Significance
Genetic linkage and association studies in obsessive-compulsive disorder (OCD) implicate SLC1A1 (encoding the neuronal glutamate transporter excitatory amino acid transporter 3, EAAT3), and neuroimaging studies demonstrate abnormal basal ganglia circuit function in OCD. However, no previous studies have investigated the role of EAAT3 in these circuits or tested its impact on repetitive behavior. Using a combined genetic and pharmacological challenge approach, we have demonstrated that ablated expression of EAAT3 diminishes basal ganglia-mediated repetitive behavior in mice. Targeted rescue of midbrain expression points to an impact of EAAT3 on dopaminergic neuron function, suggesting a model for synthesizing glutamate and dopamine effects on stereotypic behavior. These findings provide evidence that EAAT3 impacts basal ganglia-dependent repetitive behavior and suggest a potential target for drug development.
Keywords: obsessive-compulsive disorder, Tourette, basal ganglia, dopamine, EAAC1
Abstract
Obsessive-compulsive disorder (OCD) is a chronic, disabling condition with inadequate treatment options that leave most patients with substantial residual symptoms. Structural, neurochemical, and behavioral findings point to a significant role for basal ganglia circuits and for the glutamate system in OCD. Genetic linkage and association studies in OCD point to SLC1A1, which encodes the neuronal glutamate/aspartate/cysteine transporter excitatory amino acid transporter 3 (EAAT3)/excitatory amino acid transporter 1 (EAAC1). However, no previous studies have investigated EAAT3 in basal ganglia circuits or in relation to OCD-related behavior. Here, we report a model of Slc1a1 loss based on an excisable STOP cassette that yields successful ablation of EAAT3 expression and function. Using amphetamine as a probe, we found that EAAT3 loss prevents expected increases in (i) locomotor activity, (ii) stereotypy, and (iii) immediate early gene induction in the dorsal striatum following amphetamine administration. Further, Slc1a1-STOP mice showed diminished grooming in an SKF-38393 challenge experiment, a pharmacologic model of OCD-like grooming behavior. This reduced grooming is accompanied by reduced dopamine D1 receptor binding in the dorsal striatum of Slc1a1-STOP mice. Slc1a1-STOP mice also exhibit reduced extracellular dopamine concentrations in the dorsal striatum both at baseline and following amphetamine challenge. Viral-mediated restoration of Slc1a1/EAAT3 expression in the midbrain but not in the striatum results in partial rescue of amphetamine-induced locomotion and stereotypy in Slc1a1-STOP mice, consistent with an impact of EAAT3 loss on presynaptic dopaminergic function. Collectively, these findings indicate that the most consistently associated OCD candidate gene impacts basal ganglia-dependent repetitive behaviors.
Obsessive-compulsive disorder (OCD) is a common neuropsychiatric condition that ranks among the top 10 causes of disability worldwide (1, 2). Primary forms of therapy include serotonin-reuptake inhibitors (SRIs) and cognitive behavioral therapy; however, only 50–60% of patients exhibit adequate response to current treatment approaches, and most patients have clinically significant residual symptoms (1, 3). Other agents, including antipsychotic medications and glutamatergic agents, have been investigated to augment SRIs but have shown limited evidence of efficacy to date (4, 5). Surgical intervention with deep-brain stimulation in the ventral capsule/ventral striatum or subthalamic nucleus shows promise but is reserved for the most severely ill patients (6, 7). New treatments based on greater understanding of pathophysiology are therefore needed.
Multiple lines of evidence indicate that the basal ganglia are critically affected in OCD. Structural neuroimaging studies demonstrate altered caudate volume in OCD (8, 9), and functional imaging has identified hyperactivity in cortico-striatal circuits, both at baseline and with symptom provocation (10). Interestingly, some reports using magnetic resonance spectroscopy describe elevated striatal glutamatergic signal in the caudate (11, 12), suggesting increased intracellular glutamate and/or GABA. Recent work in genetic and optogenetic mouse models of SRI-sensitive compulsive-like grooming behavior have also implicated cortico-striatal signaling, suggesting the relevance of dysfunctional basal ganglia signaling to repetitive behavior across species (13–16).
Family studies support a significant role for genetics in OCD, with increased heritability in early-onset OCD (17). Suggestive linkage to the chromosome region 9p24, which contains SLC1A1 in addition to other genes, was initially established in a genome-wide linkage scan of OCD and then was independently replicated (18, 19). Analysis of SLC1A1, which codes for the neuronal glutamate transporter EAAT3 (excitatory amino acid transporter 3), identified significant association in the 3′ region, with stronger evidence in males (20–22). Some, but not all, subsequent studies also identified an association with polymorphisms in the 3′ gene region, with the greatest evidence for association with the rs301430C allele (21–26), which is linked to elevated SLC1A1 expression in lymphoblastoid cells, human postmortem brain, and a luciferase reporter assay (25). Taken together, these data suggest that OCD susceptibility may result from elevated SLC1A1 expression and that decreasing EAAT3 activity therefore could be a therapeutic target. Association findings, gene-expression differences, and deletions of SLC1A1 have also been reported in schizophrenia and bipolar disorder (27–29), indicating a potential role for EAAT3 in a broader array of neuropsychiatric disorders.
SLC1A1 mRNA and EAAT3 protein are strongly expressed in the cortex and the striatum and in mesolimbic and nigrostriatal dopaminergic neurons (30–33). EAAT3 localizes to peri- and postsynaptic regions (32), where it serves three apparent functions: (i) buffering glutamate concentrations around peri/extrasynaptic NMDA and metabotropic glutamate receptors (34); (ii) taking up glutamate as an intracellular precursor for GABA synthesis (35); and (iii) taking up cysteine for glutathione synthesis and protection from oxidative stress (36, 37).
One logical place for SLC1A1/EAAT3 to impact cortico-striatal signaling is in the GABAergic medium spiny neurons (MSNs) of the striatum, which receive glutamatergic inputs from the cortex and provide output to the thalamus via the direct and indirect pathways of the basal ganglia. EAAT3 activity limits NR2B-containing NMDA receptor-dependent signaling at hippocampal glutamatergic synapses (38) and could similarly impact postsynaptic signaling in striatal MSNs. In addition, recent reports suggest that EAAT3 is expressed in dopaminergic neurons projecting from the ventral tegmental area (VTA) and the substantia nigra (SN) to the ventral and dorsal striatum, respectively (30). It therefore is possible that the subpopulation of EAAT3 in midbrain dopaminergic neurons could impact OCD-implicated basal ganglia circuitry via neuromodulation. This possibility is especially relevant in light of recent evidence that amphetamine elicits endocytosis of EAAT3 and causes elevated signaling at glutamate receptors in dopaminergic neurons (30, 39).
Despite its genetic association with OCD, no studies have addressed the functional impact of Slc1a1/EAAT3 on OCD-relevant brain circuits or on OCD-like behaviors. To explore these questions, we used a flexible knockin approach (40) to generate mice with constitutively reduced Slc1a1/EAAT3 expression (Slc1a1-STOP mice, hereafter “ST mice”). We hypothesized that these animals would show decreased liability to repetitive behaviors, based on the implication of increased SLC1A1 expression in OCD risk. Beyond assessing spontaneous repetitive behaviors, which occur at low baseline frequency, we examined sensitivity to pharmacologically induced compulsive-like behaviors using amphetamine (which causes dopamine efflux and increased synaptic dopamine levels) and the dopamine D1 receptor agonist SKF-38393. Our flexible knockin approach also permitted targeted excision of the STOP cassette, allowing us to localize the impact of EAAT3 loss on repetitive behaviors.
Results
Slc1a1/EAAT3 Expression and Transporter Function Are Reduced in ST Mice.
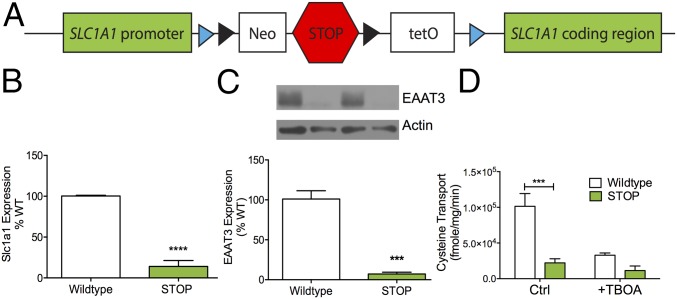
To investigate the functional impact of Slc1a1/EAAT3 expression in OCD-relevant behavioral assays, we used the flexible accelerated STOP tetracycline (FAST) operator knockin system (40) to create a knockin mouse with globally reduced Slc1a1/EAAT3 expression (Fig. 1A). As expected, Slc1a1 mRNA was reduced in ST mice relative to WT littermate controls (Fig. 1B) via quantitative RT-PCR (qRT-PCR) of the dorsal striatum (unpaired t test; P < 0.0001, n = 5 per genotype). Immunoblots of whole-striatum synaptosomes also demonstrated ablated EAAT3 protein expression in ST mice compared with WT littermate controls (P = 0.0001, n = 6 per genotype) (Fig. 1C).
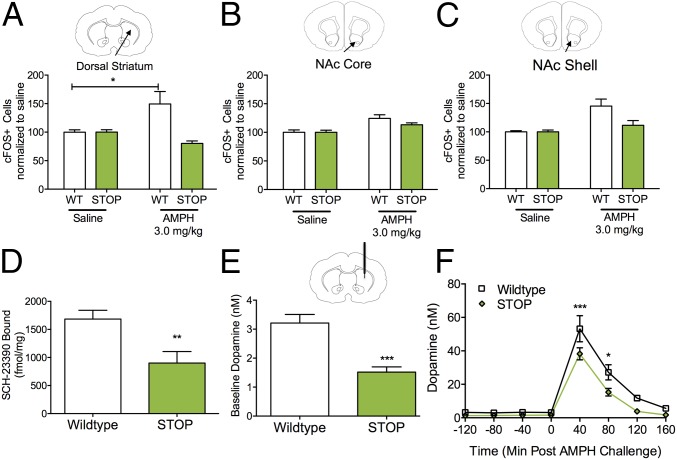
Fig. 1.
ST mice have reduced Slc1a1/EAAT3 protein expression and function. (A) Schematic of the Slc1a1-STOP construct. Black triangles represent flippase recognition target sequences, and blue triangles represent LoxP sites. Neo, PGK-EM7-NEO minigene; STOP, Stop signal; tetO, tetracycline operon. (B) ST mice have reduced Slc1a1 mRNA expression as measured by qRT-PCR of dorsal striatum (unpaired t test; t = 11.81, ****P < 0.0001, n = 5 per genotype). (C) EAAT3 protein expression is reduced in striatal synaptosome preparations from ST mice (lanes 2 and 4) relative to WT mice (lanes 1 and 3) (unpaired t test; t = 8.84, ***P = 0.0001, n = 6 per genotype). The figure is representative of three separate experiments. Average protein expression is demonstrated in the bar graph. (D) Na+-dependent uptake of l-cysteine (50 μM) is abolished in striatal synaptosome preparations from ST mice. The nonselective EAAT inhibitor threo-β-benzyloxyaspartate (TBOA) (100 μM) reduced WT synaptosome uptake but did not affect uptake from ST synaptosomes [two-way ANOVA; inhibitor × genotype F(1,20) = 8.361, P = 0.009; inhibitor F(1,20) = 15.7, P = 0.0008; genotype F(1,20) = 25.34, P < 0.0001, n = 6 per genotype; post hoc Sidak’s multiple comparison test, ***P < 0.05]. The figure is representative of three separate experiments.
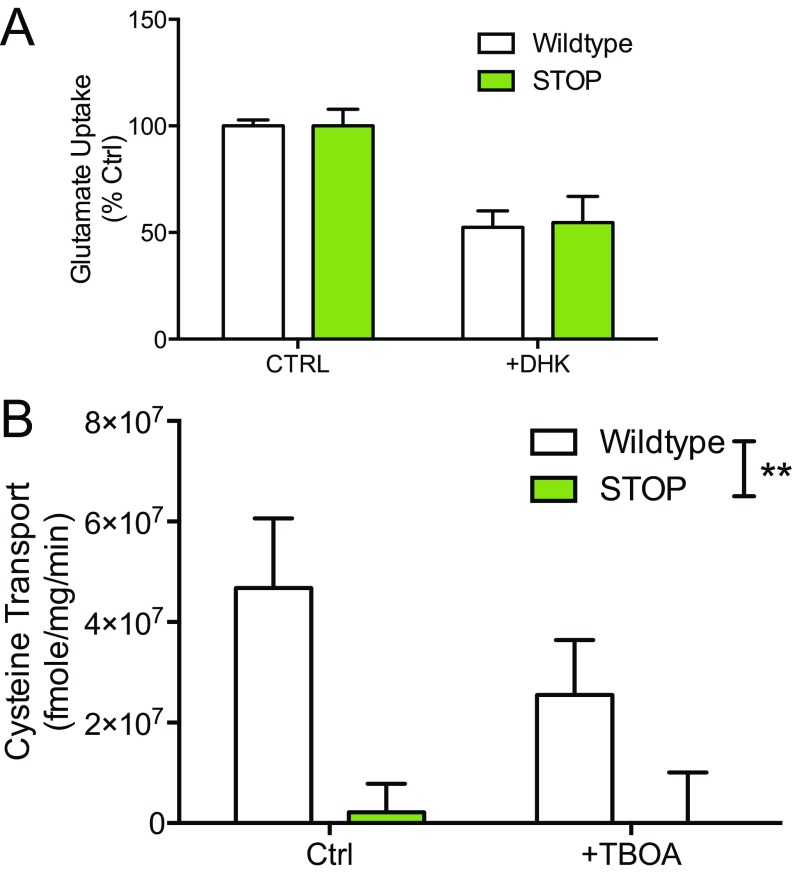
We next probed the functional consequences of reduced EAAT3 expression using striatal synaptosome transport assays (32). Because EAAT3 is the primary source for neuronal cysteine (37), [35S]cysteine was used as the substrate for EAAT3 synaptosome uptake. Na+-dependent uptake of cysteine in synaptosomes prepared from ST mice was ablated relative to WT synaptosomes [two-way ANOVA; inhibitor × genotype F(1,20) = 8.361, P = 0.009; inhibitor F(1,20) = 15.7, P = 0.0008; genotype F(1,20) = 25.34, P < 0.0001, n = 6 per genotype; post hoc Sidak’s multiple comparison test, P < 0.05] (Fig. 1D). As expected from previous reports (31), we were unable to detect a difference in Na+-dependent glutamate uptake in striatal synaptosomes from ST mice relative to WT littermate controls in either the presence or absence of the EAAT inhibitor dihydrokainic acid (DHK) (Fig. S1A).
