Significance
Recognition of pathogen-associated molecular patterns (PAMPs) by the host immune system is essential for activation of innate immunity in plants and animals. In plants, the receptor kinase FLAGELLIN SENSING 2 (FLS2) recognizes flagellin, a PAMP from bacteria. Here, we found that the glycosylphosphatidylinositol (GPI)-anchored protein LORELEI-LIKE GPI-ANCHORED PROTEIN 1 (LLG1) associates with FLS2 and regulates plant immunity by modulating FLS2 accumulation and signaling. LLG1 functions as a chaperone and coreceptor of FERONIA, a key component in pollen reception and development. This work identifies a shared component for immunity and development, and indicates that modulation of receptor kinases by membrane-localized GPI proteins could be a general mechanism for plants to respond to environmental and developmental cues.
Keywords: plant innate immunity, pattern recognition receptors, LLG1, FLS2, FERONIA
Abstract
Plants detect and respond to pathogen invasion with membrane-localized pattern recognition receptors (PRRs), which recognize pathogen-associated molecular patterns (PAMPs) and activate downstream immune responses. Here we report that Arabidopsis thaliana LORELEI-LIKE GPI-ANCHORED PROTEIN 1 (LLG1), a coreceptor of the receptor-like kinase FERONIA, regulates PRR signaling. In a forward genetic screen for suppressors of enhanced disease resistance 1 (edr1), we identified the point mutation llg1-3, which suppresses edr1 disease resistance but does not affect plant growth and development. The llg1 mutants show enhanced susceptibility to various virulent pathogens, indicating that LLG1 has an important role in plant immunity. LLG1 constitutively associates with the PAMP receptor FLAGELLIN SENSING 2 (FLS2) and the elongation factor-Tu receptor, and forms a complex with BRASSINOSTEROID INSENSITIVE 1-ASSOCIATED RECEPTOR KINASE 1 in a ligand-dependent manner, indicating that LLG1 functions as a key component of PAMP-recognition immune complexes. Moreover, LLG1 contributes to accumulation and ligand-induced degradation of FLS2, and is required for downstream innate immunity responses, including ligand-induced phosphorylation of BOTRYTIS-INDUCED KINASE 1 and production of reactive oxygen species. Taken together, our findings reveal that LLG1 associates with PAMP receptors and modulates their function to regulate disease responses. As LLG1 functions as a coreceptor of FERONIA and plays central roles in plant growth and development, our findings indicate that LLG1 participates in separate pathways, and may suggest a potential connection between development and innate immunity in plants.
Plant immune receptors detect pathogen infection (1–3). For example, pattern recognition receptors (PRRs) on the plant plasma membrane recognize pathogen-associated molecular patterns (PAMPs) to activate basal defenses, also known as PAMP-triggered immunity (4). Examples of PAMPs include bacterial flagellin, elongation factor-Tu (EF-Tu), lipopolysaccharides, peptidoglycans, and fungal chitins (5). Most PRRs in plants are receptor-like kinases containing extracellular domains for ligand recognition, transmembrane domains, and cytoplasmic kinase domains (4). PRRs include the Arabidopsis leucine-rich repeat receptor kinases FLAGELLIN SENSING 2 (FLS2) and EF-TU RECEPTOR (EFR), which recognize bacterial flagellin and EF-Tu, respectively (6, 7). Upon binding with flagellin or EF-Tu, FLS2 and EFR rapidly form complexes with the coreceptor BRASSINOSTEROID INSENSITIVE 1-ASSOCIATED RECEPTOR KINASE 1 (BAK1), another leucine-rich repeat receptor-like kinase, to activate downstream immune responses (8–10). FLS2 undergoes clathrin-dependent endocytosis and subcellular compartmentalization depending on activation by flg22, which is critical for plant immunity (11, 12).
A number of proteins, including BOTRYTIS-INDUCED KINASE1 (BIK1) and BRASSINOSTEROID SIGNALING KINASE1 (BSK1), function downstream of PAMP perception (13, 14). BIK1 associates with FLS2 and EFR, and dissociates from the complex after ligand recognition, then phosphorylates RbohD to regulate production of reactive oxygen species (ROS) (15, 16). Furthermore, other components, such as STOMATAL CYTOKINESIS-DEFECTIVE1 (SCD1) and GLYCINE-RICH PROTEIN 7 (GRP7), also interact with FLS2 and modulate innate immunity responses (17, 18). In Arabidopsis, the LysM domain receptor kinase CERK1 binds to fungal chitin and dimerizes upon chitin treatment; CERK1 dimerization can efficiently trigger downstream innate immunity responses in a BAK1-independent manner (19–21). Upon perception of pathogens by immune receptors, plants mount multilayered defense responses, including an ROS burst, activation of a MAPK cascade, up-regulation of defense gene expression, and accumulation of the defense hormone salicylic acid (SA); these responses can result in disease resistance (3, 8, 22).
ENHANCED DISEASE RESISTANCE 1 (EDR1), a Raf-like MAPK kinase kinase, plays a negative role in plant immunity (23). EDR1 interacts with MKK4 and MKK5, and negatively regulates the MKK4/MKK5–MPK3/MPK6 cascade (24). The resistance in edr1 mutants requires the RING E3 ligase KEEP ON GOING (KEG) and HYPERRECOMBINATION 1 (HPR1), a component of the putative Arabidopsis THO/TREX complex, as well as BSK1 (13, 25, 26). EDR1 also associates with an E3 ubiquitin ligase ARABIDOPSIS TOXICOS EN LEVADURA1 (ATL1) and is recruited to the fungal penetration site in an EDR4-dependent manner (27, 28).
To identify new components in plant innate immunity, we performed a screen for suppressors of the edr1 mutant phenotype (26). Here we report that mutations in LORELEI-LIKE GPI-ANCHORED PROTEIN1 (LLG1), which encodes a glucosylphosphatidylinositol (GPI)-anchored protein, suppressed the edr1 phenotypes. Recent work showed that LLG1 acts as a chaperone and coreceptor for the receptor kinase FERONIA (FER) and regulates plant growth and development (29). We found that the llg1 mutants show enhanced susceptibility to a wide range of pathogens. LLG1 constitutively interacts with the PAMP receptors FLS2 and EFR, and also associates with BAK1 upon flg22 treatment. Moreover, LLG1 contributes to FLS2 accumulation, ligand-induced degradation and signaling, and is indispensable for PAMP-induced phosphorylation of BIK1 and production of ROS. Taking these results together, our study indicates that LLG1 associates with PAMP receptors and plays an important role in plant innate immunity.
Results
The llg1-3 Mutation Suppresses Disease Resistance in edr1 Mutants.
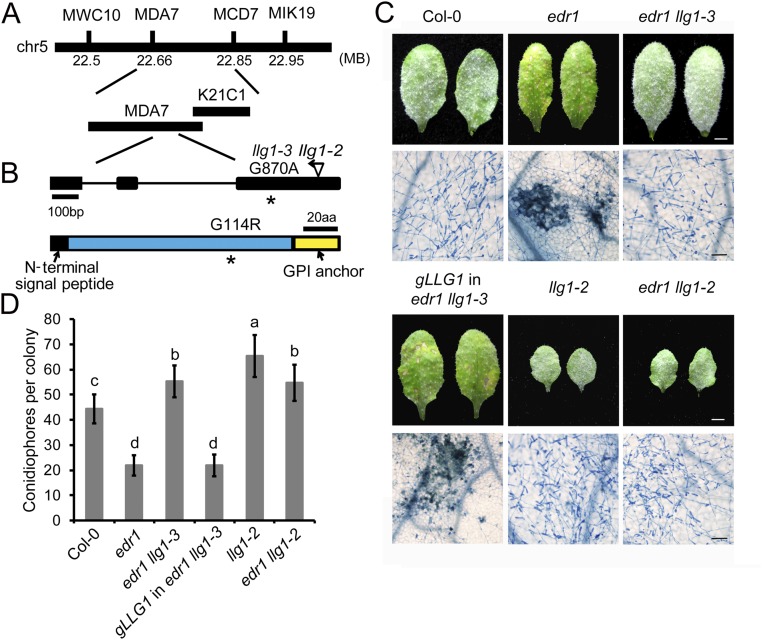
To search for new components that regulate plant immunity, we screened for mutations that suppress the disease-resistance phenotypes of edr1 (26). We selected a mutant for further study and, based on subsequent characterization, we named this edr1 suppressor mutation llg1-3. At 7 d post infection (dpi), the edr1 plants exhibited cell death and disease resistance to the virulent powdery mildew strain Golovinomyces cichoracearum UCSC1, whereas the edr1 llg1-3 mutant showed a susceptible phenotype (Fig. 1A). Trypan blue staining showed that llg1-3 suppressed the powdery mildew-induced lesions in edr1 (Fig. 1B). To quantify fungal growth, we counted the conidiophores per colony in wild-type, edr1, and edr1 llg1-3 at 5 dpi. The edr1 plants supported fewer conidiophores and showed mildew-induced lesions. However, the edr1 llg1-3 plants supported significantly more conidiophores than edr1 and wild-type (Fig. 1C), indicating that the llg1-3 mutation fully suppressed the powdery mildew resistance phenotype of edr1.
