Abstract
Introduction:
Blood pressure is lower in females than males. Angiotensin II type-2 receptor (AT2R) induces vasodilation. This study determined whether sex differences in vascular AT2R expression occur and if androgens exert control on AT2R expression in the vasculature.
Methods:
AT2Rs in the aorta of male and female Sprague-Dawley rats were examined following alteration in androgen levels by gonadectomy or hormone supplementation.
Results:
AT2R mRNA and protein expression levels were lower in the aortas of males than females. In males, testosterone withdrawal by castration significantly elevated AT2R mRNA and protein levels and testosterone replacement restored them. In females, increasing androgen levels decreased AT2R mRNA and protein expression and this was attenuated by androgen receptor blocker flutamide. Ex vivo, dihydrotestosterone downregulated AT2R in endothelium-intact but not endothelium-denuded aorta. Dihydrotestosterone-induced AT2R downregulation in isolated aorta was blocked by an androgen receptor antagonist. Furthermore, blockade of ERK1/2 but not p38 MAP kinase or TGFβ signaling with specific inhibitors abolished dihydrotestosterone-induced AT2R downregulation.
Conclusion:
Androgens downregulate AT2R expression levels in aorta, in vivo and ex vivo. The androgen receptor-mediated ERK1/2 MAP kinase-signaling pathway may be a key mechanism by which testosterone downregulates AT2R expression, implicating androgens’ contributing role to gender differences in vascular AT2R expression.
Keywords: Endothelium, ERK, gender difference, blood pressure, testosterone, vascular
Introduction
The sex difference in blood pressure (BP) has long been recognized between premenopausal women and age-matched men.1 Before menopause, women have lower BP and are protected from most cardiovascular events compared with age-matched men, and postmenopausal women are at increased risk of cardiovascular complications compared with premenopausal women.2 The pathophysiological mechanisms have been extensively explored, and increasing evidence has shown that sex hormones are one of the major contributors to the above phenomena.3 Among different mechanisms, the interaction between sex hormones and the renin-angiotensin system (RAS) is shown to play important role in regulating cardiovascular function and BP.4,5 Angiotensin II is the main effector of RAS, and it regulates BP through its effect on the angiotensin II type-1 receptor (AT1R) and angiotensin II type-2 receptor (AT2R).6,7 The AT1R promotes antinatriuresis, proliferation, inflammation, and vasoconstriction.8 AT2Rs are generally assumed to oppose AT1R-mediated responses, for example, by evoking vasorelaxation, natriuresis, antigrowth, and anti-inflammatory effects.9 Increasing evidence has shown that the AT2R is a key player in lowering BP in females but not in males.10,11 This enhanced BP-lowering effect of the AT2R in females is attributed to increased expression of the AT2R in females compared to age-matched males. For example, higher AT2R levels are observed in the female brain,12 kidney,13 and liver14 compared to males. However, it is not known if there are differences in expression pattern of AT2Rs in the vasculature between the males and females. The vascular AT2Rs are key elements in homeostatic regulation of the cardiovascular system. Therefore, elucidating the expression patterns of the AT2R in the vasculature would provide evidence for understanding the possible roles of AT2Rs in regulating BP.
Sex steroid hormones, particularly estrogens, are attributed to the greater BP lowering effect15,16 and enhanced tissue expression of AT2R in females.17,18 However, estrogen is shown to exert a tissue-specific effect by regulating AT2R expression in the kidneys17,18 but not in lungs,19 urethra,20 and blood vessels.17 On the other hand, whether androgens exert control on AT2R expression is unknown. Studies show that androgens can regulate RAS components.21,22 Therefore, we hypothesized that testosterone (T) is involved in the control of AT2R expression in the vasculature. We investigated (a) whether there are sex differences in vascular AT2R expression, (b) if AT2R is influenced by alterations in androgen status in male and female rats, and (c) the underlying mechanism of AT2R regulation by androgens.
Materials and methods
Animals and institutional animal care and use committee (IACUC) approval
All experimental procedures were carried out in accordance with the National Institutes of Health guidelines (NIH publication no. 85–23 (revised 1996)) with approval by the Animal Care and Use Committee at The University of Texas Medical Branch at Galveston. Three-month-old male and female Sprague-Dawley rats were purchased from Harlan Laboratories, Inc. (Houston, Texas, USA). Rats were housed in a temperature-controlled room (23°C), with a 12L:12D cycle, and with food and water available ad libitum. After one week acclimatization, male rats were divided into three groups: (a) intact, (b) castrated, and (c) castrated with testosterone replacement using subcutaneous implanted pellets (25 mg, 21 day release, Innovative Research of America, Sarasota, Florida, USA). Females were divided into four groups: (a) control, (b) treated with dihydrotestosterone (DHT) using pellets (2.5 mg, 21 day release), (c) DHT plus flutamide (100 mg, 21 day release), and (d) flutamide alone. DHT was used in females to overcome its aromatization to estradiol. Castration was done by standard procedures as described in our previous studies.23,24 The doses of the pellets were chosen to mimic physiologic hormone levels as reported previously23-27 and were further confirmed by hormone assays. After the 21-day treatment, BP was measured and then animals were euthanized by CO2 inhalation, and blood was collected for hormone assays. The thoracic aorta was isolated and either immediately frozen in liquid nitrogen for mRNA and protein analysis or used for ex vivo-signaling studies.