Fig. S1.
There were no differences in glutamate uptake between WT and ST mice; high-concentration cysteine reveals abolished transporter function in ST mice (related to Fig. 1). (A) Na+-dependent uptake of l-glutamate (100 μM) is not altered in synaptosome preparations from ST mice [two-way ANOVA; genotype F(1,8) = 0.02, P = 0.9, n = 3]. EAAT inhibitor: DHK (100 μM). The figure is representative of three separate experiments. (B) Na+-dependent uptake of l-cysteine (200 μM) is absent in striatal synaptosome preparations from ST mice [two-way ANOVA; genotype F(1,20) = 10.89, P = 0.0036, n = 6 per genotype]. The figure is representative of three separate experiments.
ST Mice Show No Changes in Spontaneous Behavior.
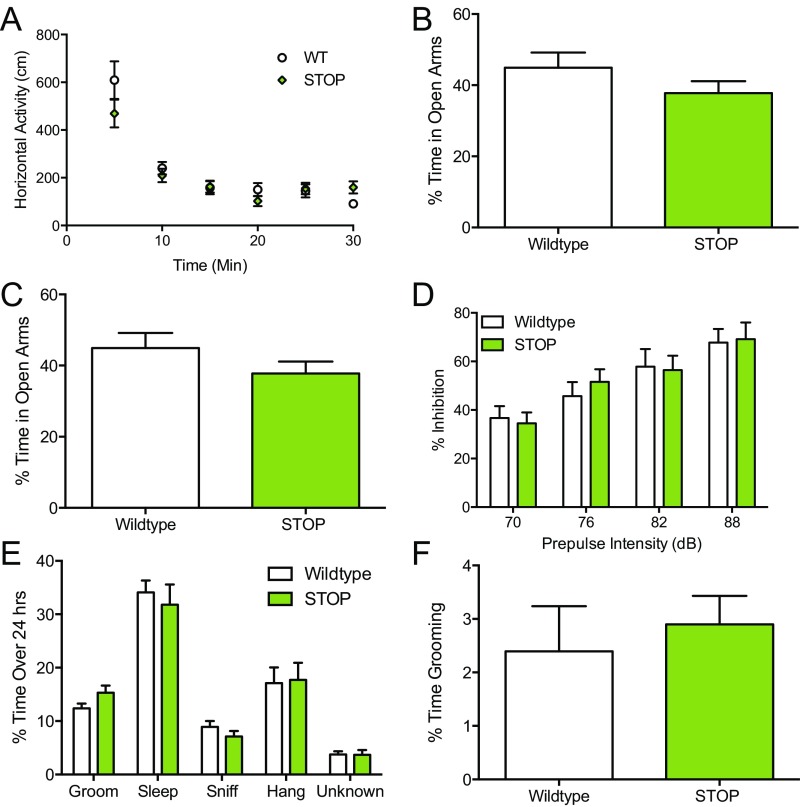
ST mice and littermate controls were subjected to a battery of behavioral tasks to determine if baseline behavioral differences were present. No anxiety-like phenotypes, compulsive-like phenotypes, or deficits in sensorimotor gating (41) were observed in ST mice relative to WT littermate controls as measured by changes in open-field activity, time spent in the open arms of the elevated zero maze, light–dark emergence, prepulse inhibition, or spontaneous grooming (Fig. S2).
Fig. S2.
ST mice show no changes in spontaneous behavior (related to Fig. 2). ST (STOP) mice display no behavioral abnormalities in assays relevant to anxiety or OCD-like behavior. (A) Spontaneous locomotor behavior [two-way ANOVA; genotype F(1,53) = 0.4378; P = 0.44, n = 14 per genotype]. (B) Elevated zero maze (unpaired t test; t = 1.3, P = 0.20, n = 14 per genotype). (C) Light–dark emergence (unpaired t test; t = 0.5, P = 0.63, n = 14 per genotype). (D) Prepulse inhibition [two-way ANOVA; genotype F(1,91) = 0.05; P = 0.83, n = 14 per genotype]. (E) Home cage scan (unpaired t test; grooming P = 0.08, P > 0.05 for all other behaviors analyzed separately, n = 14 per genotype). (F) Spontaneous grooming (unpaired t test; t = 0.5, P = 0.62, n = 14 per genotype).
Pharmacological Probing of Basal Ganglia Circuitry Reveals Reductions in Basal Ganglia-Dependent Repetitive Behavior in ST Mice.
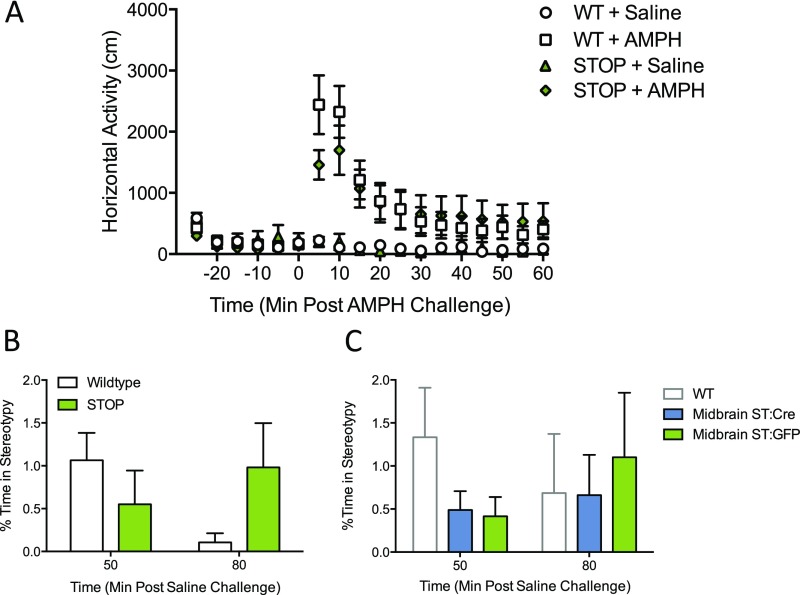
To induce basal ganglia-mediated locomotor and repetitive behaviors, d-amphetamine was administered acutely in ST mice and WT littermate controls. At a low dose (1.8 mg/kg), amphetamine-induced locomotion was significantly attenuated in ST mice relative to controls [curve–fit analysis; F(4,496) = 6.89, P < 0.0001] (Fig. 2A). A moderate dose (3.0 mg/kg) further accentuated this difference [curve–fit analysis; F(4,496) = 13.32, P < 0.0001] (Fig. 2B). At the highest amphetamine dose tested (8.0 mg/kg), at which stereotypic behavior dominates over locomotion (42), there were no differences in overall locomotor behavior (Fig. S3A). However, when a separate cohort of animals was tested at this 8.0-mg/kg dose, blinded video scoring of stereotypic behavior at 50 and 80 min postamphetamine revealed a main effect of genotype in ST and WT littermate controls [two-way ANOVA; genotype F(1,42) =12.09, P = 0.0012, n = 12 per genotype] (Fig. 2C and Movie S1). Mice did not exhibit stereotypic behavior following saline challenge (Fig. S3B).
Fig. 2.
ST mice have attenuated locomotor response to low- and moderate-dose amphetamine challenge. (A and B) Following acute d-amphetamine challenge (1.8 and 3.0 mg/kg), WT and ST mice demonstrate locomotor hyperactivity, which was attenuated in ST mice at both 1.8 mg/kg (A) [curve–fit analysis, t = 0–60; F(4,496) = 6.891, ****P < 0.0001, n = 14 per genotype] and 3.0 mg/kg (B) [curve–fit analysis, t = 0–60; F(4,496) = 13.32, ****P < 0.0001, n = 14 per genotype]. (C) A main effect of genotype is observed on stereotypy following high-dose amphetamine challenge (8.0 mg/kg) in ST mice and WT littermate controls. Stereotypic behavior was scored by trained, blinded, independent observers at 50 and 80 min post challenge [two-way ANOVA; genotype F(1,42) = 12.09, **P = 0.001, n = 12 per genotype]. (D) SKF-38393 challenge reveals a main effect of drug and genotype for grooming behavior following agonist challenge in ST mice and WT controls. Grooming behavior was scored by trained, blind, independent observers [two-way repeated-measures ANOVA; drug × genotype, F(1,26) = 0.74, P = 0.40; drug, F(1,26) = 28.5, P < 0.0001; genotype, F(1,26) = 7.95, **P = 0.0091; n = 14 per genotype].
Fig. S3.
High-dose amphetamine locomotor response is not altered in ST mice relative to WT mice, and stereotypic behavior is not observed following saline administration (related to Fig. 2). Following acute d-amphetamine challenge (8.0 mg/kg), WT and ST mice initially exhibited locomotor activation and then transitioned to stationary stereotypy. (A) The 8.0 mg/kg d-amphetamine (i.p.) [curve–fit analysis; F(4,496) = 1.17, P = 0.32, n = 14 per genotype]. Saline-treated mice do not exhibit phenotypic shuffling or sniffing stereotypy behavior. All mice in B and C were vehicle treated (i.e., received only saline). (B) Saline-induced stereotypy [two-way ANOVA: time × genotype F(1,44) = 0.75, P = 0.39; time F(1,44) = 0.41, P = 0.52, genotype F(1,44) = 0.42, P = 0.42; n = 12 per genotype]. (C) Saline-induced stereotypy [two-way ANOVA; time × virus F(1,50) = 0.32, P = 0.58; time F(1,50) = 0.89, P = 0.35; virus F(1,50) = 0.69, n = 12 ST:GFP, 15 ST:Cre].
To examine the impact of ablated EAAT3 expression independent of presynaptic dopamine release triggered by amphetamine administration, we acutely challenged ST mice with the dopamine D1 receptor (D1) agonist SKF-38393 (10 mg/kg, i.p.) to induce perseverative grooming (43, 44). Via two-way ANOVA, a main effect of genotype was identified following SKF-38393 challenge in ST mice and controls (two-way repeated-measures ANOVA; drug, F(1,26) = 28.5, P < 0.0001; genotype, F(1,26) = 7.95, P = 0.0091, n = 14 per genotype) (Fig. 2D).
Amphetamine-Dependent cFos+ Induction Is Decreased in the Dorsal Striatum of ST Mice.
Amphetamine-induced locomotion and stereotypy are dependent on discrete subregions of the striatum (45, 46). We therefore quantified cFos immunoreactivity in the dorsal striatum and the nucleus accumbens (NAc) core and shell in response to amphetamine (3.0 mg/kg). In the dorsal striatum, a main effect of genotype was observed in cFos+ cells, with an amphetamine-induced increase in cFos+ cells in WT littermate controls that was absent in ST mice [two-way ANOVA; drug × genotype; F(1,17) = 9.91, P = 0.006; genotype; F(1,17) = 9.91, P = 0.006; n = 5 or 6 per genotype; post hoc Sidak’s multiple comparison test, P < 0.05] (Fig. 3A and Fig. S4A). A similar but less robust main effect of amphetamine was observed in the NAc core [two-way ANOVA; drug × genotype; F(1,12) = 1.55, P = 0.24; drug; F(1,12) = 17.52, P = 0.001; n = 4 per genotype] (Fig. 3B and Fig. S4B) and shell [two-way ANOVA; drug × genotype F(1,12) = 4.74, P = 0.05; drug F(1,12) = 13.59, P < 0.003; n = 4 per genotype] in WT and ST mice (Fig. 3C and Fig. S4C).
Fig. 3.
EAAT3 loss affects amphetamine-induced cFos expression, dopamine receptor membrane density, and extracellular dopamine concentrations in the dorsal striatum. (A–C) Quantification of cFos+ cells was performed in the dorsal striatum (A), NAc core (B), and NAc shell (C). (A) Staining for cFos+ cells reveals an amphetamine (3.0 mg/kg, i.p.)-dependent increase in the dorsal striatum of WT mice that is absent in ST mice (two-way ANOVA; drug × genotype; F(1,17) = 9.91, P = 0.006; genotype; F(1,17) = 9.91, P = 0.006; n = 5 or 6 per genotype; post hoc Sidak’s multiple comparison test, *P < 0.05]. (B) Staining for cFos+ cells reveals a main effect of amphetamine on cFos+ cells in the NAc core of WT and ST mice (two-way ANOVA; drug, F(1,12) = 17.52, P < 0.01; n = 4 per genotype]. (C) Staining for cFos+ cells reveals a main effect of amphetamine on cFos+ cells in the NAc shell of WT and ST mice and a trend-level interaction and genotype effect [two-way ANOVA; drug × genotype and genotype F(1,12) = 4.74, P = 0.05; drug F(1,12) = 13.59, P < 0.01; n = 4 per genotype]. (D) Dopamine D1 receptor density estimated with [3H]-SCH-23390 binding in dorsal striatum membrane preparations (unpaired t test; t = 3.1, **P = 0.008, n = 8 WT, n = 6 STOP). (E) ST mice have significantly lowered dopamine levels at baseline as measured by the extrapolation of linear regression using no-net-flux microdialysis (unpaired t test, t = 4.89, ***P = 0.0006, n = 6 per genotype). (F) Dorsal striatal dopamine levels are significantly reduced in ST mice relative to WT controls following systemic administration of amphetamine (3 mg/kg, i.p.) [two-way repeated-measures ANOVA; time × genotype, F(7,70) = 2.52, P = 0.0226; genotype F(1,10) = 9.34, P = 0.01; Sidak’s multiple comparison; ***P < 0.001,*P < 0.05; n = 6 per genotype].
