Significance
Teleost fishes have evolved a wonderful array of diverse dentitions. The highly derived order Tetraodontiformes exhibits the most unique dental forms among teleosts. The novel beak-like dentition of the pufferfish develops through a drastic shift in dental morphology during ontogeny. A simple first-generation tooth set is followed by the repetitive development of multiple elongated jaw-length tooth bands, which fuse together over time to form the characteristic beak. A restriction of the tooth-regenerative process in all but four tooth sites, coupled with the maintenance of lifelong stem cells for perpetual tooth development, is essential for the formation of this unique dentition. In pufferfish, regeneration plays a vital role in producing this novel dental form from highly conserved developmental underpinnings.
Keywords: tooth development, diversity, dental regeneration, stem cells, novelty
Abstract
Vertebrate dentitions are extraordinarily diverse in both morphology and regenerative capacity. The teleost order Tetraodontiformes exhibits an exceptional array of novel dental morphologies, epitomized by constrained beak-like dentitions in several families, i.e., porcupinefishes, three-toothed pufferfishes, ocean sunfishes, and pufferfishes. Modification of tooth replacement within these groups leads to the progressive accumulation of tooth generations, underlying the structure of their beaks. We focus on the dentition of the pufferfish (Tetraodontidae) because of its distinct dental morphology. This complex dentition develops as a result of (i) a reduction in the number of tooth positions from seven to one per quadrant during the transition from first to second tooth generations and (ii) a dramatic shift in tooth morphogenesis following the development of the first-generation teeth, leading to the elongation of dental units along the jaw. Gene expression and 1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) lineage tracing reveal a putative dental epithelial progenitor niche, suggesting a highly conserved mechanism for tooth regeneration despite the development of a unique dentition. MicroCT analysis reveals restricted labial openings in the beak, through which the dental epithelium (lamina) invades the cavity of the highly mineralized beak. Reduction in the number of replacement tooth positions coincides with the development of only four labial openings in the pufferfish beak, restricting connection of the oral epithelium to the dental cavity. Our data suggest the spatial restriction of dental regeneration, coupled with the unique extension of the replacement dental units throughout the jaw, are primary contributors to the evolution and development of this unique beak-like dentition.
Given the known conservation of developmental signaling, morphological novelties raise important questions about their evolutionary and developmental origin (1). Vertebrate groups exhibit profound dental morphological variation, making teeth interesting models for the study of evolutionary developmental biology (2). Dental morphology is highly specialized to diet, and prey preference can shift throughout ontogeny. The ability to regenerate and replace teeth provides the opportunity for a change in dental morphology to occur between dental generations (3). This change in morphology allows niche partitioning, with different dental morphologies specialized to dietary preferences, which shift with age. Understanding the developmental basis of regeneration is therefore essential to understand the evolution of dental morphological variation.
Our understanding of tooth development is based largely on study of the mouse, which continuously renews its incisors but is unable to produce new tooth generations (monophyodonty) (4). In contrast, most vertebrates exhibit lifelong dental replacement (polyphyodonty) (5). Recently there has been a bid to understand such regenerative capabilities in an attempt to develop new clinical applications in humans, which lose the ability to regenerate teeth after two generations (diphyodonty) (6–9). Additionally, teeth are also useful for the evolutionary developmental study of morphological novelty; dentitions vary substantially in tooth number, shape, and generation rate but form from a highly conserved ancestral developmental framework (2, 5). To expand our knowledge of developmental dynamics and the evolution of novelty, we need to extend our research of development to new models exhibiting diverse phenotypes.
Teleosts are exceptionally diverse, as reflected in the morphological variation in their dentitions (4). The extremes of dental diversity are exemplified within the teleost order Tetraodontiformes (10–13). Four families of the Tetraodontiformes [the Triodontidae (three-toothed pufferfishes), Molidae (ocean sunfishes), Diodontidae (porcupinefishes), and Tetraodontidae (four-toothed pufferfishes)] have evolved a diverse set of oral phenotypes with a superficially and developmentally constrained beak-like appearance that develops through the modification of the dental regeneration mechanism (14). These diverse and unique dentitions have facilitated the occupation of a broad range of dietary niches, including the acquisition of hard-shelled prey (15).
The ontogenetic transition to a beaked dentition from more typical first-generation teeth in pufferfishes (14) raises interesting questions regarding the regulation of tooth regeneration and the emergence of craniofacial diversity. General vertebrate dental regeneration proceeds via the activation of epithelial dental progenitors within a dental lamina (4, 9, 16), a region of invaginated oral epithelium where new tooth generations are initiated. In vertebrates that exhibit continuous or lifelong tooth regeneration, competent dental epithelium must be maintained (tissue homeostasis) in adults. Pufferfishes develop their teeth intraosseously within an encased dental cavity (12). Given that epithelial progenitors are required for dental regeneration to take place (4), it is unclear where these cells reside in pufferfishes, because the developing teeth remain separated from the oral epithelium by an osteodentine casing fused to the jaw. Here, we define tooth regeneration as the de novo cyclical formation of tooth units from a defined epithelial dental lamina that must be continuously maintained by a progenitor cell niche (17). In this context, and for clarity, the term “tooth replacement” refers to the structural formation and positional replacement of tooth units, whereas the term “tooth regeneration” refers to the developmental process by which teeth are replaced, i.e., through the maintenance of a progenitor-rich epithelial dental lamina for de novo tooth production. Therefore, in this context, replacement is the result of regeneration.
Broadly, this study seeks to understand how novelty of form manifests in pufferfishes and the ways in which the novel dental phenotype develops over ontogenetic time. The core questions investigated in this study are (i) where epithelial progenitors required for dental regeneration in the pufferfish reside and (ii) whether the banded dental morphology arise from multiple sequential tooth germs coalescing early in dental regeneration or from a single tooth germ that develops into an elongated band in each jaw quadrant, as previously hypothesized (14). The notion that the dental morphology arises from a single tooth germ implies a loss in the number of tooth-initiation sites during the transition from the first to second dental generations. Through a combination of gene/protein expression, cell lineage tracing, morphological analyses, and small molecule inhibition assays, we sought to analyze the developmental basis of pufferfish tooth regeneration and how this process might govern the ontogenetic formation of the beak-like dentition unique to pufferfishes. Here, because of various aspects of their husbandry and accessibility for laboratory-based experimentation, and to decipher elements of diversity within the pufferfish clade, we study four species of pufferfishes: the freshwater Malabar or Dwarf Pufferfish (Carinotetraodon travancoricus), the freshwater Arrowhead pufferfish (Pao suvattii, previously Monotrete suvattii), the freshwater Hairy pufferfish (Pao baileyi), and the marine Japanese grass pufferfish (Takifugu niphobles). Uncovering key developmental drivers of dental novelty within the Tetraodontidae, we shed light on how the complex novel dentition in this highly derived teleost lineage (18) develops from a highly conserved odontogenic system.
Results
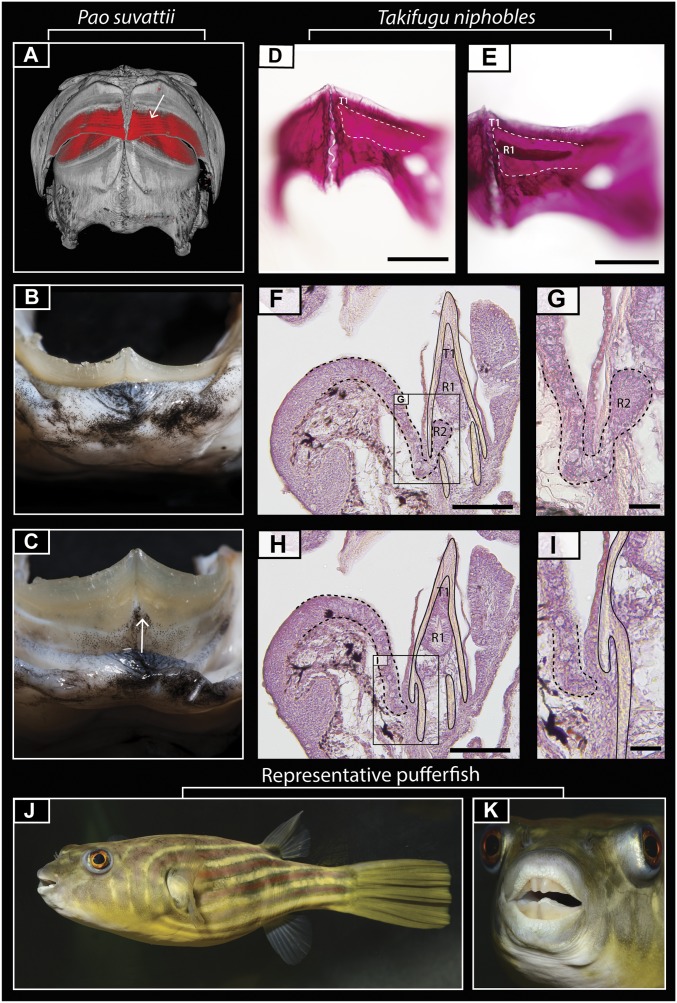
The unusual pufferfish beak is composed of four main units, one in each jaw quadrant. Each of these units is formed through the progressive accumulation of teeth, which are continuously replaced throughout life. Instead of teeth being shed, multiple generations of teeth stack together and are embedded within a compact osteodentine mass (Fig. 1A) (19), together forming the highly mineralized beak (Fig. 1 B and C). The oldest teeth are located at the top of the dentary, and the newest teeth develop at its base within a continuous (jaw-length) dental cavity (12). The dental units themselves are band-like in shape, with the teeth elongating along the length of the dental cavity from the parasymphyseal region (14). In most pufferfish species, a single tooth “band” develops in each jaw quadrant through dental generation (Fig. 1 A and E). These bands become connected into a single beak unit through interband osteodentine mineralization (14). A further osteodentine layer encases the dentine bands in both jaws and is confluent with the beak unit of the dentary in the lower and premaxilla in the upper jaw (12).