Fig. 1.
llg1-3 suppresses edr1-mediated resistance to G. cichoracearum. (A) Four-week-old plants were inoculated with G. cichoracearum. Photos were taken of leaves of Col-0, edr1, and edr1 llg1-3 plants at 7 dpi. The llg1-3 mutation suppressed the necrotic and powdery mildew-resistance phenotypes of the edr1 mutant. (Scale bar, 0.25 cm.) (B) Trypan blue staining of leaves after infection with G. cichoracearum at 7 dpi. (Scale bar, 100 μm.) (C) Quantitative analysis of conidiophore formation in Col-0, edr1, and edr1 llg1-3 plants at 5 dpi. Error bars represent SD from three independent experiments (n = 25). (D) Free SA was extracted from uninfected and infected (3 dpi) leaves of 4-wk-old plants with G. cichoracearum. Error bars represent SD from three independent experiments (n = 3). (E) The expression of PR1 was analyzed by quantitative real-time RT-PCR. mRNA was extracted from uninfected and infected (3 dpi) 4-wk-old plants infected with G. cichoracearum. ACTIN2 was used as an internal control. Error bars represent SD from three independent experiments (n = 3). Lowercase letters represent statistically significant differences (P < 0.05, nested ANOVA).
Previous work showed that the edr1 mutants accumulate higher levels of SA after powdery mildew infection (23). To examine whether llg1-3 affects SA accumulation in edr1, we measured SA levels in wild-type, edr1, and edr1 llg1-3 plants after infection with G. cichoracearum at 3 dpi. Consistent with previous findings, the edr1 plant overaccumulated free SA when challenged with powdery mildew, but the elevated level of SA in edr1 was suppressed by the llg1-3 mutation (Fig. 1D). Similarly, the llg1-3 mutation also suppressed the increased accumulation of transcripts of PR1, the SA-induced immune marker gene, in edr1 plants (Fig. 1E).
Besides powdery mildew, the edr1 mutant also displays enhanced resistance to the oomycete pathogen Hyaloperonospora arabidopsidis Noco2 and the bacterial pathogen Pseudomonas syringae pv tomato (Pto) DC3000 (24, 26). To assess whether llg1-3 affects edr1-mediated resistance to H. arabidopsidis Noco2 and Pto DC3000, we examined the resistance of edr1 llg1-3 to those two pathogens. As shown in Fig. S1, the edr1 llg1-3 plants displayed enhanced susceptibility to both H. arabidopsidis Noco2 and Pto DC3000, indicating that LLG1 contributes to edr1-mediated resistance to multiple pathogens.
Fig. S1.
llg1-3 suppresses disease resistance in edr1 mutants. (A) Growth of Pto DC3000 on the Col-0, edr1, edr1 llg1-3, and a representative LLG1 llg1-3 complementation line. Four-week-old plants were syringe-infiltrated with Pto DC3000. Bacterial growth was assessed at 0 and 3 d after inoculation. Error bars represent SD of three independent experiments (n = 3). (B) Infection assays with H. arabidopsidis (H.a.) Noco2. Two-week-old plants were sprayed with H. arabidopsidis Noco2 spores (5 × 104 spores per milliliter). Spores were counted at 7 d after inoculation. Error bars represent SD of three independent experiments (n = 4). Lowercase letters indicate statistically significant differences (P < 0.05, nested ANOVA).
The llg1-3 mutation was identified by map-based cloning (Fig. S2 A and B). The mutation resulted in a Gly to Arg substitution of the 114th amino acid of LLG1. To validate that this mutation causes the suppression of edr1, the genomic sequence of LLG1 with its native promoter was transformed into edr1 llg1-3 mutant plants. All 22 T1 transgenic plants displayed edr1-like phenotypes, showing enhanced cell death and disease resistance after powdery mildew infection (Fig. S2 C and D). In addition, we crossed edr1 with a previously characterized T-DNA insertion mutant of LLG1 (llg1-2) and infected the edr1 llg1-2 double mutant with G. cichoracearum. We found that llg1-2 could also suppress the edr1 disease resistance phenotype (Fig. S2 C and D). Taken together, these data demonstrated that the suppression of the edr1 resistance phenotype is caused by the mutation in LLG1.
Fig. S2.
Map-based cloning of llg1-3. (A) Map position of the llg1-3 mutation. Markers and BAC clones are indicated. (B) Gene structure of LLG1. The asterisk indicates the point mutation in the llg1-3 allele. The insertion position of the previously identified llg1-2 T-DNA allele is shown. Exons are indicated by black boxes and introns are indicated by lines. The LLG1 protein contains an N-terminal signal peptide and a C-terminal anchor signal. The mutation in llg1-3 causes a G to A point mutation, resulting an amino acid change from Gly to Arg. aa, amino acids. (C) Four-week-old plants were inoculated with G. cichoracearum. Photos were taken of leaves of Col-0, edr1, llg1-2, edr1 llg1-2, edr1 llg1-3, and a representative LLG1 edr1 llg1-3 complementation line at 7 dpi. The infected leaves were stained with Trypan blue to show fungal structures and dead plant cells. (Scale bars, 0.25 cm for white; 100 μm for black.) (D) Quantitative analysis of conidiophore formation at 5 dpi. Error bars represent SD of three independent experiments (n = 25). Lowercase letters represent statistically significant differences (P < 0.05, nested ANOVA).
LLG1 Is Required for Plant Innate Immunity.
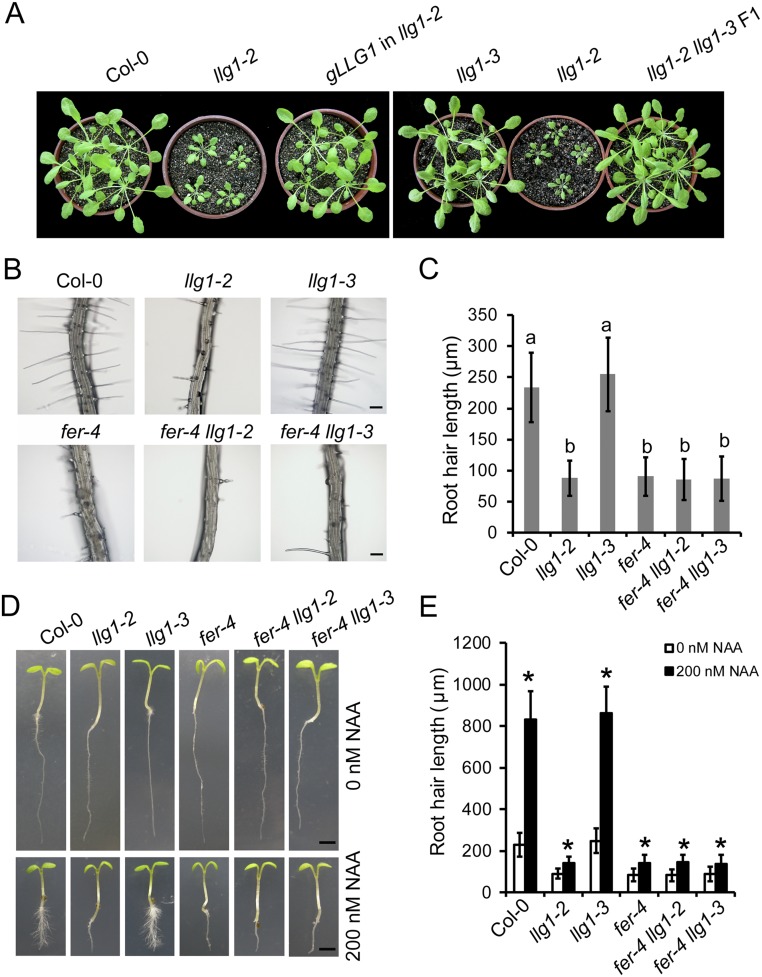
To study the role of LLG1 in plant immunity, we isolated the llg1-3 single mutant by crossing the edr1 llg1-3 mutant with Col-0 plants. The llg1-3 mutant allele identified from this study did not show growth and developmental defects, in contrast to llg1-2, which was much smaller than wild-type, as reported previously (29) (Fig. S3A). We also crossed llg1-2 with llg1-3, and the growth of the F1 plants was indistinguishable from that of llg1-3 or wild-type plants (Fig. S3A), indicating that the llg1-3 mutation only affects disease resistance, but not development: that is, the LLG1G114R protein has lost its function in immunity but may retain functions in regulating plant growth and development.
Fig. S3.
The llg1-3 plants show normal growth phenotypes. (A) Four-week-old, short-day-grown plants of Col-0, llg1-2, llg1-3, and llg1-2 llg1-3 F1 and a representative LLG1 llg1-2 complementation line. (B) Root hairs from 5-d-old Col-0, llg1-2, llg1-3, fer-4, fer-4 llg1-2, and fer-4 llg1-3 seedlings. (Scale bars, 100 μm.) The experiment was repeated three times with similar results. (C) Root hair length from 5-d-old seedlings. Error bars represent SD of three independent experiments (n = 50). Lowercase letters represent significant differences (P < 0.05, nested ANOVA). (D) Root hair growth after auxin treatment. Five-day-old Arabidopsis seedlings treated with 0 or 200 nM NAA are shown. (Scale bars, 0.2 cm.) The experiment was repeated three times with similar results. (E) Root hair length from 5-d-old seedlings treated with 0 or 200 nM NAA. Error bars represent SD of three independent experiments (n = 50). Asterisks represent significant differences (P < 0.01) using Student’s t test.
To further assess the role of LLG1 in plant immunity, we infected llg1-2 and llg1-3 plants with G. cichoracearum, H. arabidopsidis Noco2, and Pto DC3000. We found that the llg1-2 and llg1-3 mutant plants exhibited enhanced susceptibility to all three pathogens, and the complementation lines carrying the LLG1 genomic sequence showed wild-type–like phenotypes (Fig. 2 A–C). Furthermore, llg1-2 and llg1-3 mutants accumulated lower levels of SA and PR1 transcripts after G. cichoracearum infection (Fig. 2 D and E). Collectively, these data indicate that LLG1 plays an important role in plant immunity.
