Significance
The homologous recombination protein RAD51 has been extensively studied in prokaryotes and lower eukaryotes. However, there is a significant lack of knowledge of the role of this protein and its regulation in an in vivo context in vertebrates. Here we report the first viable vertebrate mutant model of rad51 in zebrafish. These mutant fish enabled us to confirm the recently discovered role of RAD51 in Fanconi anemia pathogenesis. We report that p53-linked embryonic stem cell defects directly lead to hematological impairments later in life. Comutation of rad51 with p53 rescues the observed hematological defects, but predisposes the fish to early tumor development. The application of this model opens new possibilities to advance Fanconi anemia drug discovery.
Keywords: Fanconi anemia, stem cells, hematopoiesis, cytokine effects, inflammation
Abstract
RAD51 is an indispensable homologous recombination protein, necessary for strand invasion and crossing over. It has recently been designated as a Fanconi anemia (FA) gene, following the discovery of two patients carrying dominant-negative mutations. FA is a hereditary DNA-repair disorder characterized by various congenital abnormalities, progressive bone marrow failure, and cancer predisposition. In this report, we describe a viable vertebrate model of RAD51 loss. Zebrafish rad51 loss-of-function mutants developed key features of FA, including hypocellular kidney marrow, sensitivity to cross-linking agents, and decreased size. We show that some of these symptoms stem from both decreased proliferation and increased apoptosis of embryonic hematopoietic stem and progenitor cells. Comutation of p53 was able to rescue the hematopoietic defects seen in the single mutants, but led to tumor development. We further demonstrate that prolonged inflammatory stress can exacerbate the hematological impairment, leading to an additional decrease in kidney marrow cell numbers. These findings strengthen the assignment of RAD51 as a Fanconi gene and provide more evidence for the notion that aberrant p53 signaling during embryogenesis leads to the hematological defects seen later in life in FA. Further research on this zebrafish FA model will lead to a deeper understanding of the molecular basis of bone marrow failure in FA and the cellular role of RAD51.
Fanconi anemia (FA) is a hereditary DNA-repair disorder characterized by various congenital abnormalities, progressive bone marrow failure (BMF), and cancer predisposition (1). It is caused by mutations in one of 21 genes in the FA pathway (2, 3) (www2.rockefeller.edu/fanconi/). The FA pathway has been shown to be the major route for the removal of interstrand cross-links (ICL): DNA lesions that prevent replication and transcription by inhibiting DNA strand separation (4, 5). When the pathway is defective, these structures cannot be removed, potentially leading to cell death (6). Indeed, sensitivity to cross-linking agents, such as mitomycin C (MMC), is an absolute diagnostic criterion of FA (7).
Although FA is characterized by remarkable phenotypic heterogeneity, FA patients usually succumb to the depletion of hematopoietic stem and progenitor cells (HSPCs) in their BM, leading to pancytopenia and complete BMF. Therefore, BM transplantation is the only modality that offers a potential cure of hematopoietic defects but is itself associated with considerable morbidity (8, 9). Interestingly, a decrease in HSPCs (CD34+ cells) is already apparent in FA infants even before the first hematological symptoms appear (10). This finding led to the hypothesis that FA originates from defects during the formation of the initial HSPC pool, presumably because of an overactive p53/p21 response and cell cycle arrest (10). In agreement with this, FA mice have considerably smaller fetal livers than their healthy siblings (11). It remains unclear, however, at which stage during embryonic development these defects appear and how perturbation in the production of embryonic HSPCs relates to the phenotype seen in adulthood.
Because of the role FA genes play in the repair of ICLs, DNA damaging agents causing ICLs have been proposed as a major cause of BMF, with small aldehydes being the most likely candidates. Comutation of genes in the FA pathway and aldehyde metabolizing genes (Aldh2 and Adh5) resulted in significant reduction of HSPCs and BMF in double-mutant mice (12–16). In addition, FA patients lacking ALDH2 show a more severe phenotype (17, 18). Apart from their hypersensitivity to cross-linking agents, FA cells also react excessively to proapoptotic cytokines, such as IFN-γ and TNF-α (19–24). However, the role of cytokines in the etiology of BMF remains controversial (25–28).
In the last 2 y, a novel FA subtype associated with dominant-negative mutations in RAD51 has been reported, leading to the designation of RAD51 as FANCR (29–31). It has been shown to be involved in protecting broken down replication forks from excess processing by nucleases, linking the FA pathway with RAD51/BRCA2 (29, 32). In vivo studies of Rad51 have previously been very difficult, as mice lacking the protein invariably die during early embryogenesis (33, 34).
In this study, we characterized a viable vertebrate model of Rad51 loss. Indeed, our zebrafish rad51 loss-of-function mutant recapitulates many congenital and hematological features of FA. We provide in vivo evidence that decreased HSPC numbers during embryonic development directly lead to the later BM defects in FA. Finally, we show that rad51 mutants do not overproduce inflammatory cytokines, but are more sensitive to them and that prolonged inflammatory stress can further reduce marrow cellularity.
Results
The rad51sa23805 Allele Leads to Complete Loss of Functional Rad51.
To study the function of Rad51 in hematopoiesis, we obtained fish carrying the rad51sa23805 allele from the Sanger Institute Zebrafish Mutation Project (35). The rad51sa23805 allele has a C > T mutation at codon 203 in exon 7, which leads to a premature stop codon in the region of the RecA domain. In contrast to mice lacking Rad51, which invariably die during early development (33, 34), fish carrying homozygous copies of the rad51sa23805 (referred to as rad51−/− for brevity in the text) survive to adulthood. However, all surviving adults undergo sex reversal and are infertile males, lacking mature spermatozoa in the testes (SI Appendix, Fig. S1).
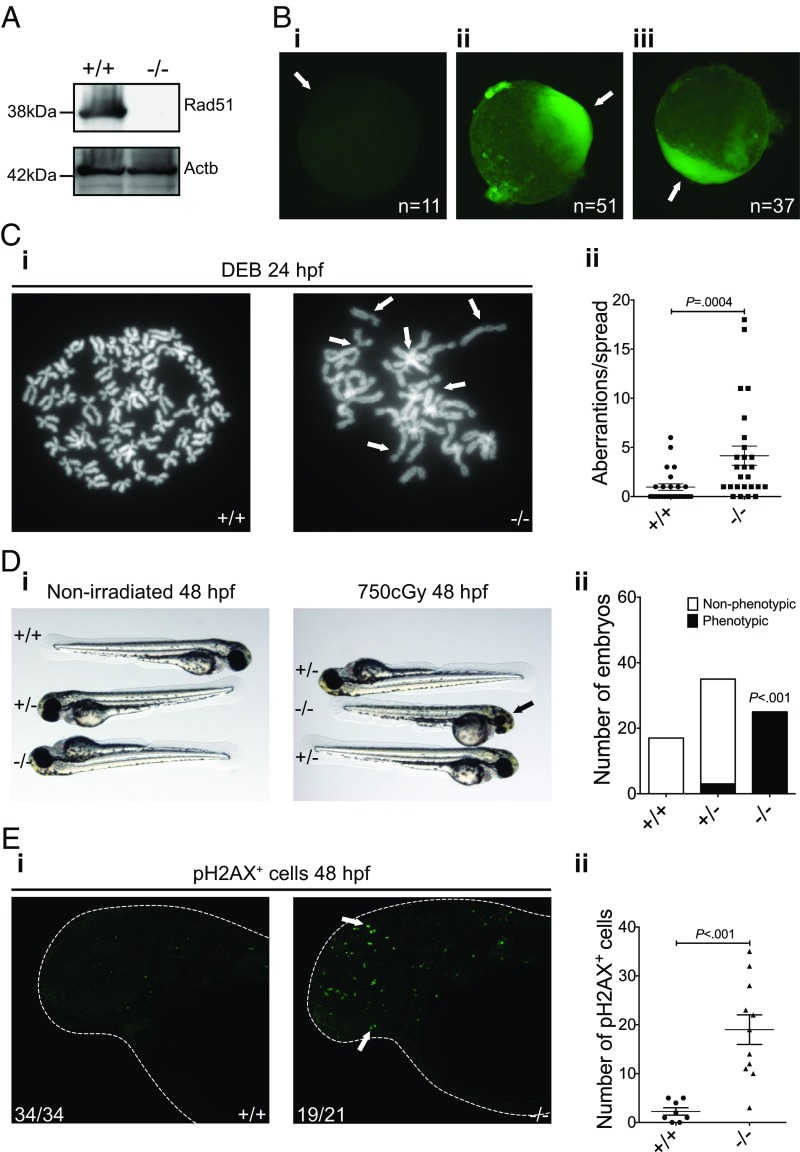
To ensure complete loss-of-function of the rad51sa23805 allele, we carried out a Western blot on testicular tissue, because Rad51 is highly expressed in germ cells in zebrafish and other species (36, 37). This approach confirmed that the full-length protein is lost in rad51−/− fish (Fig. 1A). However, immunostaining for Rad51 at the one-cell stage embryos (i.e., before maternal to zygotic transition) (38) showed diffuse staining in the cytoplasm of all embryos resulting from a rad51+/− in-cross (Fig. 1B). This finding suggested that rad51−/− embryos have maternally derived Rad51.