Fig. 1.
Pufferfish dental morphology. (A) Reconstructed microCT scan of adult P. suvattii reveals a banded dentition encased in osteodentine. (B and C) Digital photos show the fleshy lip of P. suvattii and its attachment to the beaked dentition at the fused beak/dentary boundary. A cleft can be seen at the symphysis of the jaw, with the attachment of the labial oral epithelium to the beak following the contour of the cleft (white arrow in C). (D and E) Alizarin red staining of early embryonic T. niphobles samples reveals a single developing band in each jaw quadrant. (F–I) Hematoxylin-stained sagittal paraffin sections reveal a gubernacular opening at the osteodentine/mandibular boundary in each jaw quadrant (F and G) (depicted by dashed lines in H) that is absent in serial sections of the adjacent areas (H and I). G and I are close-up images of the boxed regions in F and H, respectively. In the histological sections, a continuous stream of epithelium connects the oral epithelium to the regenerating teeth (R1 and R2) within the dental cavity. (J and K) Digital photographs of Tetraodon lineatus showing the overall morphology of a “typical” pufferfish. R1-2, replacement tooth generations; T1, first tooth generation. (Scale bars: 250 µm in D and E; 100 µm in F and H; 50 µm in G and I.)
The generation of new teeth requires a source of epithelial progenitors (16, 17). To maintain this ability, polyphyodonts must retain a population of these cells throughout life. Epithelial dental progenitors have been localized to the dental lamina in polyphyodonts (7, 8, 16, 17, 20), with the microenvironment maintaining these cells in an otherwise dynamic and proliferative system. Pufferfishes regenerate and replace their teeth intraosseously, within the dental cavity (12). This region is isolated from the oral epithelium (Fig. 1), but epithelial cells within the dental cavity are a prerequisite for regeneration (21), so the question arises: Where are the dental progenitors located in this system? There are two mechanisms that could lead to the presence of dental epithelium in the dental cavity: (i) the preceding tooth generation might provide a source of epithelial cells for newly developing teeth in a cervical loop-like fashion, as in the mouse (22), or (ii) gaps within the beak casing might enable epithelium from the externally situated dental lamina to enter the dental cavity. To understand the temporal developmental dynamics of the pufferfish replacement dentition and to determine the location of the epithelial progenitors, we investigated the development of its dentition in sagittal sections.
Source of Epithelial Dental Progenitors in Intraosseous Pufferfish Dental Regeneration.
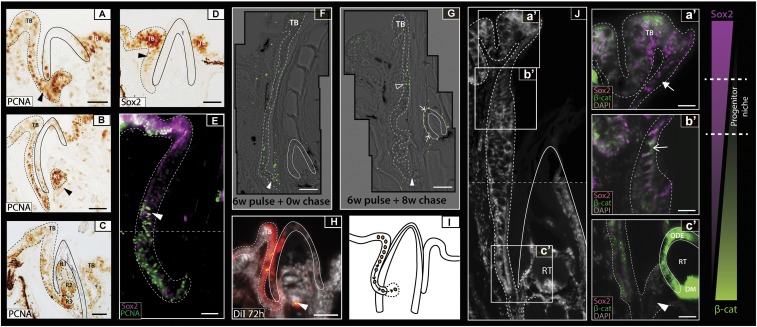
Hematoxylin staining of juvenile sagittal sections (Fig. 1 F–I) and reconstructed micro-CT scans (Fig. 1A) of adult pufferfishes reveal a gubernacular opening within the osteodentine beak casing of each jaw quadrant through which the juxtaposed labial oral epithelium connects to the dental cavity (Fig. 1 F and G). This opening suggests that a connection between the dental cavity and the oral epithelium is maintained throughout development. Proliferative cell nuclear antigen (PCNA) immunohistochemistry during second-generation tooth initiation highlights high levels of cellular proliferation within the oral epithelium (Fig. 2 A–C). Notably, a continuous stream of proliferative cells connects the labial oral epithelium and the developing tooth bud in the dental cavity (arrowhead in Fig. 2A); this in-folded labial epithelial sheet is the pufferfish dental lamina (Fig. 2 A–C). Emerging tooth generations bud from the distal (relative to the oral surface) end of the dental lamina (Fig. 2 A–C). Developing teeth are then positioned orally relative to their initiation site and continue morphogenesis inside the confines of the dental cavity (R1–R3 in Fig. 2C).
Fig. 2.
Localization of a dental progenitor cell niche within the pufferfish dental lamina. (A–C) P. baileyi PCNA immunohistochemistry reveals high levels of cellular proliferation within the oral epithelium. As replacement teeth progress from late-initiation (A) to morphogenesis (C), the new tooth generation (R1) buds from the dental lamina (B). Successive rounds of replacement show that the dental generations stack on one another within an enameloid outer casing (black line) (C). (D) Sox2 immunohistochemical labeling during dental replacement initiation depicts high levels of Sox2 within both the developing taste buds (TB) and the dental progenitor site located within the labial oral epithelium (dental lamina) (black arrowhead). (E) Double immunofluorescence treatment for Sox2/PCNA in T. niphobles shows low levels of PCNA expression within the Sox2+ cells of the presumptive dental progenitor niche and cells within the aboral dental lamina exhibiting high levels of PCNA. The horizontal dashed line depicts image stitching of two adjacent images. White arrowhead marks region of overlapping PCNA/Sox2 expression. (F) BrdU pulse/chase experiments (0.2 mM) show the incorporation of BrdU into dividing cells after 6 wk of treatment, with high levels of incorporation noted in the distal dental lamina next to the base of the beak (white arrowhead). (G) After a further 8-wk chase, label-retaining cells were found in the most superficial dental lamina cells (open arrowhead) but not in the distal dental lamina (white arrowhead). Label-retaining cells found in the dental epithelium of the developing tooth are indicated by a white arrow. Images in F and G are composites of multiple images taken at high magnification and stitched together. (H) DiI labeling of the labial oral epithelium in P. suvattii highlighted this region as a presumptive source of dental progenitor cells. DiI was detected within the outer dental epithelium of the tooth (white arrowhead) 72 h post DiI treatment. (I) As summarized in a schematic representation, we observed a continuous field of Sox2+ cells between the labial taste bud and the dental progenitor site, with cells from the latter migrating and contributing to the new dental generations. Black arrows represent the direction of cell movement. (J) Sox2/ABC double immunohistochemical labeling on adult C. travancoricus highlights epithelial Sox2+/ABC− (a′, white filled arrow), Sox2+/ABC+ (b′, white arrow), and Sox2−/ABC+ (c′, white arrowhead) regions within the dental lamina. Coexpression of these markers marks the site of activation of putative dental progenitors within the oral epithelium. Dashed line across (J) depicts image stitching of two adjacent images. Images are orientated with labial to the left and oral to the top. The dotted line in all images depicts the boundary of the oral epithelium and the end of the dental lamina. DM, dental mesenchyme; ODE, outer dental epithelium; R1–3, replacement tooth generations; RT, regenerating tooth; TB, labial taste bud. (Scale bars: 25 µm in A–E; 20 µm in F, a′–F, c′; 50 µm in G and H; 15 µm in I.)