Fig. S4.
Representative coronal sections of dorsal striatum, NAc, and somatosensory cortex for cFos+ quantification after amphetamine challenge (related to Fig. 3). Low- and high-magnification images are in the left and right columns, respectively. WT and ST mice were challenged with saline or amphetamine and then were subjected to cFos+ immunohistochemistry. The region of quantification is indicated by a black box in the low-magnification images. (Scale bars: 250 μM and 15 μM on low- and high-magnification images, respectively.) (A) Dorsal striatum (AP +0.9 mm, ML ±1.5 mm, DV −3 mm). (B) NAc core (AP +1.2 mm, ML ±0.75 mm, DV −4.5 mm). (C) NAc shell (AP +1.2 mm, ML ±0.5 mm, DV −4.5 mm). (D) Analysis of cFos+ staining in the somatosensory cortex (AP +0.9 mm, ML ±3.0 mm, DV −3.0 mm) was performed as a negative control for both amphetamine and genotype. Analysis reveals no main effects on cFos+ cells in the somatosensory cortex of WT and ST mice [two-way ANOVA; drug × genotype F(1,12) = 0.36, P = 0.56; drug F(1,12) = 0.13, P = 0.72; genotype F(1,12) = 0.36, P = 0.56; n = 4 per genotype].
Dopamine Receptor Density Is Reduced in Striatal Membranes of ST Mice.
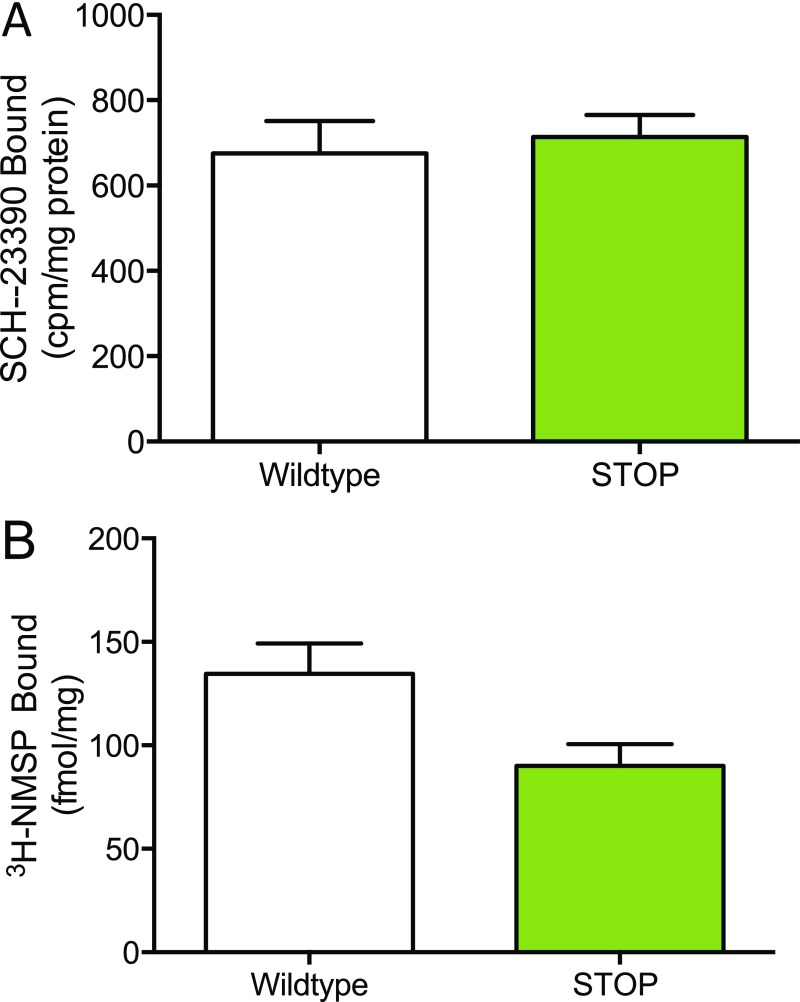
To assess whether the blunted dopamine agonist response in ST mice could be explained by changes in striatal dopamine receptor density, we performed binding experiments in striatal membrane preparations from both dorsal and ventral striatum using a D1 antagonist, [3H]-SCH-23390. D1 binding was decreased in membranes isolated from the dorsal striatum of ST mice relative to WT littermate controls (unpaired t test; P = 0.008, n = 6 WT mice, n = 8 ST mice) (Fig. 3D); however, binding estimates of D1 density in the ventral striatum were not affected (Fig. S5A). We also measured dorsal striatal membrane binding of the D2 receptor using a D2 antagonist, [3H]-methylspiperone (NMSP) and observed a trend toward decreased binding (unpaired t test; P = 0.058, n = 6 WT mice, n = 8 ST mice) (Fig. S5B).
Fig. S5.
D1 receptor density is not affected in the ventral striatum of ST mice, but D2 receptor density is reduced at a trend level in the dorsal striatum (related to Fig. 3). (A) Dopamine D1 receptor density estimated with [3H]-SCH-23390 binding in ventral striatum membrane preparations (unpaired t test; t = 0.39, P = 0.7, n = 9 WT, n = 7 ST). (B) Dopamine D2 receptor density estimated with NMSP binding in dorsal striatum membrane preparations (unpaired t test; t = 2.1, P = 0.06, n = 9 WT, n = 6 ST).
Decreased Striatal Dopaminergic Transmission in ST Mice.
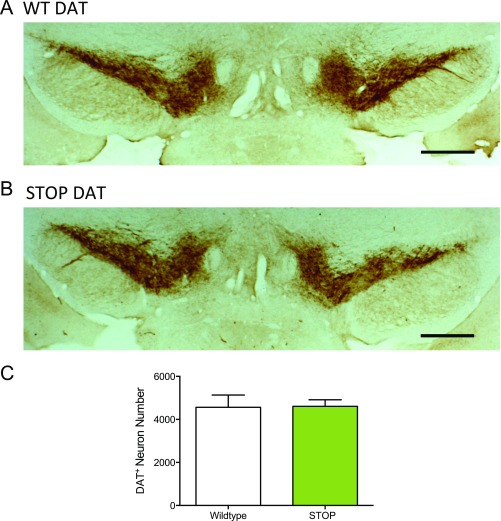
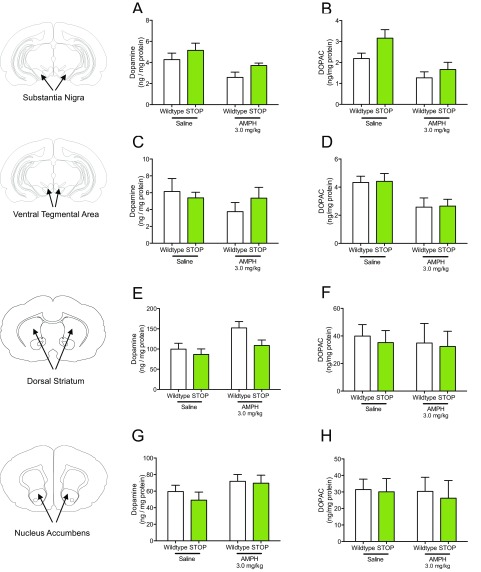
To corroborate a previous report of no change in dopamine neuron numbers or morphology in Slc1a1-null mice at 3 mo of age (47), we performed dopamine transporter (DAT) immunohistochemistry in the midbrain of 10- to 12-wk-old ST mice and littermate controls and found no differences in stereological DAT+ cell number estimates (Fig. S6). To explore presynaptic mechanisms that could account for altered response to dopamine agonists in the ST mice, we measured tissue levels of dopamine and its major metabolite, 3,4-Dihydroxyphenylacetic acid (DOPAC), 30 min after amphetamine (3.0 mg/kg i.p.) or saline injection. Via two-way ANOVA, significant drug effects were observed on SN dopamine and DOPAC, VTA DOPAC, and dorsal striatum dopamine (Fig. S7). A main effect of genotype was observed only in SN DOPAC (Fig. S7).
Fig. S6.
No differences in DAT staining were identified in the midbrain of ST mice (related to Figs. 2–4). (A and B) Representative midbrain coronal sections of DAT staining in WT (A) and ST (B) mice. AP −3.1 mm. (Scale bars: 500 μM.) (C) Stereological analysis of DAT+ staining revealed no difference in the midbrain of WT and ST mice using the optical fractionator method. Coronal midbrain sections used for analysis of DAT staining were from −2.9 to −3.4 mm relative to bregma (n = 4 WT and ST).
Fig. S7.
Effects of amphetamine on tissue levels of dopamine and DOPAC in ST mice (related to Figs. 2–4). (A) A main effect of amphetamine is observed in a two-way ANOVA of amphetamine’s effect on dopamine levels in the SN of WT and ST mice [two-way ANOVA; drug × genotype F(1,23) = 0.05, P = 0.83; drug F(1,23) = 7.95, P < 0.01; genotype F(1,23) = 3.29, P = 0.08. n = 7 per genotype for each treatment]. (B) A main effect of amphetamine and genotype is observed in a two-way ANOVA of amphetamine’s effect on DOPAC levels in the SN of WT and ST mice [two-way ANOVA; drug × genotype, F(1,23) = 0.78, P = 0.39; drug F(1,23) = 13.54, P < 0.01; genotype F(1,23) = 4.35, P < 0.05. n = 7 per genotype for each treatment]. (C) Neither amphetamine nor genotype had a significant effect on tissue levels of dopamine in the VTA [two-way ANOVA; drug × genotype F(1,23) = 0.99, P = 0.33; genotype F(1,23) = 0.1294, P = 0.72, n = 7 per genotype for each treatment]. (D) A main effect of amphetamine is observed in a two-way ANOVA of amphetamine’s effect on DOPAC levels in the VTA of WT and ST mice [two-way ANOVA; drug × genotype F(1,23) < 0.0001, P = 0.99; drug F(1,23) = 10.3, P < 0.01, n = 7 per genotype for each treatment]. (E) A main effect of amphetamine is observed in a two-way ANOVA of amphetamine’s effect on dopamine levels in the dorsal striatum (DS) of WT and ST mice [two-way ANOVA; drug × genotype F(1,24) = 1.13, P = 0.30; drug F(1,24) = 6.8, P = 0.01; genotype F(1,24) = 3.92, P = 0.06; n = 7 per genotype for each treatment]. (F) Neither amphetamine nor genotype had a significant effect on dorsal striatum DOPAC levels [two-way ANOVA; drug × genotype F(1,24 ) = 0.01, P = 0.92; genotype F(1,24) = 0.11, P = 0.74, n = 7 per genotype for each treatment]. (G) Neither amphetamine nor genotype had a significant effect on NAc dopamine tissue levels [two-way ANOVA; drug × genotype F(1,24) = 0.2, P = 0.65; drug F(1.24) = 3.43, P = 0.08; genotype F(1,23) = 0.51, P = 0.48, n = 7 per genotype for each treatment]. (H) Neither amphetamine nor genotype had a significant effect on NAc DOPAC tissue levels [two-way ANOVA; drug × genotype F(1,24) = 0.03, P = 0.87; genotype F(1,24) = 0.11, P = 0.75, n = 7 per genotype for each treatment].