Fig. 1.
The rad51sa23805 allele leads to loss of Rad51 protein and causes DNA damage sensitivity. (A) Western blot showing the expression of Rad51 in testes extracts of WT and mutant zebrafish. β-Actin was used as a loading control. (B) Representative Rad51 immunostained embryos derived from a rad51+/− in-cross. Secondary only (i), Abcam primary (ii), AnaSpec primary (iii). (Magnification, 65×.) (C) Chromosome spreads of 24-hpf WT and mutant embryos treated with 1 µg/mL DEB for 20 h taken using a 100× oil-immersion objective. White arrows indicate characteristic damage (chromosome breaks and radial structures) in response to cross-linking agents (i). Quantification of the damage (ii). Mann–Whitney test, P = 0.0004, n+/+ = 25, n−/− = 26. (D) Comparison of the response of 48-hpf WT and mutant embryos to irradiation (i). (Magnification, 15×.) The black arrow indicates the small head and eye phenotype, which is quantified in ii. Two-tailed Fisher’s exact test pooling WT and heterozygotes as control group, P < 0.001, n = 67. (E) Immunostaining for pH2AX in WT and mutant embryos with pictures of representative embryos taken with a 63× water-immersion objective (i) and quantification of foci (ii). White arrows indicate example foci. Two-tailed Student’s t test, P < 0.0001, n+/+ = 8, n−/− = 11.
Because of the early embryonic lethality of murine Rad51 mutants (33, 34), we decided to look at the functional redundancy between rad51 and its paralogues. To do this, we knocked out rad51l1, the fish ortholog of the human RAD51B gene using CRISPR. Initially, we raised 204 embryos resulting from a rad51+/−, rad51l1+/− in-cross to adulthood. However, of these none were double mutants, even though 12.75 would be expected from Mendelian inheritance. We repeated the same cross and collected embryos at 4 d postfertilization (dpf). Again, 0 of 44 embryos were double mutants, even though 2.75 would have been expected. Finally, we repeated the cross and genotyped at 6 h postfertilization (hpf; the earliest point the genotyping works reliably). Of 47 embryos, none were double mutants. This early embryonic synthetic lethality of comutation indicates that rad51 and rad51l1 have at least some redundant functionality.
Lack of Rad51 in Embryos Leads to Increased DNA Damage Sensitivity.
Rad51 is essential for the repair of ICLs and double-stranded breaks via homologous recombination (HR). In ICL repair, it plays a role in both HR-linked processes, as well as HR-independent steps (29–31). To investigate the role of Rad51 in the repair of ICLs, we treated embryos resulting from an in-cross of rad51+/− parents with the cross-linking agents diepoxybutane (DEB) and MMC, as well as the topoisomerase I inhibitor camptothecin (CPT) and the poly(ADP-ribose) polymerase (PARP)-1 inhibitor 1,5-isoquinolinediol (DiQ). After treatment, the tails of the embryos were used for chromosome spreads, whereas the heads were kept for genotyping. The spreads revealed that DEB induced defects characteristic for FA, including breaks and radial structures (Fig. 1C). MMC induced many of the same features, but also led to considerably more premature chromatid separation events (SI Appendix, Fig. S2A). Similarly, CPT induced high levels of chromosome breaks, but few radial structures (SI Appendix, Fig. S2B). DiQ, on the other hand, was embryonic-lethal at higher concentration (100 µM), but at lower levels (10 µM) induced just a few breaks in both WT and mutant embryos (SI Appendix, Fig. S2C).
We also examined the role of Rad51 in the repair of other forms of double-stranded breaks not involving cross-linking agents. For this, we irradiated 24-h-old embryos from an in-cross of rad51+/− parents with γ-radiation and examined them at 48 hpf. The irradiated embryos were then blindly imaged, scored, and genotyped. The rad51−/− embryos developed small eyes and heads in response to radiation (Fig. 1 D, i). This phenotype was limited to the rad51−/− embryos (Fig. 1 D, ii), showing that they are more sensitive to irradiation than WT siblings. In addition, pH2AX (a marker of double-stranded breaks) immunostaining of nonirradiated embryos revealed extensive DNA damage in embryos lacking Rad51 in comparison with their WT siblings (Fig. 1E). Taken together, these data suggest that rad51−/− embryos are hypersensitive to DNA damage.
Finally, we considered the role of nonhomologous end-joining (NHEJ) in the survival of our mutant fish. To do that, we treated an in-cross of rad51+/− parents with a range of different concentrations of SCR-7, a DNA ligase IV inhibitor, and scored the embryos at 24 hpf (SI Appendix, Fig. S3). At the lowest concentration, embryos were completely unaffected, whereas at the higher concentration all embryos died. At no concentration were the mutant embryos more sensitive than their WT siblings (SI Appendix, Fig. S3), indicating that NHEJ is dispensable for the survival of rad51−/− embryos.
Rad51 Mutants Recapitulate Many Congenital and Hematological Features of FA.
RAD51 mutations were recently linked to FA in humans in two case reports (29, 30). Therefore, we examined the rad51 mutant fish for congenital and hematological features associated with FA. A common congenital symptom of FA is decreased height (1). On account of that, we measured the body length of fish with and without rad51 mutation throughout development. Although embryonic development was not affected by the mutation, the size of rad51−/− fish was decreased compared with their WT siblings, starting from around 23 dpf (SI Appendix, Fig. S4A). The size reduction during the larval period was maintained to adulthood with rad51 mutant fish being on average 10% shorter than their WT siblings (SI Appendix, Fig. S4 B and C). In addition, rad51−/− embryos and larvae developed microphthalmia, another distinct feature of FA patients (SI Appendix, Fig. S5).
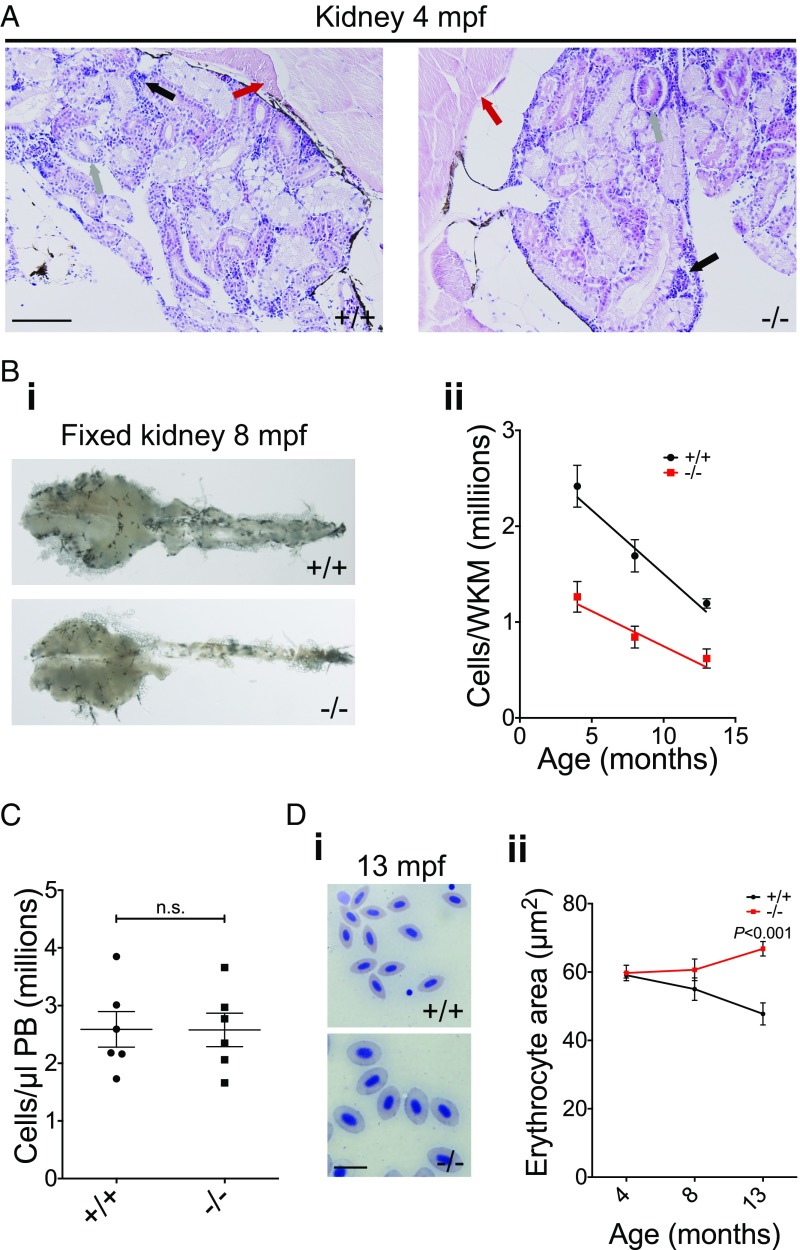
To assess whether loss of rad51 affects adult hematopoiesis, we inspected the kidney marrow (WKM) of zebrafish, which is analogous to BM in mammals. Unlike in mammals, there is only one kidney in zebrafish, which is the only site of adult hematopoiesis. Importantly, there is no space restriction of the marrow by the rigid bone, as kidney tissue is very spongy and flexible. We first obtained several H&E-stained histological sections each of the kidney of four WT and mutant fish, ranging between 4 and 8 mo of age. Representative sections showed no noticeable qualitative differences between rad51+/+ and rad51−/− fish (Fig. 2A). However, even though the morphology appeared normal, kidney size and cell number were considerably (∼50%) decreased in rad51−/− fish (Fig. 2B). The kidney cellularity of rad51−/− fish gradually decreased with aging, but at the same rate as in WT fish (Fig. 2 B, ii), suggesting that the mutants established steady-state hematopoiesis despite displaying kidney hypocellularity. Although rad51 mutants did not develop cytopenia in the peripheral blood (PB) (Fig. 2C), they did accumulate macrocytic erythrocytes in the PB (Fig. 2D), suggesting a gradual worsening of the phenotype over time.
