Significance
Narcolepsy-cataplexy is a debilitating disorder characterized by excessive daytime sleepiness (sleep attacks) and cataplexy, a sudden bilateral loss of muscle tone often triggered by emotion. The disease is caused by a selective loss of hypothalamic neurons producing the neuropeptide orexin. Currently, only symptomatic therapies are available for narcolepsy. Here, we examine the pharmacological effect of YNT-185, a nonpeptide, selective agonist for the orexin type-2 receptor in mouse models of narcolepsy-cataplexy. We show that peripheral administration of YNT-185 significantly ameliorates the narcolepsy symptoms in model mice, providing a proof-of-concept for the mechanistic treatment of narcolepsy with orexin receptor agonists. YNT-185 also promotes wakefulness in wild-type mice, suggesting that orexin receptor agonists may be useful for treating sleepiness due to other causes.
Keywords: excessive daytime sleepiness, electroencephalography, electromyography, G protein-coupled receptors, neuropeptide
Abstract
Narcolepsy-cataplexy is a debilitating disorder of sleep/wakefulness caused by a loss of orexin-producing neurons in the lateroposterior hypothalamus. Genetic or pharmacologic orexin replacement ameliorates symptoms in mouse models of narcolepsy-cataplexy. We have recently discovered a potent, nonpeptide OX2R-selective agonist, YNT-185. This study validates the pharmacological activity of this compound in OX2R-transfected cells and in OX2R-expressing neurons in brain slice preparations. Intraperitoneal, and intracerebroventricular, administration of YNT-185 suppressed cataplexy-like episodes in orexin knockout and orexin neuron-ablated mice, but not in orexin receptor-deficient mice. Peripherally administered YNT-185 also promotes wakefulness without affecting body temperature in wild-type mice. Further, there was no immediate rebound sleep after YNT-185 administration in active phase in wild-type and orexin-deficient mice. No desensitization was observed after repeated administration of YNT-185 with respect to the suppression of cataplexy-like episodes. These results provide a proof-of-concept for a mechanistic therapy of narcolepsy-cataplexy by OX2R agonists.
Orexins (orexin-A and orexin-B, also called hypocretins) are neuropeptides produced by neurons exclusively localized to the lateral hypothalamus that act on two G protein-coupled receptors termed OX1R and OX2R (1). Orexin-producing neurons send axons diffusely through the central nervous system, with especially dense innervations to several nuclei involved in sleep/wakefulness regulation (2, 3). Because orexins have been implicated in the maintenance of wakefulness, many groups are trying to develop nonpeptidic orexin receptor antagonists aiming at new medication for insomnia. Recently, suvorexant, a dual orexin receptor antagonist, was clinically approved in Japan and the United States (4, 5).
A crucial role of the orexin system in the regulation of sleep/wakefulness was initially uncovered by the discovery that OX2R-deficient dogs (6) and prepro-orexin knockout (Hcrt−/−, abbreviated as OXKO) mice (2) exhibited symptoms resembling human narcolepsy-cataplexy. According to the clinical definition by the International Classification of Sleep Disorders-Third Edition, two types of narcoleptic disorders are categorized (7). Narcolepsy type 1 is characterized by the existence of cataplexy and low levels of orexin-A in cerebrospinal fluid (CSF) (8–10). However, patients with narcolepsy type 2 show no cataplexy and normal orexin-A levels in CSF. Narcolepsy-cataplexy, or narcolepsy type 1 (11), is characterized by a marked instability of sleep/wake state transitions, resulting in nonrapid eye movement (NREM) sleep-related symptoms (e.g., excessive daytime sleepiness, “sleep attacks,” and fragmented nighttime sleep), and rapid eye movement (REM) sleep-related symptoms (e.g., cataplexy, sleep paralysis, and hypnagogic/hypnopompic hallucinations). In polysomnography, narcolepsy-cataplexy patients show markedly reduced daytime sleep latency, and sleep-onset REM periods (SOREMs). Currently available treatments for narcolepsy-cataplexy are all symptom-oriented, including psychostimulants (e.g., methylphenidate and modafinil), sedative (sodium oxybate), and tricyclic antidepressants. However, the use of these medications is often limited by adverse side effects such as headache, nausea, anxiety, irritability, and insomnia.
Murine models of narcolepsy-cataplexy include OXKO mice (2), orexin receptor-deficient (Hcrtr1−/−;Hcrtr2−/−, abbreviated as OXRDKO) mice, and the orexin/ataxin-3 transgenic mice (in which orexin neurons are genetically and postnatally ablated) (12). Tabuchi et al. created another narcolepsy mouse model, which expressed diphtheria toxin A in orexin neurons under control of the Tet-off system, leading to conditional ablation of orexin neurons (13). These mouse models exhibit full symptoms of narcolepsy-cataplexy, including frequent “behavioral arrests” in the dark (active) phase, highly fragmented wakefulness on electroencephalography/electromyography (EEG/EMG), and SOREMs. Importantly, whereas OX2R-deficient mice exhibit symptoms of narcolepsy-cataplexy, OX1R-deficient mice show no appreciable sleep/wakefulness-related phenotype, suggesting that OX2R signaling is particularly important for stabilizing sleep/wake state transitions. Symptoms of murine narcolepsy-cataplexy could be reversed either by ectopic production of orexin peptides from prepro-orexin transgene or by pharmacological administration of orexin-A (14), indicating that orexin receptors, their intracellular signaling, and the downstream neural pathways remain functionally intact even after prolonged, complete orexin deficiency. Although these results suggest that orexin replacement could be an effective mechanistic therapy, orexins do not efficiently cross the blood–brain barrier (15) and are therefore difficult to use as a clinical drug. Because deficiency of OX2R signaling is primarily responsible for sleepiness and cataplexy-like episodes (6, 16–18), nonpeptidic OX2R agonists are promising candidates for a therapeutic agent to treat narcolepsy. We recently discovered a potent nonpeptidic OX2R agonist, YNT-185 (compound 30 in ref. 19), through a lead optimization process. This compound is an orthosteric, full agonist for OX2R (19) (Fig. 1 B–D). In this study, we performed the pharmacological and electrophysiological characterization of YNT-185 and evaluated its effect on sleep/wake states in murine models of narcolepsy.
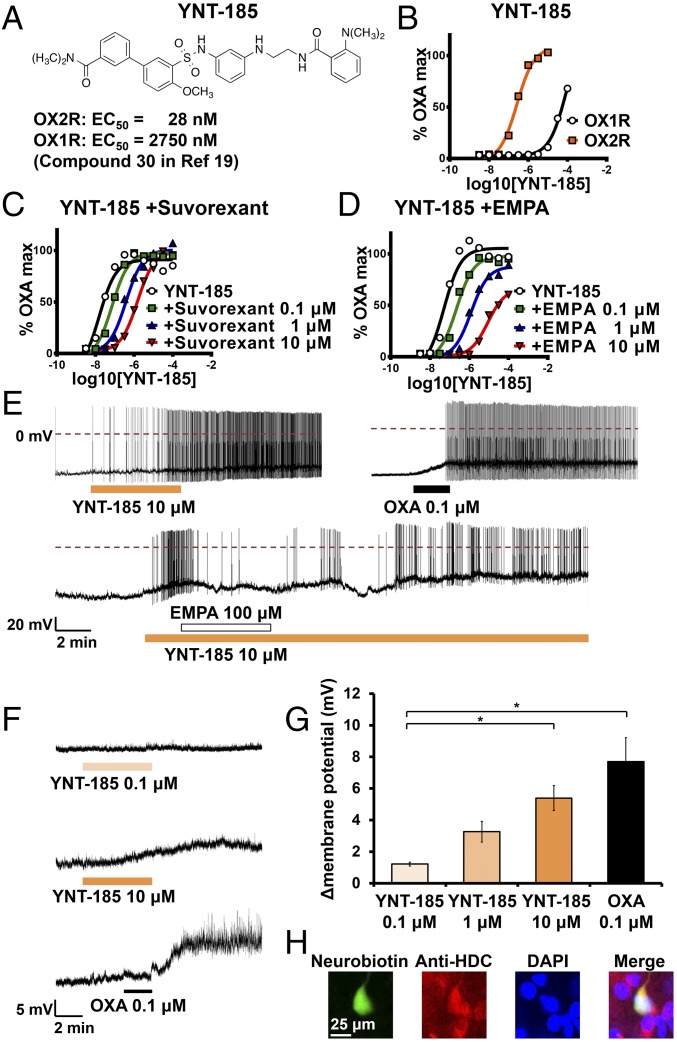
Fig. 1.