Fig. 2.
LLG1 is required for basal defenses. (A) Growth of G. cichoracearum on Col-0, llg1-2, llg1-3, and a representative LLG1 llg1-3 complementation line. Four-week-old plants were inoculated with G. cichoracearum. The number of conidiophores per colony was counted at 5 dpi. Error bars represent SD from three independent experiments (n = 25). (B) Growth of H. arabidopsidis (H.a.) Noco2 on wild-type and mutant plants. Two-week-old plants were inoculated with H. arabidopsidis Noco2 spores at a concentration of 5 × 104 spores per milliliter. Pathogen growth was scored at 7 dpi. Error bars represent SD from three independent experiments (n = 4). (C) Disease-resistance assay using Pto DC3000. Four-week-old plants were spray-inoculated with Pto DC3000 bacteria at 108 cfu/mL. Bacterial growth was scored at 4 dpi. Error bars represent SD from three independent experiments (n = 4). (D) The accumulation of PR1 transcripts was analyzed by quantitative real-time RT-PCR after infection with G. cichoracearum at 0 and 3 dpi. ACTIN2 was used as an internal control. Error bars represent SD from three independent experiments (n = 3). (E) Free SA was extracted from uninfected and infected (3 dpi) leaves of 4-wk-old plants with G. cichoracearum. Error bars represent SD from three independent experiments (n = 3). Lowercase letters indicate statistically significant differences (P < 0.05, nested ANOVA).
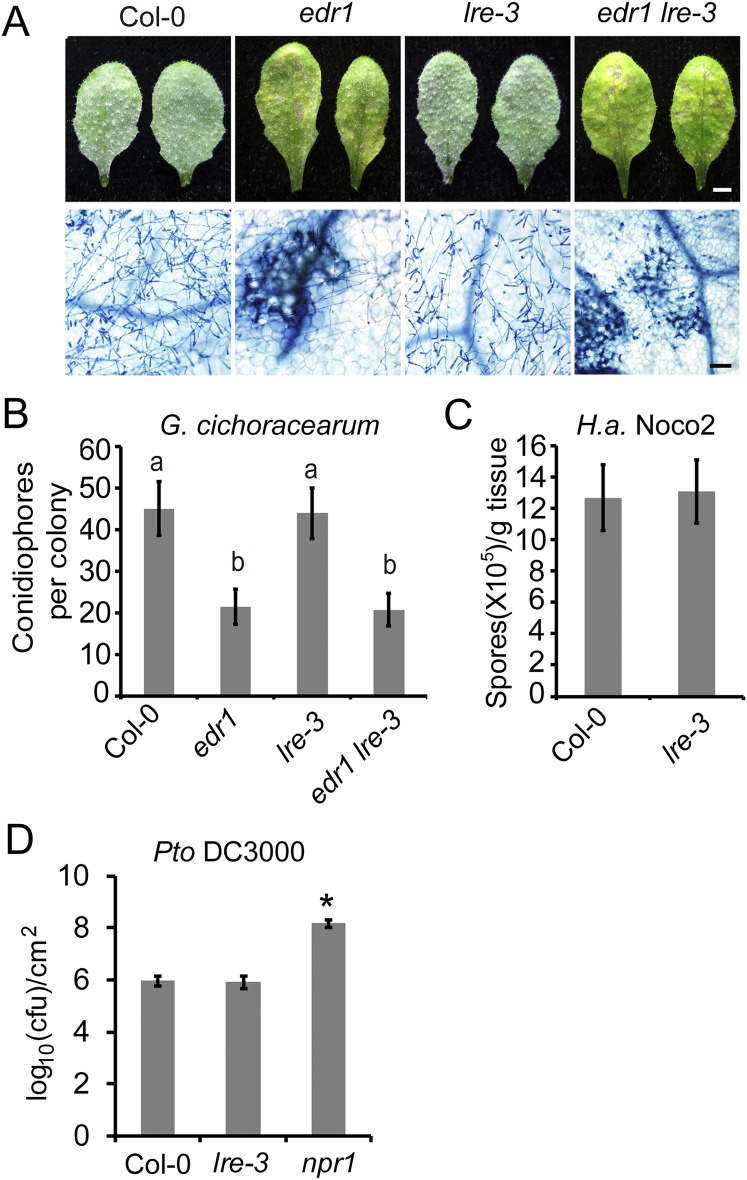
LLG1 belongs to a small gene family and LORELEI (LRE), which encodes an important regulator of pollen tube reception and double fertilization, is the closest homolog of LLG1 (30, 31). To investigate whether LRE is involved in plant immunity, we first crossed the lre-3 null mutant with edr1 to create the edr1 lre-3 double mutant, and then examined edr1 lre-3 for powdery mildew resistance. The edr1 lre-3 double mutant showed edr1-like phenotypes after G. cichoracearum infection (Fig. S4 A and B), indicating that lre-3 could not suppress the edr1 disease resistance phenotype. We then infected the lre-3 mutant with G. cichoracearum, Pto DC3000, and H. arabidopsidis Noco2. As shown in Fig. S4 B–D, the lre-3 mutant supported similar levels of pathogen growth compared with wild-type, indicating that LRE is not involved in disease resistance against those pathogens; alternatively, this may be because LRE1 is exclusively expressed in the ovule (30, 31).
Fig. S4.
LRE is not required for plant immunity. (A) Four-week-old plants were infected with G. cichoracearum and representative leaves were photographed at 7 dpi. Leaves were stained with Trypan blue to show fungal structures and dead cells. (Scale bars, 0.25 cm for white; 100 μm for black.) (B) Quantitative analysis of conidiophore formation at 5 dpi. The bars represent means and SD of three independent experiments (n = 25). Lowercase letters represent significant differences (P < 0.05, nested ANOVA). (C) Growth of H. arabidopsidis Noco2 on the Col-0 and lre-3 plants. Infections were scored at 7 dpi. Error bars represent SD of three independent experiments (n = 4). (D) Infection assays with Pto DC3000. The plants were spray-inoculated with Pto DC3000 at 108 cfu/mL. Bacterial growth was assessed at 4 d after inoculation. Error bars represent SD of three independent experiments (n = 4). Asterisk indicates significantly different from Col-0 using Student’s t test (P < 0.01).
The llg1-3 Mutation Does Not Affect Growth and Development.
A recent study showed that LLG1 acts as a chaperone and coreceptor to regulate FER localization to the plasma membrane, and fer-4 and llg1-2 mutants show similar growth defects (29); however, the llg1-3 single mutant was similar to wild-type. Furthermore, the fer-4 llg1-3 double mutant and fer-4 single mutant showed indistinguishable developmental phenotypes (Fig. S3), indicating that the llg1-3 mutation does not affect FER-dependent growth and development.
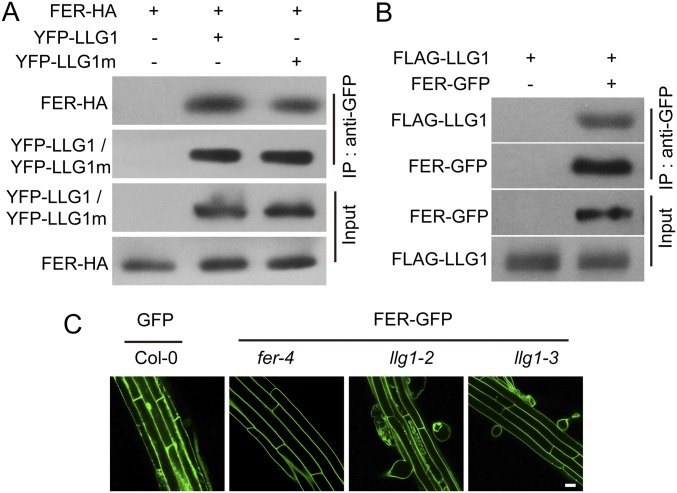
One of the important roles of LLG1 is to act as a chaperone and coreceptor for FER to modulate FER localization (29). To test whether llg1-3 affects the interaction of LLG1 and FER, or the localization of FER, we first performed coimmunoprecipitation (Co-IP) assays to test the interaction between FER and LLG1G114R. Consistent with previous reports (29), FER interacted with LLG1 in both transient assays and stably transformed plants; in addition, the G114R mutation in LLG1 did not affect the LLG1-FER interaction (Fig. S5 A and B). We next investigated whether LLG1G114R affects the plasma membrane localization of FER by introducing a FER–GFP construct into different genetic backgrounds by crossing. FER–GFP localized at the plasma membrane in both wild-type and llg1-3 backgrounds (Fig. S5C); in contrast, proper membrane localization of FER was impaired in llg1-2 mutants. This observation indicates that the LLG1G114R produced in llg1-3 mutants does not affect FER localization and LLG1G114R could still function as a chaperone for FER and guide FER localization, which is consistent with the observation that llg1-3 shows wild-type–like growth phenotypes.
Fig. S5.
LLG1 interacts with FER. (A) Co-IP of LLG1 and FER from Nicotiana benthamiana transiently expressing YFP–LLG1, YFP–LLG1m (carrying the llg1-3 mutation), and FER-HA. Total protein was extracted and subjected to IP of LLG1 protein by GFP antibody, followed by immunoblot analysis with anti-HA or anti-GFP antibody. (B) Co-IP of LLG1 and FER from transgenic Arabidopsis plants. Total protein was extracted from 3-wk-old plants and subjected to IP by anti-GFP antibody. The proteins were detected by immunoblot analysis with anti-GFP or anti-FLAG antibody. (C) Localization of GFP or FER-GFP in roots of 5-d-old Arabidopsis seedlings. (Scale bar, 20 μm.) All experiments were repeated three times with similar results.
