 6.Deb, A., M. Zamanian-Daryoush, Z. Xu, S. Kadereit, and B. R. Williams. 2001. Protein kinase PKR is required for platelet-derived growth factor signaling of c-fos gene expression via Erks and Stat3. EMBO J. 20:2487-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Benedetti, A., G. J. Williams, L. Comeau, and C. Baglioni. 1985. Inhibition of viral mRNA translation in interferon-treated L cells infected with reovirus. J. Virol. 55:588-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denzler, K. L., and B. L. Jacobs. 1994. Site-directed mutagenic analysis of reovirus sigma 3 protein binding to dsRNA. Virology 204:190-199. [DOI] [PubMed] [Google Scholar]
- 9.Duncan, R. F. 1990. Protein synthesis initiation factor modifications during viral infections: implications for translational control. Electrophoresis 11:219-227. [DOI] [PubMed] [Google Scholar]
- 10.Etchison, D., S. C. Milburn, I. Edery, N. Sonenberg, and J. W. Hershey. 1982. Inhibition of HeLa cell protein synthesis following poliovirus infection correlates with the proteolysis of a 220,000-dalton polypeptide associated with eucaryotic initiation factor 3 and a cap binding protein complex. J. Biol. Chem. 257:14806-14810. [PubMed] [Google Scholar]
- 11.Feduchi, E., M. Esteban, and L. Carrasco. 1988. Reovirus type 3 synthesizes proteins in interferon-treated HeLa cells without reversing the antiviral state. Virology 164:420-426. [DOI] [PubMed] [Google Scholar]
- 12.Francois, C., G. Duverlie, D. Rebouillat, H. Khorsi, S. Castelain, H. E. Blum, A. Gatignol, C. Wychowski, D. Moradpour, and E. F. Meurs. 2000. Expression of hepatitis C virus proteins interferes with the antiviral action of interferon independently of PKR-mediated control of protein synthesis. J. Virol. 74:5587-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furlong, D. B., M. L. Nibert, and B. N. Fields. 1988. Sigma 1 protein of mammalian reoviruses extends from the surfaces of viral particles. J. Virol. 62:246-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gale, M., Jr., S. L. Tan, and M. G. Katze. 2000. Translational control of viral gene expression in eukaryotes. Microbiol. Mol. Biol. Rev. 64:239-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gingras, A. C., B. Raught, and N. Sonenberg. 1999. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 68:913-963. [DOI] [PubMed] [Google Scholar]
- 16.Golden, J. W., J. Linke, S. Schmechel, K. Thoemke, and L. A. Schiff. 2002. Addition of exogenous protease facilitates reovirus infection in many restrictive cells. J. Virol. 76:7430-7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gradi, A., Y. V. Svitkin, H. Imataka, and N. Sonenberg. 1998. Proteolysis of human eukaryotic translation initiation factor eIF4GII, but not eIF4GI, coincides with the shutoff of host protein synthesis after poliovirus infection. Proc. Natl. Acad. Sci. USA 95:11089-11094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta, S. L., S. L. Holmes, and L. L. Mehra. 1982. Interferon action against reovirus: activation of interferon-induced protein kinase in mouse L929 cells upon reovirus infection. Virology 120:495-499. [DOI] [PubMed] [Google Scholar]
- 19.Huismans, H., and W. K. Joklik. 1976. Reovirus-coded polypeptides in infected cells: isolation of two native monomeric polypeptides with affinity for single-stranded and double-stranded RNA, respectively. Virology 70:411-424. [DOI] [PubMed] [Google Scholar]
- 20.Imani, F., and B. L. Jacobs. 1988. Inhibitory activity for the interferon-induced protein kinase is associated with the reovirus serotype 1 sigma 3 protein. Proc. Natl. Acad. Sci. USA 85:7887-7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kedl, R., S. Schmechel, and L. Schiff. 1995. Comparative sequence analysis of the reovirus S4 genes from 13 serotype 1 and serotype 3 field isolates. J. Virol. 69:552-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khabar, K. S., M. Dhalla, Y. Siddiqui, A. Zhou, M. N. Al-Ahdal, S. D. Der, R. H. Silverman, and B. R. Williams. 2000. Effect of deficiency of the double-stranded RNA-dependent protein kinase, PKR, on antiviral resistance in the presence or absence of ribonuclease L: HSV-1 replication is particularly sensitive to deficiency of the major IFN-mediated enzymes. J. Interferon Cytokine Res. 20:653-659. [DOI] [PubMed] [Google Scholar]