Pharmacological and electrophysiological verification of YNT-185 as OX2R agonist in transfected cells and in native OX2R-expressing neurons in mouse brain slices. (A) The chemical structure of YNT-185. (B) YNT-185 potently stimulated intracellular Ca2+ accumulation in CHO/hOX2R cells in a dose–response manner (n = 12 for each curve). (C and D) Effects of suvorexant and EMPA on concentration response curve to YNT-185 (n = 12 for each curve). (E) Effect of YNT-185 on the firing rate of histaminergic neurons in TMN without TTX. YNT-185 (Upper Left) and orexin-A (OXA) (Upper Right) induced firing of histaminergic neurons. EMPA inhibited the YNT185-induced firing of histaminergic neurons (Lower). Typical examples (F) and quantitative data (G) about effect of YNT-185 on resting membrane potential of histaminergic neurons in TMN with TTX (n = 4 for each condition). (H) Histological identification of histaminergic neurons in TMN. A cell injected with neurobiotin through the patch pipet was immunoreactive to anti-HDC antibody. *P < 0.05.
Results and Discussion
Activity of YNT-185 on Orexin Receptors in Transfected Cells.
We used Chinese hamster ovary (CHO) cells stably expressing human OX1R and OX2R (CHO/hOX1R, CHO/hOX2R) for evaluation of YNT-185 activity. YNT-185 induced intracellular Ca2+ mobilization in a dose-dependent manner with EC50 = 28 ± 4 nM in hOX2R and 2,750 nM in OX1R expressing cells, respectively (Fig. 1B), suggesting that the compound is selective for OX2R over OX1R by ∼100-fold (19). In CHO/hOX2R cells, suvorexant (4) caused a dose-dependent, parallel rightward shift in the dose–response curve to YNT-185 (Fig. 1C). The pA2 and Schild slope values of suvorexant are 6.8 and 0.8, respectively. The OX2R-selective antagonist EMPA (20) also caused a dose-dependent rightward shift in the concentration response curve to YNT-185 (Fig. 1D). At higher antagonist concentrations, the maximal response was slightly reduced, but Schild slope was not different from unity. The pA2 and Schild slope values of EMPA are 7.4 and 1.1, respectively. Given that EMPA and suvorexant bind to the OX2R with high affinity and antagonize orexin-induced transient increase in intracellular Ca2+ concentration through a competitive mechanism (20), these results show that YNT-185 acts as an orthosteric, full agonist for OX2R. These results are consistent with our previous report, which indicated that YNT-185 displaced [125I] orexin-A binding to OX2R in a dose-dependent manner (19).
Effects of YNT-185 on OX2R-Expressing Neurons.
Histaminergic neurons in the tuberomammillary nucleus (TMN) abundantly express OX2R, which plays an important role in the maintenance of wakefulness (16). Electrophysiological properties of histaminergic neurons in the TMN in mice have been well characterized, and orexin-A was shown to increase firing rates of these neurons by depolarizing them through OX2R (16). We examined whether YNT-185 excites histaminergic neurons by using whole-cell patch-clamp recordings in mouse brain slices. Histaminergic neurons in the TMN were identified by double staining for patch pipette-injected neurobiotin and histidine decarboxylase (HDC) after patch-clamp experiments (Fig. 1H). We first investigated the change of firing rate by adding YNT-185 in the absence of tetrodotoxin (TTX). Histaminergic neurons in the TMN showed no spontaneous firing under our recording conditions (Materials and Methods), but started firing after adding YNT-185 (10 μM) or orexin A (0.1 μM) (Fig. 1E). YNT-185–induced firing was inhibited by an OX2R antagonist, EMPA (100 μM) (Fig. 1E), suggesting that YNT-185 works as an OX2R agonist in these neurons, sharing the binding site with the endogenous ligand. Next, we investigated the change of resting membrane potential by adding YNT-185 in the presence of TTX (1 μM). Under these conditions, YNT-185 depolarized histaminergic neurons in the TMN in a dose-dependent manner (Fig. 1 F and G). These results show that YNT-185 mimics the effects of orexin on histaminergic neurons in the TMN (16).
YNT-185 Increases Wakefulness in Mice.
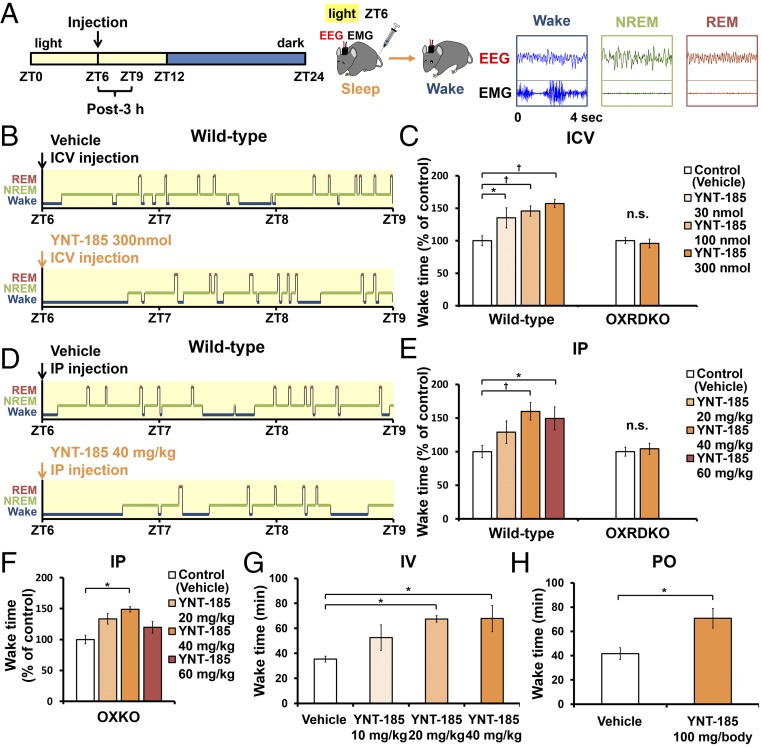
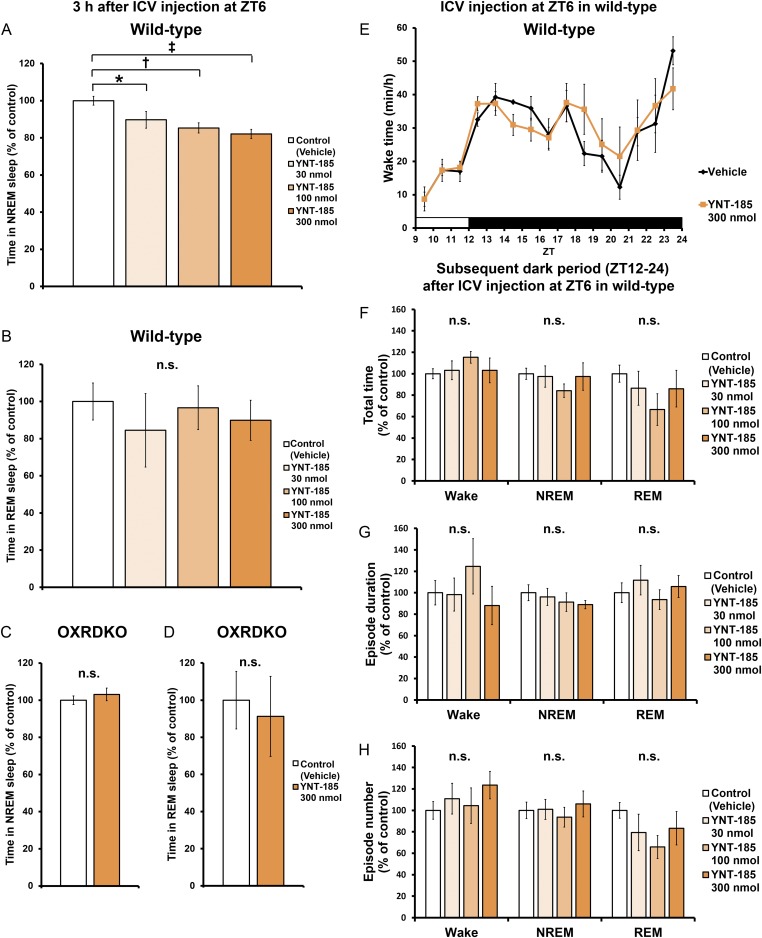
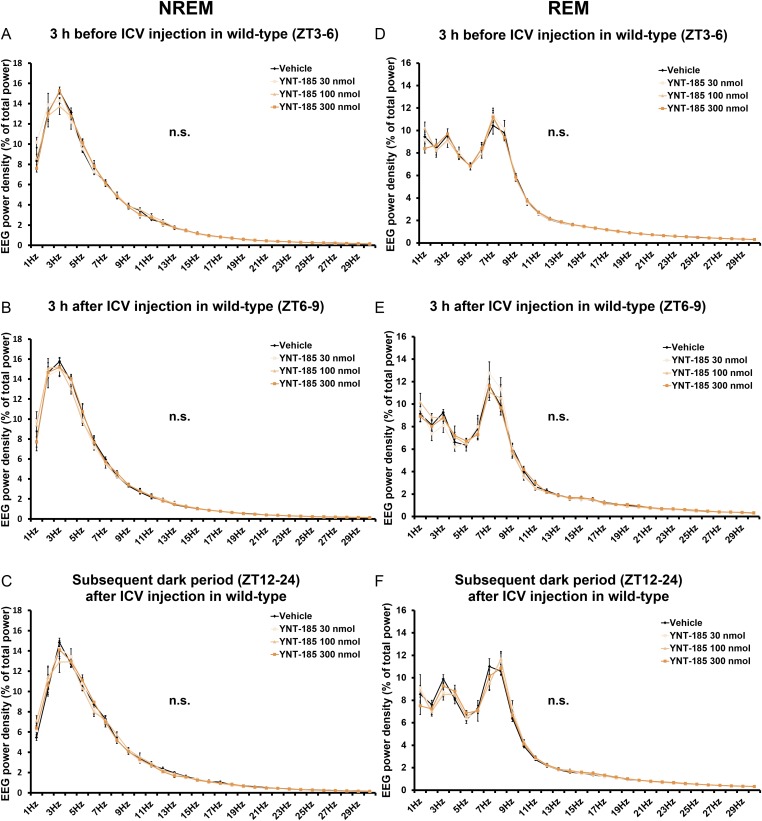
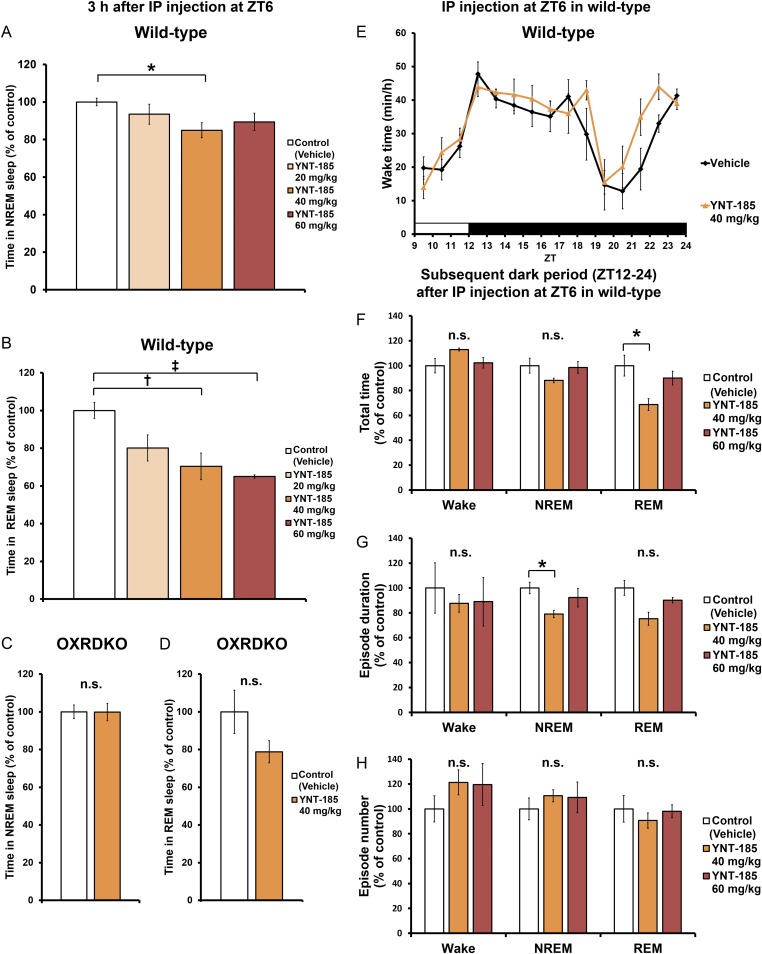
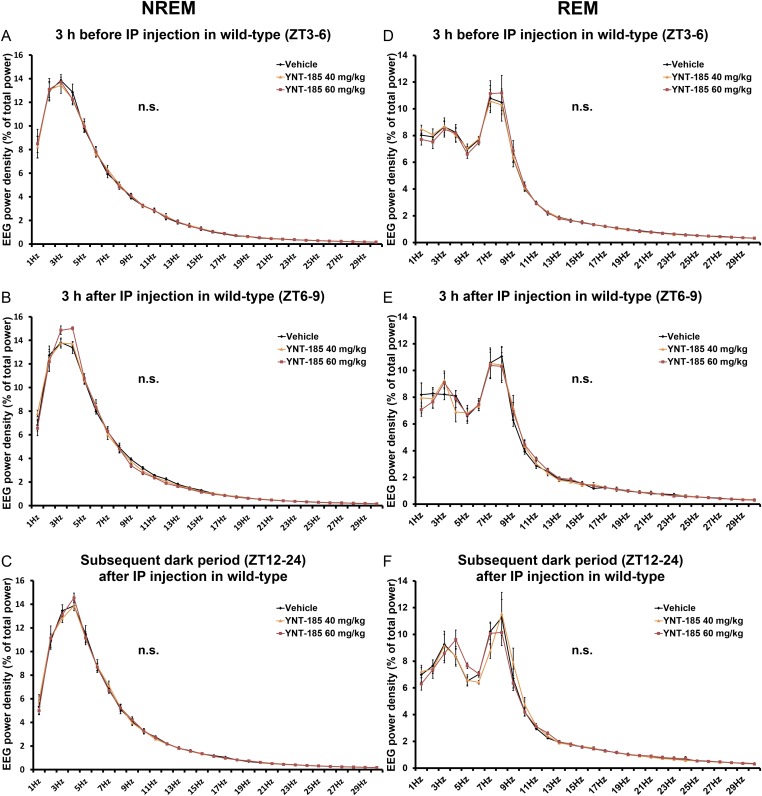
To evaluate the pharmacological effect of YNT-185 in vivo, we administered a water-soluble form of YNT-185 (YNT-185•2HCl) intracerebroventricularly (i.c.v.) in the light period (Zeitgeber time, ZT6), in which mice are mostly asleep, and monitored the sleep/wake states for 3 h by recording EEG/EMG in wild-type C57BL/6J mice (Fig. 2A). Because i.c.v.-administered orexin-A in the light period effectively increased the wake time at a dose of 3 nmol (14) and the effective dose of YNT-185 in vitro is ∼100-fold greater that of orexin-A (19), we used 30–300 nmol YNT-185 for i.c.v. administration. Fig. 2B shows hypnograms for 3 h after injection of vehicle or 300 nmol YNT-185. I.c.v. administration of YNT-185 significantly increased wake time for 3 h after administration in a dose-dependent manner, accompanied by a decrease in NREM sleep time (Fig. 2C and Fig. S1A), in wild-type mice but not in OXRDKO mice (Fig. 2C and Fig. S1C). No changes in the amount of REM sleep for 3 h after administration (Fig. S1 B and D) or rebound sleep in the remaining light period and the subsequent dark period (Fig. S1 E–H) were observed after YNT-185 administration in the light period. Power spectral analysis of EEG in NREM and REM sleep showed that EEG power density distributions in the light period before (Fig. S2 A and D) and after (Fig. S2 B and E) injection of YNT-185 were comparable. In addition, no change in EEG power density distribution in NREM and REM sleep were observed in the subsequent dark period (Fig. S2 C and F), following YNT-185 administration in the light period.
Fig. 2.
Central and peripheral administrations of YNT-185 increase wake time in the light period. (A) Time schedule of the injection and typical patterns of EEG/EMG. (B) Hypnograms after i.c.v. injections in the light period. (C) Wake time within 3 h after the i.c.v. administrations in wild-type mice (Left; n = 6) and OXRDKO mice (Right; n = 6). (D) Hypnograms after i.p. injections. (E) Wake time within 3 h after the i.p. administrations in wild-type mice (Left; n = 6), OXRDKO mice (Right; n = 6). (F) Wake time within 3 h after the i.p. administrations in OXKO mice (n = 5). (G and H) Wake time within 2 h after the i.v. (n = 5) (G) and orally (PO) (n = 3) (H) administrations of YNT-185 in wild-type mice. *P < 0.05, †P < 0.01. n.s., not significant.