BP measurement
BP was measured using a computerized CODA system (Kent Scientific, Litchfield, Connecticut, USA) as in our previous studies.28 Briefly, rats were acclimatized for a week to the measurement procedures prior to testing. Rats were held in a preheated restrainer with the tail exposed, and both an occlusion cuff and a volume pressure-recording cuff were placed close to the base of the tail. The cuff was then inflated and deflated automatically within 90 s. BP is measured during 30 consecutive, computer-automated inflation/deflation cycles of the balloon cuff (10 preliminary measurements and 20 test measurements). Unlike other tail-cuff systems, CODA uses volume pressure recording to measure both systolic and diastolic BP, which is then used by the software to calculate the mean BP. Data from the preliminary measurements are discarded and data from the test measurements are averaged. Signals were recorded and analyzed using Kent Scientific software. To minimize stress-induced variations in BP, all measurements were taken by the same person in the same peaceful environment and at the same time of the day.
Hormone assays
T and DHT were measured using enzyme-linked immunosorbent assay (ELISA) kits (T- Enzo Life Sciences, Farmingdale, New York, USA and DHT- BioVendor, Asheville, North Carolina, USA), as in our previous publications.26,29 The minimum detectable concentration of testosterone is 6 pg/ml and the intra- and interassay coefficients of variation for testosterone assay was lower than 5%. The minimum detectable concentration of DHT is 6 pg/ml and the intra- and inter-assay coefficients of variation for DHT assay were lower than 8%.
Protein extraction and western blotting
Aorta was homogenized in ice-cold radioimmunoprecipitation assay (RIPA) buffer (Cell Signaling Technology, Danvers, Massachusetts, USA) containing a protease inhibitor tablet and phosphatase inhibitor cocktail-2 and -3 (Sigma-Aldrich, St Louis, Missouri, USA). Tissue lysates were centrifuged (14000× g for 10 min at 4°C), and the protein content was measured using the bicinchoninic acid (BCA) protein assay kit (Pierce; Thermo Scientific, Grand Island, New York, USA). The supernatant was resuspended in neutral pH polyacrylamide gel electrophoresis (NuPAGE) lithium dodecyl sulfate sample buffer and reducing agent (Invitrogen; Thermo Scientific). Proteins (30 µg) alongside Precision Plus Standard (Kaleidoscope; Bio-Rad, Hercules, California, USA) were resolved on 4–12% gradient NuPAGE Bis-Tris gels (Invitrogen) at 100 V for 2 h at room temperature and then transferred onto Immobilon-P membranes (Millipore, Billerica, Massachusetts, USA) at 100 V for 1 h. The membranes were blocked with 5% non-fat dry milk for 1 h and then incubated overnight at 4°C with primary antibodies. The primary antibodies were rabbit monoclonal AT2R (1:3000 dilution; Abcam, Cambridge, Massachusetts, USA) and β-actin (1:5000 dilution; Cell Signaling Technology). After being washed, the membranes were incubated with secondary antibodies (anti-rabbit or -mouse conjugated with horseradish peroxidase) at 1:10000 dilutions and detected with the enhanced chemiluminescence (ECL) detection kits (Pierce; Thermo Scientific). Densitometric measurement was done using ImageJ software.30 Results were expressed as ratios of the intensity of a specific band to that of β-actin.
Quantitative real-time polymerase chain reaction (PCR)
Total RNA was extracted using RNeasy mini kit (QIAGEN, Valencia, California, USA) according to manufacturer’s instructions. RNA concentration and integrity was determined using DS-11 spectrophotometer (DeNovix, Wilmington, Delaware, USA). One microgram of total RNA was reverse transcribed using iScript cDNA synthesis kit (Bio-Rad). After dilution, cDNA corresponding to 100 ng of RNA was amplified by quantitative real-time (qRT)-PCR using FAM (Invitrogen) as the fluorophore in a CFX96 real-time thermal cycler (Bio-Rad). PCR conditions for TaqMan Gene Expression Assay were 2 min at 50°C and 10 min at 95°C for one cycle, then 15 s at 95°C and 1 min at 60°C for 50 cycles. Results were calculated using the 2–∆∆CT method and expressed in fold change of the gene of interest in treated versus control samples. All reactions were performed in duplicate, and β-actin was used as an internal control. TaqMan assays were carried out in 10-µl volumes for real-time PCR at a final concentration of 250 nM TaqMan probe and 900 nM of each primer. AT2R (Rn00560677_s1) and β-actin (Rn00667869_m1) assays were obtained by Assay-on-Demand (Applied Biosystems; Thermo Scientific).