- 23.Khabar, K. S., Y. M. Siddiqui, F. al-Zoghaibi, L. al-Haj, M. Dhalla, A. Zhou, B. Dong, M. Whitmore, J. Paranjape, M. N. Al-Ahdal, F. Al-Mohanna, B. R. Williams, and R. H. Silverman. 2003. RNase L mediates transient control of the interferon response through modulation of the double-stranded RNA-dependent protein kinase PKR. J. Biol. Chem. 278:20124-20132. [DOI] [PubMed] [Google Scholar]
- 24.Li, X. L., J. A. Blackford, and B. A. Hassel. 1998. RNase L mediates the antiviral effect of interferon through a selective reduction in viral RNA during encephalomyocarditis virus infection. J. Virol. 72:2752-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li, X. L., J. A. Blackford, C. S. Judge, M. Liu, W. Xiao, D. V. Kalvakolanu, and B. A. Hassel. 2000. RNase-L-dependent destabilization of interferon-induced mRNAs. A role for the 2-5A system in attenuation of the interferon response. J. Biol. Chem. 275:8880-8888. [DOI] [PubMed] [Google Scholar]
- 26.Lloyd, R. M., and A. J. Shatkin. 1992. Translational stimulation by reovirus polypeptide sigma 3: substitution for VAI RNA and inhibition of phosphorylation of the alpha subunit of eukaryotic initiation factor 2. J. Virol. 66:6878-6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maitra, R. K., and R. H. Silverman. 1998. Regulation of human immunodeficiency virus replication by 2′,5′-oligoadenylate-dependent RNase L. J. Virol. 72:1146-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mallo, M., J. Martinez-Costas, and J. Benavente. 1991. The stimulatory effect of actinomycin D on avian reovirus replication in L cells suggests that translational competition dictates the fate of the infection. J. Virol. 65:5506-5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nibert, M. L., and B. N. Fields. 1992. A carboxy-terminal fragment of protein mu 1/mu 1C is present in infectious subvirion particles of mammalian reoviruses and is proposed to have a role in penetration. J. Virol. 66:6408-6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nilsen, T. W., P. A. Maroney, and C. Baglioni. 1982. Inhibition of protein synthesis in reovirus-infected HeLa cells with elevated levels of interferon-induced protein kinase activity. J. Biol. Chem. 257:14593-14596. [PubMed] [Google Scholar]
- 31.Nilsen, T. W., P. A. Maroney, and C. Baglioni. 1982. Synthesis of (2′-5′)oligoadenylate and activation of an endoribonuclease in interferon-treated HeLa cells infected with reovirus. J. Virol. 42:1039-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohlmann, T., M. Rau, V. M. Pain, and S. J. Morley. 1996. The C-terminal domain of eukaryotic protein synthesis initiation factor (eIF) 4G is sufficient to support cap-independent translation in the absence of eIF4E. EMBO J. 15:1371-1382. [PMC free article] [PubMed] [Google Scholar]
- 33.Piron, M., P. Vende, J. Cohen, and D. Poncet. 1998. Rotavirus RNA-binding protein NSP3 interacts with eIF4GI and evicts the poly(A) binding protein from eIF4F. EMBO J. 17:5811-5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poncet, D., C. Aponte, and J. Cohen. 1993. Rotavirus protein NSP3 (NS34) is bound to the 3′-end consensus sequence of viral mRNAs in infected cells. J. Virol. 67:3159-3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ray, B. K., T. G. Brendler, S. Adya, S. Daniels-McQueen, J. K. Miller, J. W. Hershey, J. A. Grifo, W. C. Merrick, and R. E. Thach. 1983. Role of mRNA competition in regulating translation: further characterization of mRNA discriminatory initiation factors. Proc. Natl. Acad. Sci. USA 80:663-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosen, L., J. F. Hovis, F. M. Mastrota, J. A. Bell, and R. J. Huebner. 1960. Observations on a newly recognized virus (Abney) of the reovirus family. Am. J. Hyg. 71:258-265. [DOI] [PubMed] [Google Scholar]
