Significance
Epigenetic inactivation of XAF1 tumor suppressor is frequently observed in multiple human cancers. However, the mechanisms underlying its function remain largely undefined. Here, we present evidence that XAF1 plays a critical role in cell-fate decisions under heavy-metal–induced stress conditions through the mutual antagonism with MT2A. XAF1 is activated as a transcription target of MTF-1 and destabilizes MT2A through the interaction-directed lysosomal degradation, whereas it is destabilized by MT2A under cytostatic stress conditions. XAF1-mediated MT2A inactivation leads to elevation of free intracellular zinc level and up- and down-regulates p53 and XIAP, respectively, to promote apoptosis. XAF1–MT2A antagonism thus represents one critical regulator of cell-fate decisions, suggesting that it could be an attractive target for the therapeutic intervention of tumor progression.
Keywords: XAF1, metallothionein, MTF-1, heavy metal, apoptosis
Abstract
XIAP-associated factor 1 (XAF1) is a tumor suppressor that is commonly inactivated in multiple human neoplasms. However, the molecular mechanism underlying its proapoptotic function remains largely undefined. Here, we report that XAF1 induction by heavy metals triggers an apoptotic switch of stress response by destabilizing metallothionein 2A (MT2A). XAF1 directly interacts with MT2A and facilitates its lysosomal degradation, resulting in the elevation of the free intercellular zinc level and subsequent activation of p53 and inactivation of XIAP. Intriguingly, XAF1 is activated as a unique transcription target of metal-regulatory transcription factor-1 (MTF-1) in signaling apoptosis, and its protein is destabilized via the lysosomal pathway by MTF-1–induced MT2A under cytostatic stress conditions, indicating the presence of mutual antagonism between XAF1 and MT2A. The antagonistic interplay between XAF1 and MT2A acts as a key molecular switch in MTF-1–mediated cell-fate decisions and also plays an important role in cell response to various apoptotic and survival factors. Wild-type (WT) XAF1 but not MT2A binding-deficient mutant XAF1 increases the free intracellular zinc level and accelerates WT folding of p53 and degradation of XIAP. Consistently, XAF1 evokes a more drastic apoptotic effect in p53+/+ versus isogenic p53−/− cells. Clinically, expression levels of XAF1 and MT2A are inversely correlated in primary colon tumors and multiple cancer cell lines. XAF1-depleted xenograft tumors display an increased growth rate and a decreased apoptotic response to cytotoxic heavy metals with strong MT2A expression. Collectively, this study uncovers an important role for XAF1–MT2A antagonism as a linchpin to govern cell fate under various stressful conditions including heavy metal exposure.
Elimination of defective or potentially dangerous cells by apoptosis plays a fundamental role in the regulation of tissue homeostasis and prevention of malignant transformation and autoimmunity (1). X-linked inhibitor of apoptosis (XIAP)-associated factor 1 (XAF1) is a proapoptotic tumor suppressor that is identified as a negative regulator of XIAP based on its ability to bind and interfere with the anticaspase function of XIAP (2, 3). XAF1 expression is down-regulated in a broad range of human tumors mainly by aberrant promoter CpG site hypermethylation, and its reduction is associated with the advanced stage and high grade of many cancers (4–6). A recent study showed that PTEN-null mouse prostate tumors showing resistance to androgen-deprivation therapy have reduced levels of XAF1, and diminished expression of XAF1 by continuous androgen deprivation therapy is positively correlated with metastatic castrate-resistant tumor progression (7).
XAF1 sensitizes tumor cells to the proapoptotic effects of chemotherapeutic drugs, γ-irradiation, UV, H2O2, hypoxia, and growth factor deprivation, whereas its depletion increases cellular resistance to apoptotic stresses (5, 6). XAF1 was originally identified as a nuclear protein that could bind and sequester XIAP protein to the nucleus (3). It was thus proposed that loss of XAF1 may increase the functional pool of cytoplasmic XIAP, which in turn deregulates the apoptotic process and contributes to tumorigenesis (8). However, it was shown that XAF1 evokes an apoptotic effect in XIAP−/− cells to the extent comparable in XIAP+/+ cells, and XIAP is not sequestered to the nucleus in cells undergoing XAF1-induced apoptosis, indicating that XAF1’s proapoptotic function is not solely dependent on the XIAP-interfering activity (6, 9). XAF1 was also identified as an IFN-stimulated gene that contributes to IFN-dependent sensitization of cells to tumor necrosis factor (TNF)-related apoptosis inducing ligand (TRAIL)-induced apoptosis (10, 11). Recently, we reported that XAF1 forms a positive feedback loop with the p53 tumor suppressor and acts as a molecular switch in p53-mediated cell-fate decisions favoring apoptosis over cell-cycle arrest (12). In this process, XAF1 appears to promote homeodomain-interacting protein kinase 2 (HIPK2)-mediated p53 phosphorylation by interrupting the HIPK2-targeting function of Siah2 and promotes zinc finger protein 313 (ZNF313)-induced p21WAF1 ubiquitination (13). XAF1 was also shown to inhibit tumor angiogenesis by suppressing VEGF expression and regulate the cell cycle through modulating the G2/M checkpoint and interaction with checkpoint kinase 1 (14, 15).
Many heavy metals and metallic agents have biological activity but also cause many adverse health effects, such as carcinogenicity in humans (16). However, the mechanism underlying heavy-metal–induced tumorigenesis remains unclear. Metallothioneins (MTs) are low-molecular-weight (6–7 kDa), cysteine-rich, heavy-metal–binding proteins that play a crucial role in the regulation of intracellular metal metabolism and metal-induced stresses (17, 18). Upon heavy metals stimuli, MTs are rapidly transcriptionally activated by metal-regulatory transcription factor-1 (MTF-1) and function in protecting cells against heavy metals, particularly cadmium, zinc, copper, mercury, nickel, platinum, and silver primarily by acting as scavengers of toxic metal ions (19, 20). MTs also have crucial roles in cell proliferation and differentiation, and their activity to bind heavy metals and detoxify free radicals can help cancer cells to survive by inhibition of apoptosis (21, 22). Moreover, MTF-1 is elevated in human breast, lung, and cervical carcinoma-derived cell lines, supporting the importance of MTs in carcinogenesis (23).
Of the 11 human functional MT isoforms [MT-1 (A, B, E, F, G, H, M, and X), MT2A, MT-3, and MT-4], ubiquitously expressed MT1 and MT2A are extensively studied for their ability to attenuate metal-induced toxicity and as biomarkers in many human cancers (18, 24, 25). MT2A is the major MT isoform expressed in humans and has specific functions in regulating apoptosis, autophagy, and increasing risk of cancers (26–28). MT2A is up-regulated in many types of tumor tissues and cell lines, and its elevation is associated with adverse prognosis. MT2A induction decreases intracellular zinc availability whereas its silencing increases free intracellular zinc concentration, thus modulating the intracellular levels of bioavailable zinc (29). MT2A contributes to chemotherapy resistance by direct chelation of zinc and platinum-containing drugs and by indirect action on p53 zinc-dependent activity. It was reported that HIPK2 depletion induces MT2A up-regulation and p53 misfolding, but codepletion of MT2A restores p53 activity, showing that MT2A can modulate p53 through zinc exchange (30). Several studies demonstrated an association of MT2A elevation with grade and aggressiveness of tumors, including prostate cancer (27, 31, 32). Nevertheless, the molecular mechanism underlying the biological functions of MT2A and its regulation in the carcinogenic process has not been well understood.
In this study, we present evidence that XAF1–MT2A mutual antagonism via the interaction-directed lysosomal degradation plays a crucial role in cell-fate decisions under various stressful conditions, including heavy metal exposure.
Results
XAF1 Directly Interacts with and Down-Regulates MT2A.
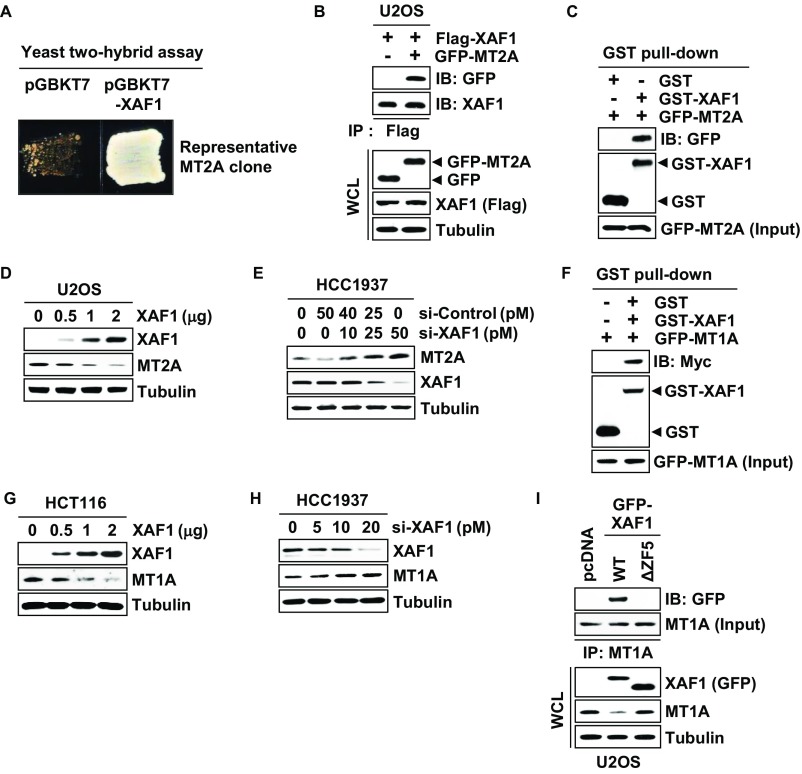
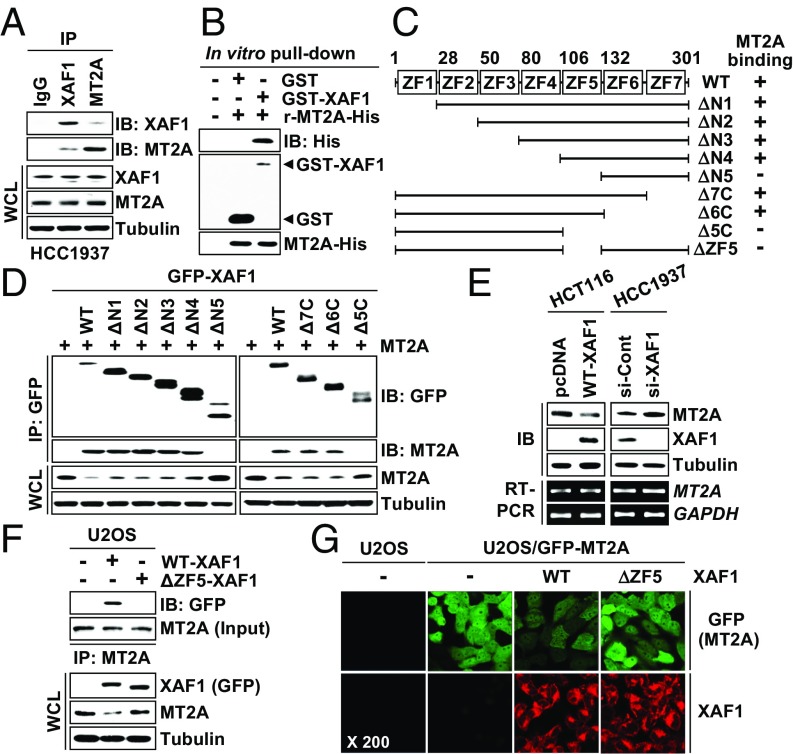
In an attempt to understand the mechanistic basis of XAF1 proapoptotic function, we initially performed a yeast two-hybrid assay and identified MT2A as a XAF1-interacting protein (Fig. S1A). The XAF1–MT2A interaction was confirmed by immunoprecipitation (IP) and GST pull-down assays (Fig. 1A and Fig. S1 B and C). An in vitro pull-down assay using purified GST-XAF1 and recombinant MT2A proteins revealed that XAF1 binds directly to MT2A (Fig. 1B). Using a series of deletion mutants, we determined that zinc finger domain 5 (ZF5) of XAF1 is essential for the interaction (Fig. 1 C and D). Moreover, the MT2A protein level was markedly decreased and increased by XAF1 expression and depletion, respectively, suggesting that XAF1 may down-regulate MT2A via direct interaction (Fig. 1E and Fig. S1 E and F). As predicted, MT2A protein level was reduced by wild type (WT)-XAF1 but not affected by ΔZF5-XAF1, a mutant XAF1 lacking the ZF5 domain (Fig. 1F). Consistently, an immunofluorescence assay using U2OS/GFP-MT2A subline cells showed a profound reduction of GFP-MT2A fluorescence by WT-XAF1 but not by ΔZF5-XAF1 (Fig. 1G). Based on these observations, we asked whether XAF1 regulates MT1A, another ubiquitously expressed MT isoform, and found that XAF1 also interacts with and down-regulates MT1A (Fig. S1 F–I).