Ex vivo treatment to aorta
Aortas from female rats were dissected, taking care to avoid stretching or compression of the tissues, and placed into ice-cold phosphate-buffered saline (PBS), cleaned of adventitia, and cut into 3–4 rings of approximately 5 mm in length. The rings were placed into 2 ml of Dulbecco’s modified eagle’s medium (DMEM) (Gibco Laboratories; Thermo Scientific) supplemented with 100 ug/ml streptomycin and 100 U/ml penicillin, 1% fetal calf serum (FCS),31 and incubated at 37°C in a humidified 5% CO2 incubator. In some experiments, the endothelium was denuded by gently rubbing the lumen with human hair. The rings were stimulated with DHT at doses of 0, 0.1, 1, and 10 nmol/l for 24 h to examine the dose response of AT2R expression. To inhibit binding of DHT to its receptor, hydroxyflutamide (1 μmol/l) was used. To inhibit DHT-induced extracellular signal-regulated kinases (ERK)1/2 mitogen activated protein (MAP) kinase, p38 MAP kinase, or transforming growth factor (TGF)β activities, inhibitors to ERK1/2 (PD98059, 10 μmol/l and U0126, 10 μmol/l), p38 (SB203580, 10 μmol/l), and TGFβ (SB431542, 10 μmol/l) were used, respectively. Each experiment was repeated at least thrice throughout the study. All chemicals were purchased from Sigma-Aldrich (St Louis, Missouri, USA) unless otherwise noted.
Statistical analysis
All data are expressed as the mean±standard error of the mean (SEM). Statistical significance was determined with one-way analysis of variance followed by Bonferroni’s post-hoc test. Comparisons between the two groups were performed using Student t tests. Differences were considered statistically significant at a value of p<0.05. Statistical analysis was conducted using GraphPad Prism (GraphPad, San Diego, California, USA).
Results
BP and hormone measurements
BP was significantly decreased in castrated rats (111.10±5.2 mm Hg; n=6; p<0.05) compared to intact controls (126.5±2.5 mm Hg; n=6) and testosterone supplementation restored BP to testis-intact controls (129.1±4.1 mm Hg; n=6). In the female rats DHT supplementation increased BP significantly (131.7±5.2; mm Hg; n=5; p<0.05) compared to controls (105.1±2.7; mm Hg; n=6).
Plasma testosterone levels were significantly decreased by castration (0.2±0.02 vs 1.4±0.07 ng/ml in intact; n=6 in each; p<0.05) and reinstated to intact levels by replacement (1.5±0.17 ng/ml). In the females, DHT levels were higher in the DHT (186±37.6 pg/ml) and DHT plus flutamide-treated group (179±25.3 pg/ml) compared to controls (111±11.6 pg/ml; n=6 in each; p<0.05). Flutamide alone to females did not alter DHT levels (107±10.4 pg/ml; n=6) compared to vehicle controls.