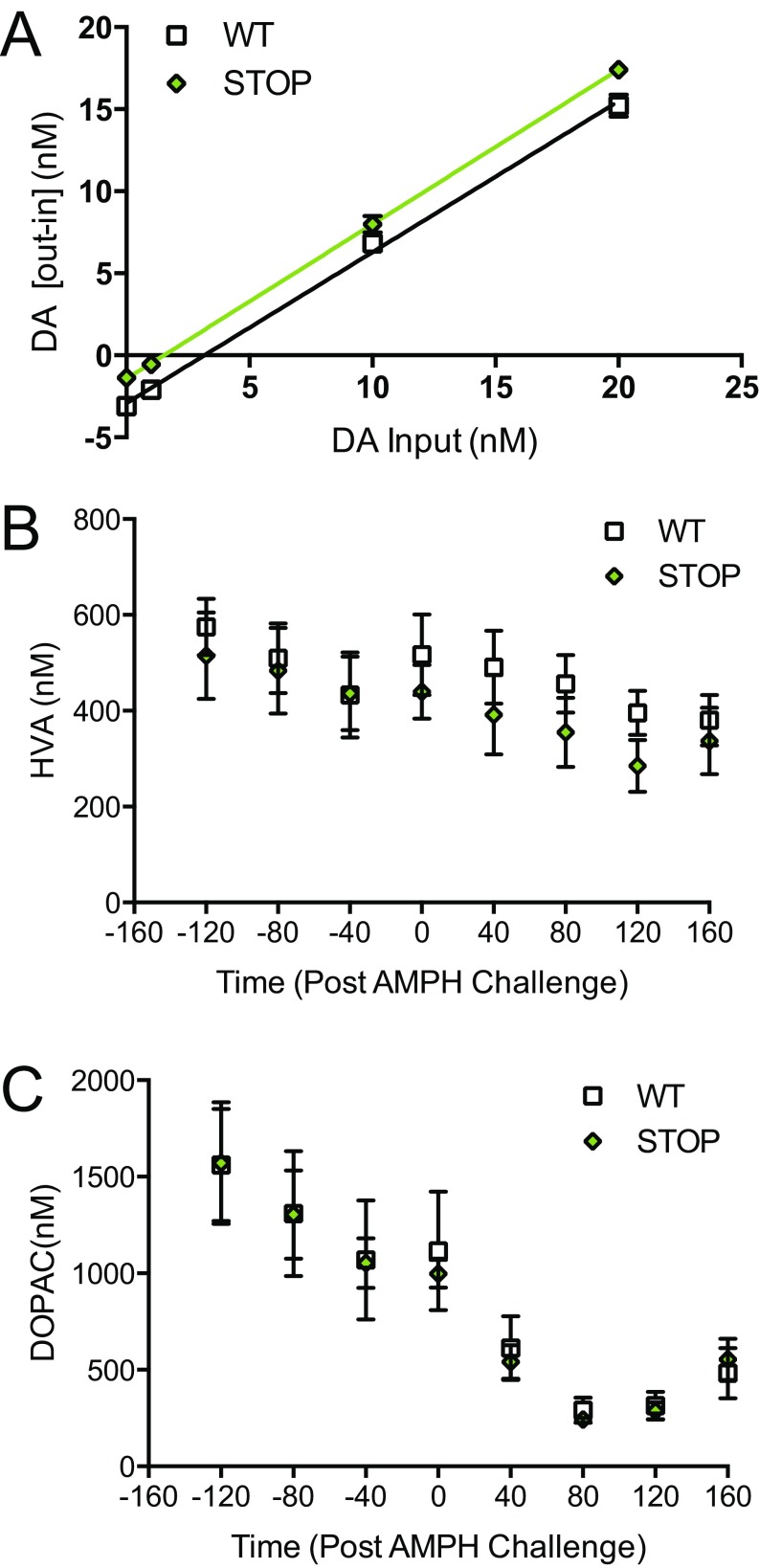
We next investigated if extracellular striatal dopamine levels were altered in ST mice relative to WT controls. Basal, steady-state extracellular dopamine levels were first measured using the quantitative technique of no-net-flux microdialysis (48). In freely moving mice, dorsal striatal extracellular dopamine levels were found to be significantly lower in ST mice than in WT littermate controls (unpaired t test; P = 0.0006, n = 6 per genotype) (Fig. 3E). No differences were observed in dopamine clearance as measured by the slope of the no-net-flux regression line (Fig. S8A). Conventional microdialysis revealed a significant elevation in dopamine levels following amphetamine (3 mg/kg, i.p.) compared with baseline levels in both WT and ST mice. However, absolute levels of dopamine after amphetamine were significantly reduced in ST mice compared with WT controls [repeated-measures two-way ANOVA; time × genotype, F(7,70) = 2.52, P = 0.0226; genotype F(1,10) = 9.34, P = 0.01; Sidak’s multiple comparison; P < 0.001; P < 0.05; n = 6 per genotype] (Fig. 3F). No genotypic differences were seen in dopamine metabolite levels at baseline or in response to amphetamine challenge (Fig. S8 B and C).
Fig. S8.
ST mice have reduced baseline dopamine as measured via no-net-flux microdialysis, and the main dopamine metabolites are not affected by amphetamine challenge (related to Fig. 3). (A) ST mice have significantly lowered dopamine levels at baseline as measured by the extrapolation of linear regression using no-net-flux microdialysis [WT slope = 0.92 ± 0.03, x-intercept = 3.17; ST slope = 0.94 ± 0.19, x-intercept = 1.52; slope comparison F(1,44) = 0.44, P = 0.51; n = 6 per genotype]. (B and C) Dorsal striatal levels of the dopamine metabolites homovanillic acid (HVA) (B) and DOPAC (C) are not different in ST mice relative to WT controls following systemic administration of amphetamine (3 mg/kg, i.p.) (B) Repeated-measures two-way ANOVA; time × genotype, F(7,70) = 0.96, P = 0.47. (C) Repeated-measures two-way ANOVA; time × genotype F(7,70) = 0.12; n = 6 per genotype.
Viral-Mediated Rescue of Slc1a1/EAAT3 in the Midbrain Attenuates Amphetamine-Induced Behavioral Deficits in ST Mice.
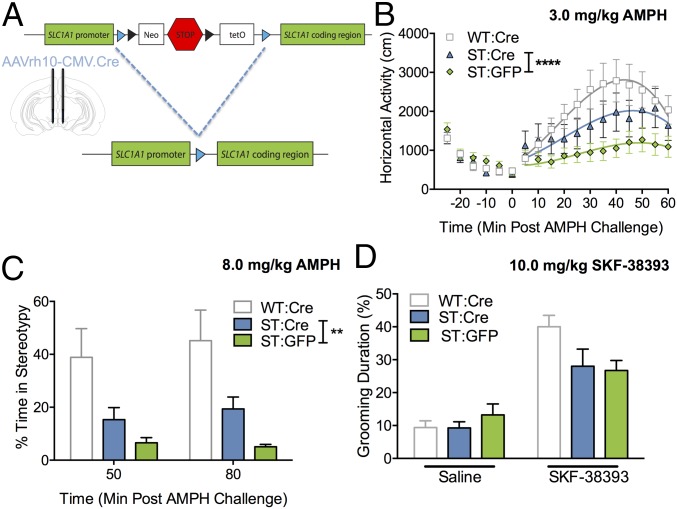
To test the hypothesis that our findings could be explained by the impact of EAAT3 ablation on midbrain dopaminergic neurons, we took advantage of the FAST construct to restore Slc1a1/EAAT3 expression in the midbrain of ST mice via Cre-Lox recombination. ST mice were bilaterally infused with either AAVrh10-CMV.Cre (ST:Cre) or AAVrh10-CMV.eGFP (ST:GFP) in the central midbrain [−3.3 mm anteroposterior (AP), ±0.4 mm mediolateral (ML), −4.5 mm dorsoventral (DV)], and WT littermate controls were bilaterally infused with AAVrh10-CMV.Cre (WT-Cre) (Fig. 4A). After a 2-wk incubation period, we found that ST:Cre animals showed a greater locomotor response to amphetamine (3.0 mg/kg) than ST:GFP control animals [curve–fit analysis; F(4,478) = 6.84, P < 0.0001, n = 12 ST:GFP, n = 15 ST:Cre] (Fig. 4B); however, their locomotor response remained less than that of WT animals. We also observed a significant main effect of Cre virus in ST:Cre and ST:GFP controls following high-dose (8.0 mg/kg) amphetamine-induced stereotypy [two-way ANOVA; Cre virus F(1,46) = 9.45, P = 0.0035] (Fig. 4C). No difference in perseverative grooming was observed between ST:GFP and ST:Cre mice after injection of the D1 agonist SKF-38393 [two-way ANOVA; drug, F(1,50) = 19.2, P < 0.0001; virus, F(1,50) = 0.13, P = 0.72; n = 12 ST:GFP mice, n = 15 ST:Cre mice) (Fig. 4D). Rescue of EAAT3 expression and viral spread in the midbrain of ST:Cre mice was confirmed via Western blot (Fig. S9) and immunohistochemistry (Fig. S10).To verify the specificity of midbrain rescue, we assessed the impact of viral Cre-mediated restoration of EAAT3 in the dorsal striatum and found no differences in amphetamine- or SKF-38393–mediated repetitive behavior in comparison with GFP controls (Fig. S11).
Fig. 4.
Viral Cre-mediated rescue of midbrain Slc1a1/EAAT3 expression rescues amphetamine but not SKF-38393 phenotypes in ST mice. (A) Schematic of Cre-mediated excision of the neo-STOP-tetO cassette in ST mice, leading to endogenous Slc1a1/EAAT3 expression. Blue triangles represent LoxP sites. The drawing indicates the injection position in the central midbrain of ST mice and WT controls (AP −3.3 mm, ML ±0.4 mm, DV −4.5 mm). (B) ST:Cre mice exhibit an increased hyperlocomotor response to 3.0 mg/kg amphetamine in comparison with ST:GFP littermate controls [curve–fit analysis, t = 0–60; F(4,478) = 6.84, ****P < 0.0001; n = 12 ST:GFP, n = 15 ST:Cre]. (C) A main effect of Cre virus is observed on stereotypy following high-dose amphetamine challenge (8.0 mg/kg) in ST:Cre and ST:GFP controls. Stereotypic behavior was scored by trained, blinded, independent observers at 50 and 80 min post challenge [two-way ANOVA; Cre virus, F(1,46) = 9.453, **P = 0.0035; n = 12 ST:GFP, n = 14 ST:Cre]. (D) ST:Cre mice showed no difference in stereotyped grooming behavior in response to 10 mg/kg SKF-38393 in comparison with ST:GFP littermate controls [two-way ANOVA; drug, F(1,50) = 19.2, P < 0.0001; virus, F(1,50) = 0.13, P = 0.72; n = 12 ST:GFP, n = 15 ST:Cre].
Fig. S9.
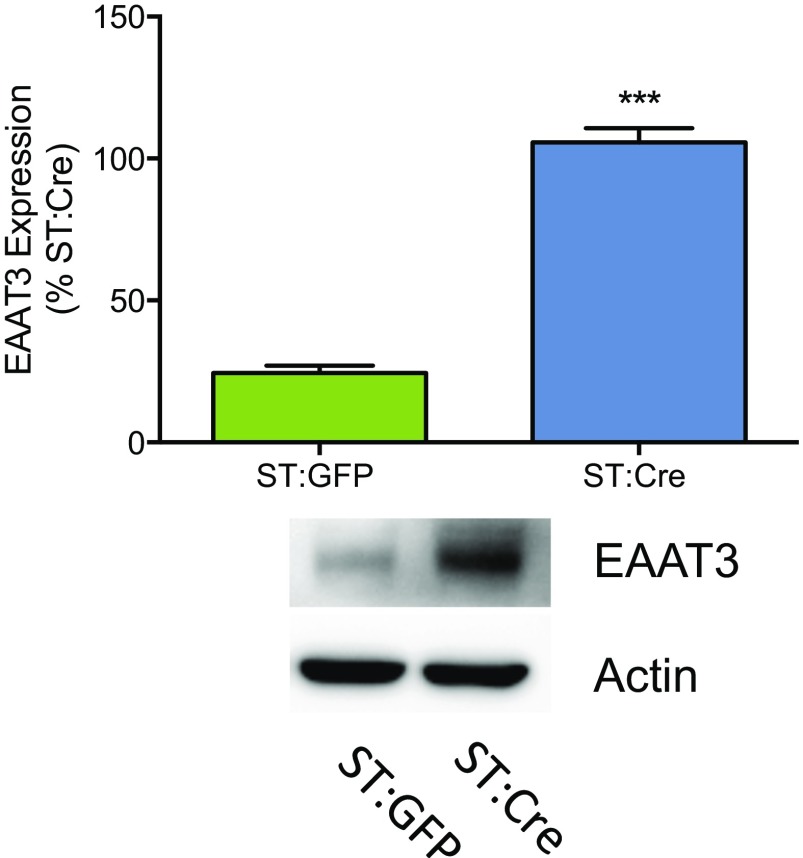
Injection of an AAV-Cre into the midbrain of ST mice results in increased EAAT3 expression (related to Fig. 4). EAAT3 protein expression is increased in midbrain synaptosome preparations from ST:Cre mice (lane 2) relative to ST:GFP mice (lane 1) (unpaired t test; t = 14.6, ***P = 0.0001, n = 3 per virus). The figure is representative of three separate experiments. Average protein expression ± SEM is demonstrated in the bar graph.
Fig. S10.
Cre expression is detected in the midbrain and striatum of ST:Cre mice (related to Fig. 4). Representative coronal sections are shown for both midbrain (−3 to −3.4 mm AP relative to bregma) (A) and striatum (1.1–0.7 mm AP relative to bregma) (B). High-magnification images were taken at 20× magnification. (Scale bars: 100 μM.)
Fig. S11.
Viral Cre-mediated rescue of dorsal striatum Slc1a1/EAAT3 expression does not rescue any of the dopaminergic agent behavioral phenotypes observed in ST mice (related to Fig. 4). (A) Cre-mediated excision of the flexible accelerated STOP-tetO knockin in ST mice, leading to endogenous Slc1a1/EAAT3 expression. Blue triangles represent LoxP sites. The drawing indicates the injection position in the dorsal striatum of ST and WT controls (AP +0.9 mm, ML ±1.5 mm, DV −3 mm). (B) ST:Cre mice exhibit a hyperlocomotor response similar to the response to 3.0 mg/kg amphetamine in comparison with ST:GFP littermate controls [curve–fit analysis t = 0–60; F(4,370) = 0.83, P = 0.50, n = 9 ST:GFP mice, n = 12 ST:Cre mice]. (C) Stereotyped behavior in ST:Cre mice in response to 8.0 mg/kg amphetamine is not different from that in ST:GFP littermate controls [two-way ANOVA; time × virus, F(1,32) = 0.14, P = 0.71; time, F(1,32) = 0.68, P = 0.42; virus, F(1,32) = 1.95, P = 0.17; n = 9 ST:GFP, n = 10 ST:Cre]. (D) ST:Cre mice showed no difference in stereotyped grooming behavior in response to 10 mg/kg SKF-38393 in comparison with ST:GFP littermate controls [two-way ANOVA; drug × virus F(1,32) = 0.45, P = 0.51; drug, F(1,32) = 29.74, P < 0.0001; virus, F(1,32) <0.0001, P = 0.99; n = 9 ST:GFP, n = 10 ST:Cre].