Fig. 2.
Adult rad51 mutant fish display kidney marrow cytopenia. (A) H&E-stained histological sections of 4-mpf WT and mutant kidneys using a 20× objective. Muscle (red arrow), ducts/tubules (gray arrow), and hematopoietic kidney marrow (black arrow) can be seen. (Scale bar, 100 µm.) (B) Fixed 8-mpf WT and mutant kidneys (i) (magnification, 10×); quantification of the number of total cells per freshly isolated kidney at different ages using a hemocytometer (ii). Two-way ANOVA was used and type III model fit [Armitage et al. (73)]. The test shows a significant influence of age [F(1, 50) = 18.23, P < 0.0001] and mutation status [F(1, 50) = 10.87, P = 0.0018] on phenotype. Four months postfertilization n+/+ = 6, n−/− = 6; 8 mpf n+/+ = 16, n−/− = 16; 13 mpf n+/+ = 6, n−/− = 4. (C) Quantification of PB cells in WT and mutant fish at 4 mpf. Two-sided t test, n+/+ = 6, n−/− = 6. n.s., not significant. (D) In i, blood smears of 13-mpf WT (Upper) and mutant fish (Lower) are compared. (Scale bar, 10 µm.) In ii, the change is quantified using two-way ANOVA and a type III model fit [Armitage et al. (73)]. There was a statistically significant interaction between age and mutation status [F(1, 28) = 12.89, P = 0.0012], no significant influence of age [F(1, 28) = 180.76, P = 0.392] and no significant influence of mutation status [F(1, 28) = 2.88, P = 0.1006]. P value shown on the graph stems from a post hoc Tukey multiple-comparison test. Four months postfertilization: n+/+ = 6, n−/− = 6; 8 mpf: n+/+ = 5, n−/− = 5; 13 mpf: n+/+ = 6, n−/− = 4. Bars represent mean ± SEM.
Rad51−/− Fish Show a Hyperproliferative Phenotype in the WKM.
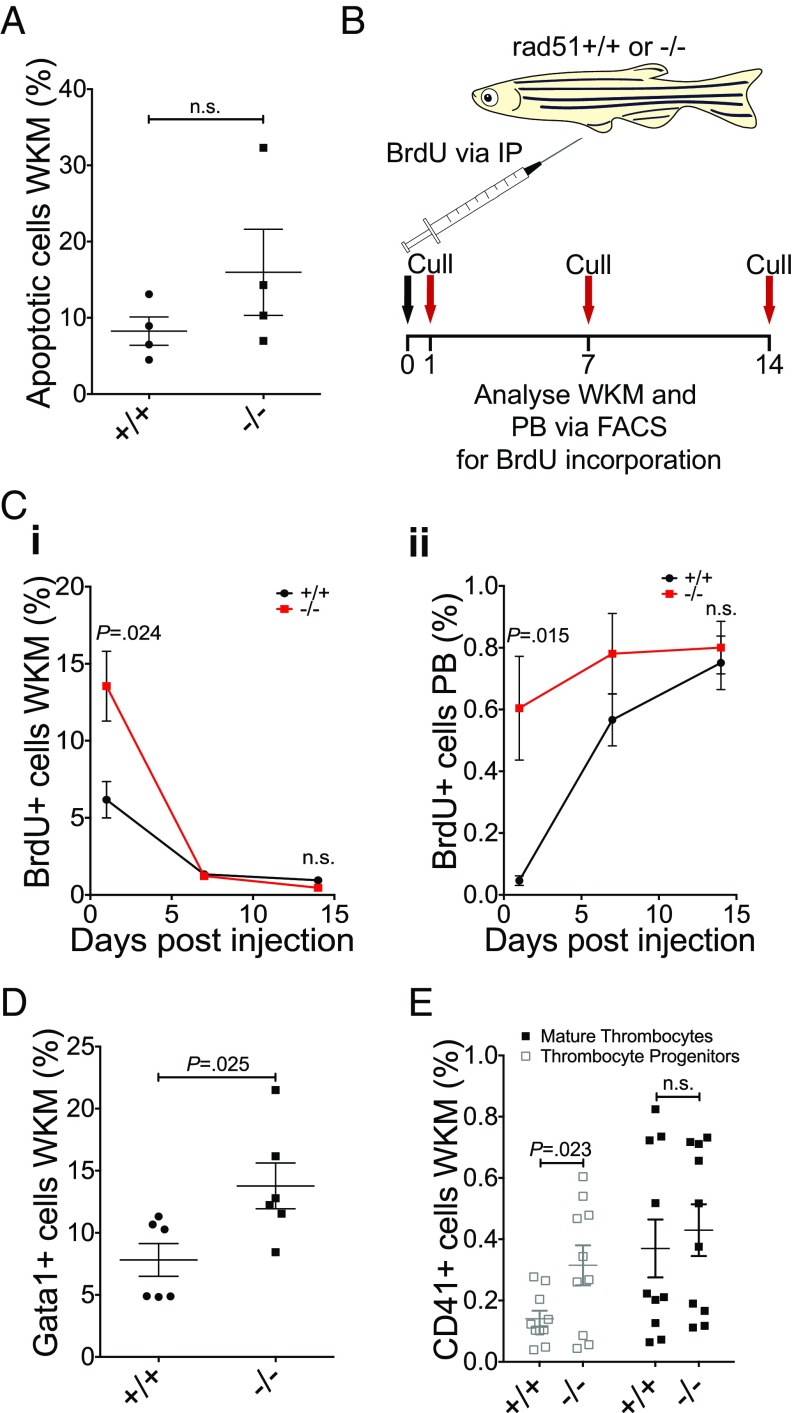
The number of cells in the WKM is determined by the balance between their proliferation and apoptosis rate, as well as their migration to the PB. To test whether the decrease in kidney cell numbers in rad51 mutants was a result of apoptosis, we carried out an Annexin V-propodium iodide (AV-PI) staining assay on kidney tissue (Fig. 3A and SI Appendix, Fig. S6 A and B). We observed no statistically significant difference between rad51+/+ and rad51−/− fish, excluding apoptosis as an initial cause for the decrease in cell numbers in the kidney. Therefore, we next focused on the proliferation of the kidney marrow cells.
Fig. 3.