Fig. S1.
I.c.v.-administered YNT-185 at ZT6 suppress sleep and has no effect on sleep/wake states of wild-type mice in the remaining light period and the subsequent dark period. (A) Amounts of time spent in NREM within 3 h after the i.c.v. administrations at ZT6 in wild-type mice (n = 5). (B) Amounts of time spent in REM within 3 h after the i.c.v. administrations at ZT6 in wild-type mice (n = 5). (C) Amounts of time spent in NREM within 3 h after the i.c.v. administrations at ZT6 in OXRDKO mice (n = 6). (D) Amounts of time spent in REM within 3 h after the i.c.v. administrations at ZT6 in OXRDKO mice (n = 6). (E) Hourly plots of wake time. Total time (F), episode duration (G), episode number (H) of sleep/wake states in the subsequent dark period after the i.c.v. administration at ZT6 in wild-type mice (n = 5). *P < 0.05, †P < 0.01, and ‡P < 0.005. n.s., not significant.
Fig. S2.
I.c.v. administrations of YNT-185 at ZT6 have no effect on EEG power density of wild-type mice. EEG power density of NREM and REM in the light period before (ZT3–6) (A and D) and after (ZT6–9) (B and E) injection and the subsequent dark period (ZT12–24) (C and F) in wild-type mice (n = 4). n.s., not significant.
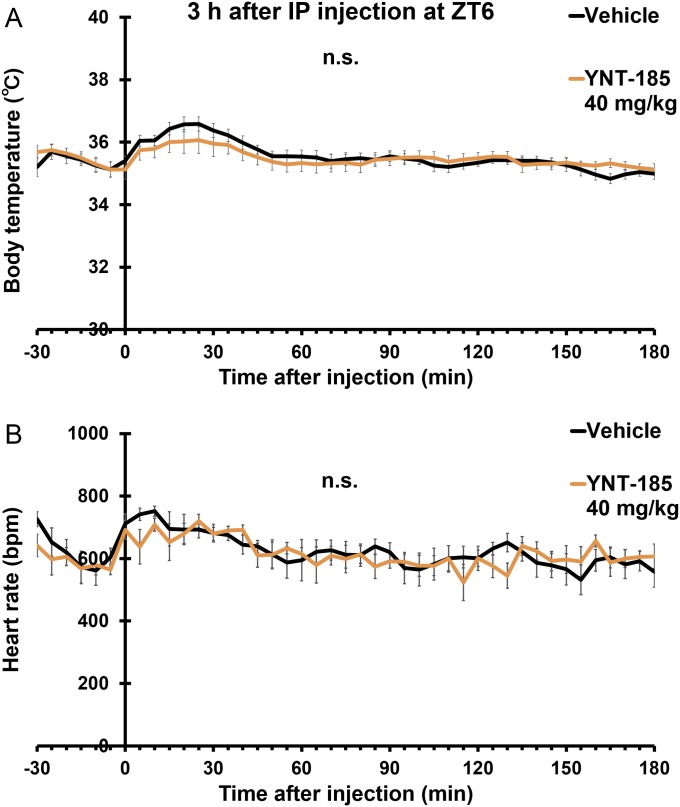
After i.p. administration of YNT-185 at 40 mg/kg body weight in the light period, wake time was also significantly increased (Fig. 2 D and E), accompanied by decreases in both NREM sleep (Fig. S3A) and REM sleep amounts (Fig. S3B) in wild-type mice but not in OXRDKO mice (Fig. 2E and Fig. S3 C and D). As with i.c.v. administration, after i.p. administration of YNT-185 (40 mg/kg, 60 mg/kg) to wild-type mice in the light period, no noticeable rebounds of sleep in the light period and the subsequent dark period were observed (Fig. S3 E–H). NREM and REM EEG power densities in the light period before (Fig. S4 A and D) and after (Fig. S4 B and E) i.p. injection of YNT-185 (40 mg/kg, 60 mg/kg) were unchanged. In the subsequent dark period, EEG power densities were not altered either (Fig. S4 C and F). Increase in wake time was also observed in OXKO mice after YNT-185 administration in the light period (Fig. 2F), with no rebound of sleep in the light period and the subsequent dark period (Fig. S5). I.p.-administered YNT-185 has no effect on body temperature or heart rate (Fig. S6).
Fig. S3.
I.p.-administered YNT-185 at ZT6 suppress sleep and has no effect on sleep/wake states of wild-type mice in the remaining light period and the subsequent dark period. (A) Amounts of time spent in NREM within 3 h after the i.p. administrations at ZT6 in wild-type mice (n = 6). (B) Amounts of time spent in REM within 3 h after the i.p. administrations at ZT6 in wild-type mice (n = 6). (C) Amounts of time spent in NREM within 3 h after the i.p. administrations at ZT6 in OXRDKO mice (n = 5). (D) Amounts of time spent in REM within 3 h after the i.p. administrations at ZT6 in OXRDKO mice (n = 5). (E) Hourly plots of wake time. Total time (F), episode duration (G), episode number (H) of sleep/wake states in the subsequent dark period after the i.p. administration at ZT6 in wild-type mice (n = 6). *P < 0.05. n.s., not significant.
Fig. S4.
I.p. administrations of YNT-185 at ZT6 have no effect on EEG power density of wild-type mice. EEG power density of NREM and REM in the light period before (ZT3–6) (A and D) and after (ZT6–9) (B and E) injection and the subsequent dark period (ZT12–24) (C and F) in wild-type mice (n = 5). n.s., not significant.
Fig. S5.
I.p. administrations of YNT-185 at ZT6 have no effect on sleep/wake states of OXKO mice in the remaining light period and the subsequent dark period. (A) Hourly plots of wake time. Total time (B), episode duration (C), and episode number (D) of sleep/wake states in the subsequent dark period after the i.p. administration at ZT6 in OXKO mice (n = 4). †P < 0.01. n.s., not significant.
Fig. S6.
Effect of YNT-185 on body temperature and heart rate in wild-type mice. (A) Body temperature of vehicle- and YNT-185–administered (40 mg/kg i.p.) wild-type mice. (B) Heart rate of vehicle- and YNT-185–administered (40 mg/kg i.p.) wild-type mice (n = 5). n.s., not significant.
YNT-185 significantly increased wake time in a dose-dependent manner for 2 h after i.v. administration (Fig. 2G). Orally (po) administered YNT-185 (100 mg per body) also increased in wake time for 2 h (Fig. 2H) after administration, suggesting that this compound is orally effective, although a high dose is required.
YNT-185 Ameliorates Narcolepsy Symptoms in Mouse Models.
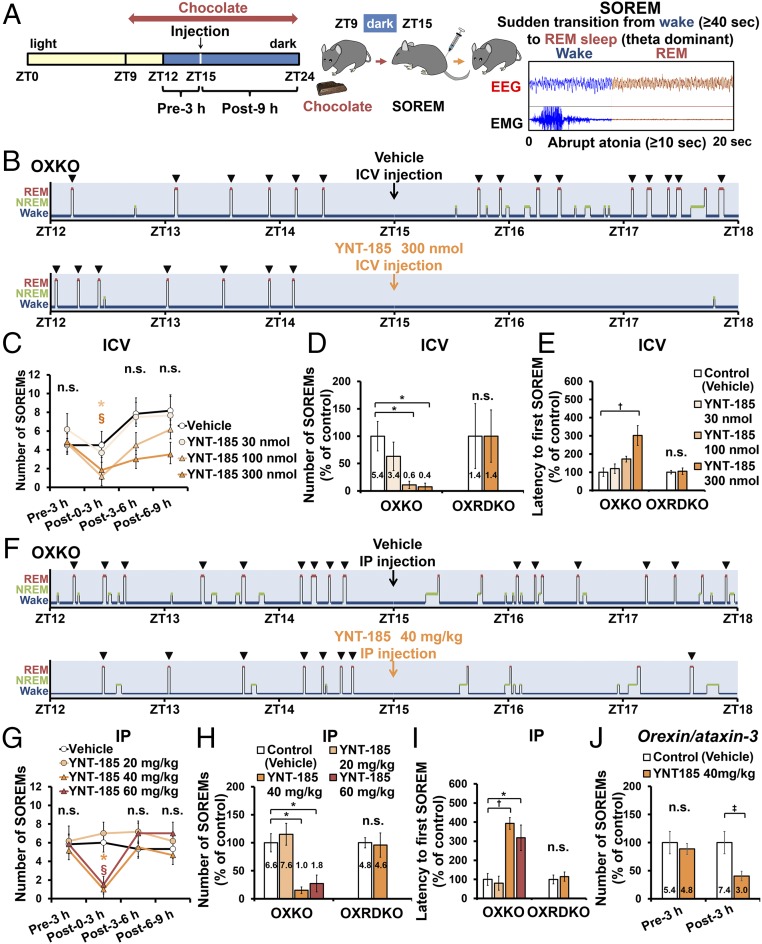
Narcoleptic humans and animals suffer from two major symptoms, i.e., sleepiness and cataplexy in the active phase, and these symptoms in mouse models are manifested as sleep/wake fragmentation and SOREMs (defined here as direct transition from wakefulness to REM sleep, equivalent to “cataplexy-like state” in ref. 21), respectively. We examined the effects of YNT-185 on these symptoms in OXKO mice after administration at the onset of the dark (active) period (Fig. 3A). Because the frequency of cataplexy-like episode increases when mice are fed with a particular kind of chocolate (22), we examined the effect under its presence. I.c.v.-administered YNT-185 (100, 300 nmol) significantly decreased the number of the chocolate-induced SOREM within 3 h in OXKO mice, but not in OXRDKO mice (Fig. 3 B–D). Moreover, 300 nmol YNT-185 significantly increased the latency from injection to the first SOREM in OXKO mice but not in OXRDKO mice (Fig. 3E).