Fig. S1.
Identification of MT2A and MT1A as a XAF1-binding protein. (A) Identification of MT2A as a XAF1-interacting protein in a yeast two-hybrid assay. (B) IP assay of the XAF1–MT2A interaction. IB, immunoblot; WCL, whole-cell lysate. (C) GST pull-down assay for the XAF1–MT2A interaction. (D) A dose-associated down-regulation of the MT2A protein level by ectopic expression of XAF1. (E) Induction of MT2A protein by siRNA-mediated depletion of XAF1 in HCC1937 cells. (F) GST pull-down assay for the XAF1-MT1A interaction. (G) A dose-associated down-regulation of the MT1A protein level by ectopic expression of XAF1. (H) Induction of MT1A protein by siRNA-mediated depletion of XAF1 in HCC1937 cells. (I) IP and IB assays showing a crucial role for the ZF5 domain of XAF1 in interaction with and down-regulation of MT1A.
Fig. 1.
XAF1 binds directly to MT2A. (A) Interaction of endogenously expressed XAF1 and MT2A. IB, immunoblot; WCL, whole-cell lysate. (B) In vitro pull-down assay showing a direct interaction between XAF1 and MT2A. (C) XAF1 constructs and its MT2A-binding status. (D) Identification of the ZF5 domain as a MT2A-binding region of XAF1. (E) XAF1 down-regulation of MT2A protein expression. (F) A crucial role for XAF1 ZF5 domain in binding and downregulating MT2A. (G) Immunofluorescence microscopic analysis showing no MT2A-reducing activity of ΔZF5-XAF1.
XAF1 Promotes Lysosomal Degradation of MTs.
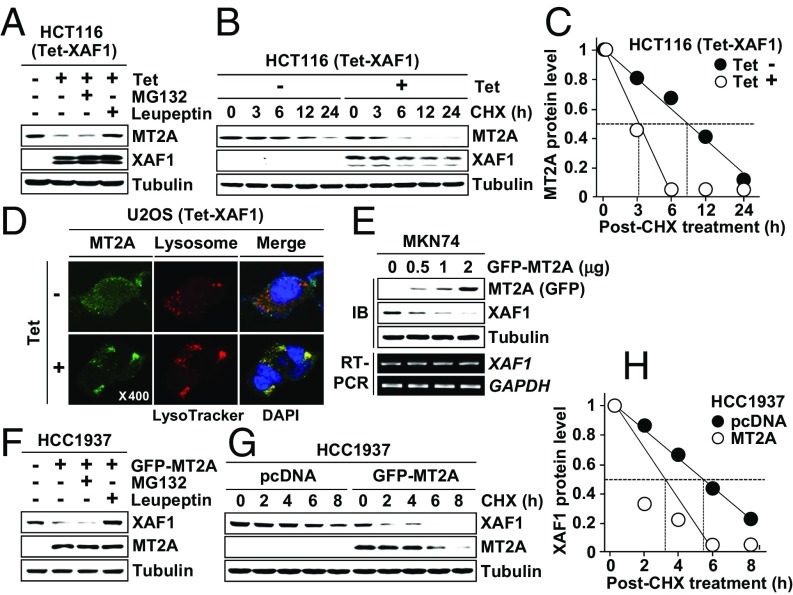
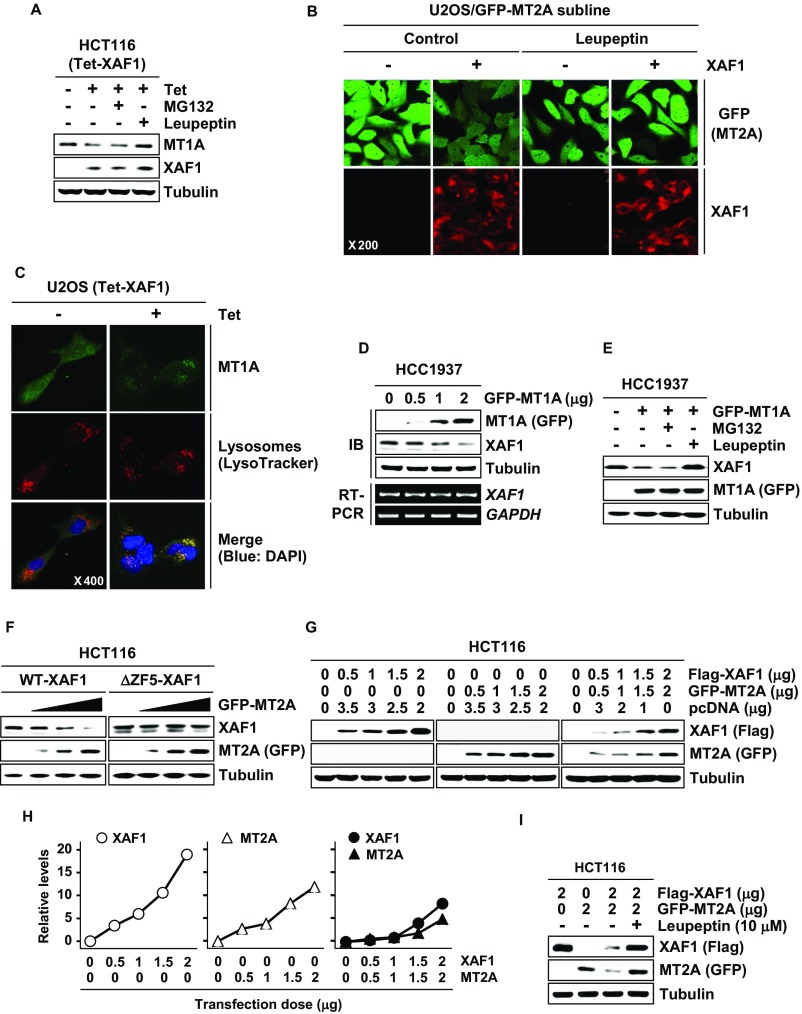
XAF1 controls proteasomal degradation of proteins through the interaction with ubiquitin E3 ligases (12, 13). Meanwhile, the protein stability of MTs has been known to be regulated mainly through the lysosomal pathway (33). We determined whether XAF1 down-regulation of MTs is affected by the proteasome inhibitor MG132 and/or the lysosome inhibitor Leupeptin. In HCT116 cells with a tetracycline-inducible system (Tet-XAF1), XAF1 down-regulation of MT2A and MT1A was blocked by Leupeptin but not affected by MG132 (Fig. 2A and Fig. S2A). A cycloheximide (CHX) chase experiment revealed that the half-life of MT2A protein is shortened from 8.9 h to 3.1 h by XAF1 induction (Fig. 2 B and C). In U2OS/GFP-MT2A cells, GFP-MT2A fluorescence was decreased by XAF1, but this effect was abolished by Leupeptin (Fig. S2B). Moreover, XAF1 was shown to stimulate the lysosomal translocation of MT2A and MT1A, supporting that XAF1 facilitates lysosomal degradation of MTs (Fig. 2D and Fig. S2C). Intriguingly, XAF1 was also down-regulated at the protein level by MT2A and MT1A, and this effect was blocked by Leupeptin, indicating that both MTs promote lysosomal degradation of XAF1 (Fig. 2 E and F and Fig. S2 D and E). The half-life of XAF1 protein was shortened from 5.7 h to 3.3 h by overexpressed MT2A (Fig. 2 F and G). MT2A failed to decrease ΔZF5-XAF1, supporting that MT2A destabilizes XAF1 through the interaction (Fig. S2F). We next assessed whether interaction of XAF1 and MT2A results in their mutual degradation. Compared with individually transfected cells, cotransfected cells showed detectable reduction of both proteins, and this reduction of both proteins was blocked by Leupeptin (Fig. S2 G–I). Together, these results support the presence of XAF1–MT2A mutual antagonism.
Fig. 2.
XAF1 promotes lysosomal degradation of MT2A. (A) Blockade of XAF1-induced MT2A reduction by Leupeptin. Cells were treated with tetracycline (1 μg/mL) and then exposed to MG132 (10 μM) or Leupeptin (10 μM) for 6 h. (B and C) A CHX chase assay showing XAF1-mediated MT2A destabilization. Cells were treated with CHX (40 μM) for the indicated hours. (D) Immunofluorescence analysis showing XAF1 stimulation of MT2A lysosomal translocation. (E) MT2A down-regulation of XAF1 protein expression. (F) Blockade of MT2A-induced XAF1 reduction by Leupeptin. (G and H) A CHX chase assay showing MT2A-mediated XAF1 destabilization (G) and comparison of XAF1 protein levels in control and MT2A-overexpressed cells following CHX treatment (H).