AT2R expression is lower in males than females
To determine whether AT2R expression in the aorta varied between the males and females, mRNA and protein levels of AT2R were determined with quantitative RT-PCR and Western blot analyses. Males had significantly lower AT2R mRNA (↓40%; Figure 1(a)) and protein (↓38%; Figure 1(b)) expression in aorta compared to females (n=6 in each group; p<0.05).
Figure 1.
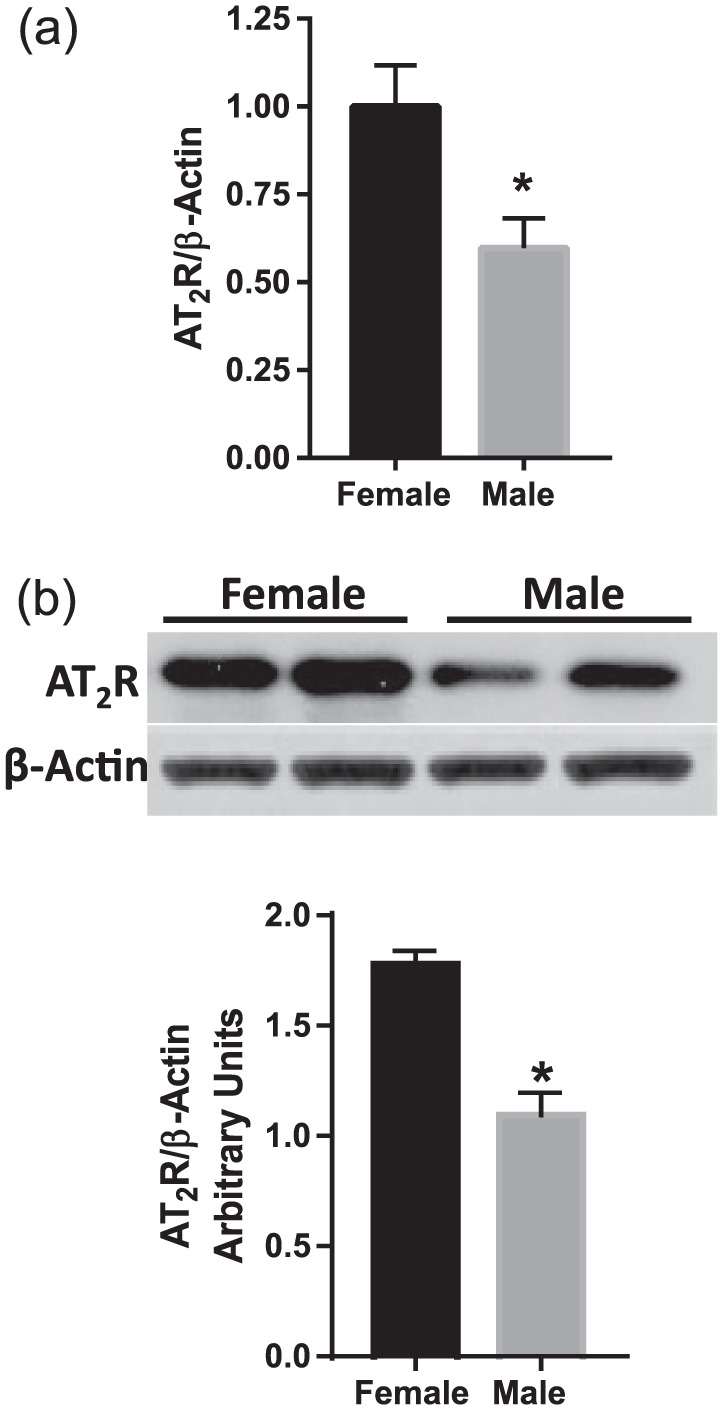
Angiotensin II type-2 receptor (AT2R) expression is lower in the aorta of male than in female rats. Expression of AT2R (a) mRNA and (b) protein was measured in aorta from three-month-old male and female rats. AT2R mRNA expression was measured by quantitative real-time polymerase chain reaction normalized relative to β-actin levels. AT2R protein expression was determined by Western blotting. Representative Western blots for AT2R and β-actin are shown at top; blot density obtained from densitometric scanning of AT2R normalized to β-actin is shown at bottom. Values are given as means±standard error of the mean (SEM) of six rats in each group. *p<0.05 vs female.
AT2R expression negatively relates to androgen levels in males and females
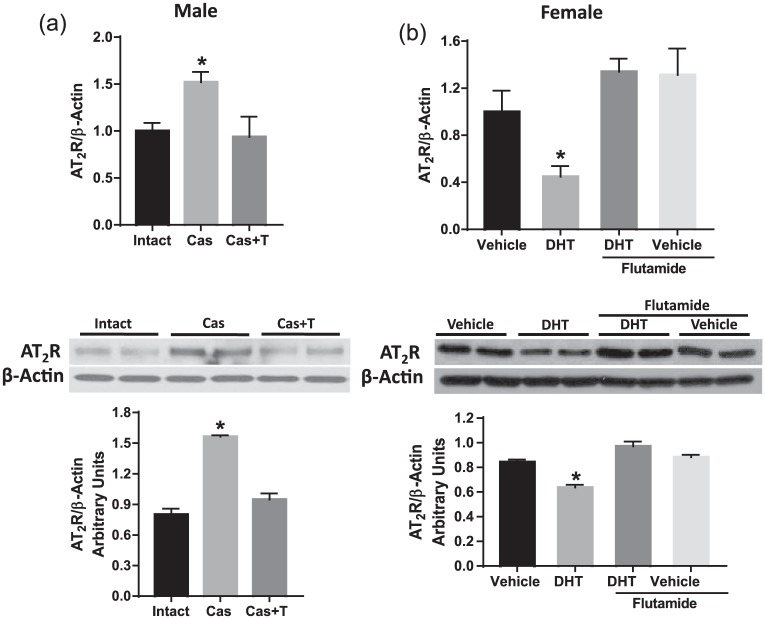
We next determined whether AT2R expression in the aorta correlated with an alteration in testosterone levels in males and females. In males, castration significantly elevated AT2R mRNA (↑52%) and protein (↑76%) expression (Figure 2(a), p<0.05, n=6) compared to intact controls. Testosterone replacement in castrated males restored AT2R mRNA and protein to levels comparable to that in intact males (Figure 2(a), p<0.05, n=6).
Figure 2.
Angiotensin II type-2 receptor (AT2R) expression in the aorta relates to androgen levels in male and female rats. AT2R mRNA (upper panel) and protein (lower panel) expression were assessed in aortas isolated from (a) male rats with testes intact, castrated, and castrated with testosterone replacement and (b) female rats treated with vehicle, dihydrotestosterone (DHT), DHT plus flutamide (antiandrogen), and flutamide alone. AT2R mRNA expression was measured by quantitative real-time polymerase chain reaction normalized relative to β-actin levels. AT2R protein expression was determined by Western blotting. Representative Western blots for AT2R and β-actin are shown at the top; blot density obtained from densitometric scanning of AT2R normalized to β-actin is shown at the bottom. Values are given as means±standard error of the mean (SEM) of six rats in each group. *p<0.05 vs vehicle and DHT plus flutamide group. Cas: castration.