The role of the dental lamina in the regeneration of teeth is well understood (2, 16, 17). Sox2 (Sex-determining region Y-box 2) is a transcription factor known for its role in maintaining cell pluripotency and stem cell renewal (23). It is expressed in epithelial dental progenitors in the dental lamina of all polyphyodont vertebrates (2, 4, 16, 17) and is thought to regulate dental progenitor cell state (16). Therefore we investigated its expression during pufferfish tooth formation to determine the location of epithelial dental progenitors in pufferfishes. Sox2 immunohistochemistry assays during second-generation dental initiation in pufferfishes highlight Sox2 expression throughout the oral epithelium and notably in the taste buds (Fig. 2D). Consistent with expression patterns described in other polyphyodonts (5, 16), we note Sox2 within the pufferfish dental lamina, with strongest expression in this region proximal to the oral surface (arrowhead in Fig. 2D). Double PCNA/Sox2 immunohistochemistry assays reveal the expression of PCNA in the aboral dental lamina cells except where Sox2 is highly expressed (Fig. 2E). These Sox2+/PCNA− cells are potentially quiescent. The continuous stream of Sox2 expression between presumptive taste bud and tooth domains (Fig. 2E) suggests that teeth and taste buds in pufferfishes develop from a common early oral epithelium, as previously shown in both cichlids (24) and sharks (17).
Sox2 alone does not confer progenitor cell identity. Therefore, we sought to identify regions of the oral epithelium containing slow-cycling cells, using BrdU pulse/chase experiments in adult C. travancoricus (Fig. 2 F and G). Carrying out this experiment in adults of this species allows the separation of rapid cellular cycling occurring during embryogenesis from that specific to lifelong cyclical dental regeneration and replacement. After a 6-wk BrdU pulse we found a high signal within the distal tip of the dental lamina (white arrowhead in Fig. 2F) corresponding to the PCNA-expressing region from which we observed new teeth developing in the pufferfish embryo of P. baileyi (Fig. 2 C and E). Although the C. travancoricus samples were treated for 6 wk, not all cells were labeled after the pulse period, perhaps because these cells were terminally differentiated and thus nondividing in the adult. After an 8-wk chase period, with samples left to develop in the absence of BrdU, signal was lost within the distal tip of the dental lamina (white arrowhead in Fig. 2G), indicating cellular proliferation or migration out of this region. The presence of BrdU within the dental epithelium of the succeeding tooth generation after an 8-wk chase (arrows in Fig. 2G), but its absence immediately after a 6-wk pulse (Fig. 2F), suggests that cells from the dental lamina contribute to the dental epithelium of the replacement dentition. Furthermore, after the chase period, there are label-retaining cells within the dental lamina region nearest the oral surface, adjacent to the first taste bud (open arrowhead in Fig. 2G). These cells are either slow cycling or have become terminally differentiated after the incorporation of BrdU during the pulse period. Given the expression of Sox2 and lack of PCNA in the equivalent dental lamina region in the pufferfish embryo (Fig. 2E), we suggest these label-retaining cells are part of a slow-cycling epithelial progenitor cell population within the dental lamina.
To establish the cellular dynamics within the dental lamina, the fluorescent lipophilic dye 1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) was administered to cells within the taste/dental lamina epithelium of the pufferfish before the initiation of dental regeneration. Seventy-two hours after labeling, we observed DiI within the dental epithelium of the developing second-generation tooth band (Fig. 2H). This result suggests that superficial taste bud/dental lamina epithelium, located labial to the beak, contributes cells for dental regeneration via migration through a gubernacular opening into the intraosseous dental cavity of the pufferfish beak (Fig. 2I).
Canonical Wnt Signaling Is Active In Dental Progenitors.
Canonical Wnt signaling is a vital and developmentally diverse set of pleiotropic molecules performing various tissue-specific functions (25). During polyphyodont odontogenesis, canonical Wnt signaling acts as a primary activator of epithelial dental progenitors in the dental lamina (26). Sox2/activated β-catenin (ABC) double immunohistochemistry assays in adult C. travancoricus reveal their coexpression in a region of the dental lamina epithelium (Fig. 2 J, b′), suggesting the involvement of canonical Wnt signaling in the activation of Sox2+ putative dental progenitors. Coupled with results from the DiI assay, the absence of ABC in a subset of Sox2+ dental lamina cells provides further evidence for a discrete epithelial progenitor niche within this region (Fig. 2 J, a′). There is an intriguing relationship between the expression of Sox2 and ABC throughout the dental lamina: ABC expression increases and Sox2 immuno-localization decreases toward the site of tooth initiation (Fig. 2 J, a′–c′). These results uncover an opposing gradient of expression of ABC and Sox2, suggesting a genetic compartmentalization of the dental lamina. The lamina therefore is a highly dynamic and complex cell layer that requires further developmental study and functional investigation.
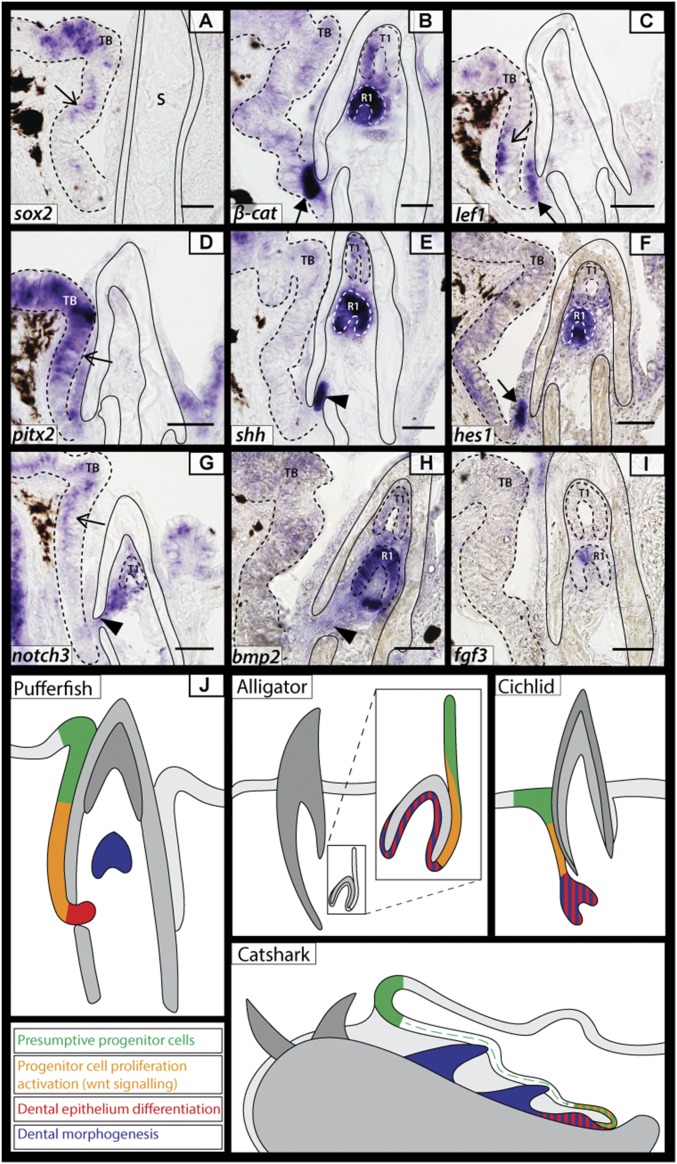
Concordant with Sox2/ABC immunohistochemistry, we observe expression of the Wnt effector lef1 (Fig. 3C) in the dental lamina in a pattern similar to the expression of ABC (Fig. 2J) and a distal subset of Sox2/sox2-expressing dental lamina cells (Figs. 2 D and E and 3A). Here, Sox2/ABC coexpression and lef1 expression in the dental lamina suggest a conserved role for canonical Wnt signaling during preinitiation progenitor regulation, in both embryo (Fig. 3) and adult (Fig. 2J) pufferfishes, suggesting important genetic and cellular maintenance of dental progenitor populations from embryo to adult in a continuously regenerating dentition.
Fig. 3.