Discussion
Multiple studies have identified linkage and association of SLC1A1/EAAT3 with OCD (20–25, 49, 50); however, this in vivo study assesses whether changes in EAAT3 expression affect repetitive behavior. By using amphetamine as well as a dopamine D1 receptor agonist, we were able to probe the effects of EAAT3 ablation on basal ganglia-mediated behavior. As hypothesized, we detected a decrease in hyperlocomotion, stereotypic behavior, and striatal dopamine concentrations in response to these drugs in ST mice (Figs. 2 and 3). Coupled with our findings of partial Cre-mediated rescue in the midbrain, this decrease in repetitive behavior is consistent with multiple previous lines of evidence supporting a role for dopaminergic pathology in compulsive-like behavior. Most immediately relevant to our findings, the Hdc-null mouse model, based upon a family demonstrating a complex neuropsychiatric phenotype including complete penetrance for Tourette syndrome and partial penetrance for OCD, displayed elevated stereotypy in response to amphetamine challenge (42). In humans, dopamine agonists, including amphetamine and the dopamine precursor l-DOPA, are well-known triggers of repetitive behavior, from simple motor movements to frankly compulsive behavior in disorders linked to altered dopamine homeostasis (51–54). Indirect evidence also suggests an interaction between the dopamine system and SLC1A1 in humans. Atypical antipsychotic medications, which act in part as dopamine receptor antagonists, trigger obsessive-compulsive symptoms in some patients, and recent evidence suggests that polymorphisms in SLC1A1 moderate susceptibility to this uncommon drug-induced compulsivity (55, 56).
Our data show that EAAT3 ablation leads to decreased immediate early gene activation in dorsal striatal neurons in response to amphetamine (Fig. 3A), in addition to reductions in extracellular dopamine concentrations in the striatum at baseline and following amphetamine challenge (Fig. 3 E–F). These data are consistent with a change in presynaptic dopaminergic neuron function as a result of EAAT3 loss and align with a recent study demonstrating that EAAT3 impacts glutamatergic input onto midbrain dopaminergic neurons (30). Specifically, Underhill and colleagues (30) found that EAAT3 is internalized in response to amphetamine, resulting in increased glutamate exposure and potentiation of AMPA and NMDA glutamate receptor-mediated synaptic transmission in midbrain dopamine neurons. Based on these convergent data, we hypothesized that chronic increases in perisynaptic glutamate levels at dopaminergic neurons may elicit a homeostatic mechanism in ST mice that underlies the attenuated response to amphetamine. In support of this idea, we found that viral-mediated rescue of midbrain Slc1a1/EAAT3 resulted in increased amphetamine-induced locomotor and stereotypy behavior compared with ST mice infused with a control virus (Fig. 4 B and C). This impact of midbrain viral rescue contrasted with rescue of Slc1a1/EAAT3 in the striatum of ST mice, which had no impact on amphetamine-induced behavior (Fig. S11). Importantly, the lack of complete rescue of amphetamine response, as well as the lack of change in D1 agonist response, suggests that EAAT3 ablation in dopaminergic neurons (at least in adult animals) may not be the only mechanism impacting response to dopamine agonists in ST mice. Further study of EAAT3 ablation, restoration, and overexpression will be needed to dissect its importance in specific brain regions, neuronal subtypes, and, importantly, within particular developmental windows.
Of note, the initial published evaluation of Slc1a1/EAAT3-null mice reported decreased activity in the open field, although this result was not consistent with a later report, which described no baseline differences but found impaired Morris Water Maze performance in aged animals as the result of oxidative stress-mediated neuronal loss (36, 57). In our baseline assessment of activity, anxiety-like behavior, and compulsive-like behavior, we found no significant changes in the ST animals. This lack of effect on baseline behavior is consistent with some data suggesting that the OCD-associated SLC1A1 alleles lead to increased, not decreased, expression; however, postmortem studies are needed to clarify the direction of the change in SLC1A1 expression in OCD (25, 58). Examining the impact of SLC1A1 overexpression on OCD-relevant behaviors in mice also would assess this hypothesis more directly. These data also may indicate the difficulty of detecting a potential decrease in low levels of spontaneous compulsive-like behavior (25). In addition, one subsequent report in Slc1a1/EAAT3-null mice described oxidative stress-mediated loss of dopamine neurons in animals at 12 mo of age but no differences at 3 mo (57). Even though our model does retain some degree of preserved EAAT3 function (Fig. 1), we therefore restricted our work to younger animals and ruled out decreases in DAT immunohistochemistry (Fig. S6) or diminished dopamine or DOPAC levels in the midbrain (Fig. S7). Furthermore, the results of our midbrain viral rescue (Fig. 4) are not consistent with dopamine neuron loss as a mechanism of altered response to amphetamine or SKF-38393.
As with many studies aimed at unraveling pathophysiology in a preclinical context, it is important not to over-interpret these data in relation to the human condition. We therefore believe that the ST mouse should be considered as a putative model of reduced liability to dopamine-induced and basal ganglia-mediated repetitive behaviors (42). Although heterozygous SLC1A1 deletions have been reported in schizophrenia and schizoaffective disorder (27, 28), our findings do not clearly indicate a psychosis-like phenotype, because of the absence of observed changes in baseline behavior or prepulse inhibition. In the context of psychotic disorders, our observation of decreased sensitivity to amphetamine could be considered the opposite of what might be expected, because amphetamine can induce psychosis in humans (59). Because of this apparent contradiction, further work using the ST mice, including heterozygous animals, is warranted to understand better the potential contribution of SLC1A1 deletions to the risk of psychosis.
In summary, we report the evaluation of the OCD candidate gene Slc1a1/EAAT3 in relation to OCD-relevant circuitry and behavior in an animal model. Using dopaminergic agonism as a probe, we demonstrate the relevance of EAAT3 to striatal dopaminergic neurotransmission and to repetitive behavior. The partial rescue of dopamine agonist response by restoration of EAAT3 expression in the midbrain demonstrates an in vivo functional impact that matches previous cell model and ex vivo reports of EAAT3 effects in dopaminergic neurons. More work is needed to examine the effects of manipulating EAAT3 expression in other proposed models of striatally mediated repetitive behavior (13, 14) and in cognitive tasks relevant to OCD (60, 61). Our results also suggest that EAAT3 antagonists should be evaluated in relation to dopamine agonist response and, perhaps, more broadly in relation to basal ganglia-mediated repetitive behavior across species.
Materials and Methods
A brief summary of experimental procedures is provided here; additional details are available in SI Materials and Methods. Raw counts of cFos+ cells following saline or amphetamine challenge in the dorsal striatum, NAc, and somatosensory cortex of WT and ST mice are given in Table S1. DAT+ stereology sampling parameters are given in Table S2.
Table S1.
Raw counts of cFos+ cells following saline or amphetamine challenge in the dorsal striatum, NAc, and somatosensory cortex of WT and ST mice
| WT mice | ST mice | ||||
| ID | Saline | Amphetamine | ID | Saline | Amphetamine |
| Dorsal striatum | |||||
| 1 | 415.5 | 344.67 | 1 | 424.00 | 319.66 |
| 2 | 397.33 | 783.50 | 2 | 340.00 | 332.25 |
| 3 | 388.75 | 568.67 | 3 | 374.25 | 329.25 |
| 4 | 366 | 706.33 | 4 | 410.25 | 243.75 |
| 5 | 324.66 | 425.00 | 5 | 341.50 | 324.75 |
| 6 | 425.50 | ||||
| NAc core | |||||
| 1 | 87.25 | 114.75 | 1 | 82.00 | 97.25 |
| 2 | 98.00 | 105.25 | 2 | 73.50 | 84.75 |
| 3 | 80.00 | 97.25 | 3 | 87.00 | 89.25 |
| 4 | 88.00 | 122.50 | 4 | 76.25 | 89.75 |
| NAc shell | |||||
| 1 | 69.25 | 108.50 | 1 | 65.50 | 86.75 |
| 2 | 67.5 | 78.25 | 2 | 74.75 | 77.25 |
| 3 | 69 | 100.75 | 3 | 71.50 | 92.00 |
| 4 | 74.25 | 119.50 | 4 | 75.50 | 64.75 |
| Somatosensory cortex | |||||
| 1 | 106.33 | 90.33 | 1 | 105.67 | 96.67 |
| 2 | 89.25 | 100.5 | 2 | 107.5 | 108 |
| 3 | 108.67 | 93.5 | 3 | 92 | 88.5 |
| 4 | 112.33 | 111 | 4 | 105.33 | 122.33 |
Coronal sections corresponding to each brain region were used for cFos+ immunostaining. Data represent the mean of three or four sections per mouse. Raw counts are averages among slices. This table is related to Fig. 3.
Table S2.
DAT+ stereology sampling parameters
| No. of mouse sections | Section evaluation interval | Distance between sections, μM | Sampling grid area, μM | Counting frame, μM | Dissector height, μM | Guard zones, μM | Average section thickness, μM (range) | CE, m = 1 | |
| WT | 15 | 3 | 40 | 170 × 170 | 45 × 45 | 14 | 2 | 18 (16–19.6) | 0.10 |
| ST | 15 | 3 | 40 | 170 × 170 | 45 × 45 | 14 | 2 | 17.8 (15–19.5) | 0.09 |
This table is related to Fig. S6.
ST Mice.
All animal care and testing were approved by the New York State Psychiatric Institute (NYSPI) or Vanderbilt Institutional Animal Care and Use Committee and were in accordance with the NIH’s Guide for the Care and Use of Laboratory Animals (62). Homologous recombination was used to introduce a floxed-NeoSTOP-tetO-Slc1a1 cassette into the native Slc1a1 locus (Fig. 1A and SI Materials and Methods). Behavioral experiments were performed in 8- to 16-wk-old ST and WT littermate control mice in the NYSPI Rodent Neuroanalytical Core or the Vanderbilt Laboratory for Neurobehavior Core Facility between 1000 and 1600 hours. A preliminary behavioral experiment at Vanderbilt (n = 6 ST mice and 6 controls) showed similar behavioral result trends; however because variances and absolute values differed from those tested at Columbia University/NYSPI, these results are not included. Viral rescue experiments used bilateral injections of 0.3 μL of AAVrh10.CMV.PI.Cre.rBG (University of Pennsylvania Vector Core) or AAVrh10.CMV.PI.eGFP.WPRE.bGH (University of Pennsylvania Vector Core) at a titer of 1 × 1013 genomic copies/mL. Animals recovered at least 2 wk before testing.
Statistical Analysis.
Data were analyzed using Prism (GraphPad). Two-tailed, unpaired Student t test or two-way ANOVA with Sidak’s posttests was used to analyze all primary data except for locomotor data, which were analyzed using nonlinear curve–fit analysis. Specific statistical analyses for each dataset are described in Results and in figure legends. In the text and figures, all data are reported and shown as the mean ± SEM.
SI Materials and Methods
Generation of the ST Mouse Line.
Generation of targeting vector.
We constructed a pNeoSTOP-tetO plasmid using BAC recombineering (40). Our targeting vector has a 10-kb 5′-homology arm, the NeoSTOPtetO cassette, a 1.7-kb 3′-homology arm, and a diphtheria toxin A subunit. We inserted the STOP-tetO cassette just upstream of the Slc1a1 translation initiation site (Fig. 1A). Multicloning site 1 (MCS1) (PacI/NotI/ BamHI), loxP, FRT, PGK-EM7-Neo-HSV thymidine kinase poly(A) minigene, STOP sequence, FRT, tetO sequence, loxP, and MCS2 (EcoRV/EcoRI/PacI/SalI) were connected in tandem. DNA fragments (400 bp) upstream and downstream of the translation initiation site were amplified with PCR primers containing appropriate restriction enzyme sites and were respectively inserted into each MCS of the pNeoSTOP-tetO plasmid. To perform BAC recombination, the linearized NeoSTOP-tetO cassette with 400-bp homology arms was transferred into bacteria carrying the pBADTcTypeG plasmid and the BAC encompassing the Slc1a1 coding frame; BAC genomic clones containing Slc1a1 promoter and regulatory regions were obtained from BacPac (RP23-475B5). The targeting vector was isolated from the recombined, kanamycin-resistant clone using a retrieving technique and was inserted into the pMCS-DTA plasmid.
Construct insertion.
Eight colonies were found to be kanamycin resistant, and two of the eight were found to contain the NeoSTOP-tetO cassette via colony direct PCR. Expected band sizes based on primer positions and on the location of the NeoSTOP-tetO sequence were seen: 685 bp (5′ arm) and 644 bp (3′ arm).
Generation of ES cells.
The targeting vector was electroporated into a 129/SvEvTac mouse ES cell line in the Duke Embryonic Stem Cell Core. Homologous recombinants were detected using PCR for the 3′ arm of the targeting vector using a primer complementary to the 3′ homology arm and an external primer complementary to the genomic sequence located 3′ of the 3′ homology arm of the targeting vector. A subset of positive clones was tested by PCR for homologous targeting of the 5′ arm. Transgene incorporation was verified using Southern blot with probe position from −11,256 to −10,717 bases upstream of the translation initiation site, outside the homology arm, which generated the predicted band sizes of 15 kb (WT) and 18 kb (Slc1a1-STOP-tetO). Positive ES clones were injected into C57BL/6J blastocysts to obtain chimeric mice, which were crossed to 129S6/SvEvTac females to obtain germline transmission. Germline-transmitted offspring were then established as Slc1a1 STOP-tetO heterozygous knockins on a pure 129S6/SvEvTac background.
qRT-PCR.