Fig. S2.
MT2A and MT1A promote lysosomal degradation of XAF1. (A) Blockade of XAF1-induced MT1A down-regulation by Leupeptin. (B) Blockade of XAF1 inhibition of GFP-MT2A fluorescence by Leupeptin. (C) Immunofluorescence microscopic analysis showing XAF1-induced lysosomal translocation of MT1A. (D) MT1A down-regulation of XAF1 protein expression. (E) Blockade of MT1A-induced XAF1 down-regulation by Leupeptin. (F) MT2A down-regulation of WT-XAF1 but not of ΔZF5-XAF1. (G–I) Cotransfection assay showing mutual degradation of XAF1 and MT2A its blockage by Leupeptin.
XAF1 Is a Unique Transcriptional Target of MTF-1 in Signaling Apoptosis.
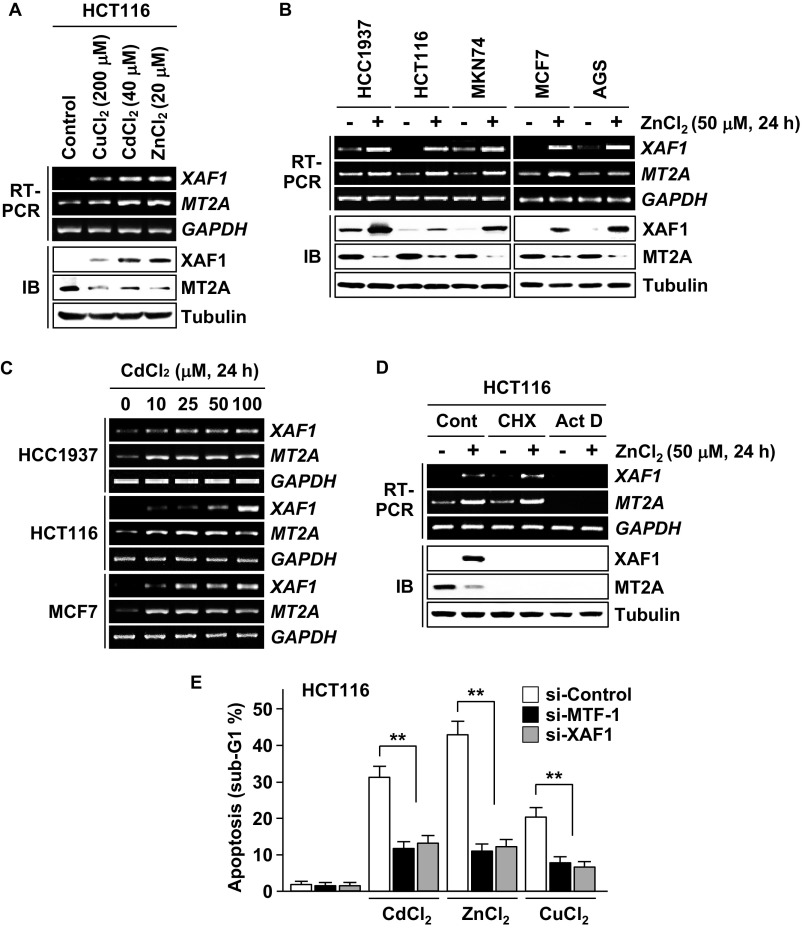
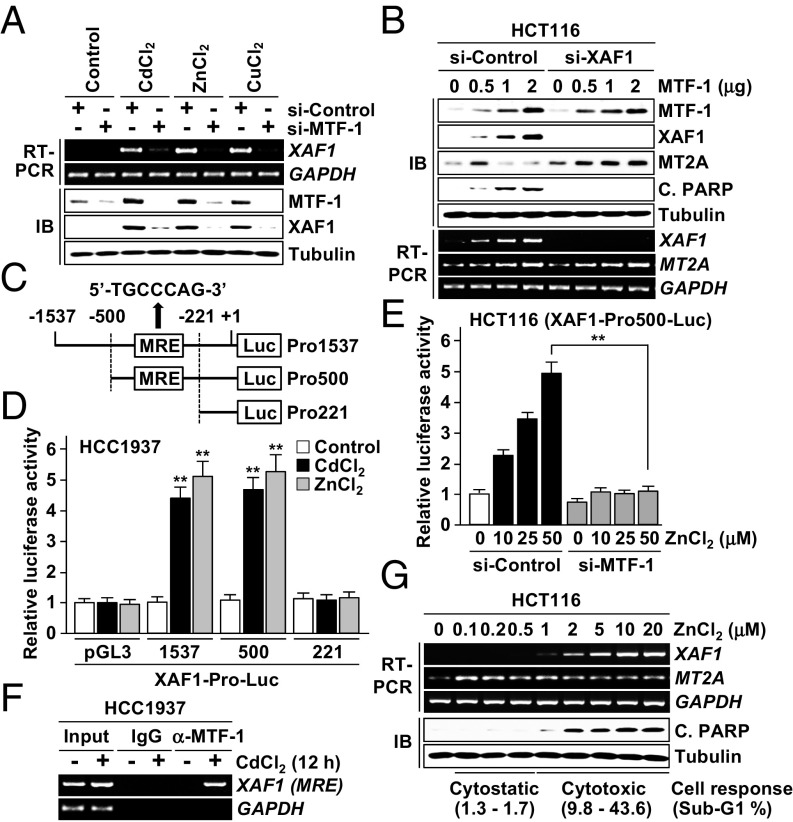
Next we examined whether XAF1 is activated in response to heavy metals. In multiple human cells exposed to CdCl2, ZnCl2, and CuCl2, XAF1 expression was markedly up-regulated at the mRNA level, and its induction showed an inverse correlation with MT2A at the protein level (Fig. S3 A–D). MTF-1 is a major transcriptional factor that protects cells from metal stress by activating metal-responsive genes, such as MTs, through the metal response element (MRE) in the promoters. Intriguingly, metal induction of XAF1 mRNA was suppressed by MTF-1 depletion, and metal-induced apoptosis was blocked by either MTF-1 or XAF1 depletion (Fig. 3A and Fig. S3E). MTF-1 overexpression led to a dose-associated elevation of XAF1 mRNA, and its apoptotic effect is abrogated if XAF1 induction is blocked (Fig. 3B). In MTF-1–overexpressed cells, XAF1 induction suppressed MT2A protein but not mRNA induction. As expected, a putative MRE was identified in the 5′ upstream region (nucleotides −283/−277 relative to ATG) of the XAF1 gene, and the reporters comprising this MRE displayed an approximately fivefold increase in luciferase activity in response to CdCl2 and ZnCl2 whereas this responsiveness was not detected in MTF-1–depleted cells (Fig. 3 C–E). A chromatin IP (ChIP) assay revealed that CdCl2 exposure induces MTF-1 binding to the MRE in the XAF1 promoter, indicating that the MRE is occupied by endogenous MTF-1 (Fig. 3F). Expression assays using increasing doses of ZnCl2 treatment showed that XAF1 expression is activated in response to cytotoxic (≥1 μM) but not cytostatic (0.1–0.5 μM) stress whereas MT2A is induced in response to both stresses (Fig. 3G).
Fig. S3.
XAF1 expression is activated in response to heavy metals. (A) XAF1 induction by heavy metals and its inverse correlation with MT2A protein level. HCT116 cells were exposed to CdCl2 (40 μM), ZnCl2 (20 μM), and CuCl2 (200 μM) for 24 h. (B) XAF1 induction by heavy metal stress in various types of human cells. Cells were exposed to ZnCl2 (50 μM) for 24 h. (C) A dose-associated induction of XAF1 mRNA expression in cells exposed to CdCl2. (D) Effect of CHX and actinomycin (Act D) on metal induction of XAF1. HCT116 cells were treated with CHX (40 μM) or Act D (1 μg/mL) and then exposed to ZnCl2 (50 μM) for 24 h. (E) Attenuation of metal-induced apoptosis by depletion of either MTF-1 or XAF1. Cells transfected with siRNA (20 pM) were exposed to the indicated heavy metals (100 μM) for 24 h. Data represent the mean ± SD. **P < 0.01 (Student t test).
Fig. 3.
Identification of XAF1 as a direct transcriptional target of MTF-1. (A) Activation of XAF1 mRNA expression by heavy metals. Cells were treated with heavy metal (100 μM) for 24 h. (B) MTF-1 induction of apoptosis and its attenuation by XAF1 depletion. (C) A MRE in the XAF1 promoter and reporter construction for luciferase assay. (D and E) Metal activation of the XAF1 promoter and its attenuation by MTF-1 depletion. (F) A ChIP assay showing MTF-1 interaction with the MRE in the XAF1 promoter. (G) Activation of XAF1 mRNA expression by cytotoxic but not cytostatic doses of ZnCl2.
XAF1–MT2A Antagonism Plays a Crucial Role in Cell-Fate Decisions Under Metal Stress Conditions.