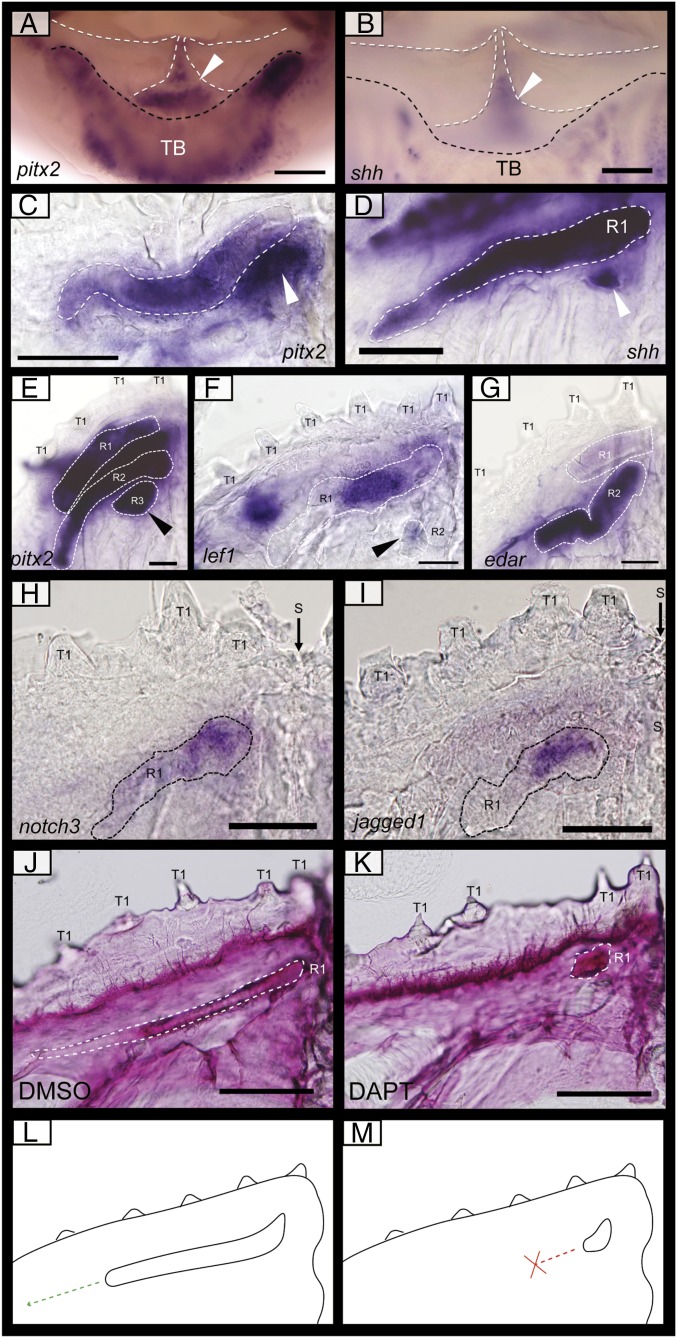
Conserved odontogenic signaling regulates dental regeneration in pufferfish. (A–I) Expression of well-documented odontogenic markers belonging to Sox (sox2, A); canonical Wnt signaling (β-catenin, B; lef1 C); Pitx (pitx2, D); Shh (E), Notch (hes1, F; notch3 G); Bmp (bmp2, H); and Fgf (fgf3, I) gene families in T. niphobles embryos. The thin arrow marks the site of presumptive dental progenitors, with expression of pitx2 (D), lef1 (C), and sox2 (A) within this region. The thick arrow marks the distal end of the dental lamina. The filled arrowhead highlights an opening within the osteodentine beak casing through which new odontogenic cells bud from the dental lamina. β-cat (B), shh (E), hes1 (F), notch3 (G), bmp2 (H), and fgf3 (I) are all expressed within the epithelium of the latest developing teeth. (J) A diagrammatic illustration of odontogenetically similar structures in various polyphyodonts [pufferfish, alligator (7, 16), cichlid (5), and catshark (43)]. Four main developmental regions are highlighted: presumptive dental progenitors, progenitor cell activation marked by the coexpression of Sox and Wnt signals, dental epithelium differentiation marked by the up-regulation of various developmental genes at the distal tip of the dental lamina and the growth of a tooth bud, and dental morphogenesis. The dotted line depicts the boundary of the oral epithelium and the end of the dental lamina. All images were taken from 14-µm sagittal paraffin-embedded sections. A, B, E, F, H, and I are from T. niphobles embryos at 50 dpf. C, D, and G are from embryos at 32 dpf. R1-2, replacement tooth generations; S, suture; TB, labial taste bud. (Scale bars: 50 µm in B–D, F, and G; 35 µm in A, E, H, and I.)
Initiation of Dental Regeneration Appears Conserved During the Development of the Pufferfish Beak.
Despite extensive vertebrate dental diversity, developmental regulation of dental regeneration is highly conserved (2). Several members of the Fgf, Hedgehog (Hh), Wnt, Bmp, and Notch signaling pathways play a role in the development and regeneration of vertebrate teeth (2, 5, 27). We selected candidates from each of these major signaling pathways and examined the expression of their pufferfish homologs to determine if their function was also conserved in the highly derived beaked dentition (Fig. 3). In pufferfishes, new tooth generations form from the distal, intraosseous tip of the dental lamina. Following the proliferation and migration of cells from the putative preinitiation progenitor cell population in the dental lamina (Fig. 2), we observe the expression of lef1 (Fig. 3C), ABC/β-catenin (Figs. 2J and 3B), the Notch target hes1 (Fig. 3F), and notch3 (Fig. 3G) restricted to a small pocket of cells within the dental lamina distal tip at the junction between the oral epithelium and the beak. These expression data identify both Wnt and Notch signaling as potential regulators of dental initiation at this site. As dental development progresses, cells of the dental lamina invade through gubernacular openings in the beak into the dental cavity where tooth morphogenesis takes place. shh is up-regulated within the dental epithelium as it extends through into the dental cavity and remains expressed in the dental epithelium throughout morphogenesis (Fig. 3E).
Bmp and Fgf signaling also have been described extensively during dental morphogenesis and are involved in reciprocal interactive signaling between the dental epithelium and neural crest-derived mesenchyme (2, 5, 27). We found fgf3 expression restricted to the apical tip of the dental epithelium of the developing tooth (Fig. 3I, R1), with bmp2 expressed throughout the dental epithelium and within the underlying condensed dental mesenchyme (Fig. 3H, R1). These expression patterns are comparable to those observed in cichlid fishes, with fgf3 and bmp2 active in both the dental epithelium and dental mesenchyme (5). These results highlight that, despite the highly derived dental morphology of the pufferfish beak, Notch, Wnt, Fgf, Hh, and Bmp signaling appear to maintain their roles in dental regeneration.
Restriction in Dental Replacement Takes Place Between the First and Second Dental Generations.
Pufferfishes typically develop a first-generation dentition composed of multiple teeth along the jaw (14), much like the dentitions observed in other larval and juvenile teleosts. The second and subsequent tooth generations in pufferfishes undergo a major transition and do not follow the pattern set by the first generation. Four tooth bands, one for each jaw quadrant (14), form per dental generation, raising the question whether these teeth form through the coalescence of teeth initiated at multiple sites along the jaw or through the loss of dental replacement at all but four sites. There is interesting morphological variation in the dentition among pufferfish species. Unlike in most pufferfish species, including P. suvattii (Fig. 1A), reconstructed T. niphobles microCT scans reveal discontinuities within the tooth band of each dental generation (Fig. S1). These discontinuities may indicate that potentially multiple teeth form with each round of replacement, instead of a single elongated dental unit in each quadrant of the jaw. However, given that at embryonic stages T. niphobles teeth develop as a single continuous banded unit (Fig. 1 D and E and Fig. S1), this observation may be an artifact of mineralization leading to multiple tooth units from a single initial tooth germ or of preservation resulting in the break-up of a continuous band. Investigating the early development of the banded teeth in T. niphobles will help clarify the way in which the teeth are replaced.
Fig. S1.
Takifugu dental morphology. (A and B) Reconstructed microCT scans of adult T. niphobles reveal discontinuous dentine bands (white arrows in A) encased in osteodentine. (C–F) Virtual slices through the dentition at both symphyseal (C and D) and lateral (E and F) regions reveal a single gubernacular opening (white arrow in F) in each jaw quadrant. An asterisk in D and F marks the dental cavity; red cross in D marks the absence of a gubernacular opening.
To understand the developmental basis of the morphological and functional shift observed in the pufferfish dentition, we examined the expression patterns of odontogenic markers during multigenerational morphogenesis using whole-mount in situ hybridization. This method enables a mediolateral view of gene-expression patterns, providing detail during dental initiation at multiple sites across the jaw simultaneously. Dissected lower jaws of pufferfish embryos reveal the expression of pitx2, shh, lef1, and edar throughout the developing tooth bands during morphogenesis (Fig. 4 C–G). These markers have previously been shown to play vital roles during differentiation and morphogenesis in other vertebrate dentitions (28–31). We observed the expression of these four markers in a single banded unit associated with the continuous and elongated dental epithelium for each tooth generation, thus suggesting that each band represents a single, highly enlarged and elongated tooth and is not formed through the coalescence of multiple teeth across the jaw.
Fig. 4.