LLG1 Interacts with FLS2 and EFR.
To assess the potential roles of LLG1 in the EDR1 pathway, we examined whether LLG1 associated with EDR1. As shown in Fig. S6, LLG1 did not interact with EDR1 in yeast two-hybrid and Co-IP assays; therefore, we did not pursue a possible link between LLG1 and the EDR1 pathway. Because LLG1 plays an important role in plant immunity against different pathogens, and also acts as a coreceptor for the receptor-like kinase FER, we hypothesized that LLG1 may associate with PAMP receptors to regulate defense responses. To test this hypothesis, we assessed whether LLG1 could associate with the PAMP receptors FLS2 and EFR. We performed Co-IP assays by coexpressing YFP–LLG1 and FLS2-YFP-HA in both Nicotiana benthamiana and transgenic Arabidopsis leaves. The YFP–LLG1 construct could complement the llg1-3 phenotypes and the YFP–LLG1 fluorescence was observed at the plasma membrane in transgenic plants, indicating that YFP–LLG1 is functional (Fig. S7). In Co-IP assays in N. benthamiana, YFP–LLG1 could only be detected in the FLS2-YFP-HA immunoprecipitate, but not in the negative control, indicating that LLG1 and FLS2 associated with each other (Fig. 3A). We further confirmed this association by Co-IP assays in stable transgenic plants expressing both YFP–LLG1 and FLS2-YFP-HA (Fig. 3B). In addition, it appears that flg22 treatment did not alter the interaction between LLG1 and FLS2. We also examined whether the G114R mutation affects the LLG1–FLS2 interaction in Co-IP assays in N. benthamiana. We did not observe differences between the association of LLG1 and LLG1G114R with FLS2 (Fig. 3A), indicating that the G114R mutation in LLG1 did not affect the interaction of LLG1 and FLS2. To further assess the role of LLG1, we also examined the interaction of LLG1 with EFR, which recognizes the bacterial PAMP elongation factor EF-Tu (7). Similarly, LLG1 also associated with EFR in Co-IP assays in N. benthamiana, and the G114R mutation in LLG1 did not affect the LLG1–EFR association (Fig. 3C). Taken together, these results indicate that LLG1 forms complexes with PRRs, such as FLS2 and EFR, in a ligand-independent manner, and the llg1-3 mutation did not affect the interaction of LLG1 with these PRRs.
Fig. S6.
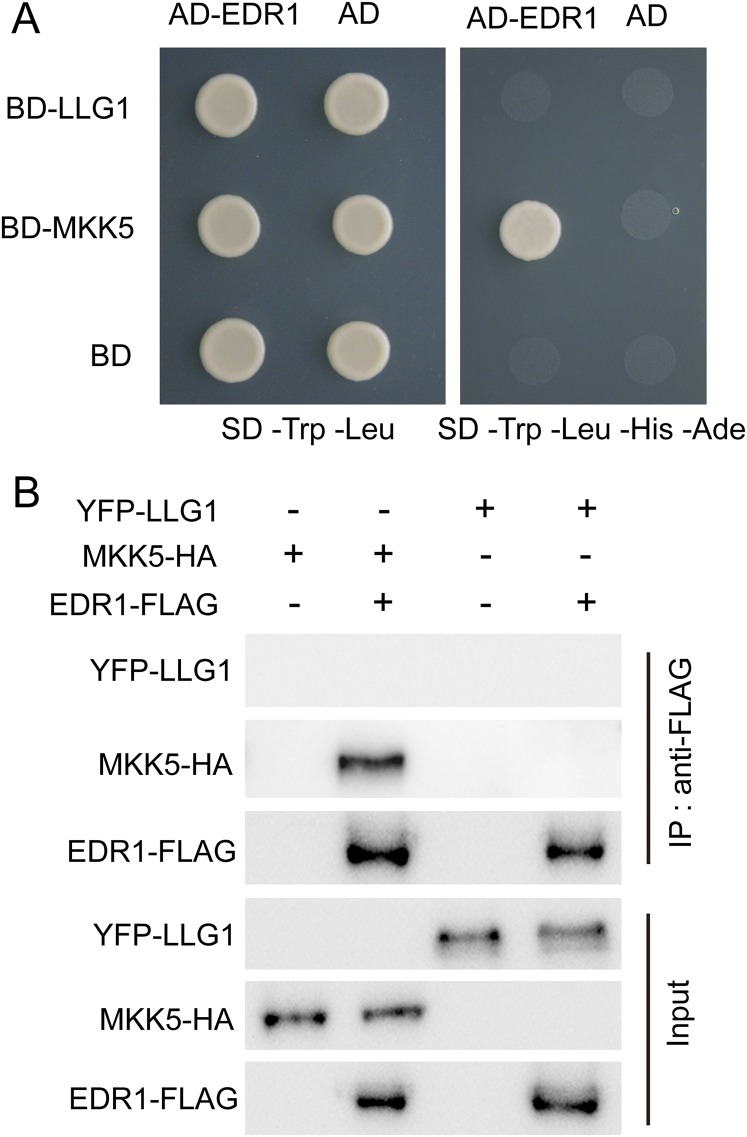
LLG1 did not interact with EDR1. (A) LLG1 did not interact with EDR1 in yeast two-hybrid assay. EDR1 and MKK5 were used as a positive control. Yeast cells containing the indicated plasmids were spotted onto the SD/-Trp/-Leu or SD/-Trp/-Leu/-His/-Ade medium as indicated. (B) LLG1 did not interact with EDR1 in Co-IP assays in N. benthamiana. EDR1-FLAG and MKK5-HA were used as a positive control. Agrobacterium containing the indicated plasmids were infiltrated into N. benthamiana, and proteins were extracted and subjected to Co-IP analysis, as indicated. All experiments were repeated three times with similar results.
Fig. S7.
The YFP–LLG1 fusion protein is functional. (A) The 35S:YFP–LLG1 construct complemented the llg1-3 phenotype. Four-week-old plants were inoculated with G. cichoracearum and the number of conidiophores per colony was counted at 5 dpi. An asterisk represent significant differences (P < 0.01) using Student’s t test. (B) YFP–LLG1 is properly expressed in 35S:YFP–LLG1 (in llg1-3 background) transgenic plants. The immunoblot analysis was performed using anti-GFP antibody in 2-wk-old plants. Ponceau S staining of the large subunit of Rubisco is shown as a loading control. (C) Confocal micrographs of YFP–LLG1 fluorescence in leaf epidermis and root cells of 35S:YFP–LLG1 transgenic plants. (Scale bar, 20 μm.) (D) Confocal micrographs of root cells of 35S:YFP–LLG1 transgenic plants stained with FM4-64. (Scale bar, 10 μm.) All experiments were repeated three times with similar results.
Fig. 3.
LLG1 interacts with FLS2. (A) Co-IP of LLG1 and FLS2 from N. benthamiana transiently expressing YFP–LLG1, YFP–LLG1m (carrying the llg1-3 mutation), and FLS2-YFP-HA. Plants were treated with 0 (−) or 1 μM (+) flg22 for 10 min. Total protein was extracted and subjected to IP by HA antibody, followed by immunoblot analysis with anti-GFP or anti-HA antibody. Plants transiently expressing both BAK1 and FLS2 were used as a positive (treated with flg22) or negative control (not treated with flg22), respectively. (B) Co-IP of LLG1 and FLS2 in Arabidopsis. Three-week-old transgenic Arabidopsis plants were treated with 0 (−) or 1 μM (+) flg22 for 10 min. Total protein was subjected to IP by anti-HA antibody, followed by immunoblot analysis with anti-HA or anti-GFP antibody. (C) Co-IP assay showing that LLG1 associated with EFR in N. benthamiana. Plants were treated with 0 (−) or 1 μM (+) elf18 for 10 min. The proteins were analyzed by immunoblot analysis with anti-GFP or anti-HA antibody after IP with HA antibody. (D) Co-IP of LLG1 and BAK1 in Arabidopsis. Three-week-old Col-0 plants and 35S:YFP-LLG1 (in the llg1-3 background) plants were treated with 0 (−) or 1 μM (+) flg22 for 10 min. YFP-LLG1 and BAK1 were detected by immunoblot analysis with anti-GFP or anti-BAK1 antibody after IP with anti-GFP antibody. (E) Yeast two-hybrid assay showing that LLG1 interacted with the extracellular juxtamembrane domains of FLS2 and EFR. exJM: extracellular juxtamembrane domain. Yeast cells containing the indicated plasmids were plated on dropout medium (-Leu-Trp) or on dropout medium (-Leu-Trp-His-Ade) supplemented with 10 mM 3-amino-1,2,4-triazole. AD represents yeast GAL4 activation domain. BD represents the GAL4 DNA binding domain. All experiments were repeated at least three times with similar results.
We next investigated whether LLG1 could associate with BAK1, a coreceptor for both FLS2 and EFR (4, 8). Co-IP assay using the YFP–LLG1 transgenic plants with BAK1 antibody indicated that YFP–LLG1 did not interact with BAK1 in nonelicited plants; however, YFP–LLG1 did associate with BAK1 after flg22 treatment (Fig. 3D). The interaction of LLG1 with FLS2 and EFR was conferred through association with the extracellular juxtamembrane region of the receptors in a yeast two-hybrid assay (Fig. 3E). These data indicate that LLG1 interacts with FLS2 constitutively, but the LLG1–BAK1 complex forms after flg22 treatment. This finding is reminiscent of the interaction of FLS2 and BAK1, which form a complex in a ligand-dependent manner.
flg22-Induced ROS Accumulation Is Impaired in llg1-2 and llg1-3 Mutants.