Increasing androgen levels by DHT administration to females significantly decreased AT2R mRNA (↓53%) and protein (↓27%) expression (Figure 2(b), p<0.05, n=6). Administration of flutamide, an androgen receptor blocker, significantly attenuated the decreased AT2R mRNA and protein in DHT-treated females (Figure 2(b), p<0.05, n=6). Flutamide by itself did not have any significant effect on AT2R expression (Figure 2(b), n=6). Thus, testosterone appears to downregulate AT2R expression in both males and females.
DHT downregulates AT2R transcription ex vivo
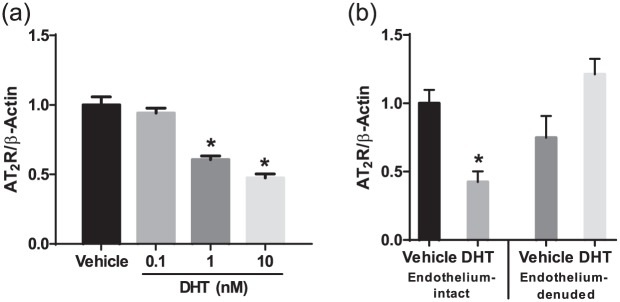
Since downregulation of AT2R by testosterone was apparent, isolated aortas from female rats were used to study the mechanisms by which AT2R expression is regulated in response to DHT. As shown in Figure 3(a), DHT induced a dose-dependent downregulation of AT2R mRNA (p<0.05, n=6). Thus, testosterone directly downregulates AT2R at a transcriptional level.
Figure 3.
Dihydrotestosterone (DHT) downregulates endothelial angiotensin II type-2 receptor (AT2R) expression in isolated aorta. Endothelium-intact and -denuded aorta from female rats were treated with DHT for 24 h, and then AT2R mRNA was measured using quantitative real-time polymerase chain reaction. (a) DHT dose-dependently downregulated AT2R transcription in endothelium-intact aorta. (b) DHT (10 nmol/l) downregulated AT2R transcription only in endothelium-intact but not endothelium-denuded aorta. Values were normalized relative to β-actin levels. Data represent the mean of four independent experiments. *p<0.05 vs vehicle control.
DHT downregulates AT2R in the endothelium but not in vascular smooth muscle
Studies show that AT2R is expressed in both the endothelium and vascular smooth muscle layer. To dissect whether the effect of DHT on AT2R expression occurs in the endothelium or vascular smooth muscle, we used endothelium-intact and endothelium-denuded aorta from female rats. As shown in Figure 3(b), DHT did not alter AT2R expression in endothelium-denuded aorta but downregulated AT2R mRNA in endothelium-intact aorta (p<0.05, n=3).
DHT downregulates AT2R transcription via androgen receptor-mediated ERK1/2-dependent mechanisms
We further tested whether activation of the androgen receptor and the downstream signaling of ERK1/2, p38 MAP kinases, and TGF-β are responsible for downregulation of AT2R expression in response to DHT. Endothelium-intact aortas from female rats were stimulated with DHT in the presence or absence of the androgen receptor antagonist and inhibitors to ERK1/2, p38, or TGF-β. As shown in Figure 4, addition of hydroxyflutamide prevented the reduction of AT2R expression in response to DHT (p<0.05, n=3). ERK1/2 inhibitor, but not p38 and TGF-β inhibitors, prevented a DHT-induced decrease in AT2R expression (Figure 5, p<0.05, n=4). Interestingly, p38 MAP kinase and TGF-β inhibitors by themselves decreased basal expression of AT2R, and DHT in presence of p38 MAP kinase and TGF-β inhibitors further decreased AT2R expression (Figure 5, p<0.05, n=4).
Figure 4.
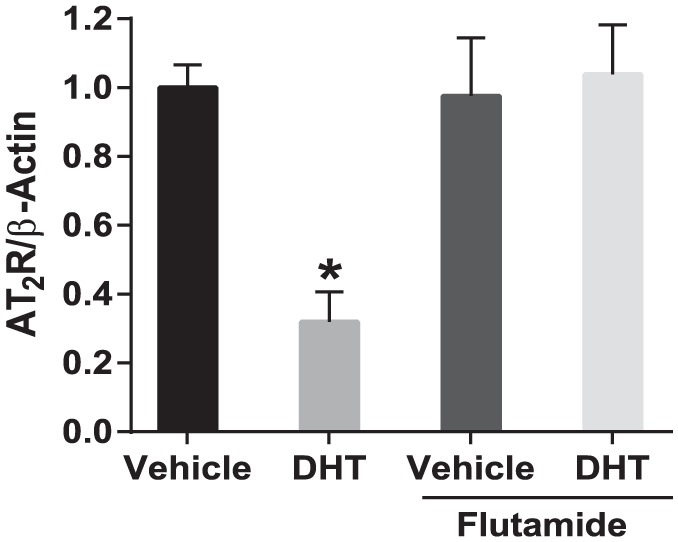
Dihydrotestosterone (DHT)-mediated downregulation of angiotensin II type-2 receptor (AT2R) transcription is blocked by androgen receptor antagonist. Aortic rings from female rats were treated with DHT (10 nmol/l) in the presence or absence of hydroxyflutamide (1 µmol/l) for 24 h and AT2R mRNA expression was analyzed using quantitative real-time polymerase chain reaction. Data represent the mean of three independent experiments. *p<0.05 vs vehicle control.