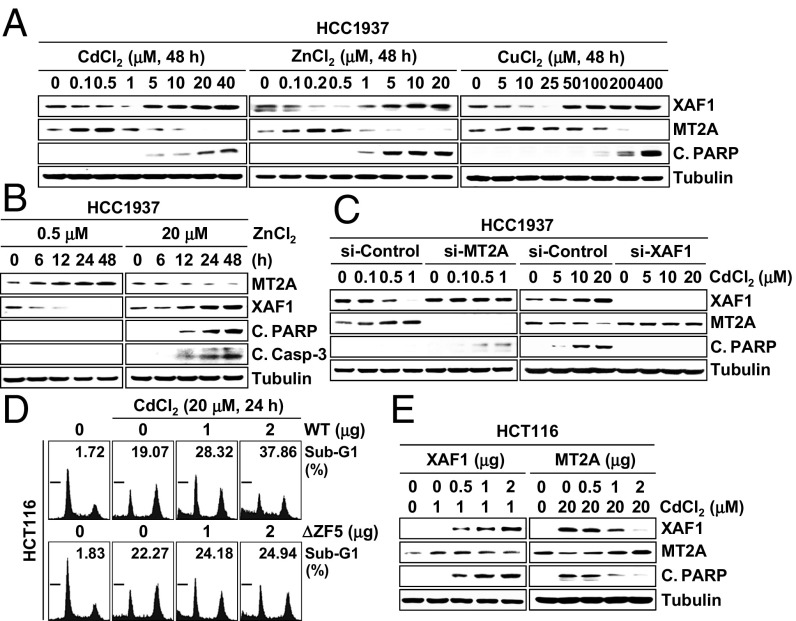
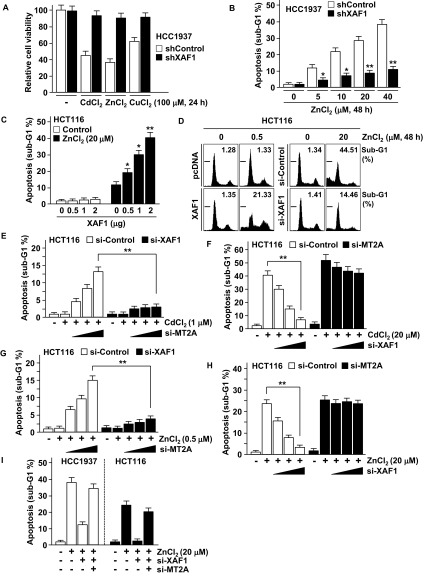
We investigated whether the XAF1–MT2A antagonism is linked to cellular response to metal stress. In cells exposed to cytostatic doses of CdCl2 (<5 μM), ZnCl2 (<1 μM), and CuCl2 (<100 μM), MT2A was up-regulated and XAF1 was down-regulated (Fig. 4 A and B). By contrast, when the cells were exposed to cytotoxic doses, XAF1 was up-regulated and MT2A was down-regulated. The metal dose-associated reduction of XAF1 and MT2A was blocked by depletion of MT2A and XAF1, respectively, indicating that the opposite responses of XAF1 and MT2A to metal stress results from their mutual antagonism (Fig. 4C). Compared with control cells, XAF1-depleted cells manifested a substantially decreased apoptotic response to cytotoxic metal stress, and XAF1-overexpressed cells showed apoptotic response to cytostatic metal stress (Fig. 4D and Fig. S4 A–D). ΔZF5-XAF1 failed to evoke this effect. Either XAF1 overexpression or MT2A depletion directed apoptotic switch of cellular response to cytostatic stress, and either MT2A overexpression or XAF1 depletion protected cells from cytotoxic stress (Fig. 4 C and E and Fig. S4D). Moreover, MT2A depletion-induced apoptotic sensitization was debilitated when XAF1 was codepleted, and XAF1 depletion-induced cell protection was abolished when MT2A was codepleted (Fig. S4 E–I).
Fig. 4.
XAF1–MT2A antagonism regulates cellular response to metal stress. (A and B) IB assays showing opposite responses of XAF1 and MT2A to cytostatic and cytotoxic metal stresses. (C) A crucial role for XAF1–MT2A mutual antagonism in cellular response to metal stress. Cells were treated with indicated doses of CdCl2 for 24 h. (D) Enhancement of metal-induced apoptosis by WT-XAF1. (E) XAF1-mediated apoptotic switch of cellular response to cytostatic metal stress and MT2A attenuation of apoptotic response to cytotoxic metal stress.
Fig. S4.
A critical role for XAF1–MT2A antagonism in cellular response to metal stress. (A–C) Cell viability and flow cytometric sub-G1 analysis showing a crucial role of XAF1 in metal-induced apoptosis. IB assay showing opposite responses of XAF1 and MT2A to increasing dose of heavy metals. (D) XAF1-induced apoptotic switch of cell response to cytostatic metal stress. (E–I) Flow cytometric sub-G1 assays showing a role for XAF1–MT2A antagonism in the regulation of cellular response to metal stress. Cells were cotransfected with si-MT2A and si-XAF1 as indicated and then exposed to CdCl2 or ZnCl2 for 48 h.
MT2A-Targeting Function Is Closely Linked to XAF1’s Apoptosis-Promoting Activity.
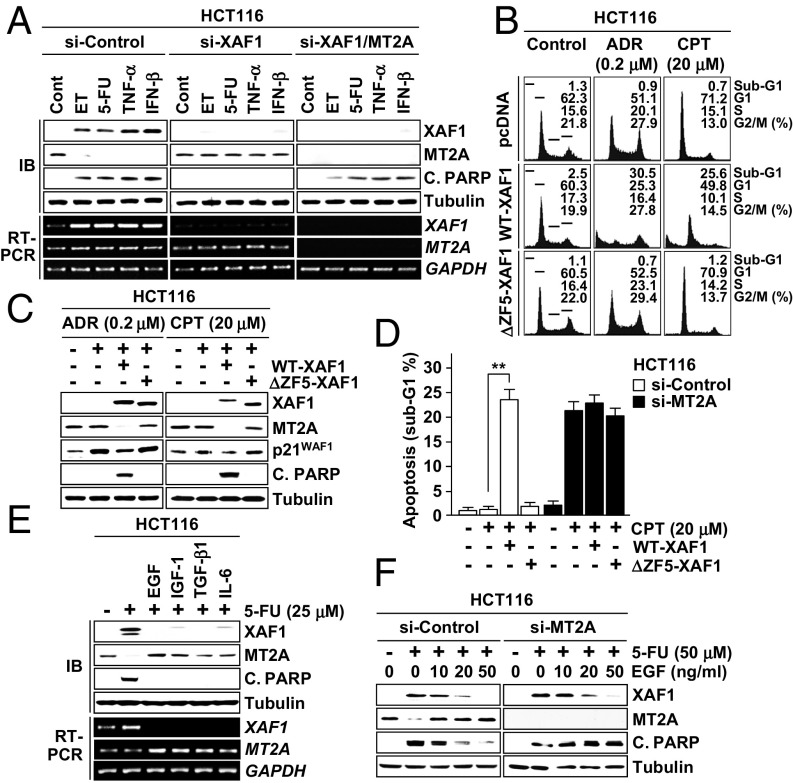
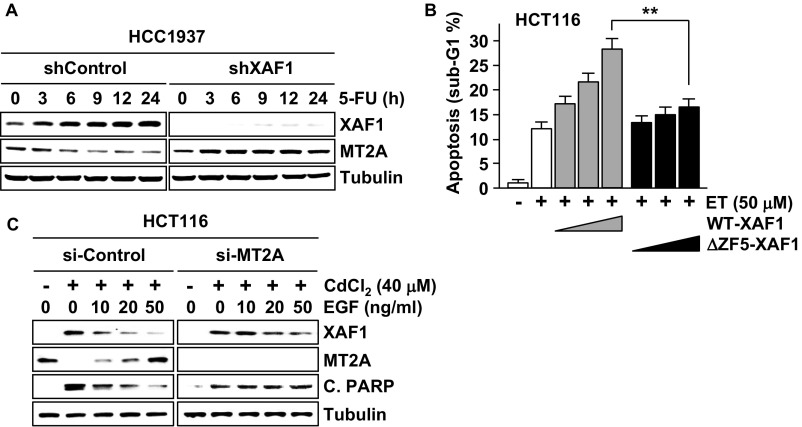
Next we assessed whether the XAF1–MT2A antagonism plays a role in cellular response to nonmetal stresses. In HCT116 cells, apoptosis induction by etoposide (ET), 5-fluorouracil (5-FU), TNF-α, and IFN-β was accompanied by XAF1 induction and MT2A reduction (Fig. 5A and Fig. S5A). However, apoptosis induction and MT2A reduction were blocked when XAF1 expression was depleted, while apoptosis occurred when MT2A was codepleted. ET-induced apoptosis was strongly promoted by WT-XAF1 but not by ΔZF5-XAF1 (Fig. S5B). Upon exposure to adriamycin (ADR) and cisplatin (CPT), HCT116 cells exhibited p21WAF1 but no XAF1 induction and manifested cytostatic response (G2 or G1 cell-cycle arrest) (Fig. 5 B and C). However, this cytostatic response was switched to apoptosis when WT-XAF1 was introduced and ΔZF5-XAF1 failed to induce this effect. Moreover, MT2A depletion caused apoptotic switch of cytostatic response, supporting that the MT2A-targeting role of XAF1 is crucial for its cell-fate–switching function (Fig. 5D). We next asked whether growth factors affect the XAF1–MT2A interplay to evoke their cell protection effect. In cells exposed to 5-FU, pretreatment with EGF, IGF1, TGF-β1, or IL-6 strongly inhibited XAF1 induction and MT2A reduction (Fig. 5E). Furthermore, XAF1 inhibition and antiapoptosis effects of EGF were impaired if MT2A restoration was blocked, indicating that growth factors exert their prosurvival effect through the regulation of the XAF1–MT2A axis (Fig. 5F and Fig. S5C).
Fig. 5.
XAF1–MT2A antagonism plays a key role in cellular response to nonmetal stresses. (A) A role for XAF1–MT2A interplay in cellular response to genotoxic and cytokine stresses. Cells were treated with ET (50 μM), 5-FU (25 μM), TNF-α (20 ng/mL), and IFN-β (200 U/mL) for 24 h. (B and C) XAF1-mediated apoptotic switch of cellular response to ADR and CPT. (D) Abrogation of XAF1-mediated switch of cell response to CPT by MT2A depletion. (E) Inhibitory effect of growth factors on 5-FU–induced XAF1 expression. Cells were pretreated with EGF (20 ng/mL), IGF1 (20 ng/mL), TGF-β1 (2 ng/mL), or IL-6 (2 ng/mL) and exposed 5-FU for 24 h. (F) Association of antiapoptotic function of EGF with its XAF1-repressing effect in 5-FU–treated cells.
Fig. S5.
Role for XAF1–MT2A antagonism in cellular response to genotoxic stress. (A) IB assay showing 5-FU–mediated XAF1 induction and effect of XAF1 depletion on MT2A expression. HCC1937-shControl and HCC1937-shXAF1 subline cells were treated with 5-FU (25 μM) for the indicated times. (B) Promotion of ET-induced apoptosis by WT-XAF1 but not by ΔZF5-XAF1. HCT116 cells transfected with increasing dose of either WT-XAF1 or ΔZF5-XAF1 were exposed to ET (50 μM) for 48 h. (C) Association of antiapoptotic function of EGF with its XAF1-downregulating effect in CdCl2-treated cells.
XAF1 Increases the Free Intracellular Zinc Level to Promote Conformational Change of p53.