To investigate the role of LLG1 in PRR-mediated signaling, we treated Col-0, llg1-2, llg1-3, and fls2 plants with 100 nM flg22 to analyze flg22-elicited responses. We found that flg22-induced ROS production and PR gene expression were reduced in llg1-2 and llg1-3 plants (Fig. 4 A and B and Fig. S8A), but Col-0 and llg1 mutant plants showed indistinguishable expression of early pattern-triggered immunity (PTI) marker genes, phosphorylation of MAPK, and inhibition of growth induced by flg22 (Fig. S8 B–D). Similarly, llg1-2 and llg1-3 mutants were also impaired in elf18-induced ROS production (Fig. 4 C and D). Taken together, our data indicate that LLG1 is required for ROS accumulation mediated by FLS2 and EFR.
Fig. 4.
LLG1 is required for the ROS burst induced by flg22 or elf18. (A) Luminescence-based assay of ROS production following treatment with 100 nM flg22 or control (H2O). (B) ROS production over 30 min of flg22 treatment, presented as total photon counts. (C) Luminescence-based assay of ROS production following treatment with 100 nM elf18 or control (H2O). (D) ROS production over 30 min of elf18 treatment, presented as total photon counts. All experiments were repeated three times with similar results. Error bars represent SD (n = 8). Lowercase letters indicate statistically significant differences (P < 0.05, nested ANOVA).
Fig. S8.
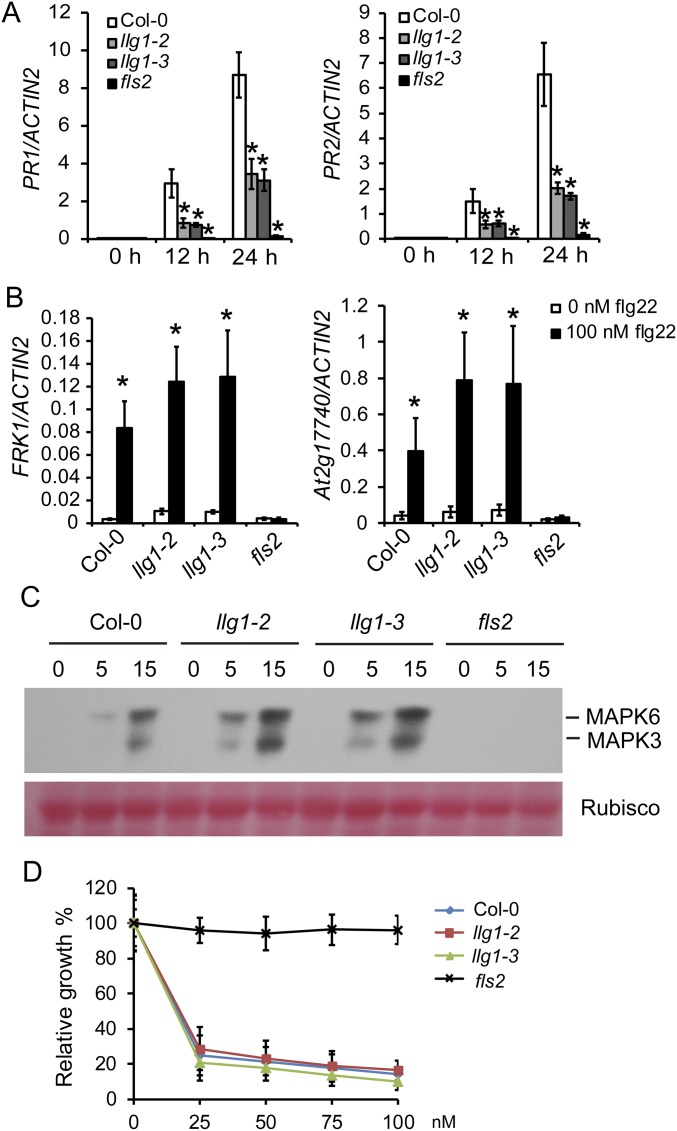
Flg22-triggered PTI responses in the llg1 mutants. (A) Quantitative real-time RT-PCR analysis of PR genes at the indicated times after 100 nM flg22 treatment. RNA was extracted from 10-d-old seedlings. (B) Quantitative real-time RT-PCR analysis of the induction of early PTI marker genes 3 h after flg22 treatment. Ten-day-old seedlings were treated with 100 nM flg22 or water (control). In A and B, ACTIN2 was used as the internal control. Error bars represent SD of three independent experiments (n = 3). Asterisks represent significant differences (P < 0.05) using Student’s t test. (C) Flg22-induced activation of MAPKs in Col-0, llg1-2, llg1-3, and fls2 plants. Ten-day-old seedlings were treated with 100 nM flg22. Samples were collected at 0, 5, and 15 min and analyzed by immunoblots using anti-p44/42-ERK antibody. Ponceau S staining of Rubisco is shown as a loading control. The experiments were repeated three times with similar results. (D) Root growth inhibition triggered by flg22 in Col-0, llg1-2, llg1-3, and fls2 plants. Seedlings were treated with 0, 25, 50, 75, or 100 nM flg22 for 8 d. Error bars represent SD of three independent experiments (n = 12).
LLG1 Regulates flg22-Induced Phosphorylation of BIK1.
To gain more insight into how LLG1 influences FLS2-mediated ROS production, we first tested whether mutations in LLG1 could affect FLS2–BAK1 association upon flg22 treatment. In Co-IP assays using FLS2-HA expressed in Arabidopsis protoplasts, we found that the flg22-induced interaction of FLS2 and BAK1 was not affected in the llg1 background (Fig. 5A), indicating that LLG1 did not play a role in the FLS2–BAK1 association. We then studied whether LLG1 contributes to flg22-induced phosphorylation of BIK1, the event that immediately follows formation of the FLS2–BAK1 complex (14). We transiently expressed BIK1-HA in Col-0, llg1-2, and llg1-3 protoplasts, and then detected BIK1-HA with anti-HA antibody before and after flg22 treatment. We found that after flg22 treatment, the phosphorylation of BIK1 (as indicated by the band shift, which was validated by phosphatase treatment) was lower in llg1 mutants, compared with wild-type (Fig. 5B), indicating that LLG1 plays an important role in flg22-induced phosphorylation of BIK1. Because BIK1 phosphorylation is required for ROS production during flg22 treatment (15), impaired BIK1 phosphorylation may cause the reduced ROS levels in llg1 mutants after flg22 treatment.
Fig. 5.
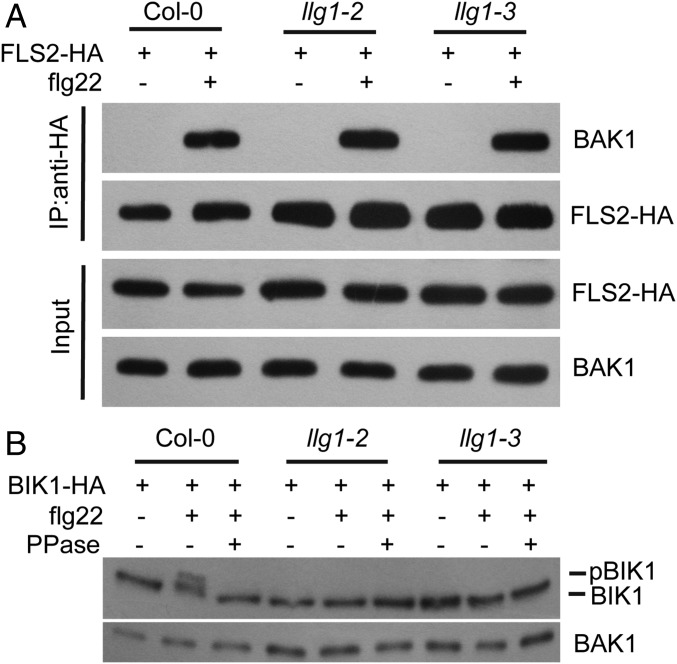
LLG1 is required for flg22-induced BIK1 phosphorylation. (A) Co-IP of FLS2-HA and BAK1 in Arabidopsis protoplasts expressing FLS2-HA before (−) or 10 min (+) after treatment with 1 μM flg22. FLS2-HA and BAK1 were detected by immunoblot analysis with anti-HA or anti-BAK1 antibody, respectively, after IP with anti-HA antibody. (B) Immunoblot analysis of BIK1-HA proteins in Arabidopsis protoplasts expressing BIK1-HA before (−) or 10 min (+) after treatment with 1 μM flg22. The proteins were detected by anti-HA antibody. PPase represents protein phosphatase. BAK1 was used as the loading control. All experiments were repeated at least three times with similar results.
LLG1 Contributes to Accumulation and flg22-Induced Degradation of FLS2.
To further investigate the role of LLG1 in plant immunity, we examined whether LLG1 is involved in accumulation and ligand-induced degradation of FLS2. Transgenic Arabidopsis plants that express 35Spro:FLS2-YFP-HA were used. The 35Spro:FLS2-YFP-HA clone was able to complement the fls2 phenotype (13). We first examined the localization and accumulation of FLS2, and no differences were observed for plasma membrane localization and total accumulation of FLS2–YFP in wild-type and llg1-3 plants but llg1-2 showed much lower FLS2–YFP signals at the plasma membrane, and reduced total FLS2-YFP accumulation (Fig. 6A and Fig. S9). We next examined flg22-induced FLS2–YFP protein degradation (32), which was wild-type–like in llg1-3 but was impaired in llg1-2 plants (Fig. 6B). Taken together, these observations indicate that LLG1 contributes to accumulation and flg22-induced degradation of FLS2 but that the LLG1–3 mutant variant uncouples its role as a chaperone from its function in FLS2 signaling.