Gene-expression patterns during dental regeneration morphogenesis and chemical inhibition of Notch signaling through small-molecule treatments. Whole-mount RNA in situ hybridization of the lower jaws of P. baileyi at 57 dpf (A and B and E and F), 53 dpf (H and I), 46 dpf (G), and T. niphobles at 50 dpf (C and D), and P. suvattii at 26 dpf (J and K). (A and B) Images taken from above the lower jaw depict expression of pitx2 (A) and shh (B) in the labial epithelium at the jaw symphysis (white arrowhead). (C–I) Images of a single lower-jaw quadrant, with the expression of pitx2 (C and E), shh (D), lef1 (F), edar (G), notch3 (H), and jagged1b (I) in the developing teeth illustrated through dotted lines (C–G). New tooth units can be seen initially developing (C–F, arrowhead) at the symphysis of the beak (right of image). (J and K) Alizarin red staining of P. suvattii embryos after treatment with 50 μM DAPT during initiation of the second-generation dentition for 72 h, followed by a 2-wk recovery period (K), and control sample treated with 1% DMSO (K). Staining reveals the mineralization of the second-generation tooth (R1, white dotted line), with the control specimens (J) elongating laterally throughout the jaw (n = 5/5). DAPT treatment (25 μM) (K) resulted in the loss of dental elongation, with the mineralized tooth restricted in size at its site of initiation (n = 7/7). (L and M) Schematic representation of the phenotypes observed in the DMSO and DAPT treatments described above. R1–3, replacement tooth generations; S, suture; T1, first tooth generation. (Scale bars: 100 µm in A–F. 50 µm in G–K.)
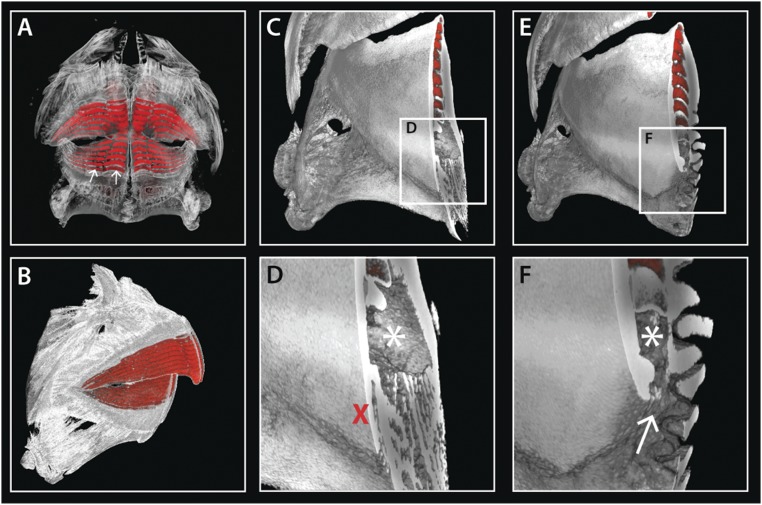
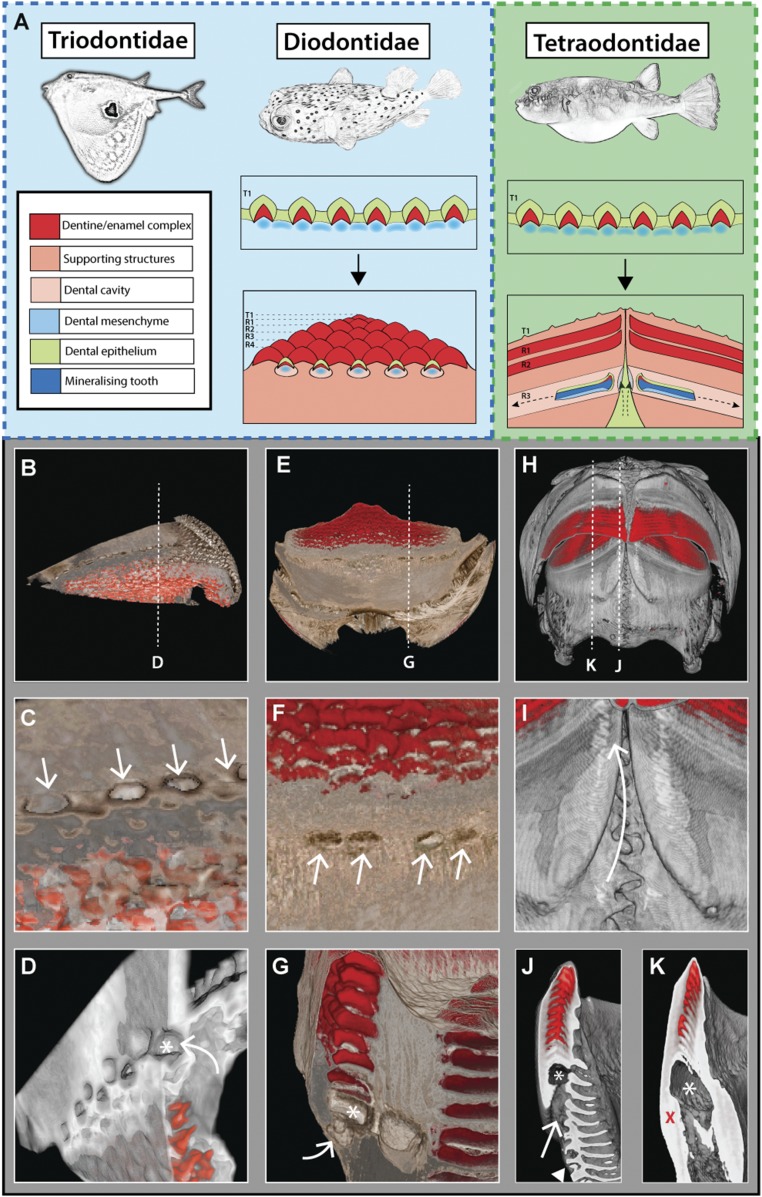
With further examination of histological serial sections in the pufferfish embryo (Fig. 1 F–I) and of reconstructed CT scans in the adult (Fig. 5 H–K), we observed four gubernacular openings, one in each jaw quadrant, restricted to the parasymphyseal region. These openings connect the developing intraosseous dental organ with the external dental lamina (5). As development progresses and generations of teeth accumulate, the pufferfish dentition becomes increasingly mineralized. The multiple tooth generations become entirely encapsulated by osteodentine, but these four openings, one in each jaw quadrant, remain throughout adulthood. Other members of the Tetraodontiformes, such as the Triodontidae and Diodontidae, form beaks superficially similar to those of pufferfishes. However, the triodontid and diodontid dentitions are formed through the replacement of all of the primary tooth sites. In this instance we observe gubernacular openings associated with each replacement tooth site (Fig. 5 C and F). These results provide evidence that the number of teeth replaced is intimately linked to the number of gubernacular openings. It is through these openings that the dental lamina is able to access the developing tooth within the dental cavity.
Fig. 5.
(A) Schematic highlighting the modes of dental replacement in Triodontidae, Diodontidae, and Tetraodontidae. Although the development of the first-generation teeth is conserved, the modes of dental replacement differ dramatically. R1–R4, replacement tooth generations; T1, first dental generation. Reconstructed microCT scans of Triodontidae (B–D) and Diodontidae (E–G) reveal gubernacular openings (C and F, white arrows) within the labial surface of the jawbone. Virtual sagittal sections through these sites show an open connection (white arrow) between the labial surface and the dental cavity (marked by an asterisk) (D and G). In Triodontidae and Diodontidae (B–G), gubernacular openings can be seen associated with multiple tooth sites along the jaw. MicroCT scans of P. suvattii (H and I) illustrate an intraosseous, banded dentition, with virtual serial slices through the dentition at the parasymphyseal region (J) and adjacent region (K) revealing gubernacular openings restricted to the parasymphyseal region (white arrow in I and J, red cross in K), with a single opening in each jaw quadrant. These openings connect the site of dental lamina attachment (white arrow in I) with the dental cavity (marked by an asterisk). The white arrowhead in J marks the interdigitating jaw suture observable at the symphysis.
The oral epithelium of pufferfishes is attached to the mandible at the junction of the dentition unit and the underlying bone (Fig. 1C). At the jaw symphysis, the left and right halves of both upper- and lower-jaw beak are separated by a cleft (Fig. 5I). The close association of the oral epithelium follows the contour of the beak, with the gubernacular openings positioned toward the top of the cleft (arrow in Fig. 5 I and J). Interestingly, we found both pitx2 and shh expressed in the oral epithelium associated with the symphyseal cleft (Fig. 4 A and B), corresponding positionally to the distal dental lamina expressing β-catenin, shh, pitx2, and hes1 (observed in section; Fig. 3). shh and pitx2 together demarcate the sites of dental initiation along the jaw (14, 32). Their expression restricted to the symphyseal epithelium may be important in regulating the reduction in tooth site number observed between the first and second dental generations (14). Furthermore, during the early growth stages of tooth formation, we observed tooth buds restricted to either side of the mandibular midline (symphysis), with one initiatory unit in each jaw quadrant, identified early in development through the expression of pitx2, shh, and lef1 within the newly developing teeth (arrowhead in Fig. 4 C–F). This observation provides further evidence that pufferfishes exhibit a loss in dental initiation at all but four parasymphyseal initiation sites during the transition from first to second dental generations (14).
Inhibition of the Notch Signaling Pathway Leads to Stunted Growth of the Elongated Banded Dentition.