HSPCs in the kidney adult rad51 mutant fish show increased proliferation. (A) AV-PI assay to assess apoptosis in the kidney. Two-sided t test, n+/+ = 4, n−/− = 4. (B) Schematic of the experimental design for the BrdU incorporation experiments. Fish were injected once with 10 mg/mL BrdU and culled after 1, 7, or 14 d to obtain the blood and kidney marrow for antibody staining and FACS analysis. (C) Percentage of BrdU+ cells in the kidney (i). Two-sided Student’s t test, P = 0.024 at 1 d postinfection (dpi) and P > 0.05 at 14 dpi; 1 dpi n+/+ = 5, n−/− = 6; 7 dpi n+/+ = 6, n−/− = 6; 14 dpi n+/+ = 5, n−/− = 5. Percentage of BrdU+ cells in the peripheral blood (ii). Two-sided Student’s t test, P = 0.0015 at 1 dpi and P > 0.05 at 14 dpi; 1 dpi n+/+ = 5, n−/− = 6; 7 dpi n+/+ = 6, n−/− = 5; 14 dpi n+/+ = 5, n−/− =5. (D) Percentage of gata1:GFP+ cells in the kidney at 4 mpf. Two-sided Student’s t test, P = 0.025, n+/+ = 6, n−/− = 6. (E) Percentage of dim and bright cd41:GFP+ cells in the kidney at 4 mpf, labeling thrombocytic progenitors, and mature thrombocytes, respectively. Two-tailed Student’s t test. Thrombocytic progenitors: P = 0.023, mature thrombocytes: P = not significant, n+/+ = 10, n−/− = 10. Bars represent mean ± SEM in all graphs; n.s., not significant.
The standard assay for assessing cell proliferation rates is the incorporation of BrdU into the DNA. To follow the kinetics of BrdU incorporation and dilution via division or migration to the circulation, we measured BrdU labeling at several time points postinjection (Fig. 3B). Because erythrocytes are nucleated in zebrafish, our analysis was not limited only to leukocytes and allowed us to robustly assess changes in the WKM as well as in the PB. The initial number of BrdU+ cells in the WKM was twofold higher in rad51−/− fish compared with the control (Fig. 3 C, i and SI Appendix, Fig. S6F), suggesting an increased proliferation rate in the mutant. This was followed by a fast dilution of the BrdU label over 2 wk, because of the increased cell division of mutant HSPCs (Fig. 3 C, i). In line with this finding, the initial number of BrdU+ cells in the PB was higher in rad51−/− fish compared with WT siblings (Fig. 3 C, ii), but then plateaued faster because of the dilution of the label in the kidney (Fig. 3 C, ii).
To show that blood, rather than other cells in the WKM, are proliferating, we generated mutants in various transgenic backgrounds. The rad51−/− Tg(gata1a:EGFP) line (39) was used to assess the erythrocytic lineage, including erythrocytic progenitors. The rad51−/− Tg(cd41/itga2b:EGFP) (40) line was used to detect thrombocytic progenitors, which are labeled in the cd41:EGFPdim subpopulation (41). Consistent with the BrdU incorporation experiments, we saw an increase in newly made gata1:EGFP+ erythrocytes in the kidney (Fig. 3D and SI Appendix, Fig. S6C), as well as an increase in cd41:EGFPdim thrombocytic progenitors (Fig. 3E and SI Appendix, Fig. S6D).
Together, this evidence shows that loss of rad51 leads to a hyperproliferation of HSPCs, possibly as a compensatory mechanism to prevent cytopenia in the PB. However, the lowered cellularity of the kidney in adult rad51−/− fish cannot be explained by this finding, suggesting that the kidney cytopenia stems from an early, possibly embryonic HSPC defect.
Lack of Rad51 Causes an HSPC Defect During Early Development.
The definitive wave of hematopoiesis starts at around 30 hpf, when long-term hematopoietic stem cells (HSCs) are formed from endothelial cells of the ventral wall of the dorsal aorta. These newly made HSCs move to the caudal hematopoietic tissue (CHT), which serves as an intermediate place of hematopoiesis in which the HSCs expand greatly, akin to the mammalian fetal liver (42).
To assess the underlying cause of the decreased number of cells in the adult kidney of FA fish, we focused on embryonic hematopoiesis. To this end, we used whole-mount in situ hybridization (ISH) using a cmyb-specific probe, which labels HSPCs. Indeed, at 2 dpf, rad51−/− embryos had a decreased number of HSPCs compared with the WT embryos from the same clutch (Fig. 4A). At 4 dpf, the difference in the number of HSPCs in the CHT of rad51−/− and WT embryos was further exacerbated (Fig. 4B).
Fig. 4.
The rad51sa23805 HSPC defect starts during embryonic development. (A) ISH using a cmyb-specific probe at 2 dpf; the arrow shows HSPCs. Representative images of the three different staining categories are shown (i) and a quantification of the different genotypes (ii) n = 119 from two clutches. (B) ISH using a cmyb-specific probe at 4 dpf; the arrow shows HSPCs. Representative images of the three different staining categories are shown (i) and a quantification of the different genotypes (ii), n = 120 from two clutches. (C) Quantification of BrdU+ cells in the tail at 2 dpf. Two-sided Student’s t test, P = 0.042, n+/+ = 3, n−/− = 3. (D) Quantification of BrdU+ cells in the CHT at 4 dpf. Two-sided Student’s t test, n+/+ = 4, n−/− = 4. Bars represent mean ± SEM in C and D. n.s., not significant. (E) Representative images of TUNEL-stained 2 dpf embryos from a rad51+/− in-cross. Dotted lines indicate the area of the CHT that was scored. Arrows indicate TUNEL+ cells. (F) Quantification of three clutches of TUNEL-stained 2 dpf rad51+/− in-crosses. Each clutch was scored blindly and consisted of 10+/+ and 10−/− embryos each. Shown is the mean of all clutches ± SEM. (Magnification, 100× in all images.)
Following up on that finding, we carried out a BrdU incorporation assay on the tail tissue of 2-dpf embryos, which showed that the proliferation rate in the tail of rad51−/− embryos was about half that of rad51+/+ embryos (Fig. 4C). Although not statistically significant, this trend was still apparent in the CHT at 4 dpf (Fig. 4D).
In addition to proliferation, we also investigated apoptosis at 2 dpf by carrying out a TUNEL assay on several crosses of rad51 heterozygotes. This process revealed a twofold increase in apoptosis in the CHT in mutants compared with WT embryos (Fig. 4 E and F). Taken together, our data imply that the cytopenia in the adult kidney is caused by an increase apoptosis of HSPCs, as well as reduced proliferation during early embryogenesis, mainly before 4 dpf.
The HSPC Defects in rad51−/− Fish Are Mediated via p53.
The defects seen in FA have recently been linked to an aggravated p53 response (10). To focus on the role of p53 in the HSPC defect, we generated a zebrafish line carrying mutations in both rad51 and p53. This double mutation was able to rescue the number of HSPCs in the CHT of 4-dpf embryos (Fig. 5A and SI Appendix, Table S3). Importantly, the number of cells in the adult WKM of p53−/− rad51−/− fish reached WT levels by 4 m postfertilization (mpf) (Fig. 5B). Along with the rescued marrow cellularity, there was no difference in the proliferation of cells in the WKM of WT and double-mutant fish at 4 mpf, as shown by a BrdU incorporation assay (Fig. 5C).
Fig. 5.
The HSPC defects in rad51sa23805 fish are rescued in a p53 mutant background. (A) Representative images of 4-dpf embryos resulting from in-crosses of p53+/− rad51+/− parents stained using a cmyb-specific probe. The total number of embryos used (all genotypes) n = 237 from four clutches. For information about all genotypes, see SI Appendix, Table S3. Arrows indicate HSPCs. (Magnification, 100×.) (B) Percentage of BrdU+ cells in the kidney at 4 mpf at 1 dpi. Two-sided Student’s t test, np53+/+ rad51+/+ = 5, np53−/− rad51−/− = 5. (C) Number of total cells per kidney at 4 mpf quantified using a hemocytometer. Analysis using one-way ANOVA [F(3, 43) = 10.45, P < 0.0001], individual P values shown in the figure are from Tukey’s post hoc test, np53+/+ rad51+/+ = 16, np53+/+ rad51−/− = 16, np53−/− rad51+/+ = 6, np53−/− rad51−/− = 8. Bars represent mean ± SEM in B and C; n.s., not significant.
We also examined the congenital phenotypes in the double mutants. The sex reversal seen in rad51 single mutants was corrected (SI Appendix, Fig. S7 A–C), but the size defect was not (SI Appendix, Fig. S7D). However, neither female, nor male double mutants were fertile (SI Appendix, Fig. S7 A–C). Taken together, our data suggest that the marrow hypocellularity in adult fish was not because of the smaller size of rad51 mutant, meaning that these two phenotypes are uncoupled. Instead, we observed a high correlation between the number of HSPCs generated early during embryonic development and the kidney marrow cellularity in adulthood. Therefore, the rescue in the embryonic definitive hematopoiesis can revert all defects observed in adult hematopoiesis in rad51 mutants.