Fig. 6.
LLG1 is required for accumulation and flg22-induced degradation of FLS2. (A) Confocal micrographs of 4-wk-old fls2, llg1-2, and llg1-3 transgenic plants expressing FLS2–YFP and showing leaf epidermal cells. Micrographs are maximum projections of 8–10 confocal optical sections taken at a 1-μm z-distance using the same microscope settings. (Scale bars, 5 μm.) Similar results were observed in at least three independent experiments. (B) Four-week-old plants were treated with 0 µM or 10 µM flg22 for 50 min. Each lane was loaded with 10 µg of total protein. FLS2–YFP was detected with anti-GFP antibody, and the molecular size for the band was indicated. H+-ATPase was used as plasma membrane protein marker. The experiment was repeated three times with similar results. FLS2–YFP protein levels were quantified with ImageJ. Error bars represent SD from three independent experiments (n = 3). Lowercase letters indicate statistically significant differences (P < 0.05, nested ANOVA). C, cytosol protein; PM, plasma membrane protein; T, total protein.
Fig. S9.
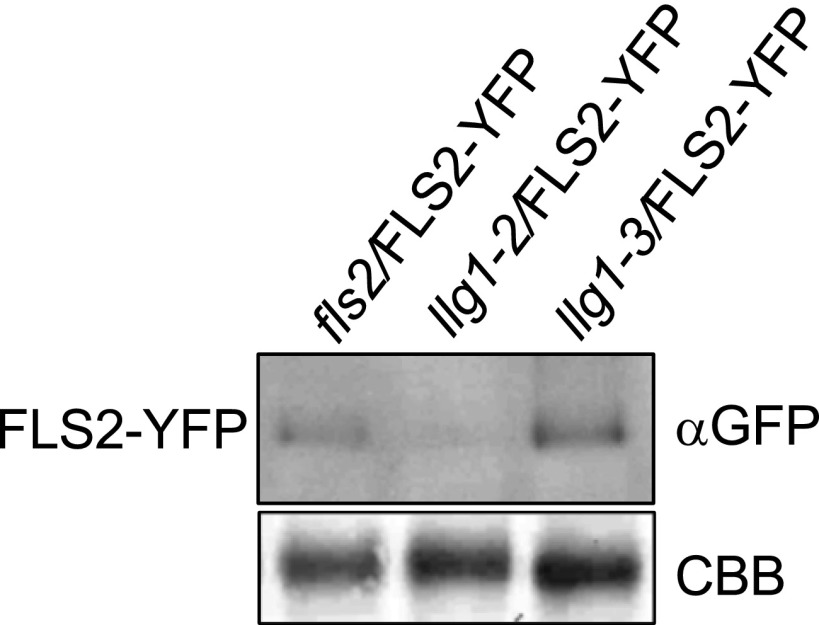
Immunoblot analysis of FLS2 protein levels. Immunoblot analysis of FLS2–YFP protein steady-state levels in 4-wk-old FLS2–YFP expressing fls2, llg1-2, and llg1-3 transgenic plants, respectively. Each lane was loaded with 20 µg of total protein. Coomassie Brilliant Blue (CCB) staining is shown for equal loading. Similar results were observed in at least two independent experiments.
Discussion
GPI-anchored proteins mainly localize to the outer leaflet of the plasma membrane and regulate a broad range of biological processes (33, 34). The Arabidopsis GPI-anchored protein LLG1 and its closest homolog LRE are involved in many developmental processes, such as the control of growth and fertilization of the oocyte (29–31). Here, we demonstrate that LLG1 functions as an important component of plant innate immunity, as it associates with PRRs to regulate PAMP-induced production of ROS and phosphorylation of BIK1.
One important question to ask is: what is the role of LLG1 in plant immunity? Plants rely on PRRs to trigger various defense responses and several proteins are involved in the regulation of receptor complexes (1, 35). For example, upon flg22 treatment, BAK1 phosphorylates BIK1, a kinase that directly phosphorylate RbohD, resulting in PAMP-induced ROS production (14–16). We found that LLG1 could constitutively interact with FLS2 and EFR, and the phosphorylation of BIK1 in response to flg22 treatment is compromised in the llg1 mutant. This finding is consistent with the observation that the llg1 mutants have reduced ROS production in response to flg22. Because FLS2-mediated ROS accumulation and BIK1 phosphorylation are impaired in llg1-3 plants without influencing accumulation or flg22-induced degradation of FLS2, LLG1-mediated chaperone function of the receptor and control of its signaling are likely to be separate. Previous work showed that correct folding of EFR requires endoplasmic reticulum (ER) chaperones, including CRT, BiP, SDF2, and ERdj3B, as well as N-glycosylation by the UGGT and OST complexes (36–39). The ER-associated quality control (ERQC) system plays important roles in proper targeting and signaling of the Arabidopsis PRRs FLS2 and EFR. PRRs that fail to fold correctly are not delivered to the functional site, but are targeted for degradation by the ER-associated degradation system. However, LLG1 is unlikely to function as an ER chaperone or in glycosylation, as it is present at the plasma membrane. Instead, LLG1 could facilitate localization of PRRs to defined plasma membrane microdomains (40), and play roles in PRR signaling. In addition to the defects in FLS2-mediated ROS production, the llg1-3 mutants accumulate lower levels of SA and have decreased SA-related induction of defenses, which may explain, at least in part, the defects in plant immunity shown by llg1-3 mutants. SA-based defense was shown to require the ERQC system (38). The llg1 mutant plants show defects in flg22-induced expression of SA marker genes (Fig. S8A), indicating a possible connection between SA defects and PTI signaling defects of llg1 plants. The ERQC system and LLG1 may be related in this aspect.
In addition, LLG1 also contributes to BIK1-dependent FLS2 signaling. Furthermore, the LLG1–3 variant did not affect FLS2 accumulation and ligand-induced degradation, thus uncoupling the role of LLG1 as a chaperone from its function in signaling. Our findings support a model in which LLG1 regulates accumulation of FLS2, its flg22-induced degradation, and signaling functions (Fig. S10), as the absence of LLG1 affects the accumulation, degradation, and signaling of FLS2. However, in the llg1-3 mutant, LLG1–3 still associates with FLS2 and functions as a chaperone, but the llg1-3 mutation may affect the conformation of LLG1–3 and FLS2, which leads to defects in activation of FLS2 signaling (Fig. S10).
Fig. S10.
A model illustrating how LLG1 differentially regulates FLS2 accumulation, flg22-induced degradation, and signaling. (Left) In the wild-type plant, LLG1 is fully functional as a chaperone for FLS2. (Center) In the absence of the LLG1 chaperone, FLS2 manifests defects in accumulation, degradation, and signaling. (Right) In the llg1-3 mutant, the LLG1–3 variant retains chaperone function, but the conformation of LLG1–3 and FLS2 may be affected, which impairs FLS2 signaling.
A previous study reported that LLG1 functions as a coreceptor of FER to regulate FER-mediated growth and developmental processes (29). The fer-4 and llg1-2 mutants show similar developmental defects, such as retarded growth and root-hair defects (29). However, llg1-3, the allele we identified, does not affect these developmental processes, suggesting that the LLG1–3 variant could function properly in FER-mediated responses during development, and that the immunity and developmental processes mediated by LLG1 can be uncoupled. Our data show that LLG1 associates with PRRs and contributes to accumulation and ligand-induced degradation of PRRs. LLG1 associates with FER, and is required for its plasma membrane localization (29). However, whether LLG1 regulates ligand-induced degradation of FER, and whether the llg1-3 mutation affects any aspects of the FER signaling remain to be determined. In conclusion, we identified LLG1 as a component of immune receptor complexes and showed that it plays crucial roles in plant immunity by modulating accumulation and signaling of the PAMP receptor FLS2.
Materials and Methods
Plant Materials and Growth Conditions.
The Arabidopsis thaliana llg1-2 (SALK_086036c) and lre-3 (SALK_040289) mutants were obtained from the Arabidopsis Biological Resource Center and have been described previously (30, 31). The edr1 llg1-3 mutant was isolated from an EMS-mutagenized edr1 population (26). Arabidopsis plants were grown under short-day conditions (8-h light, 16-h dark, 22 °C) for phenotyping and under long-day conditions (16-h light, 8-h dark, 22 °C) for seed set. For confocal microscopy, seedlings were grown 4 wk on soil under 10-h light at 22 °C and 60% humidity.
Map-Based Cloning of LLG1.
To map LLG1, we crossed the edr1 llg1-3 mutants to Landsberg erecta to generate a segregating mapping population. The llg1-3 mutation was mapped to a region in BAC clone MDA7 (GenBank accession no. AB011476) on chromosome 5. We amplified and sequenced all genes in this region to identify the llg1-3 mutation.
Pathogen Infection, SA Measurement, Plasmid Construction, RNA Extraction, qRT-PCR, Confocal Microscopy, Co-IP Assay, MAPK, and ROS Assays.
Additional detailed procedures are described in SI Materials and Methods. See Table S1 for a list of primers used in this study.
Table S1.