Fig. 3.
Effect of YNT-185 on SOREMs. (A) Time schedule of the injection and typical EEG/EMG of SOREMs (21). Hypnograms (B) and episode number (C) of SOREMs after i.c.v. administration in OXKO mice (n = 5). Number of SOREMs for 3 h (the absolute numbers are indicated in each column) (D) and latency to the first SOREM after the i.c.v. administration (each n = 5) (E). Hypnograms (F) and number of SOREMs (G) after i.p. administration in OXKO mice (n = 5). Number of SOREMs for 3 h (H) and latency to the first SOREM after the i.p. administration (each n = 5) (I). (J) Number of SOREMs for 3 h after i.p. administration in orexin/ataxin-3 mice (n = 5). (C) *P < 0.05 for vehicle versus YNT-185 100 nmol, §P < 0.05 for vehicle versus YNT-185 300 nmol. (G) *P < 0.05 for vehicle versus YNT-185 40 mg/kg, §P < 0.05 for vehicle versus YNT-185 60 mg/kg. *P < 0.05, †P < 0.01, ‡P < 0.005. n.s., not significant.
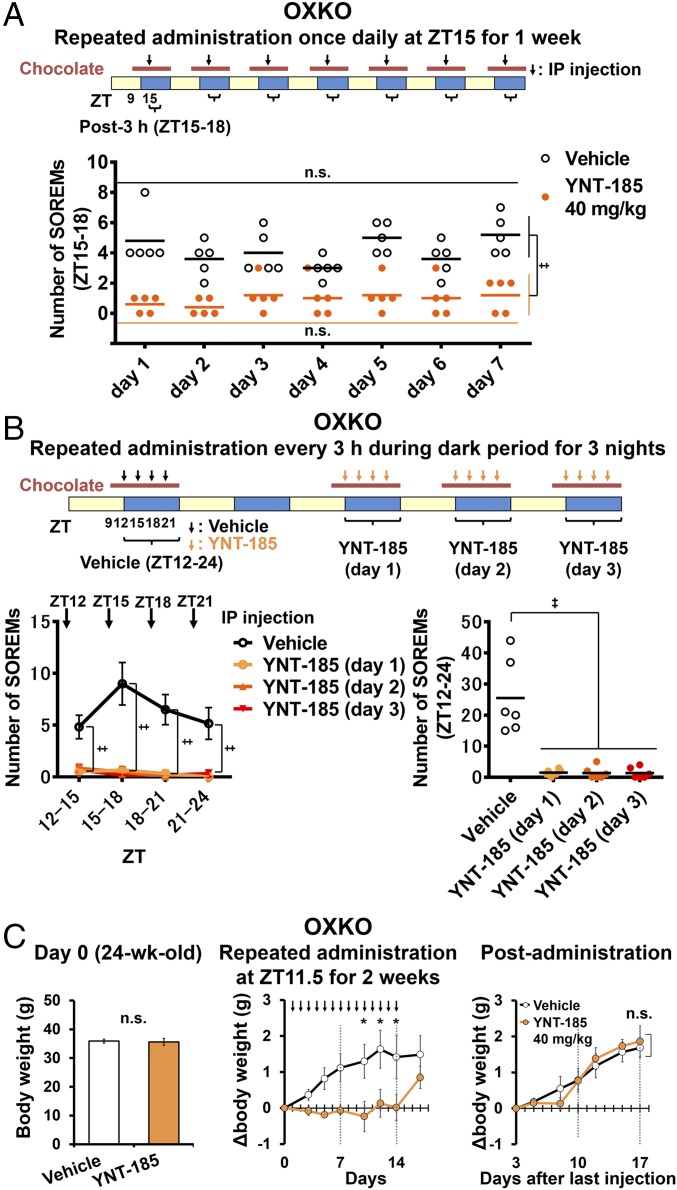
We also performed i.p. administration of YNT-185 at the onset of the dark period to narcoleptic mice and counted the number of SOREMs (Fig. 3A). I.p.-administered YNT-185 at 40 and 60 mg/kg significantly decreased the number of SOREM within 3 h compared with vehicle in OXKO mice, but not in OXRDKO mice (Fig. 3 F–H). Administration of YNT-185 at 40 and 60 mg/kg significantly increased the latency from injection to the first SOREM in OXKO mice, but not in OXRDKO mice (Fig. 3I). These results suggest that peripherally administered YNT-185 goes through the blood–brain barrier and acts on OX2R in the brain to inhibit cataplexy. We also used another narcoleptic model, orexin/ataxin-3 mice, in which orexin-producing neurons are genetically and postnatally ablated (12). I.p.-administered YNT-185 at 40 mg/kg significantly decreased the frequency of the chocolate-induced SOREMs in orexin/ataxin-3 mice for 3 h (Fig. 3J). We then investigated whether the effect of YNT-185 on SOREM are maintained after repeated administration every 24 h in OXKO mice. We did not observe a decrease in the effect on SOREMs by repeated administration of YNT-185 for 7 consecutive days (Fig. 4A). Further, we demonstrated that there was no appreciable desensitization to YNT-185 in terms of SOREM suppression in OXKO mice, when the compound was i.p. administered every 3 h during the dark period for 3 consecutive nights (Fig. 4B).
Fig. 4.
Effects of repeated YNT-185 administration on SOREMs and body weights in OXKO mice. (A) Effect of repeated once-a-day administration on SOREMs in OXKO mice (n = 5). (B) Effect of repeated administration every 3 h during the dark period for 3 consecutive nights on SOREMs in OXKO mice (n = 6). (B, Left) Number of SOREMs in each 3-h period during the dark phase, averaged over the 3 nights. (B, Right) Total number of SOREMs through 12-h dark periods for 3 consecutive nights. (C) Daily i.p. administration of YNT-185 decreased the body weight in aged OXKO mice. (C, Left) There were no difference in body weight between vehicle- and YNT-185–administered aged OXKO mice (24 wk old) before administration (n = 8). (C, Center) Increase in body weight in aged OXKO mice (24 wk old) was significantly suppressed by daily i.p. administration of YNT-185 immediately before dark period for 14 d. (C, Right) After stopping administration of YNT-185, the body weight increased in aged OXKO mice. *P < 0.05,‡P < 0.005. n.s., not significant.