Figure 5.
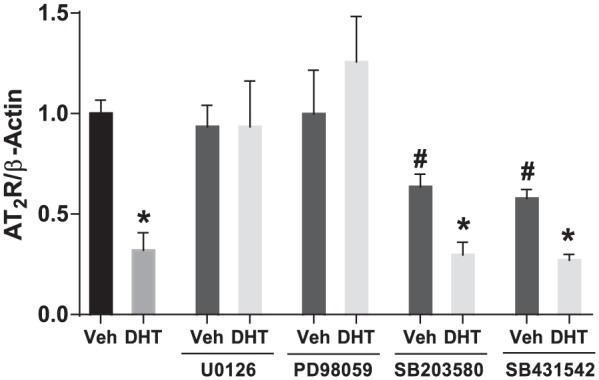
Dihydrotestosterone (DHT) mediates downregulation of angiotensin II type-2 receptor (AT2R) transcription through ERK-mediated pathways. Aortic rings from female rats were treated for 24 h with DHT (10 nmol/l) in the presence or absence of inhibitors to ERK1/2 (U0126, 10 μmol/l and PD98059, 10 μmol/l), p38 (SB203580, 10 μmol/l), and TGFβ (SB431542, 10 μmol/l). AT2R mRNA expression was analyzed using quantitative real-time polymerase chain reaction and normalized to β–actin. Data represent the mean of four independent experiments. *p<0.05 vs vehicle control, #p<0.05 vs DHT in the absence of any inhibitors. Veh: vehicle.
Discussion
To our knowledge, this is the first study that relates androgen status and vascular AT2R expression. Our main findings are that (a) AT2R expression in the aorta is significantly lower in male compared to female rats; (b) vascular AT2R expression relates to androgen status with orchiectomy increasing and testosterone replacement restoring AT2R expression in males, but in the females, increasing DHT levels decreased AT2R expression, which was prevented by blockade of the androgen receptor; (c) DHT directly decreases AT2R expression at transcriptional level in the aorta by decreasing endothelial but not vascular smooth muscle AT2R levels; and (d) DHT downregulates AT2R via androgen receptor-mediated ERK1/2 MAP kinase-dependent mechanisms.
Sex is linked to differences in cardiovascular morbidity and mortality.32,33 The RAS is an important regulatory system that is involved in the long-term control of BP. Angiotensin II is the main effector, which mediates its effect through AT1R and AT2R, which are expressed in cardiovascular system and play an opposite role in BP regulation. AT1R promotes vasoconstriction,34,35 while AT2R promotes vasodilation.36,37 In the present study, we observed that AT2R expression is higher in females compared to males, consistent with the reports in the brain, kidney, and liver.12-14 However, in contrast, spontaneously hypertensive rats (SHRs) showed no sex-dependent differences in AT2R expression in the aorta and mesenteric arteries.17 The reason for the apparent discrepancies between the above study and the present study is not entirely clear. SHRs are hypertensive rats induced by genetic modification and thus may involve different AT2R regulatory mechanisms than in normotensive animals.38,39 In line with increased vascular AT2R expression in females, studies show that AT2R-mediated relaxation is greater in women than men.40 Further experimental studies in rats and mice also support this since C21 (a AT2R agonist) induced a greater increase in renal vasodilation in females than males.13,41 In addition, angiotensin II, at low-dose, reduced pressor response in females that was inhibited by the AT2R antagonist.10 These functional reports together with our molecular finding of increased vascular AT2R expression in females suggest that AT2R may have an important role in contributing to gender differences in vascular tone and BP.
Sex hormones are shown to directly interact with the RAS.42-44 Estrogens are shown to downregulate the vasoconstrictive RAS components (i.e. angiotensin-converting-enzyme (ACE and AT1R)45,46 and upregulate the vasodilatory RAS components (i.e. AT2R and ACE2).18,47 On the other hand, testosterone is shown to upregulate the vasoconstrictive AT1R and ACE.22,48 This is the first study that shows that androgens downregulate vasodilatory AT2R, as observed by the finding that both mRNA and protein expressions of AT2R in aorta are significantly upregulated by orchiectomy and restored by testosterone replacement. In addition, increasing androgen levels in females decreased AT2R expression, and antiandrogen treatment completely normalized AT2R to control levels. Interestingly, the changes in AT2R expression is inversely related to the BP changes observed in these male and female rats. These findings indicate that androgens exert a negative modulatory effect on AT2R expression not only in males but also in females, and may play a role in influencing BP. These findings are clinically relevant since evidence indicates that androgen levels are higher in young women with conditions, such as polycystic ovary syndrome (PCOS), women after menopause,49 and African American women50,51, and the frequency of hypertension is greater in these populations.52-54 It remains to be determined if the vascular AT2R expression is altered in these population.