Finally, the tumor incidence of the double mutants was 30%, with the first tumors developing from 5 mpf [5 mo earlier than reported for p53 single mutants (43)]. The tumors resembled malignant peripheral nerve sheath tumors (MPNSTs), (SI Appendix, Fig. S7 E and F), which is the most common type of malignancy in p53−/− fish (43). None of the other fish (i.e., WT, rad51 mutants, and heterozygotes) developed any kind of noticeable tumor, including the oldest 13-mpf rad51−/− fish.
rad51−/− Fish Are More Sensitive to Inflammatory Stress.
An aberrant inflammatory response has been postulated to be one of the potential causes of the BMF in FA. This is thought to be a result of increased expression of inflammatory cytokines in FA patients and an excess apoptosis of HSPCs in response to these factors. To test this hypothesis, we developed a zebrafish model of prolonged inflammatory stress. Over a period of 4 wk, we injected rad51−/− and WT fish with the immunostimulant polyinosinic:polycytidylic acid (pI:pC) and assessed changes in the kidney cellularity, lineage output, and the expression of genes associated with inflammation (Fig. 6A).
Fig. 6.
Lack of Rad51 causes increased sensitivity to prolonged inflammatory stress. (A) Schematic of the experimental design. Both WT and rad51−/− fish were injected every 7 d with 10 µL 10 mg/mL pI:pC acid, four injections in total. All fish were culled 3 d after the last injection. Control fish were not injected; Rad51+/+, nnoninjected = 10, ninjected = 9; Rad51−/−, nnoninjected = 8, ninjected = 9. (B) Absolute number of cells belonging to different blood lineages in the kidney gained by combining FACS data with the cell counts shown in A. Statistical tests were carried out individually for each cell type, using two-way ANOVA. P value shown on the graph stems from a post hoc Šidak multiple-comparison test, comparing noninjected to injected fish within each genotype. For all groups, n is the same as in A. (C) The total number of cells in the kidney in injected and noninjected fish. Two-way ANOVA was carried out on the reciprocal of the data to fulfill the requirement of homoscedasticity as measured by Bartlett’s test (before transformation: P = 0.0002, after transformation: P = 0.095). There was a statistically significant effect of mutation status [F(1, 32) = 29.86, P < 0.0001] and of injection status [F(1, 32) = 6.778, P = 0.014]. P value shown on the graph stems from a post hoc Tukey multiple-comparison test. For all groups, n is the same as in A. (D) Viable cells as determined by PI-staining. Two-way ANOVA revealed a significant influence of injection status [F(1, 32) = 100.1, P < 0.0001]. P values on the graph stem from a post hoc Tukey multiple-comparison test. Bars represent mean ± SEM in B–D. (E) Percentage of BrdU+ cells in the WKM of WT and mutant (KO) fish in response to pI:pC. 1I, one injection; 2I, two injections; 4I, four injections. (F) Relative expression of genes linked to apoptosis and proliferation. (i) p53. P value shown on the graph stems from a post hoc Tukey multiple-comparison test. (ii) p21. Bars represent geometric mean ± 95% CI in E and F.
PI:pC resembles double-stranded RNA and is known to induce the expression of proinflammatory cytokines, such as TNF-α (44) and IL-1 (45). Therefore, it has been considered to accurately mimic viral infection (45) in murine and fish models (44–47). Indeed, a single intraperitoneal injection of pI:pC in zebrafish induced robust inflammatory response just 6 h postinjection (SI Appendix, Fig. S8A). Mutants did not express more inflammatory cytokines when unchallenged (SI Appendix, Fig. S8B). Importantly, the prolonged exposure of adult WT fish to pI:pC resulted in a twofold increased production of monocytes (Fig. 6B) but did not overtly affected kidney marrow cellularity or cell viability (Fig. 6 C and D). In addition, we observed clear up-regulation of monocyte-specific genes (SI Appendix, Fig. S8C). The skew toward monocyte production in turn decreased the erythrocyte output and to a lesser extent other lineages. In contrast, repeated pI:pC injections of rad51−/− fish led to no significant change in the number of produced monocytes (Fig. 6B) and an ∼25% decrease in the total number of cells in the kidney (Fig. 6C). As in the WT fish, the prolonged inflammation decreased the number of erythrocytes in the kidney marrow of rad51−/− fish, thus possibly contributing to the overall reduction in kidney cellularity. This was mediated by a normalization of proliferation rates in the kidneys of pI:pC-injected mutant fish (Fig. 6E and SI Appendix, Fig. S8D). Therefore, the prolonged inflammatory stress can lead to severe marrow defects in our rad51−/− zebrafish FA model not only in terms of the lineage output, but also by affecting the total marrow cellularity.
Interestingly, the unchallenged rad51 mutants down-regulated p53, but showed an exaggerated reaction in p53 expression to repeated inflammatory stress (Fig. 6 F, i). Fitting with this observation, p21 expression followed a similar trend in gene expression in noninjected and pI:pC-injected mutants (Fig. 6 F, ii).
rad51−/− WKM Cells Are Unaffected by Acetaldehyde-Induced Stress.
DNA damage induced by small aldehydes has been proposed as a major cause of exhaustion of the HSCs in the BM of FA patients (12–18). To examine the sensitivity of rad51 mutants to acetaldehyde, we exposed them to acetaldehyde-induced stress over a period of 4 wk (SI Appendix, Fig. S9A). Our analysis revealed an increase in WKM cellularity in WT fish in all blood cell types (SI Appendix, Fig. S9 B and C) upon acetaldehyde injections, whereas rad51 mutant cell numbers were unaffected. The viability of WKM cells was decreased to a similar extent in both WT and mutant fish (SI Appendix, Fig. S9D) but with no change in p53 expression in the WKM (SI Appendix, Fig. S9E).
Discussion
The FA genes encode proteins that function cooperatively in the Fanconi DNA-repair pathway. Here we characterized a viable vertebrate rad51 loss-of-function mutant. Loss of rad51 in zebrafish recapitulated many congenital features of FA, such as short stature and microphthalmia (1), as well as hematological defects, including marrow cytopenia and accumulation of macrocytic erythrocytes in circulation (1, 7). Most importantly, rad51−/− fish showed increased sensitivity to cross-linking agents, an absolute diagnostic criterion of FA (7).
Furthermore, our results show that loss of rad51 does not lead to higher sensitivity to PARP inhibitors. Previous research on mammalian cells showed that BRCA1/2 mutant cells are more sensitive to PARP inhibition. One model proposes that this is because of the importance of PARP-1 in reactivating stalled replication forks in HR-deficient cells (48). That model is not compatible with our data, suggesting that PARP-BRCA synthetic lethality stems from functions unrelated to HR. PARP1 is highly conserved between zebrafish and humans (∼70%). A small-molecule inhibitor such as DiQ should therefore be able to enter the zebrafish embryos and inhibit the enzyme the same way as in human cells. Further evidence for this is provided by the lethality of high doses of this drug in WT and rad51 mutant fish. However, we were unable to obtain brca2 mutant zebrafish to use as a positive control for DNA damage in response to PARP inhibition. This should be taken into account when interpreting our findings.
Although loss of Rad51 leads to an early embryonic death in mice (33, 34), zebrafish lacking rad51 survive to adulthood. This is not entirely surprising, as fish lacking brca2 also survive to adulthood (49, 50), whereas Brca2 mutant mice die before birth (51, 52). It has been hypothesized that maternal mRNA contributes to embryonic viability of zebrafish mutants (49). This seems likely, considering the presence of maternally derived Rad51 in rad51 mutant embryos. However, another plausible explanation is the functional redundancy between rad51 and its paralog rad51l1 in zebrafish. Our analysis suggests that rad51 and rad51l1 are able to partially compensate for each other, leading to the lethality of double mutants. In contrast, NHEJ did not appear to play a role in compensating for the loss of HR. Interestingly, rad51−/− fish, like brca2−/− fish, show sex reversal that can be rescued upon p53 comutation (49, 50). Additional p53 loss further caused development of MPNSTs in both brca2 and rad51 mutants, which occurred considerably earlier and at the higher incidence than in p53 single mutants (43, 49, 50, 53).
FA is genetically and phenotypically heterogeneous disorder, but BM failure is the most common cause of death (1), with patients having lowered CD34+ progenitor cell numbers from birth (10, 54). This led to the hypothesis that hematological defects in FA originate from an impairment of HSPCs during embryonic development, which leads to a decreased number of HSPCs at birth (10, 11). Here we provide in vivo evidence that the decrease in HSPC numbers in adult fish indeed stems from a combination of decreased proliferation and increased apoptosis during embryonic development. This defect appears to be mediated via p53 (10), as our p53/rad51 double mutants did not display any observable hematological defects in embryos or adults.