Primers used in this study
| Primer name | Purpose | Sequence (5′–3′) |
| llg1-3dcaps FP | dCAPS primer for llg1-3 | ATCTCTATGGAAAATACCCGTCT |
| llg1-3dcaps RP | dCAPS primer for llg1-3 | GTTGCTGCGTTTACCTCTGCT |
| LLG1G FP | Complementation | TCCCCCCGGGTCGTTGTCCCAGATTCGT |
| LLG1G RP | Complementation | ACATGCATGCATGGCGAGCTTATGGGTTC |
| LLG1CDS FP | YFP–LLG1 localization | CCGGAATTCTCAGATGGGGTCTTCGAATC |
| LLG1CDS RP | YFP–LLG1 localization | CCCAAGCTTGGGTCAGAACAACTTAACAAAAACCAAA |
| LLG1pro FP | YFP–LLG1 localization | CCTTAATTAAGGATGCCTTGTTACATAGTTTCGT |
| LLG1pro RP | YFP–LLG1 localization | TGCACCGGTAGAATGAAACTTGAAGAAGAGAAA |
| LLG1 FP | Gateway cloning | GGGGACAAGTTTGTACAAAAAAGCAGGCTTCATGGAGCTCCTCTCTAGAGCTCT |
| LLG1 RP | Gateway cloning | GGGGACCACTTTGTACAAGAAAGCTGGGTCTCAGAACAACTTAACAAAAACCAAAA |
| FER FP | To clone FER in pEGAD | ATTAAGAGCTCGAAGGGTGGTCACTGGTAAAGC |
| FER RP | To clone FER in pEGAD | TGCACCGGTAGACGTCCCTTTGGATTCATGATC |
| PR1 FP | Real-time PCR | GTGGGTTAGCGAGAAGGCTA |
| PR1 RP | Real-time PCR | ACTTTGGCACATCCGAGTCT |
| PR2 FP | Real-time PCR | GAATCAAGGAGCTTAGCCTCACC |
| PR2 RP | Real-time PCR | GTAGAGCCGCATTCGCTGGAT |
| FRK1 FP | Real-time PCR | GATACTCTAGTCCTTGCGCTGC |
| FRK1 RP | Real-time PCR | ATTGCAACGAAATAAACCACA |
| At2g17740 FP | Real-time PCR | TGCTCCATCTCTCTTTGTGC |
| At2g17740 RP | Real-time PCR | ATGCGTTGCTGAAGAAGAGG |
| BD-LLG1 FP | Yeast two-hybrid | CCGGAATTCAGTTTCATTTCAGATGGGGTCTT |
| BD-LLG1 RP | Yeast two-hybrid | AACTGCAGAATCACGAGGTAGTTGCTGCGTTTAC |
| AD-FLS2 FP | Yeast two-hybrid | CCGGAATTCCATGTTCCTGAATCCGGGG |
| AD-FLS2 RP | Yeast two-hybrid | CGGGATCCAACTAGGCTGATCCAAGAATAATCA |
| AD-EFR FP | Yeast two-hybrid | CCGGAATTCTTTCGAAATGCTACAGCAGTT |
| AD-EFR RP | Yeast two-hybrid | CGGGATCCAATCTAACTGACAGAGGCTTTCTCT |
| AD-FER FP | Yeast two-hybrid | CCGGAATTCGTGAATCCTCCAGAGGGAAAT |
| AD-FER RP | Yeast two-hybrid | CGGGATCCAACTATGCAATAATAGCCGTATTGCTT |
SI Materials and Methods
Pathogen Infection and SA Measurement.
The powdery mildew pathogen Golovinomyces cichoracearum UCSC1 and the oomycete pathogen Hyaloperonospora arabidopsidis Noco 2 were maintained on the highly susceptible pad4-1 mutants to generate fresh spores. The infection assays with G. cichoracearum, Pseudomonas syringae pv tomato (Pto) DC3000, and H. arabidopsidis Noco2 were performed as previously described (41). To quantify fungal growth after G. cichoracearum infection, the number of conidiophores per colony was counted at 5 dpi. Trypan blue staining was used to reveal fungal hyphae structures and dead plant cells, as described previously (42). SA extraction and measurement were performed as described previously (43).
Plasmid Construction.
For the complementation analysis, the LLG1 genomic sequence, including 1,156-bp upstream of the ATG start codon and 959-bp downstream of the stop codon, was cloned into the binary vector pCambia1300. To generate the 35S:YFP–LLG1 construct, the LLG1 coding sequence, minus the first 78-bp fragment encoding the N-terminal signal peptide, was amplified by PCR from a Col-0 cDNA library and subsequently cloned into the binary vector pEGAD-YFP between the EcoRI and HindIII sites. Then the LLG1 promoter fragment containing 1,394-bp upstream of the ATG start codon and the first 78 bp after the start codon was amplified by PCR from genomic DNA and cloned into pEGAD-YFP between the PacI and AgeI sites, resulting in the pLLG1:YFP–LLG1 construct. Then the YFP–LLG1 sequence was amplified from the pLLG1:YFP–LLG1 construct and cloned into the pDONR207 ENTRY vector, and then into the pMDC32 destination vector, according to the manufacturer’s instructions (Invitrogen). The 35S:YFP–LLG1G114R construct was made using a similar strategy, except that the LLG1G114R coding sequence was amplified from llg1-3 cDNA.
To make the pLLG1:FLAG–LLG1 construct, the FLAG–LLG1 fusion sequence was generated by gene synthesis (Genewiz) and cloned into the pEGAD vector between the AgeI and HindIII sites, then the LLG1 promoter fragment was inserted between the PacI and AgeI sites.
To make the pFER:FER-GFP and pFER:FER-HA constructs, the genomic sequence of FER (without the stop codon), including 1,640-bp upstream of the start codon, were amplified by PCR from Col-0 genomic DNA. The amplified fragments were cloned into the binary vector pEGAD-GFP and pEGAD-HA between the SacI and AgeI sites.
The constructs were introduced into the Agrobacterium tumefaciens strain GV3101 and then transformed Arabidopsis plants by the floral-dip method (44).
For the yeast two-hybrid assay, the coding sequences (70–447) of the LLG1 and LLG1G114R were amplified and cloned into pGBKT-7. The sequences encoding the extracellular juxtamembrane domains of FLS2, EFR, and FER, were amplified and cloned into pGADT-7.
RNA Extraction and qRT-PCR.
RNA extraction and qRT-PCR were performed as described previously (13). The ACTIN2 gene was used as an internal control for normalizing. Data represent duplicate technical replicates of three biological replicates.
The Statistical Analysis.
The statistical analysis was performed using a mixed-effects model for nested ANOVA as described previously (45), unless indicated otherwise.
Confocal Microscopy.
Transgenic plants expressing GFP or YFP fusion proteins were analyzed using a Zeiss LSM-710 confocal microscope (Zeiss). The GFP and YFP fluorescence was excited at a wavelength of 488 nm and detected at 505–550 nm. For colocalization experiments, Arabidopsis seedlings were mounted in 1 μM FM4-64 (Invitrogen) for 2 min to stain membranes.
For FLS2–YFP localization, standard confocal microscopy was performed using a Leica SP8 laser point scanning microscope mounted with hybrid detectors (HyD). YFP was excited at 498 nm using a white light laser source. Chloroplast autofluorescence was captured between 700 and 800 nm. Images were taken using a 63× water immersion objective and processed using the Leica Lite and FIJI software (46).
Co-IP Assay.
Co-IP assays were performed as described previously, with minor modifications (13). For anti-GFP IP, protein extracts were incubated with agarose-conjugated anti-GFP antibody (MBL) for 4 h at 4 °C with gentle rotation. For anti-HA IP or anti-FLAG IP, protein extracts were incubated with anti-HA or anti-FLAG IP antibodies (CWBIO) for 4 h at 4 °C, and then protein G agarose beads (Millipore) were added and incubated for another 3 h. The agarose beads were washed and resuspended in 80 μL PBS and 20 μL 5 × SDS/PAGE sample buffer, and boiled for 5 min. Protein samples were detected with anti-BAK1, anti-HA (CWBIO), anti-FLAG (Abmart), or anti-GFP (Abmart) immunoblot.
Plasma Membrane Protein Fractionation and Immunoblotting.
FLS2–YFP cytosol and plasma membrane protein were extracted by a Minute Plasma Membrane Protein Isolation Kit for Plants (Invent Biotechnologies). H+-ATPase was detected with anti-H+-ATPase (Agrisera).
BIK1 Phosphorylation Assay.
BIK1 phosphorylation assay was performed as described previously (47).
MAPK and ROS Assays.
MAPK and ROS assays were performed as described previously (13). For MAPK assays, 10-d-old seedlings were treated with 100 nM flg22. Proteins were detected by anti-p42/44 MAPK antibodies (Cell Signaling Technology). For ROS assays, leaf strips of 4-wk-old plants were treated with 100 nM flg22 or 100 nM elf18. Luminescence was detected with the GLOMAX 96 microplate luminometer (Promega).
Acknowledgments
We thank Ueli Grossniklaus for sharing pFER:FER-GFP/fer seeds, Alice Y. Cheung for fer-4 and pFER:FER-GFP/fer-4 seeds, and Jian-Min Zhou for BAK1 antibody and pBAK1:BAK1-FLAG, 35S:FLS2-HA, 35S:BIK1-HA constructs. The work was supported by National Science Fund for Distinguished Young Scholars of China Grant 31525019, the Ministry of Science and Technology of China Grant 2015CB910202, and the Strategic Priority Research Program of the Chinese Academy of Sciences Grant XDB11020100 (to D.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1614468114/-/DCSupplemental.