RNA isolation.
RNA from fresh striatal tissue was isolated using the Qiagen RNeasy Mini Kit with DNase I (Qiagen) treatment per the manufacturer’s instructions. RNA quality and yield were assessed by NanoDrop spectrophotometer (Thermo Scientific).
Quantitative PCR.
For quantitative PCR experiments, cDNA was generated using the High Capacity cDNA Reverse Transcription Kit (Life Technologies) per the manufacturer’s instructions. Quantitative PCR was performed using Slc1a1 (Mm00436590_m1) and Gapdh (Mm99999915_g1) TaqMan gene-expression assays run on a 7900HT system (Life Technologies). All samples were run in triplicate with nontemplate and reverse transcriptase negative controls. Differential gene expression was calculated using the ΔΔCT method with Gapdh as the endogenous control.
Immunoblotting Studies.
Brains were harvested from mice after rapid decapitation. Brains were placed immediately on an ice-cold metal platform, and the striatum was dissected. Dissected tissue was placed into 3 mL of homogenization buffer [130 mM NaCl, 1.3 mM KCl, 2.2 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 10 mM Hepes (pH 7.4), 10 mM glucose, ascorbic acid, and 0.32 M sucrose] and was homogenized using a Potter Elvehjem homogenizer (Wheaton). Protein concentrations of all samples were determined by a bicinchoninic acid (BCA) protein assay (Thermo Fisher Scientific). Equal amounts of protein were incubated with Laemmli sample buffer for 10 min at room temperature and were analyzed by SDS/PAGE and Western blotting. EAAT3 protein was visualized in samples blotted to Immobilon-P PVDF membrane (EMD Millipore) by using a rabbit anti-EAAC1/EAAT3 antibody (1:1,000 dilution) (EAAC11-A; Alpha Diagnostics). β-Actin was visualized by using a mouse anti–β-actin antibody (1:10,000 dilution) (A5316; Sigma-Aldrich) as a loading control. Appropriate HRP-conjugated, secondary antibodies were obtained from GE Healthcare Life Sciences. Secondary antibody labeling was detected by using Amersham ECL Prime Western Blotting Detection Reagents and was visualized via chemiluminescence using the FluorChem M System (Protein Simple). Multiple exposures were obtained to ensure linearity of band detection. Western blots were quantified using NIH ImageJ software.
Synaptosomal Glutamate and Cysteine Transport Assays.
Brains were harvested from mice following rapid decapitation and were placed immediately on an ice-cold metal platform so that striatum could be dissected. Striatal tissue samples were placed into 3 mL of homogenization buffer [130 mM NaCl, 1.3 mM KCl, 2.2 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 10 mM Hepes (pH 7.4), 10 mM glucose, ascorbic acid, and 0.32 M sucrose] and were homogenized by using a Potter-Elvehjem homogenizer (Wheaton). Homogenates were centrifuged at 500 × g at 4 °C for 10 min. Supernatants were removed and centrifuged at 12,000 × g at 4 °C for 10 min. The resulting synaptosome-enriched pellets were then resuspended in 2.5 mL of assay buffer [130 mM NaCl, 1.3 mM KCl, 2.2 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 10 mM Hepes (pH 7.4), 10 mM glucose, and ascorbic acid], and protein concentrations were determined by using the BCA Protein Assay (Thermo Fisher Scientific). This material (hereafter called “synaptosomes”) then was diluted to 30 μg of total protein per 100 μL. Synaptosomes were separately incubated with [3 H]-glutamate (20 μM, 10% labeled and 90% unlabeled) or with [35 S]-cysteine (50 μM or 200 μM, 1% labeled and 99% unlabeled) (Perkin-Elmer). Assays were terminated by rapid filtration over 0.3% polyethyleneimine-soaked GF/B glass microfiber filters (Whatman; GE Life Science) and were washed three times with ice-cold Krebs-Ringers-Hepes buffer [130 mM NaCl, 1.3 mM KCl, 2.2 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, and 10 mM Hepes (pH 7.4)]. Filters were placed into scintillation vials with 7 mL of Ecoscint H (National Diagnostics) scintillation fluid; vials were shaken overnight at room temperature; then radioactivity was quantified by using a TriCarb 2900TR scintillation counter (Perkin-Elmer). Specific [3 H]-glutamate and [35 S]-cysteine transport was assayed in triplicate for all samples.
Behavioral Assays.
All behavioral experiments were performed in the Rodent Neuroanalytical Core operated by the NYSPI. Behavioral testing was performed between 1000 and 1600 hours using 8- to 16-wk-old mice. Animals were habituated to testing rooms for 15–20 min before the start of each experiment.
Spontaneous Locomotor Activity.
Spontaneous locomotor activity in the open field was measured by using 27 × 27 × 20.5 cm chambers (Med-Associates) placed within light- and air-controlled sound-attenuating boxes (64 × 45 × 42 cm). Locomotion was detected by the interruption of infrared beams by the body of the mouse (16 photocells in each horizontal axis located 1 cm above the activity chamber floor and 16 photocells elevated 4 cm above the chamber floor). Data were collected and analyzed by Med Associates Activity Monitor software. Mice were acclimated to the activity chambers during a 30-min session 2–3 d before data recording began.
Elevated Zero Maze.
Anxiety behaviors were examined by using an elevated zero maze (62.5-cm outer diameter, 5-cm path width, 15-cm wall height in closed segments) (Stoelting) with recordings lasting for 5 min. At the start of each trial, the mouse was placed onto an open portion of the maze adjacent to and facing one of the closed segments. Each session was recorded by a ceiling-mounted video camera connected to a computer for digital video acquisition and analysis with ANY-maze software (Stoelting). Data analyzed include the percent of time spent in the open zone, the number of open- and closed-zones entries, and the distance traveled within the maze.
Light–Dark Emergence.
Light–dark emergence was measured by using 27 × 27 × 20.5 chambers (Med Associates) placed within light- and air-controlled sound-attenuating boxes (64 × 45 × 42 cm). An opaque black insert placed in the open-field chamber created equal-sized light and dark compartments with an open door between the compartments. Mice were placed into the dark chamber through a trap door located on top of the insert. Mouse location was detected by the interruption of infrared beams by the body of the mouse (16 photocells in each horizontal axis located 1 cm above the activity chamber floor). The distance traveled in each chamber, the total number of transitions, the time spent in the each chamber, and the latency to enter the light chamber were recorded by the Med Associates software.
Acoustic Startle and Prepulse Inhibition.
Acoustic startle response and prepulse inhibition of acoustic startle were measured by using the Acoustic Startle Reflex Test Compartments (Med Associates). Mice were acclimated to background white noise of 65 dB for 5 min in a Plexiglas holding cylinder. Mice then were presented with seven trial types in six discrete, randomized blocks of trials for a total of 42 trials with an intertrial interval of 10–20 s. One trial measured baseline movement, and one trial measured response to the 120-dB, 50-ms startle stimulus alone. The other five trials used an acoustic prepulse of 74, 78, 82, 86, or 90 dB preceding the acoustic startle stimulus by 100 ms. Startle amplitude was measured every millisecond over a 65-ms period beginning at the onset of the startle stimulus. The dependent variable was the maximum startle over the sampling period. Prepulse inhibition was calculated by dividing the difference between baseline startle and startle following prepulse by baseline startle.
Home Cage Scan.
To evaluate possible repetitive behavior, individual mice of each genotype were video-recorded alone in the home cage for 24 h while maintaining the 12-h/12-h light/dark schedule. Automated video analysis was conducted by using HomeCageScan (Clever Sys) to index time spent performing individual behaviors.
Spontaneous Grooming.
Mice were placed in an empty novel cage and allowed to habituate for 30 min. Mouse behavior then was recorded for 10 min. Time spent grooming was manually determined with a stopwatch while observing the video, with the observer blind to genotype.
Amphetamine-Induced Locomotion.
Locomotor activity in the open field was measured by using 27 × 27 × 20.5 cm chambers (Med Associates) placed within light- and air-controlled sound-attenuating boxes (64 × 45 × 42 cm). Locomotion was detected by the interruption of infrared beams by the body of the mouse (16 photocells in each horizontal axis located 1 cm above the activity chamber floor and 16 photocells elevated 4 cm above the chamber floor to detect rearing and jumping behaviors). Data were collected and analyzed by Med Associates Activity Monitor software. Mice were acclimated to the activity chambers during a 30-min session 2–3 d before data recording began. On day 1 of the experimental sequence, mice were weighed and then were placed in the activity chamber, and activity was monitored for 30 min. The mice then were removed from the activity chambers and received an i.p. injection of 0.9% saline or amphetamine (1.8, 3, or 8 mg/kg) (Sigma-Aldrich). Mice were returned to the activity chambers, and locomotor activity was recorded for 60 min. Mice were returned to their home cage for a week to allow drug washout and then were given the alternative treatment. Time-course data were analyzed by using nonlinear curve line fit analysis, and cumulative measures of behavior (i.e., total distance traveled, time in stereotypy, number of stereotype behaviors, time rearing, and number of rearing behaviors) during the entire 60-min recording were analyzed.
Amphetamine-Induced Stereotypy.
Mice were weighed 1 h before the beginning of behavioral assay and then were administered 8 mg/kg amphetamine (Sigma Aldrich). Mice were promptly placed into novel, clear, empty cages, and behavior was recorded for 90 min with a video camera. Mouse behavior was analyzed for a stationary shuffling and sniffing-like stereotypy previously observed in pilot experiments. Observers blind to genotype performed analysis by post hoc scoring of video recordings. The observer recorded the time spent performing this stationary shuffling and sniffing-like stereotypy manually with a stopwatch. Time spent engaged in stereotypy was analyzed for 2 min at 50 and 80 min post amphetamine administration. A preliminary behavioral experiment at Vanderbilt (n = 6 ST mice and 6 controls) showed similar result trends; however, because variances and absolute values differed from those tested at Columbia University/ NYSPI, these results are not included.
SKF-38393–Induced Grooming.
Mice were weighed and then were placed in an open-field apparatus consisting of 27 × 27 × 20.5 cm chambers (Med Associates) placed within light- and air-controlled sound-attenuating boxes (64 × 45 × 42 cm). Mice were allowed to habituate for 30 min and then were administered saline or SKF-38393 (10 mg/kg, i.p.) (Tocris Biosciences) and were placed back into the open field for 60 additional minutes. Behavior was recorded via cameras located above the open field for the entirety of the experiment. Following a 1-wk washout period, mice received the alternative treatment from week 1. Trained observers, blind to genotype and drug treatment, manually scored grooming duration during sample periods (the first 2 min of every 10-min bin) for 1 h following SKF-38393 administration.
cFos+ Immunohistochemistry.
To measure an indirect marker of neuronal activation, we immunostained coronal slices of mouse striatum and NAc challenged with amphetamine (3.0 mg/kg, i.p.) or saline. Mouse brains were perfused with 4% paraformaldehyde and postfixed overnight at 4 °C, transferred to 30% sucrose in 0.1 M phosphate buffer until it sank, and sectioned on a microtome to a thickness of 40 μm. Striatum-containing sections were peroxidase quenched in 0.3% H2O2 in methanol for 30 min, blocked in PBS with 5% normal donkey serum and 0.3% Triton X-100 for 2 h at room temperature, and incubated in rabbit anti-cFos primary antibody (1:5,000) (Santa Cruz) for 72 h at 4 °C. After washing in PBS with 0.3% Triton X-100, sections were incubated in biotinylated donkey anti-rabbit secondary antibody (1:500) (Jackson ImmunoResearch) for 2 h at room temperature. Washed sections were incubated with ABC reagent and DAB substrate (Vector Labs) per the manufacturer’s instructions for visualization of stained cells.
cFos+ Cell Quantification.
cFos immunostaining was identified using Stereo Investigator software (MBF Bioscience) and an Axio Imager M2 microscope (Zeiss) with a 10× objective coupled to a Retiga 2000R camera (Qimaging). Three or four coronal brain sections corresponding to dorsal medial striatum (AP +0.9 mm, ML ±1.5 mm, DV −3 mm relative to bregma), NAc core (AP +1.2 mm, ML ±0.75 mm, DV −4.5 mm relative to bregma) and NAc shell (AP +1.2 mm, ML ±0.5 mm, DV −4.5 mm relative to bregma), and somatosensory cortex (AP +0.9 mm, ML ±3.0 mm, DV −3.0 mm relative to bregma) were identified for each treatment condition and matched between genotypes using the Allen Brain Atlas (63). For each animal (n = 6 WT and 6 ST dorsal striatum, n = 4 WT and 4 ST NAc). the total number of cFos+ cells within a unilateral region of interest was estimated using a sampling frame (dorsal striatum, 90 × 67.5 μM; NAc and somatosensory cortex, 35 × 35 μM) and the cell-counter plugin for ImageJ (NIH). For each mouse, total number of cFos+ cells within the counting frame were counted, averaged within subject, and then normalized within genotype to the saline condition. Analysis was performed using two-way ANOVAs to identify interactions between drug and genotype as well as main effects. All quantification was done blind to genotype and treatment. Raw counts are included in Table S1.
Binding.