More importantly, we showed that DHT was able to downregulate AT2R ex vivo in aorta. These findings suggest that DHT directly induces a downregulation of AT2R transcription independent of any endogenous factors. Because blockade of androgen receptors abolished DHT-induced downregulation of AT2R, we suggest that the effects of testosterone are mediated through androgen receptors. In the present study, a DHT-induced decrease in AT2R transcription is observed in endothelium-intact vessels. No significant difference in DHT-induced AT2R transcription was observed in endothelium-denuded vessels. These findings indicate that testosterone induces reduction in AT2R transcription, primarily in the endothelium rather than in vascular smooth muscle. Although AT2R is expressed in both the vascular smooth muscle and endothelium,55,56 the reason why androgens specifically decrease endothelial AT2R is unclear at this time. It would have been ideal to examine if testosterone directly regulates AT2R expression in cultured endothelial cells but this is problematic because endothelial cells rapidly loose AT2R expression when put in culture,39 thus preventing a study of AT2R expression in cultured endothelial cells.
We next examined the mechanisms by which androgens can downregulate AT2R transcription. Androgens are known to activate p38 and ERK1/2 MAP kinase and TGF-β pathways in the vasculature.57-59 The finding that p38 MAP kinase and TGF-β inhibitors by themselves reduced AT2R transcription suggests that basal p38 and TGF-β activities may be important to maintain AT2R expression in unstimulated cells. The inability of p38 MAP kinase and TGF-β inhibitors to prevent a DHT-induced decrease in AT2R transcription suggests the presence of other intracellular mechanisms. Our observation that reduced AT2R expression in response to testosterone was abolished by blocking ERK1/2 suggests that androgen-induced downregulation of AT2R transcription is mediated via the ERK1/2 MAP kinase pathways. Further studies that examine the mechanism by which ERK1/2 MAP kinase downregulates AT2R transcription are warranted. Although this study used aorta, which not only functions as a channel delivering blood to the tissues but also as an important modulator of the entire cardiovascular system by buffering the intermittent pulsatile output from the heart,60 further studies are necessary to examine AT2R expression and regulation in resistance vessels which play an important role in BP control.
Conclusions
Sex differences in vascular AT2R expression is observed with lower levels in males than females. Testosterone downregulates AT2R expression levels in aorta, in vivo and ex vivo. The androgen receptor-mediated ERK1/2 MAP kinase-signaling pathway may be a key mechanism by which testosterone downregulates AT2R expression, implicating androgens’ contributing role to gender differences in vascular AT2R expression.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute of Health (NIH) through grant HL119869 is greatly appreciated. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1. Anish TS, Shahulhameed S, Vijayakumar K, et al. Gender difference in blood pressure, blood sugar, and cholesterol in young adults with comparable routine physical exertion. J Family Med Prim Care 2013; 2: 200–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maranon R, Reckelhoff JF. Sex and gender differences in control of blood pressure. Clin Sci (Lond) 2013; 125: 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Qiao GF, Li BY, Lu YJ, et al. 17Beta-estradiol restores excitability of a sexually dimorphic subset of myelinated vagal afferents in ovariectomized rats. Am J Physiol Cell Physiol 2009; 297: C654–C664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maric-Bilkan C, Manigrasso MB. Sex differences in hypertension: Contribution of the renin-angiotensin system. Gend Med 2012; 9: 287–291. [DOI] [PubMed] [Google Scholar]
- 5. Sampson AK, Hilliard LM, Moritz KM, et al. The arterial depressor response to chronic low-dose angiotensin II infusion in female rats is estrogen dependent. Am J Physiol Regul Integr Comp Physiol 2012; 302: R159–R165. [DOI] [PubMed] [Google Scholar]
- 6. Siddiqui AH, Hussain T. Enhanced AT1 receptor-mediated vasocontractile response to ANG II in endothelium-denuded aorta of obese Zucker rats. Am J Physiol Heart Circ Physiol 2007; 292: H1722-H1727. [DOI] [PubMed] [Google Scholar]
- 7. Stennett AK, Qiao X, Falone AE, et al. Increased vascular angiotensin type 2 receptor expression and NOS-mediated mechanisms of vascular relaxation in pregnant rats. Am J Physiol Heart Circ Physiol 2009; 296: H745–H755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de GM, Catt KJ, Inagami T, et al. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev 2000; 52: 415–472. [PubMed] [Google Scholar]
- 9. Verdonk K, Danser AH, van Esch JH. Angiotensin II type 2 receptor agonists: Where should they be applied? Expert Opin Investig Drugs 2012; 21: 501–513. [DOI] [PubMed] [Google Scholar]
- 10. Sampson AK, Moritz KM, Jones ES, et al. Enhanced angiotensin II type 2 receptor mechanisms mediate decreases in arterial pressure attributable to chronic low-dose angiotensin II in female rats. Hypertension 2008; 52: 666–671. [DOI] [PubMed] [Google Scholar]