References
- 1.Böhm H, Albert I, Fan L, Reinhard A, Nürnberger T. Immune receptor complexes at the plant cell surface. Curr Opin Plant Biol. 2014;20:47–54. doi: 10.1016/j.pbi.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 3.Dodds PN, Rathjen JP. Plant immunity: Towards an integrated view of plant-pathogen interactions. Nat Rev Genet. 2010;11:539–548. doi: 10.1038/nrg2812. [DOI] [PubMed] [Google Scholar]
- 4.Schwessinger B, Ronald PC. Plant innate immunity: Perception of conserved microbial signatures. Annu Rev Plant Biol. 2012;63:451–482. doi: 10.1146/annurev-arplant-042811-105518. [DOI] [PubMed] [Google Scholar]
- 5.Boller T, Felix G. A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol. 2009;60:379–406. doi: 10.1146/annurev.arplant.57.032905.105346. [DOI] [PubMed] [Google Scholar]
- 6.Zipfel C, et al. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature. 2004;428:764–767. doi: 10.1038/nature02485. [DOI] [PubMed] [Google Scholar]
- 7.Zipfel C, et al. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell. 2006;125:749–760. doi: 10.1016/j.cell.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 8.Chinchilla D, et al. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature. 2007;448:497–500. doi: 10.1038/nature05999. [DOI] [PubMed] [Google Scholar]
- 9.Sun Y, et al. Structural basis for flg22-induced activation of the Arabidopsis FLS2-BAK1 immune complex. Science. 2013;342:624–628. doi: 10.1126/science.1243825. [DOI] [PubMed] [Google Scholar]
- 10.Macho AP, et al. A bacterial tyrosine phosphatase inhibits plant pattern recognition receptor activation. Science. 2014;343:1509–1512. doi: 10.1126/science.1248849. [DOI] [PubMed] [Google Scholar]
- 11.Beck M, Zhou J, Faulkner C, MacLean D, Robatzek S. Spatio-temporal cellular dynamics of the Arabidopsis flagellin receptor reveal activation status-dependent endosomal sorting. Plant Cell. 2012;24:4205–4219. doi: 10.1105/tpc.112.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mbengue M, et al. Clathrin-dependent endocytosis is required for immunity mediated by pattern recognition receptor kinases. Proc Natl Acad Sci USA. 2016;113:11034–11039. doi: 10.1073/pnas.1606004113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi H, et al. BR-SIGNALING KINASE1 physically associates with FLAGELLIN SENSING2 and regulates plant innate immunity in Arabidopsis. Plant Cell. 2013;25:1143–1157. doi: 10.1105/tpc.112.107904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu D, et al. A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proc Natl Acad Sci USA. 2010;107:496–501. doi: 10.1073/pnas.0909705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadota Y, et al. Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol Cell. 2014;54:43–55. doi: 10.1016/j.molcel.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 16.Li L, et al. The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe. 2014;15:329–338. doi: 10.1016/j.chom.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Korasick DA, et al. Novel functions of stomatal cytokinesis-defective 1 (SCD1) in innate immune responses against bacteria. J Biol Chem. 2010;285:23342–23350. doi: 10.1074/jbc.M109.090787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicaise V, et al. Pseudomonas HopU1 modulates plant immune receptor levels by blocking the interaction of their mRNAs with GRP7. EMBO J. 2013;32:701–712. doi: 10.1038/emboj.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petutschnig EK, Jones AME, Serazetdinova L, Lipka U, Lipka V. The lysin motif receptor-like kinase (LysM-RLK) CERK1 is a major chitin-binding protein in Arabidopsis thaliana and subject to chitin-induced phosphorylation. J Biol Chem. 2010;285:28902–28911. doi: 10.1074/jbc.M110.116657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shan L, et al. Bacterial effectors target the common signaling partner BAK1 to disrupt multiple MAMP receptor-signaling complexes and impede plant immunity. Cell Host Microbe. 2008;4:17–27. doi: 10.1016/j.chom.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu T, et al. Chitin-induced dimerization activates a plant immune receptor. Science. 2012;336:1160–1164. doi: 10.1126/science.1218867. [DOI] [PubMed] [Google Scholar]
- 22.Thomma BP, Nürnberger T, Joosten MH. Of PAMPs and effectors: The blurred PTI-ETI dichotomy. Plant Cell. 2011;23:4–15. doi: 10.1105/tpc.110.082602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frye CA, Tang D, Innes RW. Negative regulation of defense responses in plants by a conserved MAPKK kinase. Proc Natl Acad Sci USA. 2001;98:373–378. doi: 10.1073/pnas.98.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao C, et al. EDR1 physically interacts with MKK4/MKK5 and negatively regulates a MAP kinase cascade to modulate plant innate immunity. PLoS Genet. 2014;10:e1004389. doi: 10.1371/journal.pgen.1004389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wawrzynska A, Christiansen KM, Lan Y, Rodibaugh NL, Innes RW. Powdery mildew resistance conferred by loss of the ENHANCED DISEASE RESISTANCE1 protein kinase is suppressed by a missense mutation in KEEP ON GOING, a regulator of abscisic acid signaling. Plant Physiol. 2008;148:1510–1522. doi: 10.1104/pp.108.127605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan H, Liu S, Tang D. HPR1, a component of the THO/TREX complex, plays an important role in disease resistance and senescence in Arabidopsis. Plant J. 2012;69:831–843. doi: 10.1111/j.1365-313X.2011.04835.x. [DOI] [PubMed] [Google Scholar]
- 27.Serrano I, Gu Y, Qi D, Dubiella U, Innes RW. The Arabidopsis EDR1 protein kinase negatively regulates the ATL1 E3 ubiquitin ligase to suppress cell death. Plant Cell. 2014;26:4532–4546. doi: 10.1105/tpc.114.131540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu G, et al. ENHANCED DISEASE RESISTANCE4 associates with CLATHRIN HEAVY CHAIN2 and modulates plant immunity by regulating relocation of EDR1 in Arabidopsis. Plant Cell. 2015;27:857–873. doi: 10.1105/tpc.114.134668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li C, et al. Glycosylphosphatidylinositol-anchored proteins as chaperones and co-receptors for FERONIA receptor kinase signaling in Arabidopsis. eLife. 2015;4:e06587. doi: 10.7554/eLife.06587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Capron A, et al. Maternal control of male-gamete delivery in Arabidopsis involves a putative GPI-anchored protein encoded by the LORELEI gene. Plant Cell. 2008;20:3038–3049. doi: 10.1105/tpc.108.061713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsukamoto T, Qin Y, Huang Y, Dunatunga D, Palanivelu R. A role for LORELEI, a putative glycosylphosphatidylinositol-anchored protein, in Arabidopsis thaliana double fertilization and early seed development. Plant J. 2010;62:571–588. doi: 10.1111/j.1365-313X.2010.04177.x. [DOI] [PubMed] [Google Scholar]
- 32.Robatzek S, Chinchilla D, Boller T. Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes Dev. 2006;20:537–542. doi: 10.1101/gad.366506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayor S, Riezman H. Sorting GPI-anchored proteins. Nat Rev Mol Cell Biol. 2004;5:110–120. doi: 10.1038/nrm1309. [DOI] [PubMed] [Google Scholar]
- 34.Schindelman G, et al. COBRA encodes a putative GPI-anchored protein, which is polarly localized and necessary for oriented cell expansion in Arabidopsis. Genes Dev. 2001;15:1115–1127. doi: 10.1101/gad.879101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith JM, et al. Loss of Arabidopsis thaliana dynamin-related protein 2B reveals separation of innate immune signaling pathways. PLoS Pathog. 2014;10:e1004578. doi: 10.1371/journal.ppat.1004578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu X, et al. Uncoupling of sustained MAMP receptor signaling from early outputs in an Arabidopsis endoplasmic reticulum glucosidase II allele. Proc Natl Acad Sci USA. 2009;106:22522–22527. doi: 10.1073/pnas.0907711106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nekrasov V, et al. Control of the pattern-recognition receptor EFR by an ER protein complex in plant immunity. EMBO J. 2009;28:3428–3438. doi: 10.1038/emboj.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saijo Y, et al. Receptor quality control in the endoplasmic reticulum for plant innate immunity. EMBO J. 2009;28:3439–3449. doi: 10.1038/emboj.2009.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Häweker H, et al. Pattern recognition receptors require N-glycosylation to mediate plant immunity. J Biol Chem. 2010;285:4629–4636. doi: 10.1074/jbc.M109.063073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keinath NF, et al. PAMP (pathogen-associated molecular pattern)-induced changes in plasma membrane compartmentalization reveal novel components of plant immunity. J Biol Chem. 2010;285:39140–39149. doi: 10.1074/jbc.M110.160531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao T, et al. A truncated NLR protein, TIR-NBS2, is required for activated defense responses in the exo70B1 mutant. PLoS Genet. 2015;11:e1004945. doi: 10.1371/journal.pgen.1004945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frye CA, Innes RW. An Arabidopsis mutant with enhanced resistance to powdery mildew. Plant Cell. 1998;10:947–956. doi: 10.1105/tpc.10.6.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gou M, et al. An F-box gene, CPR30, functions as a negative regulator of the defense response in Arabidopsis. Plant J. 2009;60:757–770. doi: 10.1111/j.1365-313X.2009.03995.x. [DOI] [PubMed] [Google Scholar]
- 44.Clough SJ, Bent AF. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 45.Liu S, Bartnikas LM, Volko SM, Ausubel FM, Tang D. Mutation of the glucosinolate biosynthesis enzyme cytochrome P450 83A1 monooxygenase increases camalexin accumulation and powdery mildew resistance. Front Plant Sci. 2016;7:227. doi: 10.3389/fpls.2016.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schindelin J, et al. Fiji: An open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Z, et al. BIK1 interacts with PEPRs to mediate ethylene-induced immunity. Proc Natl Acad Sci USA. 2013;110:6205–6210. doi: 10.1073/pnas.1215543110. [DOI] [PMC free article] [PubMed] [Google Scholar]