To determine how the highly derived elongated bands in the adult replace the relatively stereotypical first-generation teeth of pufferfishes, we sought to perturb functionally the developmental pathways known to be involved in tooth morphogenesis in other vertebrates. The Notch receptor and ligand, notch3 and jagged1b, respectively, are both expressed within the tooth-band epithelium during early morphogenesis and subsequent differentiation (Fig. 4 H and I). Given the observed Notch activity in the developing tooth and its importance for normal morphogenesis in developing mice teeth (33), we sought to determine whether changes in Notch signaling contribute to tooth elongation in pufferfishes through pathway inhibition with the small molecule inhibitor of γ-secretase complex, DAPT (Fig. 4 J–M) (5).
Embryos at 19 d post fertilization (dpf) were treated with 25 µM DAPT for a 72-h period coincident with the emergence of the second-generation dentition. During this period the dentition would normally transition from the unicuspid first-generation to the banded second-generation dentition (14). After treatment, embryos were allowed to recover for a 14-d period and then were screened for morphological shifts. Control embryos (treated with 1% DMSO) underwent normal dental replacement, with a single elongated dentine band mineralizing throughout the length of the jaw quadrant (n = 5/5) (Fig. 4J). In contrast, after DAPT treatment, dental band elongation was inhibited (n = 7/7). We observed a single truncated mineralized tooth unit, which terminated precociously near the parasymphyseal site of tooth initiation (Fig. 4K), suggesting that Notch signaling is required for the normal elongation of the replacement tooth units in pufferfishes. Furthermore, these results are in line with our idea of a single initiatory site of dental replacement in each jaw quadrant in pufferfishes, with elongation of the dental unit underlying the banded tooth morphology.
Discussion
Our results show that the primary basis of the pufferfish beak is a loss of tooth regeneration and therefore unit replacement at all but the four most parasymphyseal tooth sites. This loss occurs during the transition between the first and second dental generations (14). The developmental initiation of dental regeneration at the parasymphyseal sites in pufferfishes remains highly conserved with other polyphyodonts, despite the unique final morphology. Finally, the subsequent elongation of the dental unit leads to the banded morphology of the dentition.
The prerequisite for the formation of all vertebrate dentitions is an embryonically active dental lamina. In polyphyodont species the dental lamina must be regulated and maintained to support the production of further tooth generations (34). Pufferfishes form their teeth intraosseously and develop a dentition entirely confluent with the supporting jaw-bone, raising the question of where epithelial dental progenitors reside in a system in which the developing dentition has become spatially separated from the oral epithelium. Our Sox2/PCNA expression data and DiI cell-tracking data (Fig. 2) show that, despite the unique morphology of the Tetraodontidae beak, epithelial dental progenitors are found within the dental lamina, as in cichlids (24). The physical separation of the dental lamina from the dental cavity therefore requires a permanent connection between the two through the mineralized beak/jaw unit.
It is common in teleosts to confine epithelial cellular input of the dental lamina to openings for site-specific induction of new tooth generations. For example, in the oral jaws of cichlid fishes each functional tooth position has a neighboring gubernacular opening that allows the transfer of dental epithelial cells from the surface epithelium into the bony cavity of the jaw to initiate tooth regeneration (5). In addition to pufferfishes, other members of the Tetraodontiformes, i.e., the Molidae, Triodontidae, and Diodontidae, have also evolved beaked dentitions. The Molidae lose the ability to regenerate their teeth throughout ontogeny and are thought to develop a beak through mineralization of the jaws (12). In contrast, the Triodontidae and Diodontidae develop beaked dentitions superficially similar in shape to that of the pufferfishes; however, these dentitions form through the replacement of all the primary tooth sites (Fig. 5). Interestingly, in these systems we identified gubernacular openings associated with each tooth site that undergoes replacement. In systems that develop teeth intraosseously, we propose that the number of dental initiation sites is intimately linked with the number of sites at which the dental lamina is able to access the internal dental cavity through these openings (Fig. 5). Given the phylogenetic position of pufferfishes within the tetraodontiform lineage (18), it is reasonable to assume that the evolution of a beak composed of multiple teeth across the jaw margin (i.e., Triodontidae and Diodontidae) (Fig. 5) preceded the loss of dental replacement observed in pufferfish. Several changes must have taken place during the transition to a pufferfish beak: (i) the loss of gubernacular pores along the jaw margin at all but four sites; (ii) the restriction of dental lamina extension into the dental cavity to these pores; (iii) the establishment of an extended dental cavity that allows the growth of a banded dentition; and (iv) an elongation of the dentition. It is likely that selective pressure on feeding has driven this morphological change, leading to a change in the feeding biomechanics in the pufferfish. Further comparative study of the biomechanics in the different beaks observed in the tetraodontiform lineage could elucidate the driving force behind this evolutionary morphological novelty.
Pufferfishes have evolved a highly derived dental morphology through the subtle modification of a conserved teleost bauplan. Although the final morphology is unique, the developmental regulation shares extensive features with other polyphyodont vertebrates. We identify a putative progenitor cell niche within the dental lamina with Wnt signaling notably active in a subset of Sox2+ cells. This activity is demonstrated through the expression of ABC and lef1 and suggests a conserved role for Wnt signaling during the initiation of dental regeneration. Wnt signaling has been identified as a key regulator of dental regeneration in polyphyodonts, with its up-regulation leading to ectopic tooth germ formation in the dental lamina of snakes (35). Ectopic Wnt signaling also leads to supernumerary teeth in mice (36), with up-regulation specifically in Sox2+ dental epithelial cells sufficient to generate odontomas (37). Furthermore, active Notch, Hh, Bmp, Fgf, and Wnt signaling during dental differentiation and morphogenesis further highlights the conservation of developmental signaling during tooth development across gnathostome vertebrates. Our findings demonstrate the role that regeneration plays in the evolution of morphological novelty and how, despite a significant morphological shift, developmental signaling during regeneration remains highly conserved.
Although pufferfishes have clearly used primarily conserved odontogenic pathways, the development of a truncated tooth bud following Notch perturbation provides insights into the development of the elongated dental morphology. Our results support the hypothesis that each of the multigenerational tooth bands forms from a single dental initiatory site located on either side of the jaw symphysis (Fig. 4 J–M). The shift from a jaw-length tooth band to a small restricted tooth bud when Notch signaling is inhibited in pufferfish is remarkably similar to the mouse incisor, which develops from an elongated placode that separates into multiple smaller placodes following bmp or activin manipulation (38). However, in contrast to mice, the formation of a single, restricted symphyseal tooth, rather than multiple separated teeth, after Notch inhibition suggests that the transition from a Diodontidae/Triodontidae-like beak to a pufferfish beak did not involve the coalescence of dental placodes. Notch signaling may play an important role in the elongation of the symphyseally restricted placode. Notch is also vital for both mouse molar and incisor morphogenesis, mediating signals between the dental epithelium and the stratum intermedium that regulate both odontoblast and ameloblast differentiation in the mouse (33). When the Notch signaling pathway is disrupted, the size of mouse incisors is reduced, and there are defects in both enamel and dentine deposition (33, 39). In cichlid fishes, the inhibition of the Notch signaling pathway results in multiple phenotypes including defective mineralization of the cusps, a reduction in cusp number, and the loss of tooth replacement at multiple positions along the tooth row (5). Our intriguing result from the Notch inhibition assay could reflect either (i) the loss of an extended dental placode within the dental cavity or (ii) aborted ameloblast/odontoblast mineral secretion. Either of these factors would be expected to result in the truncation of the tooth band after DAPT treatment.
Pufferfishes provide a rare opportunity to study a vertebrate model that offers a maturing developmental system from embryo to adult, in which the processes of development and tissue homeostasis of a regenerative dentition can be investigated. The Tetraodontiformes are an extraordinarily diverse group of teleost fishes (13), ideal for the study of morphological novelty. Through the investigation of dental development, we show the existence of a highly conserved polyphyodont developmental system involved in the formation of the unique pufferfish dental morphology. Although the regulation of tooth initiation is highly conserved, the loss of dental replacement at all but the parasymphyseal tooth sites, coupled with elongation of the dental unit, has enabled pufferfishes to develop a dentition unlike any other.
Although mammals have evolved the ability to renew their dentition through continuously growing teeth (40), they have generally lost the ability to regenerate the dental unit more than once. During mammalian tooth development the dental lamina degrades, ultimately leading to a loss of polyphyodonty (34). Although pufferfishes have a spatially restricted ability to regenerate their dentition, polyphyodonty is sustained by the maintenance throughout life of a dental lamina that houses the progenitor cells required for dental regeneration to continue, thus enabling this unique dental morphology to arise. Pufferfishes represent a unique example of how the spatial restriction of dental regeneration combined with the continuous maintenance of polyphyodonty has led to morphological innovation. Further developmental study of morphological novelties resulting from the process of regeneration will provide insights into how gene-regulatory networks can be both conserved and altered while allowing novelty to develop.