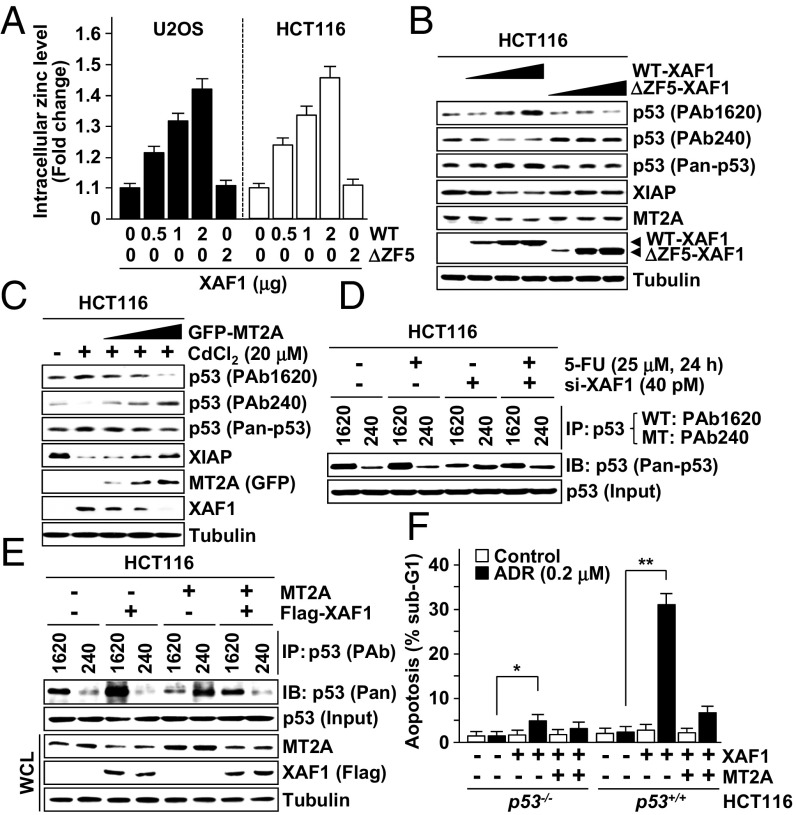
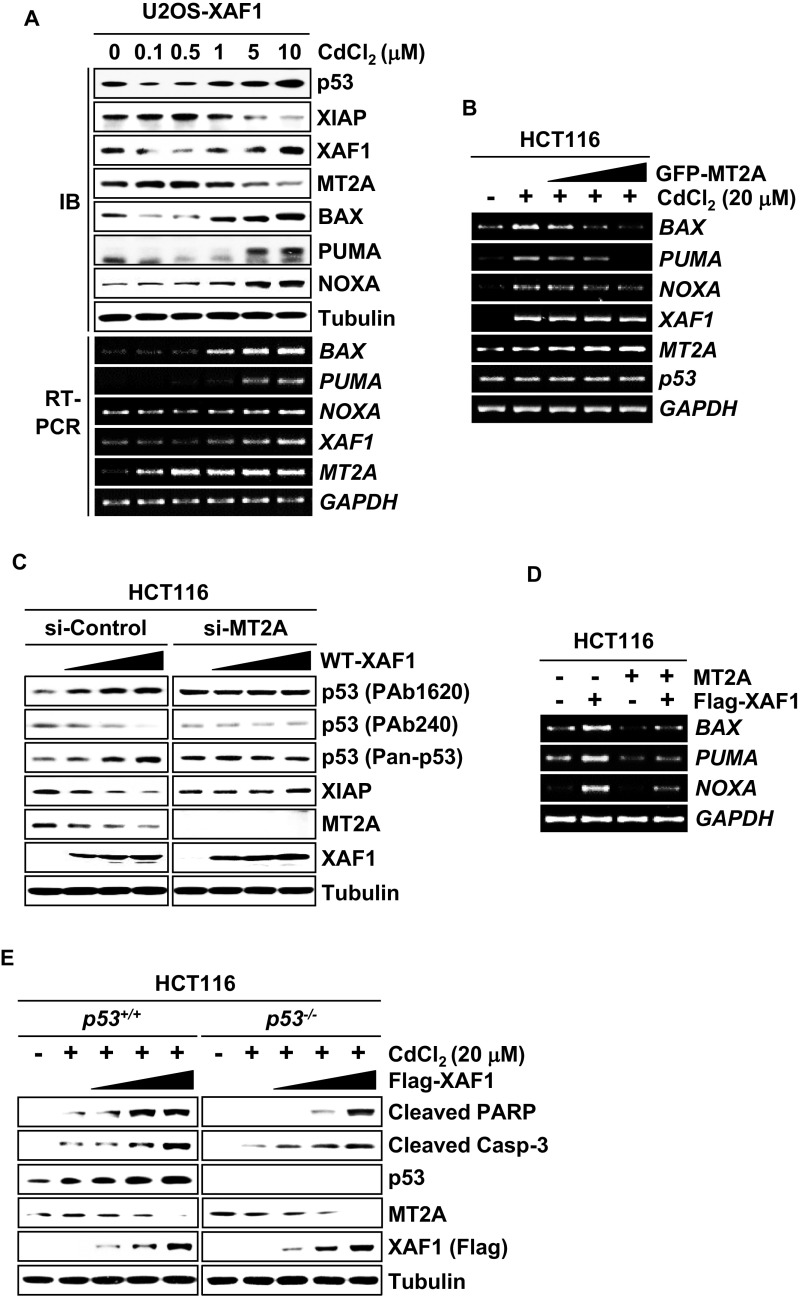
MT2A is a prototype metal-binding protein that regulates intracellular zinc homeostasis, and its cell-protective function is linked to zinc-scavenging activity. To define apoptotic signaling pathways controlled by XAF1–MT2A antagonism, we examined the effect of XAF1–MT2A antagonism on the intracellular zinc level and p53 protein, a representative apoptotic factor the conformation of which is regulated in a highly zinc-dependent manner (34). A spectrofluorimetric measurement of Zinquin revealed that the free zinc level is increased by WT-XAF1 but not by ΔZF5-XAF1 (Fig. 6A). Moreover, differential responses of XAF1 to cytostatic and cytotoxic doses were coincident with the regulation pattern of p53 and expression of its target genes, such as BAX, PUMA, and NOXA (Fig. S6A). The XAF1 response to metals also correlated inversely with the expression level of XIAP, an antiapoptotic factor the stability of which is affected by heavy metals (35, 36). Expression assays of p53 using antibodies specific for WT (PAb1620) and mutant-type (PAb240) p53 revealed that XAF1 expression up- and down-regulates WT and mutant-type p53, respectively (Fig. 6B). Moreover, elevation of WT p53 conformation and its target expression by cytotoxic metal stress were impeded by MT2A (Fig. 6C and Fig. S6B). XAF1 regulation of p53 and XIAP was not detected in MT2A-depleted cells (Fig. S6C). IP assays also showed that the 5-FU–induced conformational shift of p53 is impeded when XAF1 is depleted (Fig. 6D). The opposite effects of XAF1 and MT2A on conformational shift of p53 were abolished when they were co-overexpressed (Fig. 6E and Fig. S6D). Comparative assays of HCT116 p53+/+ and p53−/− sublines exposed to cytostatic dose of ADR revealed that the XAF1-induced apoptotic switch and its attenuation by MT2A are more drastic in p53+/+ versus p53−/− cells (Fig. 6F). Likewise, XAF1 promotion of CdCl2-induced apoptosis was more drastic in p53+/+ versus p53−/− cells, further supporting that p53 is an important target of XAF1 in evoking its proapoptotic function (Fig. S6E).
Fig. 6.
A XAF1-mediated free zinc increase drives a WT shift of p53 conformation. (A) A spectrofluorimetric measurement of Zinquin showing a XAF1 dose-associated increment of the intracellular free zinc level. (B) Up-regulation of WT p53 conformation and down-regulation of XIAP level by WT-XAF1 but not by ΔZF5-XAF1. PAb1620 and PAb240 antibodies were used to detect WT and mutant type (under nondenaturating gel conditions) of p53 conformation, respectively. Pan-p53 antibody was used to determine total p53 protein level. (C) MT2A inhibition of cytotoxic metal stress-induced p53 conformational change and XIAP reduction. (D) IP assay showing a role for XAF1 in genotoxic stress-induced p53 conformational change. (E) Opposite effects of XAF1 and MT2A on p53 conformation. (F) Comparison of XAF1 ability to induce apoptotic switch of ADR-treated HCT116 p53+/+ and p53−/− cells.
Fig. S6.
Reciprocal regulation of p53 conformation by XAF1 and MT2A. (A) Inverse response of XAF1 and MT2A to increasing doses of CdCl2 and its association with p53 and XIAP expression in U2OS-XAF1 subline cells. IB and semiquantitative RT-PCR assays were carried out to determine p53 traget gene expression after 24 h treatment. (B) A semiquantitative RT-PCR analysis showing MT2A repression of p53 target gene expression induced by cytotoxic metal stress. (C) No detectable effect of XAF1 on p53 conformation and XIAP level in MT2A-depleted cells. PAb1620 and PAb240 antibodies were used to detect WT and mutant-type (under nondenaturating gel conditions) p53 conformation, respectively. Pan-p53 antibody was used to determine total p53 protein level. (D) Opposite effects of XAF1 and MT2A on mRNA expression of p53 target genes. HCT116 cells were transfected with Flag-XAF1 and/or MT2A expression plasmids, and semiquantitative RT-PCR analysis was performed at 48 h after transfection. (E) A comparative analysis of XAF1’s apoptosis-promoting effect in p53+/+ and p53−/− cells exposed to metal stress (CdCl2, 48 h).
XAF1 and MT2A Expression Levels Correlate Inversely in Human Cancers.