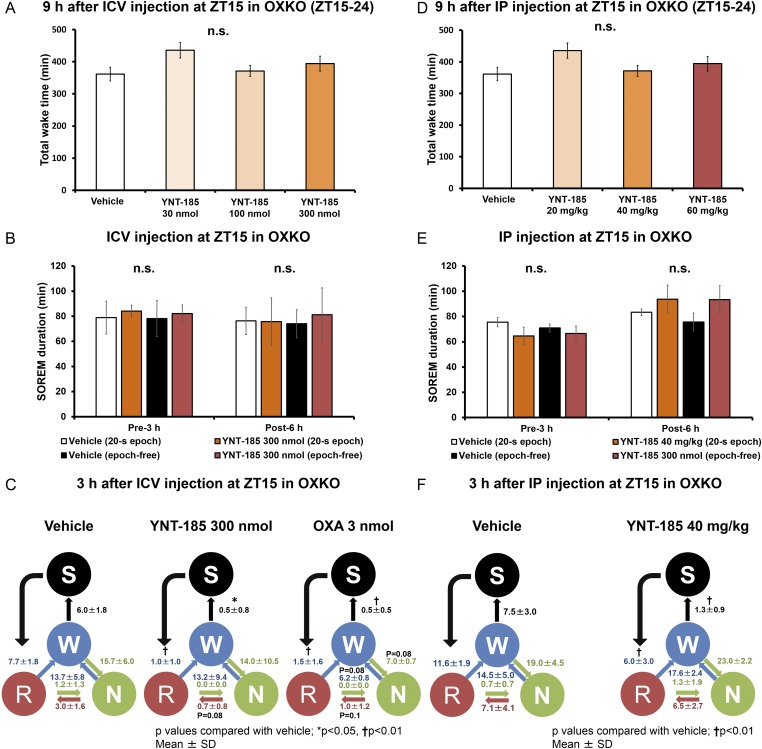
YNT-185 did not induce any alterations in the architecture of wakefulness for 9 h after i.c.v. or i.p. administration compared with vehicle administration in OXKO mice (Fig. S7 A and D). Although YNT-185 suppressed chocolate-induced SOREMs, sleep/wakefulness fragmentation was not significantly ameliorated by this compound. The increased transition frequency between NREM and wakefulness states was not changed after YNT-185 administration, whereas i.c.v.-administered orexin-A at 3 nmol tended to improve the fragmentation in OXKO mice (Fig. S7C). YNT-185 (i.p.) did not affect the number of transitions between each state in OXKO mice either (Fig. S7F). YNT-185 (i.c.v., i.p.) did not change the mean episode duration of SOREMs in OXKO mice (Fig. S7 B and E).
Fig. S7.
I.c.v. and i.p. administrations of YNT-185 have no effect on wakefulness in dark period. (A and D) Total time wakefulness in the dark period after the i.c.v. (A) and i.p. (D) administrations at ZT15 in OXKO mice (each n = 5). (B and E) Episode duration of SOREMs scored through a usual sleep staging by visual inspection of the 20-s epochs or a precise second-by-second visual inspection (n = 5). (C and F) The frequency of transitions between each stage during 3 h after i.c.v. (C) and i.p. (F) administrations (n = 5). The numbers indicate the frequency of transitions between each stage. S, SOREM; W, Wake; N, NREM; R, REM. *P < 0.05, †P < 0.01. n.s., not significant.
In a previous report (17), the targeted restoration of OX2R expression in dorsal raphe, and of OX1R in the locus coeruleus, in mice lacking orexin receptors inhibited cataplexy-like episodes and pathological fragmentation of wakefulness, respectively, suggesting a role for OX1R in the consolidation of wakefulness. Considering these results, additional activation of OX1R may be necessary for full recovery of pathological fragmentation of wakefulness in narcoleptic mice. OX2R-null mice and OXKO mice are similarly affected with behaviorally abnormal attacks of NREM sleep (“sleep attacks”) and show similar degrees of fragmentation (18). Focal restoration of OX2R in neurons of the posterior hypothalamus, including the TMN, rescued the fragmentation of wakefulness in OX2R-null mice in the presence of OX1R (16), further suggesting that simultaneous activation of both OX1R and OX2R might be important for restoring the fragmentation of wakefulness.
Most likely, the efficacy of YNT-185 was not strong enough to rescue the sleep/wake fragmentation in narcoleptic mice, given that low levels of orexin activity could inhibit cataplexy, whereas substantially higher levels of orexin activity is necessary for sleep/wake stabilization (13). A previous study showed that fragmentation of sleep/wakefulness was already observed after ∼86% of orexin neurons had been ablated, whereas a loss of 95% orexin neuron was necessary to trigger cataplexy (13), suggesting that a lower orexinergic activity can inhibit cataplexy than that required for rescue of the sleep/wake fragmentation.
Notably, YNT-185 (administered by i.c.v. or peripherally) promotes wakefulness in both wild-type and narcoleptic mice with no immediate rebound increase of sleep, suggesting that OX2R agonists might also be useful in the treatment of excessive daytime sleepiness due to other conditions, such as idiopathic hypersomnia, sleepiness accompanying depression, sleepiness due to side effects of various medicines, and jet-lag/shift work.
Metabolic Effects.
People with narcolepsy are prone to gaining weight (11). Rodent models of narcolepsy also tend to develop late onset obesity (23, 24). We examined whether administration of YNT-185 could affect the body weight in aged (24 wk old) OXKO mice, which are heavier than wild-type mice (37.4 ± 0.22 g vs. 34.3 ± 0.5 g, P = 0.01, each n = 3). Before administration, there was no difference in body weight between vehicle- and YNT-185–administered groups (Fig. 4C, Left). However, we found that the body weight of aged OXKO mice was significantly lower in the group with daily i.p. administration of YNT-185 (40 mg/kg) at the start of dark period for 14 successive days compared with vehicle-treated group (Fig. 4C, Center). No effect of YNT-185 was seen in OXRDKO mice (data not shown). These results are consistent with the previous report that peptidic OX2R-selective agonist inhibits diet-induced obesity (24). After administration of YNT-185 was terminated, the body weight gradually recovered in aged OXKO mice (Fig. 4C, Right), suggesting that the effect of YNT-185 on the body weight is reversible. Orexin plays a key role in the regulation of muscle glucose metabolism by activating muscle sympathetic nerves (25). Moreover, the amplitude of metabolic circadian rhythm is crucial for preventing hepatic insulin resistance, and sleep disturbances are associated with type 2 diabetes (26). Indeed, daily active-phase administrations of orexin-A prevented hepatic insulin resistance via bidirectional regulation of the autonomic nervous system (26), whereas daily resting-phase administrations of suvorexant for 2–4 wk improved glucose tolerance in obese mice (27). Taken together, in narcoleptic patients, daytime administration of OX2R agonist may have beneficial effects not only on promoting daytime wakefulness, but also on ameliorating metabolic abnormalities.
Because of its limited in vivo efficacy, we do not consider YNT-185 suitable for further clinical development. We are now further improving YNT-185 in terms of potency and CNS bioavailability. Nevertheless, the present study demonstrates the utility of orexin-deficient and orexin receptor-deficient mouse models to assess pharmacological and mechanistic approaches for the treatment of narcolepsy. There have been several investigations published in this regard (11, 28, 29), but our study is distinct in treating the core defect in narcolepsy as opposed to other studies that assess drug effects on narcolepsy symptomatology.
Materials and Methods
Detailed methods are described in SI Materials and Methods (also see Fig. S8). Animal experiments were carried out in a humane manner after receiving approval from the Institutional Animal Care and Use Committee of the University of Tsukuba and, thus, in accordance with the Regulation for Animal Experiments in our university and Fundamental Guideline for Proper Conduct of Animal Experiments and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology (MEXT). Increase of the intracellular Ca2+ concentration was measured by using CHO-K1 cells stably expressing human OX1R (CHO/hOX1R) or OX2R (CHO/hOX2R). Patch-clamp recordings were performed in histaminergic neurons of coronal slices (200 μm) from 3- to 5-wk-old male mice. EEG/EMG implantation was performed as described elsewhere with slight modification (30).
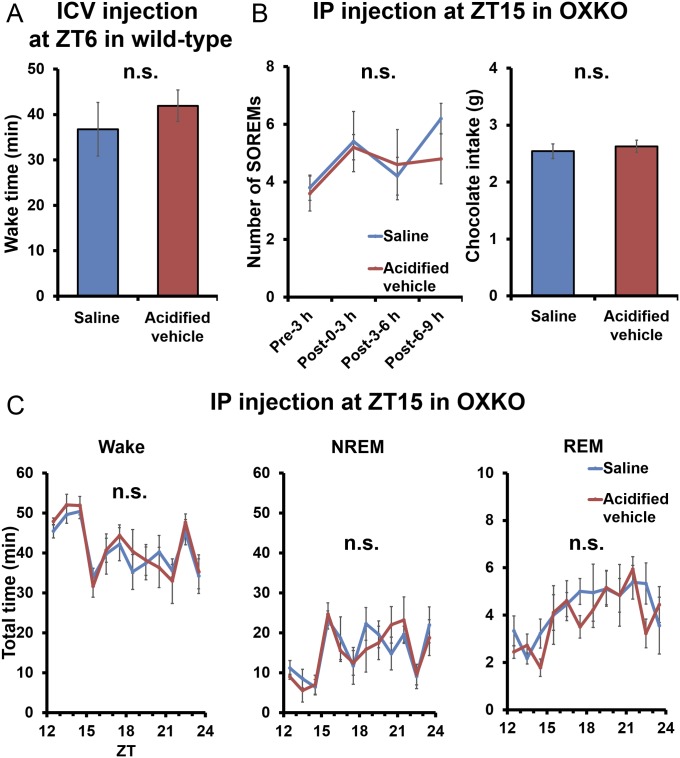
Fig. S8.