Materials and Methods
Animals.
P. baileyi embryos were raised to the required stage in a recirculating aquarium system at 20–23 °C at the Natural History Museum, London. Adult P. suvattii and C. travancoricus were maintained in a recirculating aquarium system at 26 °C at the University of Sheffield. P. suvattii embryos were collected and raised to the required stage at 26 °C. Fertilized T. niphobles eggs were obtained through induced insemination of adults collected on Arai beach, Kanagawa prefecture, Japan. Embryos were raised to the desired stage in fresh seawater at 20 °C. Adult C. travancoricus and larval P. suvattii, P. baileyi, and T. niphobles were anesthetized with MS-222 and fixed in 4% paraformaldehyde overnight at 4 °C. Samples then were dehydrated through a graded MeOH series and stored at −20 °C.
CT Scanning.
Specimens of T. niphobles (accession no. 1905.2.4.493) and P. suvattii preserved in EtOH were obtained from collections at the Natural History Museum, London. Samples were scanned using the Metris X-Tek HMX ST 225 CT scanner (Imaging and Analysis Centre, Natural History Museum, London). 3D volume renderings of the microCT scans were carried out using DRISHTI (https://github.com/nci/drishti).
Histology and Clearing and Staining.
For histological study, samples were decalcified with 0.5 M EDTA in water for 24 h, further dehydrated in isopropanol for paraffin embedding, cleared with xylene, and subsequently embedded in paraffin. Sagittal paraffin sections (14 µm) were cut using a Leica RM2145 microtome. Slides were stained with 50% hematoxylin for 10 min. Stained slides were mounted with Fluoromount (Sigma) and imaged using a BX51 Olympus compound microscope fitted with an Olympus DP71 camera. Juvenile pufferfishes were cleared and stained with alizarin red (bone and dentine) according to the protocol in ref. 41.
DiI Lineage Tracing.
The labial oral epithelium of P. suvattii embryos at 19 dpf was superficially labeled with DiI (V22885; Thermo) using a microinjection capillary needle and aspirator tube assembly. Embryos were anesthetized in MS222 (60 mg/mL) in freshwater during treatment. Embryos then were raised for a further 3 d in freshwater before fixation and standard paraffin embedding and sectioning.
cDNA and Riboprobes.
Cloned cDNA sequences used to generate Digoxigenin (DIG)-labeled antisense riboprobes from P. suvattii, P. baileyi, and T. niphobles were identified through the genome database available from the International Fugu Genome Consortium (www.fugu-sg.org). cDNA clones for pufferfish shh, pitx2, sox2, β-catenin, lef1, jagged1b, hes1, fgf3, bmp2, notch3, and edar homologs were isolated through PCR, with forward and reverse primers designed from the Takifugu rubripes genomic sequence. PCR clones were ligated into pGEM-T-Easy Vectors (Promega) and used as a template for probe synthesis. DIG-labeled antisense RNA probes were synthesized through in vitro transcription of the PCR template with T7/SP6 RNA polymerase (Promega) and DIG RNA labeling mix (Roche).
Forward and Reverse Primer Sequences.
sox2:
5′-CCAGAGGAGGAAGATGGCGCAA-3′
5′-CATGTGTAGCCTGGTCTGGGC-3′
Shh:
5′-GAAGGCAAGATCACAAGAAACTC-3′
5′-ACGTTCCCACTTGATAGAGGAG-3′
pitx2:
5′-TCTATGAGGGAACCCTTGAATATAG-3′
5′-CTGCTTGGCTTTCAGTCTCAG-3′
β-catenin:
5′-CCCTGAGGAAGATGATGTGGACAA-3′
5′-ACAGTTCTGGACCAGTCTCTGGCTG-3′
lef1:
5′-CAGTCCCAAATACCAGATTCATATC-3′
5′-TCTTCTTCTTTCCATAGTTGTCTCG-3′
notch3:
5′-TCTGACTACACTGGAAGCTATTGTG-3′
5′-TTGCAGTCAAAGTTGTCATAGAGAC-3′
jagged1b:
5′-CACCTGCGTCTGTAAAGAAGGCTG-3′
5′-ACGACACCACGTACGCGGCG-3′
hes1:
5′-GACAGCCTCCGAGCACAGAAAGTC-3′
5′-AGTCAAACCGCTGGGACCACT-3′
fgf3:
5′-GTTGAATTTGTTGGATCCGGTTAG-3′
5′-TGACCTTCGTCTCTTAACTCTCTTG-3′
bmp2:
5′-TTAGAAGCTTTCACCATGAAGAGTC-3′
5′-TTCATCCAGGTAGAGTAAGGAGATG-3′
Edar:
5′-GTACTCCAAAGGGAAGTACGAAATC-3′
5′-AAGATCTTTCTCCTCCGACTCTG-3′
Immunohistochemistry.
Peroxidase-labeled section immunohistochemistry was carried out according to the manufacturer’s instructions (Abcam) using mouse anti-PCNA (1:5,000) (ab29; Abcam) and peroxidase-labeled anti-mouse IgG (1:250) (DAKO) or rabbit anti-Sox2 (1:500) (ab97959; Abcam) and peroxidase-labeled anti-rabbit IgG (1:250) (DAKO). The color reaction was carried out using 3,3′-diaminobenzidine-tetrahydrochloride (DAB) (DAKO). Rabbit anti-Sox2/mouse anti-PCNA and rabbit anti-Sox2/mouse anti-active β-catenin (1:500) (05-665; Merck) double immunofluorescence was carried out as described by Martin et al. (17). Goat anti-rabbit Alexa-Fluor 647 (1:250) (A-20721245; Thermo) and goat anti-mouse Alexa-Fluor 488 (1:250) (A-11-001; Thermo) secondary antibodies were used for immunodetection. Images were taken on an Olympus BX51 upright epifluorescent microscope.
Whole-Mount in Situ Hybridization.
Whole-mount in situ hybridization was performed according to ref. 42 with modified proteinase K treatment. Samples were treated for 1 h at room temperature with 1–10 µg/mL proteinase K depending on the developmental stage.
Section in Situ Hybridization.
Sagittal paraffin sections (14 µm) were obtained as previously described. Slides were deparaffinized with xylene, rehydrated, and superheated with 0.01 M citric acid in diethylpyrocarbonate (DEPC)-treated double-distilled H2O (pH 6) for 15 min. Slides were incubated in hybridization buffer [50% formamide, 5× saline sodium citrate (SSC), 500 mg/mL yeast tRNA, 50 mg/mL heparin, and 0.1% Tween-20, pH 6.0] containing the DIG-labeled antisense RNA probe at 61 °C overnight. The following day slides underwent stringent washes in 2× SSC containing 0.01% Tween-20 (SSCT) and 0.2× SSCT at 51 °C and 1-h incubation in blocking solution [2% Blocking Reagent (Roche) in maleic acid buffer containing Tween 20 (MABT)] at room temperature. Slides were antibody-labeled overnight at 4 °C with anti–DIG-ALP (0.2 µL/mL) (Roche). Following extensive MABT washes, slides were color-reacted with BM Purple (Roche) at room temperature until staining allowed the visualization of gene expression but minimum background. Slides then were washed and mounted with Fluoroshield with DAPI (Sigma), and images were obtained with a BX51 Olympus compound microscope.
Treatment with Small Molecules.
DAPT (MedChem Express) stock solution was prepared using DMSO as a solvent. Treatment concentration was based on and adapted from ref. 5. P. suvattii embryos were raised to 19 dpf and treated with 25 µM DAPT in freshwater for 3 d. DMSO (25 µM) was used as a control treatment. After treatment, embryos were washed and raised for 2 wk in freshwater before fixation and clear staining.
Acknowledgments
We thank Kyle Martin for comments on the manuscript; members of the G.J.F. laboratory and Nathan Jeffery for discussions; Serina Hayes for laboratory assistance; Martin Garlovsky for digital photography; Hiroyuki Doi and Toshiaki Ishibashi for the donation of embryos; and the Light Microscopy Facility, the Bateson Centre, and the Molecular Ecology laboratory at the University of Sheffield for support. MicroCT imaging was carried out with assistance from Farah Ahmed and Amin Garbout of the Imaging and Analysis Centre (Natural History Museum, London). This work was generously funded by Leverhulme Trust Research Project Grant RPG-211 (to G.J.F.), Natural Environment Research Council (NERC) Standard Grant NE/K014595/1 (to G.J.F.), Royal Society Research Grant RG120160 (to G.J.F.), The Great Britain Sasakawa Foundation (G.J.F. and T.S.), the Daiwa Anglo-Japanese Foundation (G.J.F.), and by “Adapting to the Challenges of a Changing Environment”, NERC-funded Doctoral Training Partnership NE/L002450/1 (to A.P.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1702909114/-/DCSupplemental.