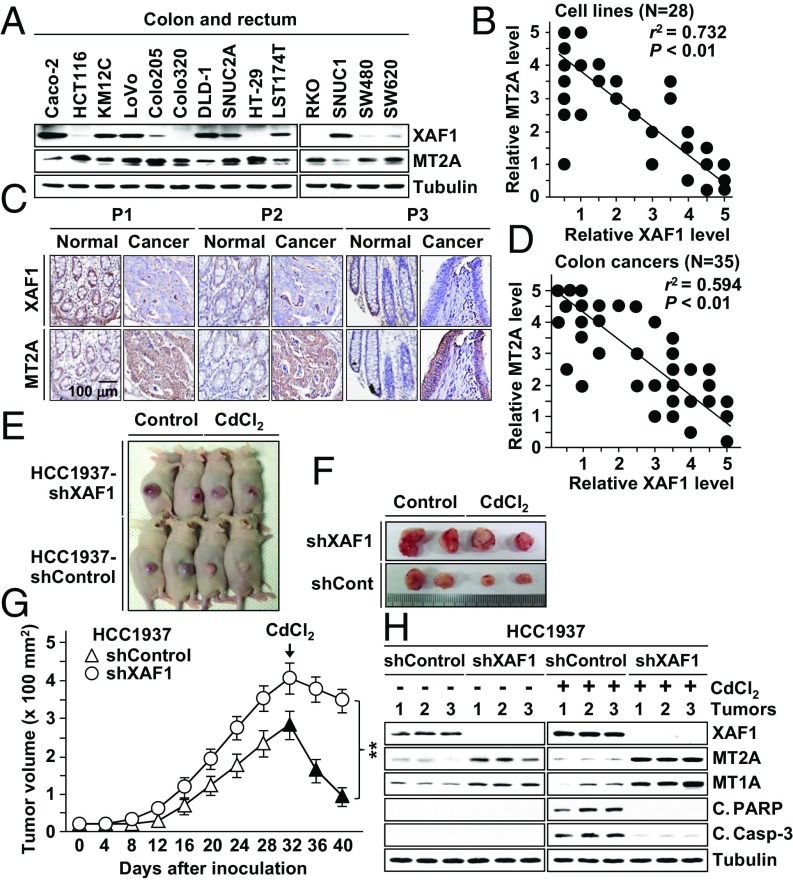
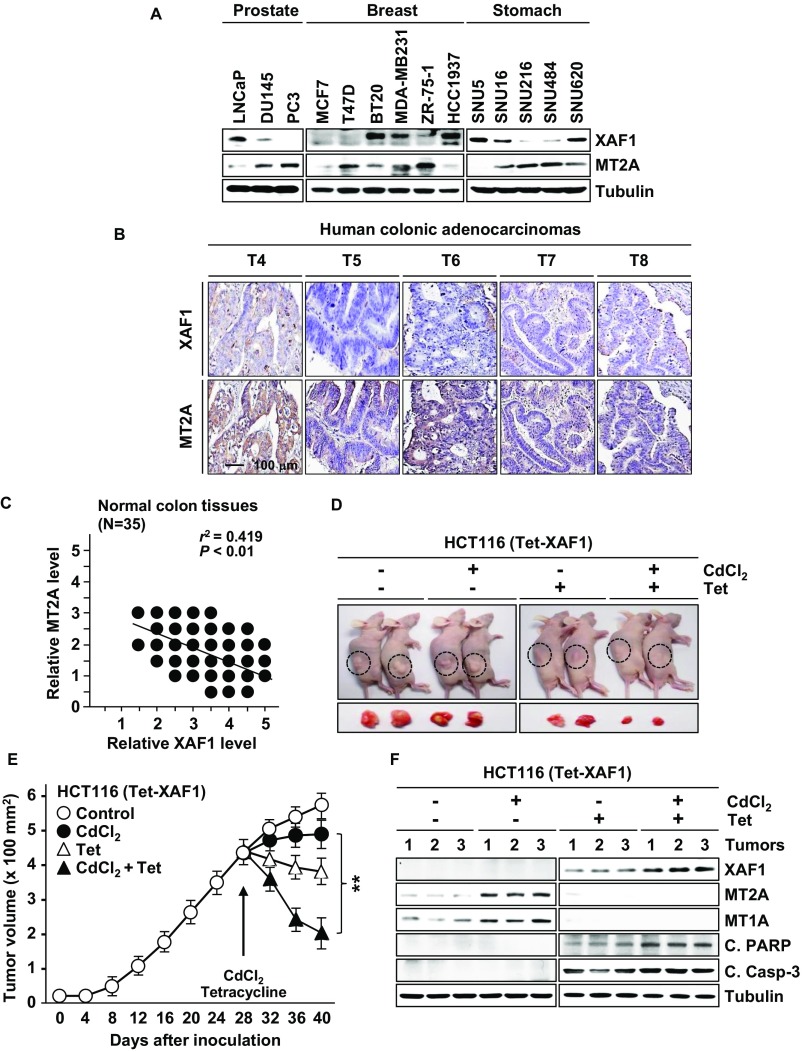
To delineate the XAF1–MT2A interpaly in human tumors, we characterized their expression status in 28 cancer cell lines of multiple tissue origins and 35 primary colon tumor tissues. A substantial fraction of cell lines and tumor tissues exhibited extremely low XAF1 and high MT2A expression, and a strong inverse correlation was detected between their levels (Fig. 7 A–D and Fig. S7 A–C). Compared with 35 normal tissues that we examined, 10 (28.6%) and 14 (40%) tumors were shown to express abnormally low XAF1 and high MT2A (Fig. 7D and Fig. S7C).
Fig. 7.
Inverse correlation of XAF1 and MT2A expression in human cancers and XAF1 effect on tumor growth and response to metal stress. (A and B) Expression status of XAF1 and MT2A in human cancer cell lines. Relative expression levels were classified as levels 0–5. r, Pearson’s correlation coefficient. (C) Immunohistochemical analysis of XAF1 and MT2A in human primary colon tumor and matched normal tissues. P, patient. (D) Inverse correlation of XAF1 and MT2A immunoreactivity in colon cancer tissues. Relative staining levels were classified as levels 0–5. (E–G) Mouse tumor xenograft assays showing the XAF1 depletion effect on tumor growth and response to metal stress. Data represent the mean ± SD (n = 6 per group; **P < 0.01). (H) IB assay of xenograft tumor tissues showing the XAF1 depletion effect on MT2A and cleaved PARP and caspase-3 levels.
Fig. S7.
XAF1 and MT2A expression in human cancers. (A) Expression of XAF1 and MT2A in human cancer cell lines. (B) Immunohistochemical analysis of XAF1 and MT2A in primary colon cancers. T, tumor. (C) Immunoreactivity status of XAF1 and MT2A in 35 normal colon tissues. Relative staining levels were classified as levels 0–5. r, Pearson’s correlation coefficient. (D and E) Mouse tumor xenograft assays showing effect of XAF1 expression on tumor response to cytostatic metal stress (CdCl2, 0.02 mg/g). Data represent the mean ± SD (n = 3 per group; **P < 0.01). (F) IB assay of xenograft tumor tissues showing XAF1 effect on MT2A and MT1A and cleaved PARP and caspase-3 levels.
Moreover, high MT2A was detected in 8 of 10 (80%) low-XAF1 tumors but only in 6 of 26 (23.1%) normal XAF1 tumors, and low XAF1 was detected in 9 of 14 (64.3%) high-MT2A tumors but only in 2 of 21 (9.5%) normal MT2A tumors. Mouse tumor xenograft assays using XAF1-depleted HCC1937 subline cells revealed that XAF1-depleted tumors show substantially increased growth rate and delayed regression following injection of a cytotoxic dose of CdCl2 (0.05 mg/g) (Fig. 7 E–G). Compared with control tumors, XAF1-depleted tumors showed higher MT2A and MT1A and lower cleaved PARP and caspase-3 levels (Fig. 7H). We next asked whether XAF1 induction could drive a switch of tumor response to cytostatic metal stress using HCT116 (Tet-XAF1) xenograft tumors. Upon exposure to low dose of CdCl2 (0.02 mg/g), XAF1-induced tumors displayed drastic regression and undetectably low MT2A whereas control tumors showed only a delayed growth and elevated MT2A expression (Fig. S7 D–F).
Discussion
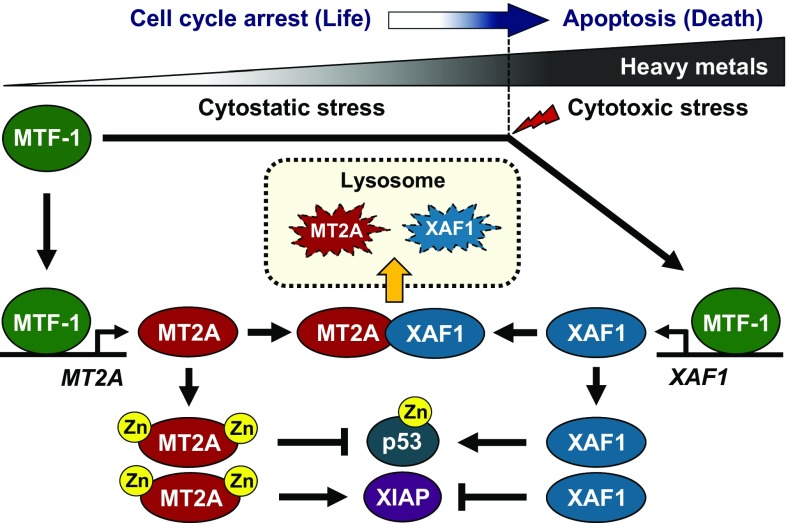
Upon heavy metal exposure, MT2A activated by MTF-1 sequesters metals to protect cells from metal cytotoxicity (19–22). However, the molecular mechanism by which MT2A is controlled under cytotoxic metal stress conditions remains undefined. MT2A degradation takes place mainly in the lysosomes, but the signaling pathways involved in the lysosomal trafficking of MT2A have been poorly understood (33). In the present study, we demonstrate first that XAF1 is a negative regulator of MT2A, the activation of which directs the apoptotic switch of cellular response to heavy metals. XAF1 has been shown to regulate protein stability through interaction with ubiquitin E3 ligases such as XIAP, cIAP1/2, ZNF313, and Siah2 (2, 12, 13, 37). Our data provide evidence that XAF1 binds to MT2A and accelerates its lysosomal relocalization, identifying its involvement in both the proteasomal and lysosomal degradation pathways. Intriguingly, it was also found that MT2A destabilizes XAF1 through the lysosomal pathway and that this activity is linked to its cell protection function. Therefore, our data support the notion that XAF1–MT2A antagonism functions as a linchpin to govern cell fate under metal stress conditions (Fig. 8).
Fig. 8.
Schematic representation of the antagonistic interplay of XAF1 and MT2A under metal stress conditions and its role in cell-fate decisions.
XAF1 is a direct transcription target of p53 and IFN regulatory factor 1 (IRF-1), which is up-regulated by various apoptotic stresses (10–12, 38). In this study, we found that XAF1 is a unique transcription target of MTF-1 in signaling apoptosis and counteracts MT2A, a prosurvival target of MTF-1. Given that MTF-1 can induce apoptosis in a highly XAF1-dependent fashion, it is plausible that MTF-1 provokes both prosurvival and proapoptotic effects depending on its target selectivity. In this context, MTF-1 might be modified differentially by cytostatic and cytotoxic stresses, allowing its selective or preferential binding to a subset of target promoters. Considering that both MTF-1 and MT2A are up-regulated and that XAF1 is down-regulated in many human cancers, alteration of the MTF-1–XAF1 and/or XAF1–MT2A axes might contribute to tumor resistance in heavy-metal–induced apoptosis.