In agreement with our study, knockdown of fancd2 in zebrafish embryos causes massive apoptosis in the whole body, associated with up-regulation of genes in the p53 pathway, as well as decreased expression of cyclins. Strikingly, co-knockdown of p53 rescued both the cyclin down-regulation, as well as apoptosis (55), resembling the situation in our fish and providing further evidence for the importance of p53 signaling. This phenotype is, however, only partly consistent with what was observed in murine fetal Fancd2−/− cells. In the murine model the reduced number of HSPCs was set off by p38-mediated reduced proliferation, without any involvement of apoptosis or p53 signaling (56). More research into the causes of the early HSPC defects and how they can be mitigated is warranted. Because of the external development and their transparency, zebrafish embryos would be an ideal tool to discover compounds that can alleviate these defects.
The decreased WKM cellularity in rad51 zebrafish mutants also mirrors defects seen in Lig4 (a protein involved in NHEJ) mutant mice, which show decreased BM cell numbers, coupled to an approximately twofold increase in proliferation of long-term HSCs (57). This underlines the importance of repairing double-stranded breaks in HSC maintenance and suggests hyperproliferation of blood progenitors might be a common mechanism to cope with decreased cell numbers in the kidney/BM. However, like most murine FA models (58, 59), the fish never progressed to pancytopenia or spontaneous BMF/kidney marrow failure, which is probably because of species differences in the HSC compartment, lifespan, and rearing conditions. Nevertheless, together with the clinical data (29, 30), these facts provide further evidence for the designation of RAD51 as FANCR and show that our rad51−/− fish are a suitable model for FA.
The progressive decline of HSC numbers in FA patients leads to BMF and there is considerable evidence for an overproduction of inflammatory cytokines in patients and mouse models (26, 60–64). Furthermore, inflammatory stress (for example by repeated pI:pC injections) can induce BMF in mouse models of FA (65, 66). We did not observe an increase in inflammatory cytokines in our unchallenged rad51 mutants, ruling out cytokine overproduction as a cause for hematological defects. We did, however, observe a 25% decrease in WKM cellularity after repeated of pI:pC injections, possibly because of decreased proliferation compared with unchallenged fish. Unlike WT fish, rad51 mutants were unable to respond appropriately to inflammation and increase monocyte production. The decrease in WKM cellularity as a result of inflammatory stress was accompanied by an up-regulation of p53, again underlining the high importance of this pathway in the etiology of FA and explaining the normalized proliferation. Interestingly, we were unable to elicit similar changes using acetaldehyde, indicating that damage induced by small aldehydes is not a major factor in FA pathogenesis, at least after birth.
Our study characterized a viable vertebrate model of RAD51 loss, which recapitulates many human FA symptoms and is thus also a zebrafish model of the disease. Further study of this mutant will increase our knowledge of the cellular roles of RAD51 in vivo and will deepen our understanding of the molecular pathology of FA. Transparency of zebrafish embryos, their high fecundity, and the existence of transgenic lines labeling various blood lineages makes them very amenable for high-throughput screening in comparison with other model organisms. The application of the rad51 mutant line will significantly impact the development of novel therapeutics to improve HSPC function in FA patients, by screening for molecules that can alleviate the HSPC reduction in embryos.
Methods
Zebrafish Care and Strains.
Fish lines were maintained in the Sanger Institute zebrafish facility according to European Union regulations. WT fish were of the Tübingen long-fin strain. Fish were genotyped as described previously (35).
Western Blotting.
Western blotting was carried out as described previously (67). Antibodies can be found in SI Appendix, Table S1.
Embryo Irradiation.
Embryos were irradiated at 24 hpf in a Gammacell 1000 Elite Blood Irradiator (MDS Nordiron) at 750 cGy.
Immunostaining.
Staining was carried out as described previously (68). We used Hoechst 33342 as nuclear stain. Embryos were imaged on a Leica SP-5 confocal microscope using a 40× water-immersion lens. Antibodies can be found in SI Appendix, Table S1.
Chromosome Spreads.
Embryos were treated with 1 µg/mL DEB (Sigma Aldrich), 5 µg/mL MMC (Sigma Aldrich), 1 nM CPT (Sigma Aldrich), or 10 µM 1,5-isoquinolinediol (Sigma Aldrich) in egg water between 4 and 24 hpf. From here on we followed The Zebrafish Book, 4th edition (69), but kept the head of each embryo for genotyping. VECTASHIELD mounting medium with DAPI was used to visualize the spreads.
NHEJ Inhibition.
Embryos were treated with 1, 10, 25, 50, 75, and 100 µM SCR-7 (Sigma Aldrich) at 4 hpf. Embryos were scored for defects at 24 hpf.
CRISPR-Cas9.
Mutations in rad51l1 were induced at exon two, leading to a 7-bp deletion (478-484delTGGGTCC in the cDNA). The targeted DNA sequence was 5′-GGATGTCCTGTCGGTCACCCAGG-3′. ssDNA oligonucleotides 5′-TAGGATGTCCTGTCGGTCACCC-3′ and 5′-AAACGGGTGACCGACAGGACAT-3′ (Sigma-Aldrich) were annealed and ligated with pDR274 vector (Addgene) linearized with BsaI (New England Biolabs) to make guide RNA (gRNA) expression vectors. gRNA was prepared with MAXIscript T7 kit (Life Technologies) using DraI-linearized gRNA expression vector as a template, and Cas9 mRNA was synthesized using mMESSAGE mMACHINE T7 kit (Ambion) and pMLM3613 expression vector (Addgene) linearized with PmeI (New England Biolabs). Zebrafish embryos were injected at the one-cell stage with 12.5 pg of gRNA and 160 pg of Cas9 mRNA. For a detailed zebrafish CRISPR methodology, see Brocal et al. (70).
Histology.
Formalin-fixed tissues were processed and sectioned using standard techniques (71), followed by staining with Harris H&E.
AV-PI Assay.
We used the Alexa Fluor-488 Annexin V/Dead Cell Apoptosis Kit (Thermo Fisher) according to the manufacturer’s instructions.
BrdU Assay on Adults.
Fish were injected with 10 µL of 10 mg/mL BrdU (Sigma Aldrich) and culled at 1, 7, or 14 d postinjection. We extracted kidney and blood, made single-cell suspensions, and fixed cells in 70% EtOH overnight. BrdU immunostaining was carried out as described previously (67), after which the cells were resuspended in PBS and analyzed using FACS.
BrdU Assay on Embryos.
Embryos were chilled on ice for 15 min. This was followed by a 20-min incubation in 10 mM BrdU on ice. After a 3-h recovery in egg water, embryos were fixed in 4% PFA. Heads from embryos were used for genotyping. For 2-dpf embryos, the whole tails were pooled according to genotype, whereas for 4-dpf embryos just the CHT was dissected. Samples were treated with 10 mM DTT in 1× Danieau’s solution for 30 min at room temperature, followed by incubation in liberase (Roche) in PBS for 3 h at 37 °C. The reaction was stopped by replacing the solution with 5% FBS/PBS. Single-cell suspensions were fixed in 70% ethanol overnight. From here on, the staining process and analysis was identical to cells obtained from adults. Antibodies can be found in SI Appendix, Table S1.
TUNEL Assays.
Embryos for TUNEL assays were fixed and stored as for ISH. Staining was carried out using the In-Situ Cell Death Detection Kit, AP (Roche), according to the manufacturer’s instructions.
Kidney FACS.
Dissected kidneys were placed in 5% FBS/PBS and processed to single-cell suspensions. Dead cells were excluded using DAPI (Sigma-Aldrich) or PI. Flow cytometry was carried out on a MoFlo XDP (Beckman Coulter), a BD LSRFortessa, or a BD Influx (BD Biosciences).
In Situ Hybridization.
ISH was carried out as described previously (72). We used a cmyb antisense probe. Embryos were blindly sorted into high-, medium-, and low-staining conditions followed by genotyping.
Long-Term pI:pC Injections.
Fish were injected with 10 mg/mL pI:pC (Sigma-Aldrich) once a week, totaling four injections. Fish were culled 1 or 3 d after the last injection. Optionally, 10 mg/mL BrdU were added to the last injection. Kidneys were processed to single-cell suspensions for FACS, as described above, and cell numbers counted. Remaining cell suspensions were used for gene-expression analysis.
Long-Term Acetaldehyde Injections.
For long-term acetaldehyde injections, the same protocol as for the pI:pC injections was followed, but using 10 µL 1% acetaldehyde (Sigma Aldrich) in PBS instead for the injections.
qPCRs.