Fresh striatal tissue was dissected and frozen as noted above and then was placed in 2 mL of binding buffer [50 mM Tris (pH 7.4), 5 mM MgCl2, 5 mM KCl, 1 mM EDTA, and 120 mM NaCl] and homogenized by using an Omni-Tip handheld homogenizer (Omni International) at 10,000–12,000 × g for 10–15 s. Homogenates then were centrifuged at 20,000 × g at 4 °C for 20 min. Membrane pellets were resuspended in 3 mL of binding buffer and were homogenized again as described above. Homogenates were preincubated at 37 °C for 15 min and then were centrifuged at 20,000 × g at 4 °C for 20 min. Membrane pellets were resuspended again in 3 mL of binding buffer and were homogenized as described above. Protein concentration was determined by BCA protein assay (Thermo Fisher Scientific). Samples were stored at −80 °C until used for binding assays. Membrane samples (100–150 μg for striatum) then were thawed and incubated in a final reaction volume of 1 mL at room temperature for 75 min in the presence of 3 nM [3 H]-SCH 23390 (84.3 Ci/mmol) (Perkin-Elmer) for D1 reporter assays or for 90 min in the presence of 3 nM [3 H]-methylspiperone (84.3 Ci/mmol) (Perkin-Elmer) for D2 reporter assays. Binding reactions were terminated by the addition of 8 mL of ice-cold wash buffer [50 mM Tris (pH 7.4)] followed by rapid filtration over a water-moistened S&S filter (no. 5; Schleicher and Schuell Bioscience) or a GF/B glass fiber filter (Whatman) by using a Millipore vacuum manifold. Each filter then was washed two times with 8 mL of ice-cold wash buffer. Filters were placed in scintillation vials, and 10 mL of Bio-Safe II scintillation fluid (Research Products International) was added to each vial. Vials were shaken overnight at room temperature, and then radioactivity was counted by using a TriCarb 2900TR scintillation counter (Perkin-Elmer). Nonspecific binding was determined by using parallel incubations as above with the addition of 2 μM butaclamol to samples; binding values from these samples were subtracted from radioligand-only samples to determine specific binding. All samples (total and nonspecific binding) were assayed in triplicate.
No-Net-Flux Microdialysis.
Isoflurane-anesthetized 6- to 8-wk-old mice were implanted unilaterally with an MBR-5 microdialysis guide cannula (MBR-2255; Bioanalytical Systems, Inc.) using standard stereotactic techniques (AP +0.9 mm, ML 1.7 mm, DV −2.0 mm relative to bregma). Microdialysis was performed following a recovery period of 3 d. On the day of microdialysis an MBR-2-5 brain microdialysis probe (MB-2212; Bioanalytical Systems, Inc.) with a confirmed in vitro recovery of >7% was lowered into the guide cannula. The probe then was perfused with artificial cerebral spinal fluid (aCSF) (145 mM NaCl, 2.7 mM KCl, 1.0 mM MgCl2, 1.2 mM CaCl2, pH 7.4) at a rate of 1 µL/min for a period of 120 min before the first sample was collected. Following this equilibration period, dialysis samples were collected into vials containing 5 µL of HeGA preservative every 40 min. Once stable basal dopamine levels were established, four different concentrations of dopamine (0, 1, 10, or 20 nM) in aCSF were perfused in a random order, and loss or gain of dopamine was measured. Following completion of this no-net-flux protocol, aCSF was again perfused until steady levels of dopamine were established; at that point 3 mg/kg amphetamine was administered i.p. Four 40-min samples were collected. Dialysis samples were analyzed immediately via HPLC using a Pursuit XRs C18100 × 3.0 mm column (Agilent Technologies) and a mobile phase consisting of 1.5% acetonitrile, 7% methanol, 75 mM sodium phosphate monobasic monohydrate, 25 µM EGTA, and 2.28 mM 1-octanesulfonic acid soap with a pH of 3.9 prepared no more than 24 h before the run. Dopamine and metabolite peaks were detected using an electrochemical microdialysis cell (model 5014B; ESA) coupled to a CoulArray detector (model 5600A; ESA) with CoulArray software. After the experiments, mice were deeply anesthetized and transcardially perfused, brains were sliced, and probe positions were confirmed.
DAT and Cre Immunohistochemistry.
Brains from 12-wk-old mice (for DAT) or 16- to 24-wk-old mice(for Cre) were perfused with 4% paraformaldehyde and postfixed overnight at 4 °C, transferred to 30% sucrose in 0.1 M phosphate buffer until they sank, and sectioned on a cryostat to a thickness of 40 μm. Midbrain-containing sections were washed with PBS and 0.3% Triton X-100 three times for 10 min each washing, peroxidase quenched in 0.3% H2O2 in methanol for 30 min, and then blocked in PBS with 5% normal donkey serum and 0.3% Triton X-100 for 1 h at room temperature. Sections then were incubated overnight at 4 °C with either rat anti-DAT (1:1,000) (Millipore) or mouse anti-Cre (1:1,000) (Millipore) antibody. After washing in PBS with 0.3% Triton X-100, sections were incubated in biotinylated donkey anti-mouse (1:500) (Jackson ImmunoResearch) (for Cre) or in biotinylated donkey anti-rat (1:500) (Jackson ImmunoResearch) (for DAT) secondary antibody for 2 h at room temperature. Washed sections were incubated with ABC reagent and DAB substrate (Vector Labs) per the manufacturer’s instructions for visualization of stained cells. DAT and Cre immunostaining was identified using Stereo Investigator software (MBF Biosciences) and an Axio Imager M2 microscope (Zeiss) coupled to a Retiga 2000R camera (Qimaging). Areas corresponding to the VTA (AP −2.9 to 0.3.4 mm; ML ± 0.4 mm; DV −4.5 mm relative to bregma) were identified (Allen Brain Atlas) (62).
DAT Stereological Quantification.
Unbiased stereology was used to estimate the mean total number of DAT+ neurons in the midbrain via the optical fractionator method (64–66). This design-based method allows an estimation of cell number that is independent of volume estimates. Data collection was performed using the Stereo Investigator program (MBF Biosciences). The system used an X-Y-Z motorized stage. The program was integrated with an Axio Imager M2 microscope (Zeiss) coupled to a Retiga 2000R camera (Qimaging). The region of interest (ROI) was outlined at low power using a 2.5× objective. ROIs were determined based on stereotactic coordinates provided by Paxinos and Franklin’s atlas (63) at −2.9 to −3.5 mm from bregma for the midbrain (VTA and substantia nigra pars compacta). Cells were counted using a 20× objective (N.A. 0.5). Sampling scheme, sampling grid sizes (170 × 170 μM), and counting frames (45 × 45 μM) were optimized to obtain individual estimates of DAT+ neuron number with a mean Gundersen coefficient of error (CE) ≤0.1 (65). Four mice per genotype (WT and ST), obtained via heterozygous breeding, were analyzed using Stereo Investigator. Sampling parameters are listed below. Fifteen sections per animal were used for estimates, with the first section being selected randomly within the first three sections. Section thickness was measured at every fifth sampling location, and the mean thickness was used for computation of DAT+ neuron estimates. At each sampling location, the microscope was focused down through the dissector sample to count any cell according to dissector counting rules. Because the fractionator method does not require a measurement of tissue volume or any other dimensional quality, the cell number estimate is valid, even if the tissue volume changes during processing (66).
HPLC Analysis of Tissue Biogenic Amines.
Neurochemical measures were obtained in the Neurochemistry Core Facility at Vanderbilt University Medical Center operated by the Vanderbilt Brain Institute. Brain regions, harvested and dissected as noted above, were flash-frozen in liquid nitrogen and stored at −80 °C. Frozen brain tissue was homogenized by using a tissue dismembrator (Misonix XL-2000; Qsonica) in 100–750 μL of a solution containing 100 mM trichloroacetic acid (TCA), 10 mM NaC2H3O2, 100 μM EDTA, 5 ng/mL isoproterenol (an internal standard), and 10.5% (vol/vol) methanol (pH 3.8). Samples were spun in a microcentrifuge at 10,000 × g for 20 min, and the supernatants were stored at −80 °C until assayed. Before assay, thawed supernatants were centrifuged at 10,000 × g for 20 min before being analyzed by HPLC. Twenty microliters of each sample were injected by using a Waters 2707 autosampler onto a Phenomenex Kintex (2.6 μm, 100 Å) C18 HPLC column (100 × 4.6 mm). Biogenic amines were eluted with a mobile phase [89.5% 100 mM TCA, 10 mM NaC2H3O2, 100 μM EDTA, and 10.5% methanol (pH 3.8)] delivered at 0.6 mL/min by using a Waters 515 HPLC pump. Analytes were detected by using a Decade II (oxidation: 0.4) (3 mm glassy carbon working electrode, HYREF) electrochemical detector (Antec Scientific) operated at 33 °C. HPLC instrument control and data acquisition were managed by Empower software.
In Vivo Viral Infusion.
Stereotaxic surgery was performed following standard procedures under sterile conditions. Adult (8- to 12-wk-old) male ST homozygous mice and WT controls were anesthetized with an i.p. injection of xylazine (10 mg/kg) (Sigma-Aldrich) and ketamine (100 mg/kg) (Hospira) and were placed into a mouse stereotaxic apparatus. A 5-uL Hamilton syringe attached to a Nano syringe pump (KDS310; KD Scientific) was lowered through a burr hole in the skull into the VTA (AP −3.3 mm, ML ±0.4 mm, DV −4.5 mm relative to bregma) or dorsal striatum (AP +0.9 mm, ML ±1.7 mm, DV −2.5 mm relative to bregma) with reference to the mouse brain atlas of Paxinos (63). A total of 0.3 μL of virus was infused bilaterally at a flow rate of 0.1 μL/min. The syringe was left in place for 10 min after completion of infusion to eliminate backflow and then was withdrawn slowly. This process was repeated on the other side to produce bilateral viral injections. The cranial incision was closed with Vetbond (3M), and mice were returned to their home cages for a minimum of 2 wk postsurgery.
Mice received either AAVrh10.CMV.PI.Cre.rBG (University of Pennsylvania Vector Core) or AAVrh10.CMV.PI.eGFP.WPRE.bGH (University of Pennsylvania Vector Core) at a titer of 1 × 1013 genomic copies/mL.
Statistical Analysis.
Data were analyzed using Prism (GraphPad). Two-tailed, unpaired Student t test or two-way ANOVA with Sidak’s posttests was used to analyze the primary data, except for locomotor data, which were analyzed using nonlinear curve–fit analysis. Specific statistical analyses for each dataset are described in Results and in the figure legends. In the text and figures, all data are reported as the mean ± SEM. Bar graphs depict the mean ± SEM.
Supplementary Material
Acknowledgments
We thank Carly Wender and Danielle Garfunkel for assistance with general laboratory support; Dr. James Bodfish for expert advice on the video analysis of stereotypy, and Dr. Ariel Deutch for advice on the interpretation of tissue dopamine levels and dopamine receptor-binding results. This work was funded, in part, by a National Association for Research on Schizophrenia and Depression Young Investigator Grant (to J.V.) and NIH Grant MH096200 (to S.E.A. and J.V.).
Footnotes
Conflict of interest statement: C.K. has received research funding from Forest. C.K.J. has received research funding from AstraZeneca, Johnson & Johnson, Bristol-Myers Squibb, and Seaside Therapeutics. J.V. has consulted or served on advisory boards for Roche, Novartis, and SynapDx and has received research funding from Roche, Novartis, SynapDx, Seaside Therapeutics, and Forest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1701736114/-/DCSupplemental.