Wake time, the number of SOREMs, and chocolate intake in saline- and HCl-acidified vehicle administered mice. (A) Wake time in wild-type mice (n = 5). (B) The number of SOREMs and chocolate intake in OXKO mice (n = 5). (C) Hourly plots of wake, NREM, and REM after administration. n.s., not significant.
SI Materials and Methods
Animals.
For in vivo tests, we used male wild-type C57BL/6J mice and narcoleptic model mice from two genotypes on a C57BL/6J background (Hcrt−/−, those lacking the orexin ligand and abbreviated as OXKO, and Hcrtr1−/−;Hcrtr2−/−, those lacking both orexin receptors and abbreviated as OXRDKO) (2). We also used male transgenic orexin/ataxin-3 mice in which orexin-producing neurons are postnatally ablated (12). Mice were from 8 to 12 wk old, except that orexin/ataxin-3 mice were 15 wk old when the ablation was complete. To analyze the effects of YNT-185 electrophysiologically, 3- to 5-wk-old mice were used. To examine the effects of YNT-185 on body weights, 24-wk-old OXKO and OXRDKO mice were used. Experimental mice were individually housed and kept on a 12 h:12 h light:dark schedule at an ambient temperature of 23 ± 1 °C and under specific pathogen-free conditions. Mice were randomly assigned to experimental groups. We used male mice in all experiments, because female mice have an estrus cycle and there are variations in the action of drugs. The lack of data from female mice is a limitation in the present study.
Solutions and Materials.
We used YNT-185•2HCl that was dissolved in saline for in vivo test. As a vehicle, we used HCl-acidified saline with equal pH (pH 2.3) and isotonic (325 mOsm) to YNT-185•2HCl solution. There were no differences in wake time, the number of SOREMs, and chocolate intake after administration of saline or HCl-acidified saline (Fig. S8). Human orexin-A (Peptide Institute) was dissolved in saline. For in vitro tests, orexin-A was dissolved in 0.1% BSA (Sigma-Aldrich)/PBS. YNT-185, suvorexant, and EMPA (Tocris Chemicals) were dissolved in dimethyl sulfoxide (DMSO: Nakarai Tesque) solution and readjusted by adding these solutions into each experimental solution (final concentration of DMSO is 1%).
In Vitro Ca2+ Mobilization Assay.
CHO-K1 cells stably expressing human OX1R (CHO/hOX1R) or OX2R (CHO/hOX2R) were seeded in a 96-well-plate (10,000 cells per well) and incubated with 5% FBS (Corning)/Dulbecco’s modified Eagle’s medium (DMEM: WAKO) at 37 °C for 48 h. Then, cells were loaded with 5 µM fluorescent calcium indicator Fura 2-AM (Cayman Chemical) in HBSS (GIBCO) including 20 mM Hepes (Sigma-Aldrich), 2.5 mM Probenecid (WAKO), 5% CremophorEL (Fluka), and 0.1% BSA at 37 °C for 1 h. The cells were washed once and 75 µL of HBSS buffer was added. Then, plates were loaded in the Functional Drug Screening System 7000 (Hamamatsu Photonics), and cells were treated with 25 µL of various concentrations of test compounds or orexin-A (diluted in 0.1% BSA/PBS), and the increase of the intracellular Ca2+ concentration was measured from the ratio of fluorescence emission at 510 nm by excitation at 340 or 380 nm. Suvorexant or EMPA was added to the cell 15 min before YNT-185. The EC50 values of YNT-185, and IC50 and pA2 values of suvorexant or EMPA against YNT-185, were calculated by using GraphPad Prism 5.0 (Graph Pad). For each dose–response curve, data from three experiments were combined, with each experiment conducted in quadruplicate wells.
Patch-Clamp Electrophysiology.
Coronal slices (200 μm) of 3- to 5-wk-old male mice were cut with a vibrating microtome in ice-cold cutting solution (KCl 2.5 mM, NaH2PO4 1.25 mM, MgCl2 5 mM, CaCl2 0.5 mM, NaHCO3 26 mM, glucose 11 mM, sucrose 210 mM, pH 7.4). Slices were incubated for 30 min in oxygenated artificial CSF (NaCl 125 mM, KCl 2.5 mM, NaH2PO4 1.25 mM, MgCl2 1 mM, CaCl2 2 mM, NaHCO3 26 mM, glucose 11 mM, pH 7.4) at room temperature, and then were recorded, submerged, and perfused with oxygenated artificial CSF (2 mL/min) maintained at room temperature. Patch-clamp recordings were performed in whole-cell, current-clamp mode by using a Multiclamp 700B amplifier (Molecular Devices) in current-clamp mode. Signals were low-pass filtered at 5 kHz and digitized at 50 kHz by using a Digidata1322A interface and acquired by using Clampex 10.2 software (Molecular Devices). Data were analyzed by using Clampfit 9.0 (Molecular Devices). Patch electrodes were filled with a pipette solution (K-gluconate 125 mM, KCl 10 mM, MgATP 4 mM, Hepes 10 mM, Na-GTP 0.3 mM, phosophocreatine-Na2 8 mM, EGTA 0.5 mM, pH 7.2). Neurobiotin (0.1%) was also added to the pipette solution to label the patched-clamp cells. We assessed pharmacological effects on the change of firing rate in the absence of tetrodotoxin (TTX, Nakarai Tesque) and on the change of resting potential in the presence of TTX (1 μM). YNT-185, EMPA, and orexin-A were dissolved with DMSO, and TTX was dissolved with distilled water and adjusted with artificial CSF. TMN neurons were identified by a depolarizing sag during hyperpolarizing pulses produced by activation of a hyperpolarization-activated current (16). After patch-clamp experiments, slices were fixed and the neurobiotin in the patched cells was identified with streptavidin conjugated with Alexa Fluor 488 (1:1,000; Invitrogen). These histaminergic neurons were identified with guinea pig anti-histidine decarboxylase (HDC) antibody (1:1,000; EuroProxima, catalog no. 2263B-GP265-1) and anti-guinea pig IgG antibody conjugated with Alexa Flour 594 (1:1,000; Invitrogen). Finally, slices were mounted with Vectashield H-1200 (Vector) and examined by using an LSM700 microscope (Zeiss).
Implantation of EEG/EMG Electrode and i.c.v/i.v. Catheters.
The implant comprised two stainless steel screws (1 mm diameter) serving as EEG electrodes, one of which was placed epidurally over the right frontal cortex (0.6 mm anterior and 1.5 mm lateral to bregma) and the other over the right parietal cortex (0.6 mm anterior and 1.5 mm lateral to lambda) under stereotaxic control. Two flexible wires for EMG recording were implanted into both trapezius muscles (left/right). The whole assembly was then attached to the skull with dental cement. After recovery from anesthesia, the mice were housed individually, let to recover for at least 1 wk and then connected to a tether hung from a counterbalanced arm (Instech Laboratories) that allowed the free movement for habituation to the recording conditions for another 1 wk. For i.c.v. administration, mice were simultaneously implanted with a guide cannula into left lateral ventricle (0.3 mm anterior and 0.9 mm lateral to bregma) as described (14, 19). For i.v. administration, mice were implanted with a catheter into the right jugular vein attached with s.c. access port (Primtech). Port was flushed with saline containing heparin every day. One week after the i.v. surgery, mice were implanted with EEG/EMG electrode.
I.c.v., i.p., i.v., and po Administration.
I.c.v. administration was performed through a cannula connected to an oil-filled syringe (Hamilton) by using an automated syringe pump (Harvard Apparatus) under anesthesia with isoflurane. YNT-185 or orexin-A were injected into the left lateral ventricle in 5 μL over 10 min. This flow rate (0.5 μL/min) is close to the physiological CSF flow rate in mice (∼0.4 μL/min) and, therefore, could be a limitation of the present study (31). During the second week after surgery, to acclimate mice, we performed i.p., i.v., and po administration of 100 μL of saline on every other day for 1 wk. From the third week, mice were administered with 100 μL of compound solution and the EEG/EMG were measured as mentioned below.
Time Schedule of Injection.
To assess waking effects, vehicle or compound solution was administered at ZT6 (in the light period) and wake time was evaluated for 3 h. To evaluate SOREMs, we gave milk chocolates (Hershey) to mice at ZT9 to increase cataplexy during the measurement of EEG/EMG. Vehicle or YNT-185 was administered at ZT15, and SOREMs were evaluated for 9 h. To assess the desensitization to YNT-185, OXKO mice were i.p. administered with 40 mg/kg YNT-185 repeatedly as shown in the diagrams (Fig. 4 A and B).