In addition to its essential role for the sequestration of heavy metals away from critical macromolecules, MT2A contributes to chemotherapy resistance through the indirect action on p53 zinc-dependent activity (29, 30). MT2A is increased by HIPK2 knockdown, and this elevation correlates with p53 misfolding and cellular resistance to adriamycin-driven apoptosis (30). We have shown that XAF1 activates p53 by interfering with MDM2 interaction with p53 and also by activating HIPK2 (12). Our present study shows that XAF1 increases the free intercellular zinc level and activates p53 through MT2A destabilization. It is thus conceivable that XAF1 allows rapid amplification of the apoptotic program through Siah2-HIPK2 axis activation and MT2A inactivation. Consistently, we observed that the cell-fate decision function of the XAF1–MT2A axis is more drastic in p53+/+ versus isogenic p53−/− cells.
XAF1 is originally identified to interact with and sequester XIAP to the nucleus (2, 3). However, XAF1’s proapoptotic function is not solely dependent on the XIAP-interfering activity (6, 9). Our study shows that XAF1 induction by heavy metals leads to a drastic reduction of XIAP in a highly MT2A-dependent manner. Studies have shown that intracellular copper accumulation causes XIAP reduction through the copper-induced conformational change and that cadmium down-regulates XIAP via a proteasome-mediated mechanism, and this reduction coincides with an increased cellular sensitivity to TNF-α–induced apoptosis (35, 36). Given that MT2A presents a high affinity for copper, our study raises the possibility that XAF1-mediated MT2A destabilization increases the free intracellular copper level and thereby leads to conformational change and degradation of XIAP, lowering the apoptotic threshold and sensitizing the cell to apoptosis (39). Our study thus identifies an alternative mechanism of XAF1 inhibition of XIAP by attenuating MT2A stabilization of XIAP.
Collectively, this study demonstrates that XAF1–MT2A mutual antagonism represents one crucial molecular switch in cell-fate decisions under stressful conditions, adding a layer of complexity to the mechanisms by which stress response is regulated. Our data suggest that disruption of XAF1–MT2A interplay may contribute to the development and progression of various diseases, including malignant tumors.
Materials and Methods
Details regarding human tissues, cancer cell lines, cellular assays of cell cycle and apoptosis, expression constructs, ChIP, immunofluorescence, immunoblotting, GST pull-down, measurement of intracellular zinc concentration, and semiquantitative RT-PCR analysis are available in SI Materials and Methods. All animal studies were performed with the approval of Korea University Institutional Animal Care and Use Committee and Korea Animal Protection Law.
SI Materials and Methods
Human Cell Lines and Reagents.
Human cancer cell lines (HCT116, HCC1937, U2OS, MKN74, AGS, and MCF7) were purchased from the American Type Culture Collection (ATCC) and the Korea Cell Line Bank and maintained in RPMI medium supplemented with 10% FBS (GIBCO BRL) at 37 °C in a humidified atmosphere with 5% CO2. All these cell lines were authenticated by short tandem repeat profiling at the Korea Cell Line Bank before use. Allelic score data revealed a pattern related to the scores reported by the ATCC and consistent with their presumptive identity. The HCT116 (Tet-XAF1) cells were generated as described previously (12). U2OS sublines with shRNA-mediated knockdown of MT2A were established by transfection of a shMT2A (SC-93491-SH) construct (Santa Cruz Biotechnology) and Zeocin selection. Flag-XAF1 and GFP-MT2A stable sublines of U2OS were selected by G418 (200–600 µg/mL). Heavy metals (CdCl2, CuCl2, and ZnCl2), cycloheximide, and Leupeptin were purchased from Sigma Aldrich.
Expression Plasmids and siRNA.
Expression vectors for XAF1, MT2A, MT1A, and MTF-1 were constructed using a PCR-based approach as previously described (5, 6). siRNA duplexes against XAF1 (5ʹ-AUGUUGUCCAGACUCAGAG-3ʹ) and MT2A (5′-CCGGUUCCUGCAAAUGCAA-3ʹ and 5′-CUGGACUUCCAGAAGAACA-3′) were synthesized by Bioneer. The siRNA duplex against MTF-1 (SC-43939) and the control siRNA duplex that served as a negative control were purchased from Santa Cruz Biotechnology and Dharmacon Research. Transfection of siRNAs or expression plasmids was performed using the Neon Transfection System (Thermo Fisher Scientific) or Turbofect in Vitro Transfection Reagent (Pierce Biotechnology).
Reporter Constructs and Luciferase Assay.
The XAF1 promoter region was cloned into the pGL3-basic vector (Promega). Cells were transfected with 500 ng of reporter constructs using the Neon Transfection System (Thermo Fisher Scientific). After normalization of each extract for protein content, luciferase activity was measured by using the Luciferase Assay System (Promega).
ChIP.
Cells were incubated in 1% formaldehyde solution for 20 min. The cells were lysed, and the pellet was resuspended in nuclei lysis buffer and sonicated to yield chromatin fragments. A ChIP assay was carried out using a Simple ChIP Enzymatic Chromatin IP Kit (Cell Signaling Technology) and antibody specific for MTF-1 (H-300) or rabbit IgG antibody. PCR was done using primers P3 (sense 5ʹ-GAACAGCATTCAAGCTCAACATGG-3ʹ) and P4 (antisense 5ʹ-CGTCATTCTTTGCTGCTGTCACAG-3ʹ) for the XAF1 promoter region comprising the putative MRE site.
Immunoblot and Immunoprecipitation.
Immunoblot and immunoprecipitation assays were performed as described (12). Antibodies specific for XAF1 (SC-19194), MT2A (H00004502), MT1A (H00004489), MTF-1 (SC-365090), p53 (PAb1620 and PAb240), cleaved PARP (9541), cleaved caspase-3 (9661), GFP (SC-9996), Flag (SC-166384), GST (SC-33613), HA (SC-7392), and β-tubulin (T0198) were purchased from Santa Cruz Biotechnology, Abnova, Cell Signaling Technology, and Abcam.
Immunofluorescence and Immunohistochemistry.
Immunofluorescence assay was performed as described (6, 12). Briefly, cells transfected with GFP-MT2A and Flag-XAF1 expression vectors were fixed with 4% paraformaldehyde–Tris⋅HCl-buffered saline (TBS), permeabilized with 0.5% Triton X-100-TBS, and blocked with 5% BSA–TBS. The slides were incubated with anti-MT2A or anti-XAF1 antibody for 24 h, washed with TBS, and stained with secondary antibodies for 1 h. DAPI was used for the counterstaining of nuclei. Fluorescent imaging was performed with a confocal laser-scanning microscope (Carl Zeiss). Image acquisition was performed with ZEN 2012 LSM 700 software. An immunohistochemistry assay for human colon tissues was performed using tissue arrays (TMA) slides (US Biomax) and a Vectastain ABC (avidin–biotin–peroxidase) kit (Vector Laboratories) as described previously (7).
Protein Pull-Down and in Vitro Binding Assay.
GST pull-down assays were performed as described (7, 12, 40). For the in vitro binding assay, GST- or 6His-fused recombinant proteins overexpressed by isopropyl β-d-1-thiogalactopyranoside in Escherichia coli strain BL21DE3 were purified using Glutathione Sepharose 4B (GE Healthcare) or Ni-NTA agarose (Qiagen). The purified GST-XAF1 and recombinant MT2A proteins were incubated with binding assay buffer for 6 h at 4 °C. Immunocomplexes were separated by incubation with protein-A/G Sepharose for 1 h, heated at 95 °C in SDS sample buffer, and subjected to SDS/PAGE for immunoblot analysis.
Measurement of Intracellular Zinc Concentration.
Intracellular free zinc concentration was measured by a fluorimetric probe assay using the specific probe Zinquin (Sigma Aldrich). Briefly, cells were incubated with Zinquin (25 μM) for 40 min in the dark, and fluorescence was measured using a Hidex Sense Microplate Reader (Hidex) at a wavelength of λex 330 nm and λem 485 nm.
Semiquantitative RT-PCR.
Our strategies for the semiquantitative RT-PCR analysis were previously described (4, 12). Briefly, 1 μg of DNase1-treated RNA was converted to cDNA by reverse transcription using random hexamer primers and MoMuLV reverse transcriptase (Life Technologies). PCR was initially performed over a range of cycles (20–40 cycles) using serially diluted cDNA, and 1:4 diluted cDNA (12.5 ng/50 μL PCR) undergoing 24–40 cycles was found within the logarithmic phase of amplification with primers used for XAF1A-S (5′-CAGAAGTCCTCGCTGGAGTTTC-3′) and XAF1A-AS (5′-TTCAGGAGCTGAAATTCTTTCC-3′) for XAF1, MT2A-S (5′-CCGACTCTAGCCGCCTCTT-3′) and MT2A-AS (5′-GTGGAAGTCGCGTTCTTTACA-3′) for MT2A, MTF1-S (5′-CTTTCTGTTGTTGCTGGGGC-3′) and MTF1-AS (5′-ATGCCTCTTCTTGTTTGATGATG-3′) for MTF1, and the endogenous expression standard gene GAPDH. Quantitation was achieved by densitometric scanning of the ethidium bromide-stained gels, and analysis was performed using the Molecular Analyst software program (Bio-Rad).
Flow Cytometry Analysis.
Cells were seeded at the density of 5 × 104 cells and transfected with expression vector or siRNA. For sub-G1 fraction analysis, cells were fixed with 70% ethanol and resuspended in 1 mL of PBS containing 100 mg/mL RNase and 50 mg/mL propidium iodide. The assay was performed on a FACSCalibur flow cytometer (BD Bioscience), and the sub-G1 fraction was analyzed using MultiCycle software (Phoenix Flow Systems).
Human Tissues.
Human tissue arrays were obtained from US Biomax, and immunostaining was performed by using the Vectastain ABC (avidin–biotin–peroxidase) kit (Vector Laboratories).
Animal Studies.
A mouse tumor xenograft assay was carried out as described (12). Six-week-old immunodeficient nude mice (nu/nu) mice (Orient Bio) were maintained in pressurized ventilated cages. HCC1937 or HCT116 (Tet-XAF1) cells (1 × 107) were injected subcutaneously into mice, and tumor growth was monitored periodically. Tumor volume (V) was calculated by using the following modified ellipsoidal formula: V = 0.5 × length × (width)2. Tumor volume was measured at the beginning of treatment and then monitored regularly. CdCl2 (0.05 mg/g for HCC1937 tumors and 0.02 mg/g for HCT116 tumors) was administered by intratumoral injection, and tumor response was monitored regularly for 8–12 d after treatment. All studies were performed with the approval of Korea University Institutional Animal Care and Use Committee and Korea Animal Protection Law.