References
- 1.Koran LM, Hanna GL, Hollander E, Nestadt G, Simpson HB. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164(7) Suppl:5–53. [PubMed] [Google Scholar]
- 2.Michael S, Ritsner AGA. New Perspectives on Research and Treatment. Springer International Publishing; Cham, Switzerland: 2007. Quality of life impairment in schizophrenia, mood and anxiety disorders. [Google Scholar]
- 3.Dougherty DD, Rauch SL, Jenike MA. Pharmacotherapy for obsessive-compulsive disorder. J Clin Psychol. 2004;60:1195–1202. doi: 10.1002/jclp.20083. [DOI] [PubMed] [Google Scholar]
- 4.Simpson HB, et al. Cognitive-behavioral therapy vs risperidone for augmenting serotonin reuptake inhibitors in obsessive-compulsive disorder: A randomized clinical trial. JAMA Psychiatry. 2013;70:1190–1199. doi: 10.1001/jamapsychiatry.2013.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarris J, et al. N-acetyl cysteine (NAC) in the treatment of obsessive-compulsive disorder: A 16-week, double-blind, randomised, placebo-controlled study. CNS Drugs. 2015;29:801–809. doi: 10.1007/s40263-015-0272-9. [DOI] [PubMed] [Google Scholar]
- 6.Aouizerate B, et al. Deep brain stimulation of the ventral caudate nucleus in the treatment of obsessive-compulsive disorder and major depression. Case report. J Neurosurg. 2004;101:682–686. doi: 10.3171/jns.2004.101.4.0682. [DOI] [PubMed] [Google Scholar]
- 7.Fontaine D, et al. Effect of subthalamic nucleus stimulation on obsessive-compulsive disorder in a patient with Parkinson disease. Case report. J Neurosurg. 2004;100:1084–1086. doi: 10.3171/jns.2004.100.6.1084. [DOI] [PubMed] [Google Scholar]
- 8.Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. 2009;195:393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg DR, et al. Frontostriatal measurement in treatment-naive children with obsessive-compulsive disorder. Arch Gen Psychiatry. 1997;54:824–830. doi: 10.1001/archpsyc.1997.01830210068007. [DOI] [PubMed] [Google Scholar]
- 10.Menzies L, et al. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: The orbitofronto-striatal model revisited. Neurosci Biobehav Rev. 2008;32:525–549. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starck G, et al. A 1H magnetic resonance spectroscopy study in adults with obsessive compulsive disorder: Relationship between metabolite concentrations and symptom severity. J Neural Trans. 2008;115:1051–1062. doi: 10.1007/s00702-008-0045-4. [DOI] [PubMed] [Google Scholar]
- 12.Whiteside SP, Port JD, Deacon BJ, Abramowitz JS. A magnetic resonance spectroscopy investigation of obsessive-compulsive disorder and anxiety. Psychiatry Res. 2006;146:137–147. doi: 10.1016/j.pscychresns.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Welch JM, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shmelkov SV, et al. Slitrk5 deficiency impairs corticostriatal circuitry and leads to obsessive-compulsive-like behaviors in mice. Nat Med. 2010;16:598–602, 591p following 602. doi: 10.1038/nm.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmari SE, et al. Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science. 2013;340:1234–1239. doi: 10.1126/science.1234733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burguiere E, Monteiro P, Feng G, Graybiel AM. Optogenetic stimulation of lateral orbitofronto-striatal pathway suppresses compulsive behaviors. Science. 2013;340:1243–1246. doi: 10.1126/science.1232380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pauls DL. The genetics of obsessive compulsive disorder: A review of the evidence. Am J Med Genet C Semin Med Genet. 2008;148C:133–139. doi: 10.1002/ajmg.c.30168. [DOI] [PubMed] [Google Scholar]
- 18.Hanna GL, et al. Genome-wide linkage analysis of families with obsessive-compulsive disorder ascertained through pediatric probands. Am J Med Genet. 2002;114:541–552. doi: 10.1002/ajmg.10519. [DOI] [PubMed] [Google Scholar]
- 19.Willour VL, et al. Replication study supports evidence for linkage to 9p24 in obsessive-compulsive disorder. Am J Hum Genet. 2004;75:508–513. doi: 10.1086/423899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veenstra-VanderWeele J, et al. Genomic organization of the SLC1A1/EAAC1 gene and mutation screening in early-onset obsessive-compulsive disorder. Mol Psychiatry. 2001;6:160–167. doi: 10.1038/sj.mp.4000806. [DOI] [PubMed] [Google Scholar]
- 21.Arnold PD, Sicard T, Burroughs E, Richter MA, Kennedy JL. Glutamate transporter gene SLC1A1 associated with obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63:769–776. doi: 10.1001/archpsyc.63.7.769. [DOI] [PubMed] [Google Scholar]
- 22.Dickel DE, et al. Association testing of the positional and functional candidate gene SLC1A1/EAAC1 in early-onset obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63:778–785. doi: 10.1001/archpsyc.63.7.778. [DOI] [PubMed] [Google Scholar]
- 23.Stewart SE, et al. Association of the SLC1A1 glutamate transporter gene and obsessive-compulsive disorder. Am J Med Gen. 2007;144B:1027–1033. doi: 10.1002/ajmg.b.30533. [DOI] [PubMed] [Google Scholar]
- 24.Shugart YY, et al. A family-based association study of the glutamate transporter gene SLC1A1 in obsessive-compulsive disorder in 378 families. Am J Med Gen. 2009;150B:886–892. doi: 10.1002/ajmg.b.30914. [DOI] [PubMed] [Google Scholar]
- 25.Wendland JR, et al. A haplotype containing quantitative trait loci for SLC1A1 gene expression and its association with obsessive-compulsive disorder. Arch Gen Psychiatry. 2009;66:408–416. doi: 10.1001/archgenpsychiatry.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart SE, et al. Meta-analysis of association between obsessive-compulsive disorder and the 3′ region of neuronal glutamate transporter gene SLC1A1. Am J Med Genet B Neuropsychiatr Genet. 2013;162B:367–379. doi: 10.1002/ajmg.b.32137. [DOI] [PubMed] [Google Scholar]
- 27.Afshari P, et al. Characterization of a novel mutation in SLC1A1 associated with schizophrenia. Mol Neuropsychiatry. 2015;1:125–144. doi: 10.1159/000433599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myles-Worsley M, et al. Deletion at the SLC1A1 glutamate transporter gene co-segregates with schizophrenia and bipolar schizoaffective disorder in a 5-generation family. Am J Med Genet B Neuropsychiatr Genet. 2013;162B:87–95. doi: 10.1002/ajmg.b.32125. [DOI] [PubMed] [Google Scholar]
- 29.Horiuchi Y, et al. Association of SNPs linked to increased expression of SLC1A1 with schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:30–37. doi: 10.1002/ajmg.b.31249. [DOI] [PubMed] [Google Scholar]
- 30.Underhill SM, et al. Amphetamine modulates excitatory neurotransmission through endocytosis of the glutamate transporter EAAT3 in dopamine neurons. Neuron. 2014;83:404–416. doi: 10.1016/j.neuron.2014.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holmseth S, et al. The density of EAAC1 (EAAT3) glutamate transporters expressed by neurons in the mammalian CNS. J Neurosci. 2012;32:6000–6013. doi: 10.1523/JNEUROSCI.5347-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nieoullon A, et al. The neuronal excitatory amino acid transporter EAAC1/EAAT3: Does it represent a major actor at the brain excitatory synapse? J Neurochem. 2006;98:1007–1018. doi: 10.1111/j.1471-4159.2006.03978.x. [DOI] [PubMed] [Google Scholar]
- 33.Bjorn-Yoshimoto W, Underhill SM. The importance of the excitatory amino acid transporter 3 (EAAT3) Neurochem Int. 2016 doi: 10.1016/j.neuint.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otis TS, Brasnjo G, Dzubay JA, Pratap M. Interactions between glutamate transporters and metabotropic glutamate receptors at excitatory synapses in the cerebellar cortex. Neurochem Int. 2004;45:537–544. doi: 10.1016/j.neuint.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Mathews GC, Diamond JS. Neuronal glutamate uptake Contributes to GABA synthesis and inhibitory synaptic strength. J Neurosci. 2003;23:2040–2048. doi: 10.1523/JNEUROSCI.23-06-02040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aoyama K, et al. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci. 2006;9:119–126. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- 37.Watts SD, Torres-Salazar D, Divito CB, Amara SG. Cysteine transport through excitatory amino acid transporter 3 (EAAT3) PLoS One. 2014;9:e109245. doi: 10.1371/journal.pone.0109245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scimemi A, Tian H, Diamond JS. Neuronal transporters regulate glutamate clearance, NMDA receptor activation, and synaptic plasticity in the hippocampus. J Neurosci. 2009;29:14581–14595. doi: 10.1523/JNEUROSCI.4845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li MH, et al. Amphetamine and methamphetamine increase NMDAR-GluN2B synaptic currents in midbrain dopamine neurons. Neuropsychopharmacol. 2017 doi: 10.1038/npp.2016.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka KF, et al. Flexible accelerated STOP tetracycline operator-knockin (FAST): A versatile and efficient new gene modulating system. Biol Psychiatry. 2010;67:770–773. doi: 10.1016/j.biopsych.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmari SE, Risbrough VB, Geyer MA, Simpson HB. Impaired sensorimotor gating in unmedicated adults with obsessive-compulsive disorder. Neuropsychopharmacol. 2012;37:1216–1223. doi: 10.1038/npp.2011.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castellan Baldan L, et al. Histidine decarboxylase deficiency causes tourette syndrome: Parallel findings in humans and mice. Neuron. 2014;81:77–90. doi: 10.1016/j.neuron.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor JL, Rajbhandari AK, Berridge KC, Aldridge JW. Dopamine receptor modulation of repetitive grooming actions in the rat: Potential relevance for Tourette syndrome. Brain Res. 2010;1322:92–101. doi: 10.1016/j.brainres.2010.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Starr BS, Starr MS. Grooming in the mouse is stimulated by the dopamine D1 agonist SKF 38393 and by low doses of the D1 antagonist SCH 23390, but is inhibited by dopamine D2 agonists, D2 antagonists and high doses of SCH 23390. Pharmacol Biochem Behav. 1986;24:837–839. doi: 10.1016/0091-3057(86)90421-1. [DOI] [PubMed] [Google Scholar]
- 45.Rebec GV, White IM, Puotz JK. Responses of neurons in dorsal striatum during amphetamine-induced focused stereotypy. Psychopharmacology (Berl) 1997;130:343–351. doi: 10.1007/s002130050249. [DOI] [PubMed] [Google Scholar]
- 46.Ikemoto S. Ventral striatal anatomy of locomotor activity induced by cocaine, D-amphetamine, dopamine and D1/D2 agonists. Neuroscience. 2002;113:939–955. doi: 10.1016/s0306-4522(02)00247-6. [DOI] [PubMed] [Google Scholar]
- 47.Berman AE, et al. N-acetylcysteine prevents loss of dopaminergic neurons in the EAAC1−/− mouse. Ann Neurol. 2011;69:509–520. doi: 10.1002/ana.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chefer VI, Zapata A, Shippenberg TS, Bungay PM. Quantitative no-net-flux microdialysis permits detection of increases and decreases in dopamine uptake in mouse nucleus accumbens. J Neurosci Methods. 2006;155:187–193. doi: 10.1016/j.jneumeth.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 49.Veenstra-VanderWeele J, et al. Functional studies and rare variant screening of SLC1A1/EAAC1 in males with obsessive-compulsive disorder. Psychiatr Genet. 2012;22:256–260. doi: 10.1097/YPG.0b013e328353fb63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Samuels J, et al. Comprehensive family-based association study of the glutamate transporter gene SLC1A1 in obsessive-compulsive disorder. Am J Med Gen. 2011;156B:472–477. doi: 10.1002/ajmg.b.31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Varley CK, Vincent J, Varley P, Calderon R. Emergence of tics in children with attention deficit hyperactivity disorder treated with stimulant medications. Compr Psychiatry. 2001;42:228–233. doi: 10.1053/comp.2001.23145. [DOI] [PubMed] [Google Scholar]
- 52.Madruga-Garrido M, Mir P. Tics and other stereotyped movements as side effects of pharmacological treatment. Int Rev Neurobiol. 2013;112:481–494. doi: 10.1016/B978-0-12-411546-0.00016-0. [DOI] [PubMed] [Google Scholar]
- 53.Parraga HC, Parraga MI, Harris DK. Tic exacerbation and precipitation during atomoxetine treatment in two children with attention-deficit hyperactivity disorder. Int J Psychiatry Med. 2007;37:415–424. doi: 10.2190/PM.37.4.e. [DOI] [PubMed] [Google Scholar]
- 54.Voon V, et al. Chronic dopaminergic stimulation in Parkinson’s disease: From dyskinesias to impulse control disorders. Lancet Neurol. 2009;8:1140–1149. doi: 10.1016/S1474-4422(09)70287-X. [DOI] [PubMed] [Google Scholar]
- 55.Kwon JS, et al. Association of the glutamate transporter gene SLC1A1 with atypical antipsychotics-induced obsessive-compulsive symptoms. Arch Gen Psychiatry. 2009;66:1233–1241. doi: 10.1001/archgenpsychiatry.2009.155. [DOI] [PubMed] [Google Scholar]
- 56.Cai J, et al. Influence of polymorphisms in genes SLC1A1, GRIN2B, and GRIK2 on clozapine-induced obsessive-compulsive symptoms. Psychopharmacology (Berl) 2013;230:49–55. doi: 10.1007/s00213-013-3137-2. [DOI] [PubMed] [Google Scholar]
- 57.Peghini P, Janzen J, Stoffel W. Glutamate transporter EAAC-1-deficient mice develop dicarboxylic aminoaciduria and behavioral abnormalities but no neurodegeneration. EMBO J. 1997;16:3822–3832. doi: 10.1093/emboj/16.13.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Porton B, et al. Isoforms of the neuronal glutamate transporter gene, SLC1A1/EAAC1, negatively modulate glutamate uptake: Relevance to obsessive-compulsive disorder. Transl Psychiatry. 2013;3:e259. doi: 10.1038/tp.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murray RM, Paparelli A, Morrison PD, Marconi A, Di Forti M. What can we learn about schizophrenia from studying the human model, drug-induced psychosis? Am J Med Genet B Neuropsychiatr Genet. 2013;162B:661–670. doi: 10.1002/ajmg.b.32177. [DOI] [PubMed] [Google Scholar]
- 60.Krebs G, Heyman I. Obsessive-compulsive disorder in children and adolescents. Arch Dis Child. 2015;100:495–499. doi: 10.1136/archdischild-2014-306934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dittrich WH, Johansen T. Cognitive deficits of executive functions and decision-making in obsessive-compulsive disorder. Scand J Psychol. 2013;54:393–400. doi: 10.1111/sjop.12066. [DOI] [PubMed] [Google Scholar]
- 62.Franklin KBJ, Paxinos G. 2013. Paxinos and Franklin's The Mouse Brain in Stereotaxic Coordinates (Academic, an imprint of Elsevier, Amsterdam), 4th Ed, Vol 1 (unpaged)
- 63.National Research Council . Guide for the Care and Use of Laboratory Animals. 8th Ed National Academies Press; Washington, DC: 2011. [Google Scholar]
- 64.Furness DN, et al. A quantitative assessment of glutamate uptake into hippocampal synaptic terminals and astrocytes: New insights into a neuronal role for excitatory amino acid transporter 2 (EAAT2) Neuroscience. 2008;157:80–94. doi: 10.1016/j.neuroscience.2008.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pakkenberg B, Gundersen HJ. New stereological method for obtaining unbiased and efficient estimates of total nerve cell number in human brain areas. Exemplified by the mediodorsal thalamic nucleus in schizophrenics. APMIS. 1989;97:677–681. doi: 10.1111/j.1699-0463.1989.tb00462.x. [DOI] [PubMed] [Google Scholar]
- 66.Gondre-Lewis MC, Darius PJ, Wang H, Allard JS. Stereological analyses of reward system nuclei in maternally deprived/separated alcohol drinking rats. J Chem Neuroanat. 2016;76:122–132. doi: 10.1016/j.jchemneu.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.