References
- 1.Wagner GP, Lynch VJ. Evolutionary novelties. Curr Biol. 2010;20:R48–R52. doi: 10.1016/j.cub.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Jernvall J, Thesleff I. Tooth shape formation and tooth renewal: Evolving with the same signals. Development. 2012;139:3487–3497. doi: 10.1242/dev.085084. [DOI] [PubMed] [Google Scholar]
- 3.Sire JY, Davit-Beal T, Delgado S, Van Der Heyden C, Huysseune A. First-generation teeth in nonmammalian lineages: Evidence for a conserved ancestral character? Microsc Res Tech. 2002;59:408–434. doi: 10.1002/jemt.10220. [DOI] [PubMed] [Google Scholar]
- 4.Tucker AS, Fraser GJ. Evolution and developmental diversity of tooth regeneration. Semin Cell Dev Biol. 2014;25-26:71–80. doi: 10.1016/j.semcdb.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Fraser GJ, Bloomquist RF, Streelman JT. Common developmental pathways link tooth shape to regeneration. Dev Biol. 2013;377:399–414. doi: 10.1016/j.ydbio.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchtová M, et al. Initiation and patterning of the snake dentition are dependent on Sonic hedgehog signaling. Dev Biol. 2008;319:132–145. doi: 10.1016/j.ydbio.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Wu P, et al. Specialized stem cell niche enables repetitive renewal of alligator teeth. Proc Natl Acad Sci USA. 2013;110:E2009–E2018. doi: 10.1073/pnas.1213202110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Handrigan GR, Leung KJ, Richman JM. Identification of putative dental epithelial stem cells in a lizard with life-long tooth replacement. Development. 2010;137:3545–3549. doi: 10.1242/dev.052415. [DOI] [PubMed] [Google Scholar]
- 9.Smith MM, Fraser GJ, Mitsiadis TA. Dental lamina as source of odontogenic stem cells: Evolutionary origins and developmental control of tooth generation in gnathostomes. J Exp Zoolog B Mol Dev Evol. 2009;312B:260–280. doi: 10.1002/jez.b.21272. [DOI] [PubMed] [Google Scholar]
- 10.Cuvier G. Leçons d’Anatomie Comparee: La Première Partie des Organes de la Digestion. Crochard; Paris: 1805. [Google Scholar]
- 11.Owen R. Odontography. Hippolyte Bailliere; London: 1840–1845. [Google Scholar]
- 12.Andreucci RD, Britski HA, Carneiro J. Structure and Evolution of Tetraodontoid Teeth - an Autoradiographic Study (Pisces, Tetraodontiformes) J Morphol. 1982;171:283–292. doi: 10.1002/jmor.1051710304. [DOI] [PubMed] [Google Scholar]
- 13.Tyler JC. Osteology, Phylogeny, and Higher Classification of the Fishes of the Order Plectognathi (Tetraodontiformes) US Dept. of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service; Washington, DC: 1980. [Google Scholar]
- 14.Fraser GJ, Britz R, Hall A, Johanson Z, Smith MM. Replacing the first-generation dentition in pufferfish with a unique beak. Proc Natl Acad Sci USA. 2012;109:8179–8184. doi: 10.1073/pnas.1119635109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turingan RG. Ecomorphological relationships among Caribbean tetraodontiform fishes. J Zool (Lond) 1994;233:493–521. [Google Scholar]
- 16.Juuri E, et al. Sox2 marks epithelial competence to generate teeth in mammals and reptiles. Development. 2013;140:1424–1432. doi: 10.1242/dev.089599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin KJ, et al. Sox2+ progenitors in sharks link taste development with the evolution of regenerative teeth from denticles. Proc Natl Acad Sci USA. 2016;113:14769–14774. doi: 10.1073/pnas.1612354113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santini F, Sorenson L, Alfaro ME. A new phylogeny of tetraodontiform fishes (Tetraodontiformes, Acanthomorpha) based on 22 loci. Mol Phylogenet Evol. 2013;69:177–187. doi: 10.1016/j.ympev.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Britski HAA, Andreucci RD, Menezes NA, Carneiro J. Coalescence of teeth in fishes. Rev Bras Zool. 1985;2:459–482. [Google Scholar]
- 20.Handrigan GR, Richman JM. A network of Wnt, hedgehog and BMP signaling pathways regulates tooth replacement in snakes. Dev Biol. 2010;348:130–141. doi: 10.1016/j.ydbio.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Trapani J. Position of developing replacement teeth in teleosts. Copeia. 2001;(1):35–51. [Google Scholar]
- 22.Harada H, et al. Localization of putative stem cells in dental epithelium and their association with Notch and FGF signaling. J Cell Biol. 1999;147:105–120. doi: 10.1083/jcb.147.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnold K, et al. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9:317–329. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bloomquist RF, et al. Coevolutionary patterning of teeth and taste buds. Proc Natl Acad Sci USA. 2015;112:E5954–E5962. doi: 10.1073/pnas.1514298112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 26.Richman JM, Handrigan GR. Reptilian tooth development. Genesis. 2011;49:247–260. doi: 10.1002/dvg.20721. [DOI] [PubMed] [Google Scholar]
- 27.Thesleff I, Sharpe P. Signalling networks regulating dental development. Mech Dev. 1997;67:111–123. doi: 10.1016/s0925-4773(97)00115-9. [DOI] [PubMed] [Google Scholar]
- 28.Tucker AS, Headon DJ, Courtney JM, Overbeek P, Sharpe PT. The activation level of the TNF family receptor, Edar, determines cusp number and tooth number during tooth development. Dev Biol. 2004;268:185–194. doi: 10.1016/j.ydbio.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Lin CR, et al. Pitx2 regulates lung asymmetry, cardiac positioning and pituitary and tooth morphogenesis. Nature. 1999;401:279–282. doi: 10.1038/45803. [DOI] [PubMed] [Google Scholar]
- 30.Dassule HR, Lewis P, Bei M, Maas R, McMahon AP. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development. 2000;127:4775–4785. doi: 10.1242/dev.127.22.4775. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki T, et al. LEF1 is a critical epithelial survival factor during tooth morphogenesis. Dev Biol. 2005;278:130–143. doi: 10.1016/j.ydbio.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 32.Fraser GJ, Bloomquist RF, Streelman JT. A periodic pattern generator for dental diversity. BMC Biol. 2008;6:32. doi: 10.1186/1741-7007-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitsiadis TA, Graf D, Luder H, Gridley T, Bluteau G. BMPs and FGFs target Notch signalling via jagged 2 to regulate tooth morphogenesis and cytodifferentiation. Development. 2010;137:3025–3035. doi: 10.1242/dev.049528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buchtová M, Stembírek J, Glocová K, Matalová E, Tucker AS. Early regression of the dental lamina underlies the development of diphyodont dentitions. J Dent Res. 2012;91:491–498. doi: 10.1177/0022034512442896. [DOI] [PubMed] [Google Scholar]
- 35.Gaete M, Tucker AS. Organized emergence of multiple-generations of teeth in snakes is dysregulated by activation of Wnt/beta-catenin signalling. PLoS One. 2013;8:e74484. doi: 10.1371/journal.pone.0074484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang XP, et al. Apc inhibition of Wnt signaling regulates supernumerary tooth formation during embryogenesis and throughout adulthood. Development. 2009;136:1939–1949. doi: 10.1242/dev.033803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xavier GM, et al. Activated WNT signaling in postnatal SOX2-positive dental stem cells can drive odontoma formation. Sci Rep. 2015;5:14479. doi: 10.1038/srep14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munne PM, et al. Splitting placodes: Effects of bone morphogenetic protein and Activin on the patterning and identity of mouse incisors. Evol Dev. 2010;12:383–392. doi: 10.1111/j.1525-142X.2010.00425.x. [DOI] [PubMed] [Google Scholar]
- 39.Jheon AH, et al. Inhibition of Notch signaling during mouse incisor renewal leads to enamel defects. J Bone Miner Res. 2016;31:152–162. doi: 10.1002/jbmr.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Renvoisé E, Michon F. An Evo-Devo perspective on ever-growing teeth in mammals and dental stem cell maintenance. Front Physiol. 2014;5:324. doi: 10.3389/fphys.2014.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor WR, Van Dyke GC. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium. 1985;9:107–119. [Google Scholar]
- 42.Shono T, Kurokawa D, Miyake T, Okabe M. Acquisition of glial cells missing 2 enhancers contributes to a diversity of ionocytes in zebrafish. PLoS One. 2011;6:e23746. doi: 10.1371/journal.pone.0023746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rasch LJ, et al. An ancient dental gene set governs development and continuous regeneration of teeth in sharks. Dev Biol. 2016;415:347–370. doi: 10.1016/j.ydbio.2016.01.038. [DOI] [PubMed] [Google Scholar]