The qPCR reaction used SYBR green (Thermo Fisher) and run on a QuantStudio 3 (Thermo Fisher) qPCR machine. Statistics were carried out on raw ΔCt values. Primers used are listed in SI Appendix, Table S2.
Supplementary Material
Acknowledgments
We thank the Sanger Institute Zebrafish Mutation Project for supplying the rad51sa23805 allele; Sebastian Gerety for supplying the tp53zdf1 line; Yvette Hooks for her help with histology; and the Sanger Institute FACS core facility and Charlotte Labalette for their experimental help. This work was supported by Cancer Research UK Grant C45041/A14953 (to A.C.); a core support grant from the Wellcome Trust and Medical Research Council to the Wellcome Trust–Medical Research Council Cambridge Stem Cell Institute; and a European Hematology Association–Jose Carreras Foundation Young Investigator Award and Isaac Newton Trust grant (to A.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620631114/-/DCSupplemental.
References
- 1.Shimamura A, Alter BP. Pathophysiology and management of inherited bone marrow failure syndromes. Blood Rev. 2010;24:101–122. doi: 10.1016/j.blre.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park JY, et al. Complementation of hypersensitivity to DNA interstrand crosslinking agents demonstrates that XRCC2 is a Fanconi anaemia gene. J Med Genet. 2016;53:672–680. doi: 10.1136/jmedgenet-2016-103847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bluteau D, et al. Biallelic inactivation of REV7 is associated with Fanconi anemia. J Clin Invest. 2016;126:3580–3584. doi: 10.1172/JCI88010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deans AJ, West SC. DNA interstrand crosslink repair and cancer. Nat Rev Cancer. 2011;11:467–480. doi: 10.1038/nrc3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim H, D’Andrea AD. Regulation of DNA cross-link repair by the Fanconi anemia/BRCA pathway. Genes Dev. 2012;26:1393–1408. doi: 10.1101/gad.195248.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schärer OD. DNA interstrand crosslinks: Natural and drug-induced DNA adducts that induce unique cellular responses. ChemBioChem. 2005;6:27–32. doi: 10.1002/cbic.200400287. [DOI] [PubMed] [Google Scholar]
- 7.Auerbach AD. Fanconi anemia and its diagnosis. Mutat Res. 2009;668:4–10. doi: 10.1016/j.mrfmmm.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butturini A, et al. Hematologic abnormalities in Fanconi anemia: An International Fanconi Anemia Registry study. Blood. 1994;84:1650–1655. [PubMed] [Google Scholar]
- 9.Kutler DI, et al. A 20-year perspective on the International Fanconi Anemia Registry (IFAR) Blood. 2003;101:1249–1256. doi: 10.1182/blood-2002-07-2170. [DOI] [PubMed] [Google Scholar]
- 10.Ceccaldi R, et al. Bone marrow failure in Fanconi anemia is triggered by an exacerbated p53/p21 DNA damage response that impairs hematopoietic stem and progenitor cells. Cell Stem Cell. 2012;11:36–49. doi: 10.1016/j.stem.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamimae-Lanning AN, Goloviznina NA, Kurre P. Fetal origins of hematopoietic failure in a murine model of Fanconi anemia. Blood. 2013;121:2008–2012. doi: 10.1182/blood-2012-06-439679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langevin F, Crossan GP, Rosado IV, Arends MJ, Patel KJ. Fancd2 counteracts the toxic effects of naturally produced aldehydes in mice. Nature. 2011;475:53–58. doi: 10.1038/nature10192. [DOI] [PubMed] [Google Scholar]
- 13.Garaycoechea JI, et al. Genotoxic consequences of endogenous aldehydes on mouse haematopoietic stem cell function. Nature. 2012;489:571–575. doi: 10.1038/nature11368. [DOI] [PubMed] [Google Scholar]
- 14.Oberbeck N, et al. Maternal aldehyde elimination during pregnancy preserves the fetal genome. Mol Cell. 2014;55:807–817. doi: 10.1016/j.molcel.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pontel LB, et al. Endogenous formaldehyde is a hematopoietic stem cell genotoxin and metabolic carcinogen. Mol Cell. 2015;60:177–188. doi: 10.1016/j.molcel.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosado IV, Langevin F, Crossan GP, Takata M, Patel KJ. Formaldehyde catabolism is essential in cells deficient for the Fanconi anemia DNA-repair pathway. Nat Struct Mol Biol. 2011;18:1432–1434. doi: 10.1038/nsmb.2173. [DOI] [PubMed] [Google Scholar]
- 17.Hira A, et al. Variant ALDH2 is associated with accelerated progression of bone marrow failure in Japanese Fanconi anemia patients. Blood. 2013;122:3206–3209. doi: 10.1182/blood-2013-06-507962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yabe M, et al. The phenotype and clinical course of Japanese Fanconi anaemia infants is influenced by patient, but not maternal ALDH2 genotype. Br J Haematol. 2016;175:457–461. doi: 10.1111/bjh.14243. [DOI] [PubMed] [Google Scholar]
- 19.Haneline LS, et al. Multiple inhibitory cytokines induce deregulated progenitor growth and apoptosis in hematopoietic cells from Fac-/- mice. Blood. 1998;91:4092–4098. [PubMed] [Google Scholar]
- 20.Rathbun RK, et al. Inactivation of the Fanconi anemia group C gene augments interferon-gamma-induced apoptotic responses in hematopoietic cells. Blood. 1997;90:974–985. [PubMed] [Google Scholar]
- 21.Rathbun RK, et al. Interferon-gamma-induced apoptotic responses of Fanconi anemia group C hematopoietic progenitor cells involve caspase 8-dependent activation of caspase 3 family members. Blood. 2000;96:4204–4211. [PubMed] [Google Scholar]
- 22.Wang J, et al. Overexpression of the fanconi anemia group C gene (FAC) protects hematopoietic progenitors from death induced by Fas-mediated apoptosis. Cancer Res. 1998;58:3538–3541. [PubMed] [Google Scholar]
- 23.Li X, et al. Continuous in vivo infusion of interferon-gamma (IFN-gamma) preferentially reduces myeloid progenitor numbers and enhances engraftment of syngeneic wild-type cells in Fancc-/- mice. Blood. 2004;104:1204–1209. doi: 10.1182/blood-2004-03-1094. [DOI] [PubMed] [Google Scholar]
- 24.Si Y, et al. Continuous in vivo infusion of interferon-gamma (IFN-gamma) enhances engraftment of syngeneic wild-type cells in Fanca-/- and Fancg-/- mice. Blood. 2006;108:4283–4287. doi: 10.1182/blood-2006-03-007997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarkies P, et al. FANCJ coordinates two pathways that maintain epigenetic stability at G-quadruplex DNA. Nucleic Acids Res. 2012;40:1485–1498. doi: 10.1093/nar/gkr868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosselli F, Sanceau J, Gluckman E, Wietzerbin J, Moustacchi E. Abnormal lymphokine production: A novel feature of the genetic disease Fanconi anemia. II. In vitro and in vivo spontaneous overproduction of tumor necrosis factor alpha. Blood. 1994;83:1216–1225. [PubMed] [Google Scholar]
- 27.Matsui K, Giri N, Alter BP, Pinto LA. Cytokine production by bone marrow mononuclear cells in inherited bone marrow failure syndromes. Br J Haematol. 2013;163:81–92. doi: 10.1111/bjh.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garaycoechea JI, Patel KJ. Why does the bone marrow fail in Fanconi anemia? Blood. 2014;123:26–34. doi: 10.1182/blood-2013-09-427740. [DOI] [PubMed] [Google Scholar]
- 29.Wang AT, et al. A dominant mutation in human RAD51 reveals its function in DNA interstrand crosslink repair independent of homologous recombination. Mol Cell. 2015;59:478–490. doi: 10.1016/j.molcel.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ameziane N, et al. A novel Fanconi anaemia subtype associated with a dominant-negative mutation in RAD51. Nat Commun. 2015;6:8829. doi: 10.1038/ncomms9829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Long DT, Räschle M, Joukov V, Walter JC. Mechanism of RAD51-dependent DNA interstrand cross-link repair. Science. 2011;333:84–87. doi: 10.1126/science.1204258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlacher K, Wu H, Jasin M. A distinct replication fork protection pathway connects Fanconi anemia tumor suppressors to RAD51-BRCA1/2. Cancer Cell. 2012;22:106–116. doi: 10.1016/j.ccr.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim DS, Hasty P. A mutation in mouse rad51 results in an early embryonic lethal that is suppressed by a mutation in p53. Mol Cell Biol. 1996;16:7133–7143. doi: 10.1128/mcb.16.12.7133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsuzuki T, et al. Targeted disruption of the Rad51 gene leads to lethality in embryonic mice. Proc Natl Acad Sci USA. 1996;93:6236–6240. doi: 10.1073/pnas.93.13.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kettleborough RNW, et al. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature. 2013;496:494–497. doi: 10.1038/nature11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bezzubova O, Shinohara A, Mueller RG, Ogawa H, Buerstedde JM. A chicken RAD51 homologue is expressed at high levels in lymphoid and reproductive organs. Nucleic Acids Res. 1993;21:1577–1580. doi: 10.1093/nar/21.7.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmed EA, et al. Differences in DNA double strand breaks repair in male germ cell types: Lessons learned from a differential expression of Mdc1 and 53BP1. DNA Repair (Amst) 2007;6:1243–1254. doi: 10.1016/j.dnarep.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Lee MT, Bonneau AR, Giraldez AJ. Zygotic genome activation during the maternal-to-zygotic transition. Annu Rev Cell Dev Biol. 2014;30:581–613. doi: 10.1146/annurev-cellbio-100913-013027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Long Q, et al. GATA-1 expression pattern can be recapitulated in living transgenic zebrafish using GFP reporter gene. Development. 1997;124:4105–4111. doi: 10.1242/dev.124.20.4105. [DOI] [PubMed] [Google Scholar]