EEG/EMG Analysis.
The vigilance state in each 20-s epoch was classified as NREM sleep, REM sleep, or wakefulness. Our definition of SOREM here is equivalent to “cataplexy-like state” as defined in ref. 21. EEG signals were subjected to a fast Fourier transform analysis from 1 to 30 Hz with 1-Hz bin by using MatLab-based custom software. Total time spent in wakefulness, NREM, and REM sleep were derived by summing the total number of 20-s epochs in each stage. Mean episode durations were determined by dividing the total time spent in each stage by the number of episodes of that state. We have analyzed the episode duration of SOREMs by using two different methods: (i) by visual inspection of the 20-s epochs used for sleep staging; and (ii) precise second-by-second measurement of SOREM episode durations through visual inspections of atonic EMG accompanying theta-rich REM-like EEG (Fig. S7 B and E). There was no appreciable difference in the mean SOREM episode durations between the two evaluation methods, indicating that there are no significant quantizing artifacts due to the use of 20-s epochs. We analyzed EEG power density (power of each frequency bin divided by total EEG power) of vehicle- or YNT-185–injected mice in the light period before (ZT3–6) and after (ZT6–9) administration and the subsequent dark period (ZT12–24).
Effects of YNT-185 on Body Temperature and Heart Rate.
To investigate the effect of YNT-185 on body temperature and heart rate of mice continuously every 5 min, the mice were s.c. implanted with a telemetry probe (TA10ETA-F20; Data Science International). Mice were allowed a 1-wk postsurgical recovery period before use in experiments.
Effects of YNT-185 on Body Weight of OXKO and OXRDKO Mice.
Twenty-four-week-old OXKO and OXRDKO mice were group-housed (4 mice per cage), and injected with saline once a day for 7 d to acclimate and fed a normal diet (MF, 5% of total fat content) for 14 d. Every 3 d, body weight was measured. After stopping administration of YNT-185, body weight was measured for 14 d.
Data Analysis.
All values are expressed as mean ± SEM. To compare two groups, data were analyzed with unpaired Student t test. To compare more than two groups, data were analyzed with a one-way ANOVA, and individual group means were then compared with Bonferroni’s test. To compare the time elapsed data, data were analyzed with a repeated-measurement two-way ANOVA, and individual group means were compared with Bonferroni’s test by using Graph Pad Prism 5.0 (Graph Pad). Differences were considered significant when P < 0.05.
Acknowledgments
We thank Dr. Tito Akindele for reading the manuscript and J. Fukuoka, A. Jingu, D. Nakatsuka, and H. Muta for technical assistance. This work was supported in part by World Premier International Research Center Initiative, MEXT, Japan; Japan Society for the Promotion of Science KAKENHI Grants 26220207, 16K08541, 16H05098, 15K16557, and 17H06049; the Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST Program); and Japan Foundation for Applied Enzymology Grant 16T007.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1700499114/-/DCSupplemental.
References
- 1.Sakurai T, et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 2.Chemelli RM, et al. Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 3.Peyron C, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox CD, et al. Discovery of the dual orexin receptor antagonist [(7R)-4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl][5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl]methanone (MK-4305) for the treatment of insomnia. J Med Chem. 2010;53:5320–5332. doi: 10.1021/jm100541c. [DOI] [PubMed] [Google Scholar]
- 5.Uslaner JM, et al. Orexin receptor antagonists differ from standard sleep drugs by promoting sleep at doses that do not disrupt cognition. Sci Transl Med. 2013;5:179ra44. doi: 10.1126/scitranslmed.3005213. [DOI] [PubMed] [Google Scholar]
- 6.Lin L, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 7.Sateia MJ. International classification of sleep disorders-third edition: Highlights and modifications. Chest. 2014;146:1387–1394. doi: 10.1378/chest.14-0970. [DOI] [PubMed] [Google Scholar]
- 8.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 9.Peyron C, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 10.Thannickal TC, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scammell TE. Narcolepsy. N Engl J Med. 2015;373:2654–2662. doi: 10.1056/NEJMra1500587. [DOI] [PubMed] [Google Scholar]
- 12.Hara J, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 13.Tabuchi S, et al. Conditional ablation of orexin/hypocretin neurons: A new mouse model for the study of narcolepsy and orexin system function. J Neurosci. 2014;34:6495–6509. doi: 10.1523/JNEUROSCI.0073-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mieda M, et al. Orexin peptides prevent cataplexy and improve wakefulness in an orexin neuron-ablated model of narcolepsy in mice. Proc Natl Acad Sci USA. 2004;101:4649–4654. doi: 10.1073/pnas.0400590101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujiki N, Yoshida Y, Ripley B, Mignot E, Nishino S. Effects of IV and ICV hypocretin-1 (orexin A) in hypocretin receptor-2 gene mutated narcoleptic dogs and IV hypocretin-1 replacement therapy in a hypocretin-ligand-deficient narcoleptic dog. Sleep. 2003;26:953–959. doi: 10.1093/sleep/26.8.953. [DOI] [PubMed] [Google Scholar]
- 16.Mochizuki T, et al. Orexin receptor 2 expression in the posterior hypothalamus rescues sleepiness in narcoleptic mice. Proc Natl Acad Sci USA. 2011;108:4471–4476. doi: 10.1073/pnas.1012456108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasegawa E, Yanagisawa M, Sakurai T, Mieda M. Orexin neurons suppress narcolepsy via 2 distinct efferent pathways. J Clin Invest. 2014;124:604–616. doi: 10.1172/JCI71017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willie JT, et al. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: Molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron. 2003;38:715–730. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 19.Nagahara T, et al. Design and synthesis of non-peptide, selective Orexin receptor 2 agonists. J Med Chem. 2015;58:7931–7937. doi: 10.1021/acs.jmedchem.5b00988. [DOI] [PubMed] [Google Scholar]
- 20.Malherbe P, et al. Biochemical and behavioural characterization of EMPA, a novel high-affinity, selective antagonist for the OX(2) receptor. Br J Pharmacol. 2009;156:1326–1341. doi: 10.1111/j.1476-5381.2009.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scammell TE, Willie JT, Guilleminault C, Siegel JM. International Working Group on Rodent Models of Narcolepsy A consensus definition of cataplexy in mouse models of narcolepsy. Sleep. 2009;32:111–116. [PMC free article] [PubMed] [Google Scholar]
- 22.Oishi Y, et al. Role of the medial prefrontal cortex in cataplexy. J Neurosci. 2013;33:9743–9751. doi: 10.1523/JNEUROSCI.0499-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujiki N, et al. Sex difference in body weight gain and leptin signaling in hypocretin/orexin deficient mouse models. Peptides. 2006;27:2326–2331. doi: 10.1016/j.peptides.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Funato H, et al. Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell Metab. 2009;9:64–76. doi: 10.1016/j.cmet.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiuchi T, et al. Hypothalamic orexin stimulates feeding-associated glucose utilization in skeletal muscle via sympathetic nervous system. Cell Metab. 2009;10:466–480. doi: 10.1016/j.cmet.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Tsuneki H, et al. Hypothalamic orexin prevents hepatic insulin resistance via daily bidirectional regulation of autonomic nervous system in mice. Diabetes. 2015;64:459–470. doi: 10.2337/db14-0695. [DOI] [PubMed] [Google Scholar]
- 27.Tsuneki H, et al. Nighttime administration of nicotine improves hepatic glucose metabolism via the hypothalamic orexin system in mice. Endocrinology. 2016;157:195–206. doi: 10.1210/en.2015-1488. [DOI] [PubMed] [Google Scholar]
- 28.Black SW, Schwartz MD, Chen TM, Hoener MC, Kilduff TS. Trace amine-associated receptor 1 agonists as narcolepsy therapeutics. Biol Psych. October 18, 2016 doi: 10.1016/j.biopsych.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Black SW, et al. GABAB agonism promotes sleep and reduces cataplexy in murine narcolepsy. J Neurosci. 2014;34:6485–6494. doi: 10.1523/JNEUROSCI.0080-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lazarus M, Huang ZL, Lu J, Urade Y, Chen JF. How do the basal ganglia regulate sleep-wake behavior? Trends Neurosci. 2012;35:723–732. doi: 10.1016/j.tins.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Rudick RA, Zirretta DK, Herndon RM. Clearance of albumin from mouse subarachnoid space: A measure of CSF bulk flow. J Neurosci Methods. 1982;6:253–259. doi: 10.1016/0165-0270(82)90088-7. [DOI] [PubMed] [Google Scholar]