Statistical Analysis.
Reporter luciferase, flow cytometry, cell viability, and apoptosis assays were performed in triplicate, and statistical values were presented as mean ± SD. The Student t test was used to determine the statistical significance. Pearson correlation coefficient (r) was used to measure the strength of the association between XAF1 and MT2A expression levels in cell lines and tissues. A P value of less than 0.05 was considered significant.
Acknowledgments
We thank Dr. Bert Vogelstein (Johns Hopkins University) for HCT116 p53+/+ and p53−/− cells. This work was supported in part by National Research Foundation of Korea Grants NRF-2015R1A2A1A01005389 (to S.-G.C.) and NRF-2015R1D1A401016836 (to M.-G.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1700861114/-/DCSupplemental.
References
- 1.Roos WP, Thomas AD, Kaina B. DNA damage and the balance between survival and death in cancer biology. Nat Rev Cancer. 2016;16:20–33. doi: 10.1038/nrc.2015.2. [DOI] [PubMed] [Google Scholar]
- 2.Fong WG, et al. Expression and genetic analysis of XIAP-associated factor 1 (XAF1) in cancer cell lines. Genomics. 2000;70:113–122. doi: 10.1006/geno.2000.6364. [DOI] [PubMed] [Google Scholar]
- 3.Liston P, et al. Identification of XAF1 as an antagonist of XIAP anti-Caspase activity. Nat Cell Biol. 2001;3:128–133. doi: 10.1038/35055027. [DOI] [PubMed] [Google Scholar]
- 4.Byun DS, et al. Hypermethylation of XIAP-associated factor 1, a putative tumor suppressor gene from the 17p13.2 locus, in human gastric adenocarcinomas. Cancer Res. 2003;63:7068–7075. [PubMed] [Google Scholar]
- 5.Lee MG, et al. Promoter CpG hypermethylation and downregulation of XAF1 expression in human urogenital malignancies: Implication for attenuated p53 response to apoptotic stresses. Oncogene. 2006;25:5807–5822. doi: 10.1038/sj.onc.1209867. [DOI] [PubMed] [Google Scholar]
- 6.Chung SK, et al. Frequent alteration of XAF1 in human colorectal cancers: Implication for tumor cell resistance to apoptotic stresses. Gastroenterology. 2007;132:2459–2477. doi: 10.1053/j.gastro.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 7.Lunardi A, et al. A co-clinical approach identifies mechanisms and potential therapies for androgen deprivation resistance in prostate cancer. Nat Genet. 2013;45:747–755. doi: 10.1038/ng.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng KCP, Campos EI, Martinka M, Li G. XAF1 expression is significantly reduced in human melanoma. J Invest Dermatol. 2004;123:1127–1134. doi: 10.1111/j.0022-202X.2004.23467.x. [DOI] [PubMed] [Google Scholar]
- 9.Xia Y, Novak R, Lewis J, Duckett CS, Phillips AC. Xaf1 can cooperate with TNFalpha in the induction of apoptosis, independently of interaction with XIAP. Mol Cell Biochem. 2006;286:67–76. doi: 10.1007/s11010-005-9094-2. [DOI] [PubMed] [Google Scholar]
- 10.Leaman DW, et al. Identification of X-linked inhibitor of apoptosis-associated factor-1 as an interferon-stimulated gene that augments TRAIL Apo2L-induced apoptosis. J Biol Chem. 2002;277:28504–28511. doi: 10.1074/jbc.M204851200. [DOI] [PubMed] [Google Scholar]
- 11.Micali OC, et al. Silencing of the XAF1 gene by promoter hypermethylation in cancer cells and reactivation to TRAIL-sensitization by IFN-beta. BMC Cancer. 2007;7:52–59. doi: 10.1186/1471-2407-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee MG, et al. XAF1 directs apoptotic switch of p53 signaling through activation of HIPK2 and ZNF313. Proc Natl Acad Sci USA. 2014;111:15532–15537. doi: 10.1073/pnas.1411746111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han J, et al. ZNF313 is a novel cell cycle activator with an E3 ligase activity inhibiting cellular senescence by destabilizing p21(WAF1.) Cell Death Differ. 2013;20:1055–1067. doi: 10.1038/cdd.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu LM, et al. Tumor suppressor XAF1 induces apoptosis, inhibits angiogenesis and inhibits tumor growth in hepatocellular carcinoma. Oncotarget. 2014;5:5403–5415. doi: 10.18632/oncotarget.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, et al. Identification of XAF1 as a novel cell cycle regulator through modulating G(2)/M checkpoint and interaction with checkpoint kinase 1 in gastrointestinal cancer. Carcinogenesis. 2009;30:1507–1516. doi: 10.1093/carcin/bgp155. [DOI] [PubMed] [Google Scholar]
- 16.Bishak YK, Payahoo L, Osatdrahimi A, Nourazarian A. Mechanisms of cadmium carcinogenicity in the gastrointestinal tract. Asian Pac J Cancer Prev. 2015;16:9–21. doi: 10.7314/apjcp.2015.16.1.9. [DOI] [PubMed] [Google Scholar]
- 17.Kimura T, Kambe T. The functions of metallothionein and ZIP and ZnT transporters: An overview and perspective. Int J Mol Sci. 2016;17:336. doi: 10.3390/ijms17030336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heger Z, et al. Metallothionein as a scavenger of free radicals: New cardioprotective therapeutic agent or initiator of tumor chemoresistance? Curr Drug Targets. 2016;17:1438–1451. doi: 10.2174/1389450116666151001113304. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki K, Koizumi S. Individual metal responsive elements of the human metallothionein-IIA gene independently mediate responses to various heavy metal signals. Ind Health. 2000;38:87–90. doi: 10.2486/indhealth.38.87. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi S. Positive and negative regulators of the metallothionein gene (review) Mol Med Rep. 2015;12:795–799. doi: 10.3892/mmr.2015.3459. [DOI] [PubMed] [Google Scholar]
- 21.McGee HM, Woods GM, Bennett B, Chung RS. The two faces of metallothionein in carcinogenesis: Photoprotection against UVR-induced cancer and promotion of tumour survival. Photochem Photobiol Sci. 2010;9:586–596. doi: 10.1039/b9pp00155g. [DOI] [PubMed] [Google Scholar]
- 22.Theocharis SE, Margeli AP, Koutselinis A. Metallothionein: A multifunctional protein from toxicity to cancer. Int J Biol Markers. 2003;18:162–169. doi: 10.1177/172460080301800302. [DOI] [PubMed] [Google Scholar]
- 23.Shi Y, et al. The metal-responsive transcription factor-1 protein is elevated in human tumors. Cancer Biol Ther. 2010;9:469–476. doi: 10.4161/cbt.9.6.10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theocharis SE, Margeli AP, Klijanienko JT, Kouraklis GP. Metallothionein expression in human neoplasia. Histopathology. 2004;45:103–118. doi: 10.1111/j.1365-2559.2004.01922.x. [DOI] [PubMed] [Google Scholar]
- 25.Cherian MG, Jayasurya A, Bay BH. Metallothioneins in human tumors and potential roles in carcinogenesis. Mutat Res. 2003;533:201–209. doi: 10.1016/j.mrfmmm.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Ma H, et al. HMBOX1 interacts with MT2A to regulate autophagy and apoptosis in vascular endothelial cells. Sci Rep. 2015;5:15121. doi: 10.1038/srep15121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krześlak A, et al. Effect of metallothionein 2A gene polymorphism on allele-specific gene expression and metal content in prostate cancer. Toxicol Appl Pharmacol. 2013;268:278–285. doi: 10.1016/j.taap.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Krześlak A, et al. Metallothionein 2A genetic polymorphisms and risk of ductal breast cancer. Clin Exp Med. 2014;14:107–113. doi: 10.1007/s10238-012-0215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Habel N, et al. Zinc chelation: A metallothionein 2A’s mechanism of action involved in osteosarcoma cell death and chemotherapy resistance. Cell Death Dis. 2013;4:e874. doi: 10.1038/cddis.2013.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puca R, et al. Restoring wtp53 activity in HIPK2 depleted MCF7 cells by modulating metallothionein and zinc. Exp Cell Res. 2009;315:67–75. doi: 10.1016/j.yexcr.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 31.Garrett SH, et al. Metallothionein isoform 1 and 2 gene expression in the human prostate: Downregulation of MT-1X in advanced prostate cancer. Prostate. 2000;43:125–135. doi: 10.1002/(sici)1097-0045(20000501)43:2<125::aid-pros7>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 32.Jin R, et al. Metallothionein 2A expression is associated with cell proliferation in breast cancer. Carcinogenesis. 2002;23:81–86. doi: 10.1093/carcin/23.1.81. [DOI] [PubMed] [Google Scholar]
- 33.Klaassen CD, Choudhuri S, McKim JM, Jr, Lehman-McKeeman LD, Kershaw WC. In vitro and in vivo studies on the degradation of metallothionein. Environ Health Perspect. 1994;102:141–146. doi: 10.1289/ehp.94102s3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kruse JP, Gu W. Modes of p53 regulation. Cell. 2009;137:609–622. doi: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mufti AR, et al. XIAP is a copper binding protein deregulated in Wilson’s disease and other copper toxicosis disorders. Mol Cell. 2006;21:775–785. doi: 10.1016/j.molcel.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 36.Golovine K, et al. Cadmium down-regulates expression of XIAP at the post-transcriptional level in prostate cancer cells through an NF-kappaB-independent, proteasome-mediated mechanism. Mol Cancer. 2010;9:183. doi: 10.1186/1476-4598-9-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arora V, et al. Degradation of survivin by the X-linked inhibitor of apoptosis (XIAP)-XAF1 complex. J Biol Chem. 2007;282:26202–26209. doi: 10.1074/jbc.M700776200. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, et al. c-Jun N-terminal kinase (JNK1) upregulates XIAP-associated factor 1 (XAF1) through interferon regulatory factor 1 (IRF-1) in gastrointestinal cancer. Carcinogenesis. 2009;30:222–229. doi: 10.1093/carcin/bgn271. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura S, et al. Implication of the differential roles of metallothionein 1 and 2 isoforms in the liver of rats as determined by polyacrylamide-coated capillary zone electrophoresis. Biochem Biophys Res Commun. 2004;320:1193–1198. doi: 10.1016/j.bbrc.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 40.Lee MG, et al. RASSF1A directly antagonizes RhoA activity through the assembly of a Smurf1-mediated destruction complex to suppress tumorigenesis. Cancer Res. 2016;76:1847–1859. doi: 10.1158/0008-5472.CAN-15-1752. [DOI] [PubMed] [Google Scholar]