- 40.Lin HF, et al. Analysis of thrombocyte development in CD41-GFP transgenic zebrafish. Blood. 2005;106:3803–3810. doi: 10.1182/blood-2005-01-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macaulay IC, et al. Single-cell RNA-sequencing reveals a continuous spectrum of differentiation in hematopoietic cells. Cell Reports. 2016;14:966–977. doi: 10.1016/j.celrep.2015.12.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murayama E, et al. Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity. 2006;25:963–975. doi: 10.1016/j.immuni.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 43.Berghmans S, et al. tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc Natl Acad Sci USA. 2005;102:407–412. doi: 10.1073/pnas.0406252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 45.Fortier ME, et al. The viral mimic, polyinosinic:polycytidylic acid, induces fever in rats via an interleukin-1-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2004;287:R759–R766. doi: 10.1152/ajpregu.00293.2004. [DOI] [PubMed] [Google Scholar]
- 46.Magee WE, Griffith MJ. The liver as a site for interferon production in response to poly I:poly C. Life Sci II. 1972;11:1081–1086. doi: 10.1016/0024-3205(72)90216-0. [DOI] [PubMed] [Google Scholar]
- 47.Xiong R, Nie L, Xiang LX, Shao JZ. Characterization of a PIAS4 homologue from zebrafish: Insights into its conserved negative regulatory mechanism in the TRIF, MAVS, and IFN signaling pathways during vertebrate evolution. J Immunol. 2012;188:2653–2668. doi: 10.4049/jimmunol.1100959. [DOI] [PubMed] [Google Scholar]
- 48.Helleday T. The underlying mechanism for the PARP and BRCA synthetic lethality: Clearing up the misunderstandings. Mol Oncol. 2011;5:387–393. doi: 10.1016/j.molonc.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shive HR, et al. brca2 in zebrafish ovarian development, spermatogenesis, and tumorigenesis. Proc Natl Acad Sci USA. 2010;107:19350–19355. doi: 10.1073/pnas.1011630107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodríguez-Marí A, et al. Roles of brca2 (fancd1) in oocyte nuclear architecture, gametogenesis, gonad tumors, and genome stability in zebrafish. PLoS Genet. 2011;7:e1001357. doi: 10.1371/journal.pgen.1001357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharan SK, et al. Embryonic lethality and radiation hypersensitivity mediated by Rad51 in mice lacking Brca2. Nature. 1997;386:804–810. doi: 10.1038/386804a0. [DOI] [PubMed] [Google Scholar]
- 52.Hakem R, de la Pompa JL, Mak TW. Developmental studies of Brca1 and Brca2 knock-out mice. J Mammary Gland Biol Neoplasia. 1998;3:431–445. doi: 10.1023/a:1018792200700. [DOI] [PubMed] [Google Scholar]
- 53.Shive HR, West RR, Embree LJ, Golden CD, Hickstein DD. BRCA2 and TP53 collaborate in tumorigenesis in zebrafish. PLoS One. 2014;9:e87177. doi: 10.1371/journal.pone.0087177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kelly PF, et al. Stem cell collection and gene transfer in Fanconi anemia. Mol Ther. 2007;15:211–219. doi: 10.1038/sj.mt.6300033. [DOI] [PubMed] [Google Scholar]
- 55.Liu TX, et al. Knockdown of zebrafish Fancd2 causes developmental abnormalities via p53-dependent apoptosis. Dev Cell. 2003;5:903–914. doi: 10.1016/s1534-5807(03)00339-3. [DOI] [PubMed] [Google Scholar]
- 56.me Yoon Y, Storm KJ, Kamimae-Lanning AN, Goloviznina NA, Kurre P. Endogenous DNA damage leads to p53-independent deficits in replicative fitness in fetal murine Fancd2−/− hematopoietic stem and progenitor cells. Stem Cell Rep. 2016;5:840–853. doi: 10.1016/j.stemcr.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nijnik A, et al. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. 2007;447:686–690. doi: 10.1038/nature05875. [DOI] [PubMed] [Google Scholar]
- 58.Parmar K, D’Andrea A, Niedernhofer LJ. Mouse models of Fanconi anemia. Mutat Res. 2009;668:133–140. doi: 10.1016/j.mrfmmm.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bakker ST, de Winter JP, te Riele H. Learning from a paradox: Recent insights into Fanconi anaemia through studying mouse models. Dis Model Mech. 2013;6:40–47. doi: 10.1242/dmm.009795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ibáñez A, et al. Elevated levels of IL-1beta in Fanconi anaemia group A patients due to a constitutively active phosphoinositide 3-kinase-Akt pathway are capable of promoting tumour cell proliferation. Biochem J. 2009;422:161–170. doi: 10.1042/BJ20082118. [DOI] [PubMed] [Google Scholar]
- 61.Dufour C, et al. TNF-alpha and IFN-gamma are overexpressed in the bone marrow of Fanconi anemia patients and TNF-alpha suppresses erythropoiesis in vitro. Blood. 2003;102:2053–2059. doi: 10.1182/blood-2003-01-0114. [DOI] [PubMed] [Google Scholar]
- 62.Brégnard C, et al. Upregulated LINE-1 activity in the Fanconi anemia cancer susceptibility syndrome leads to spontaneous pro-inflammatory cytokine production. EBioMedicine. 2016;8:184–194. doi: 10.1016/j.ebiom.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garbati MR, et al. FANCA and FANCC modulate TLR and p38 MAPK-dependent expression of IL-1β in macrophages. Blood. 2013;122:3197–3205. doi: 10.1182/blood-2013-02-484816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sumpter R, Jr, et al. Fanconi anemia proteins function in mitophagy and immunity. Cell. 2016;165:867–881. doi: 10.1016/j.cell.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walter D, et al. Exit from dormancy provokes DNA-damage-induced attrition in haematopoietic stem cells. Nature. 2015;520:549–552. doi: 10.1038/nature14131. [DOI] [PubMed] [Google Scholar]
- 66.Zhang H, et al. TGF-β inhibition rescues hematopoietic stem cell defects and bone marrow failure in Fanconi anemia. Cell Stem Cell. 2016;18:668–681. doi: 10.1016/j.stem.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bielczyk-Maczyńska E, et al. The ribosome biogenesis protein Nol9 is essential for definitive hematopoiesis and pancreas morphogenesis in zebrafish. PLoS Genet. 2015;11:e1005677. doi: 10.1371/journal.pgen.1005677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kagawa H, et al. A novel signaling pathway mediated by the nuclear targeting of C-terminal fragments of mammalian Patched 1. PLoS One. 2011;6:e18638. doi: 10.1371/journal.pone.0018638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Westerfield M. A Guide for the Laboratory Use of Zebrafish (Danio rerio). The Zebrafish Book. Univ of Oregon Press; Eugene, OR: 2000. pp. 4–5. [Google Scholar]
- 70.Brocal I, et al. Efficient identification of CRISPR/Cas9-induced insertions/deletions by direct germline screening in zebrafish. BMC Genomics. 2016;17:259. doi: 10.1186/s12864-016-2563-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fischer AH, Jacobson KA, Rose J, Zeller R. Paraffin embedding tissue samples for sectioning. CSH Protoc. 2008 doi: 10.1101/pdb.prot4989. [DOI] [PubMed] [Google Scholar]
- 72.Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc. 2008;3:59–69. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- 73.Armitage P, Matthews JNS, Berry G. Statistical Methods in Medical Research. John Wiley and Sons; New York: 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.