- 37.Samuel, C. E., R. Duncan, G. S. Knutson, and J. W. Hershey. 1984. Mechanism of interferon action. Increased phosphorylation of protein synthesis initiation factor eIF-2 alpha in interferon-treated, reovirus-infected mouse L929 fibroblasts in vitro and in vivo. J. Biol. Chem. 259:13451-13457. [PubMed] [Google Scholar]
- 38.Scheuner, D., B. Song, E. McEwen, C. Liu, R. Laybutt, P. Gillespie, T. Saunders, S. Bonner-Weir, and R. J. Kaufman. 2001. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell 7:1165-1176. [DOI] [PubMed] [Google Scholar]
- 39.Schiff, L. A., M. L. Nibert, M. S. Co, E. G. Brown, and B. N. Fields. 1988. Distinct binding sites for zinc and double-stranded RNA in the reovirus outer capsid protein sigma 3. Mol. Cell. Biol. 8:273-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmechel, S., M. Chute, P. Skinner, R. Anderson, and L. Schiff. 1997. Preferential translation of reovirus mRNA by a sigma3-dependent mechanism. Virology 232:62-73. [DOI] [PubMed] [Google Scholar]
- 41.Sharpe, A. H., and B. N. Fields. 1982. Reovirus inhibition of cellular RNA and protein synthesis: role of the S4 gene. Virology 122:381-391. [DOI] [PubMed] [Google Scholar]
- 42.Silverman, R. H. 1997. 2-5A-Dependent RNase L: a regulated endoribonuclease in the interferon system, p. 515-551. In G. D'Alessio and J. Riordan (ed.), Ribonucleases: structure and function. Academic Press, Inc., San Diego, Calif.
- 43.Skup, D., and S. Millward. 1980. mRNA capping enzymes are masked in reovirus progeny subviral particles. J. Virol. 34:490-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strong, J. E., M. C. Coffey, D. Tang, P. Sabinin, and P. W. Lee. 1998. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 17:3351-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vende, P., M. Piron, N. Castagne, and D. Poncet. 2000. Efficient translation of rotavirus mRNA requires simultaneous interaction of NSP3 with the eukaryotic translation initiation factor eIF4G and the mRNA 3′ end. J. Virol. 74:7064-7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Virgin, H. W. T., R. Bassel-Duby, B. N. Fields, and K. L. Tyler. 1988. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing). J. Virol. 62:4594-4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walden, W. E., T. Godefroy-Colburn, and R. E. Thach. 1981. The role of mRNA competition in regulating translation. I. Demonstration of competition in vivo. J. Biol. Chem. 256:11739-11746. [PubMed] [Google Scholar]
- 48.Williams, B. R. 2001. Signal integration via PKR. Sci. STKE 2001:RE2. [DOI] [PubMed] [Google Scholar]
- 49.Xu, Z., and B. R. Williams. 2000. The B56alpha regulatory subunit of protein phosphatase 2A is a target for regulation by double-stranded RNA-dependent protein kinase PKR. Mol. Cell. Biol. 20:5285-5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang, Y. L., L. F. Reis, J. Pavlovic, A. Aguzzi, R. Schafer, A. Kumar, B. R. Williams, M. Aguet, and C. Weissmann. 1995. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 14:6095-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng, X., R. H. Silverman, A. Zhou, T. Goto, B. S. Kwon, H. E. Kaufman, and J. M. Hill. 2001. Increased severity of HSV-1 keratitis and mortality in mice lacking the 2-5A-dependent RNase L gene. Investig. Ophthalmol. Vis. Sci. 42:120-126. [PubMed] [Google Scholar]
- 52.Zhou, A., J. Paranjape, T. L. Brown, H. Nie, S. Naik, B. Dong, A. Chang, B. Trapp, R. Fairchild, C. Colmenares, and R. H. Silverman. 1997. Interferon action and apoptosis are defective in mice devoid of 2′,5′-oligoadenylate-dependent RNase L. EMBO J. 16:6355-6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou, A., J. M. Paranjape, S. D. Der, B. R. Williams, and R. H. Silverman. 1999. Interferon action in triply deficient mice reveals the existence of alternative antiviral pathways. Virology 258:435-440. [DOI] [PubMed] [Google Scholar]
- 54.Zweerink, H. J., and W. K. Joklik. 1970. Studies on the intracellular synthesis of reovirus-specified proteins. Virology 41:501-518. [DOI] [PubMed] [Google Scholar]