- 11. Sandberg K, Ji H. Why can’t a woman be more like a man?: Is the angiotensin type 2 receptor to blame or to thank? Hypertension 2008; 52: 615–617. [DOI] [PubMed] [Google Scholar]
- 12. Dai SY, Peng W, Zhang YP, et al. Brain endogenous angiotensin II receptor type 2 (AT2-R) protects against DOCA/salt-induced hypertension in female rats. J Neuroinflammation 2015; 12: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hilliard LM, Chow CL, Mirabito KM, et al. Angiotensin type 2 receptor stimulation increases renal function in female, but not male, spontaneously hypertensive rats. Hypertension 2014; 64: 378–383. [DOI] [PubMed] [Google Scholar]
- 14. Yu L, Zheng M, Wang W, et al. Developmental changes in AT1 and AT2 receptor-protein expression in rats. J Renin Angiotensin Aldosterone Syst 2010; 11: 214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fung MM, Poddar S, Bettencourt R, et al. A cross-sectional and 10-year prospective study of postmenopausal estrogen therapy and blood pressure, renal function, and albuminuria: The Rancho Bernardo Study. Menopause 2011; 18: 629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Issa Z, Seely EW, Rahme M, et al. Effects of hormone therapy on blood pressure. Menopause 2015; 22: 456–468. [DOI] [PubMed] [Google Scholar]
- 17. Silva-Antonialli MM, Tostes RC, Fernandes L, et al. A lower ratio of AT1/AT2 receptors of angiotensin II is found in female than in male spontaneously hypertensive rats. Cardiovasc Res 2004; 62: 587–593. [DOI] [PubMed] [Google Scholar]
- 18. Armando I, Jezova M, Juorio AV, et al. Estrogen upregulates renal angiotensin II AT(2). receptors. Am J Physiol Renal Physiol 2002; 283: F934–F943. [DOI] [PubMed] [Google Scholar]
- 19. Brosnihan KB, Hodgin JB, Smithies O, et al. Tissue-specific regulation of ACE/ACE2 and AT1/AT2 receptor gene expression by oestrogen in apolipoprotein E/oestrogen receptor-alpha knock-out mice. Exp Physiol 2008; 93: 658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ramos-Filho AC, Faria A, Calmasini FB, et al. The renin-angiotensin system plays a major role in voiding dysfunction of ovariectomized rats. Life Sci 2013; 93: 820–829. [DOI] [PubMed] [Google Scholar]
- 21. Freshour JR, Chase SE, Vikstrom KL. Gender differences in cardiac ACE expression are normalized in androgen-deprived male mice. Am J Physiol Heart Circ Physiol 2002; 283: H1997–H2003. [DOI] [PubMed] [Google Scholar]
- 22. Leung PS, Wong TP, Chung YW, et al. Androgen dependent expression of AT1 receptor and its regulation of anion secretion in rat epididymis. Cell Biol Int 2002; 26: 117–122. [DOI] [PubMed] [Google Scholar]
- 23. Chinnathambi V, Balakrishnan M, Yallampalli C, et al. Prenatal testosterone exposure leads to hypertension that is gonadal hormone-dependent in adult rat male and female offspring. Biol Reprod 2012; 206: 507.e1–507e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. More AS, Mishra JS, Gopalakrishnan K, et al. Prenatal testosterone exposure leads to gonadal hormone-dependent hyperinsulinemia and gonadal hormone-independent glucose intolerance in adult male rat offspring. Biol Reprod 2016; 94: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sathishkumar K, Balakrishnan M, Chandrasekhar Y. Enhanced Mesenteric Arterial Responsiveness to Angi-otensin II Is Androgen Receptor-Dependent in Prenatally Protein-Restricted Adult Female Rat Offspring. Biol Reprod 2015; 92: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blesson CS, Chinnathambi V, Hankins GD, et al. Prenatal testosterone exposure induces hypertension in adult females via androgen receptor-dependent protein kinase Cdelta-mediated mechanism. Hypertension 2014; 65: 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hurliman A, Keller BJ, Maille N, et al. Hyperandrogenism and insulin resistance, not changes in body weight, mediate the development of endothelial dysfunction in a female rat model of polycystic ovary syndrome (PCOS). Endocrinology 2015; 156: 4071–4080. [DOI] [PubMed] [Google Scholar]
- 28. Sathishkumar K, Elkins R, Yallampalli U, et al. Protein restriction during pregnancy induces hypertension in adult female rat offspring – influence of oestradiol. Br J Nutr 2011; 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gopalakrishnan K, Mishra JS, Chinnathambi V, et al. Elevated testosterone reduces uterine blood flow, spiral artery elongation, and placental oxygenation in pregnant rats. Hypertension 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 Years of image analysis. Nat Methods 2012; 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McKenna TM. Prolonged exposure of rat aorta to low levels of endotoxin in vitro results in impaired contractility. Association with vascular cytokine release. J Clin Invest 1990; 86: 160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hudson M, Rahme E, Behlouli H, et al. Sex differences in the effectiveness of angiotensin receptor blockers and angiotensin converting enzyme inhibitors in patients with congestive heart failure–a population study. Eur J Heart Fail 2007; 9: 602–609. [DOI] [PubMed] [Google Scholar]
- 33. Bubb KJ, Khambata RS, Ahluwalia A. Sexual dimorphism in rodent models of hypertension and atherosclerosis. Br J Pharmacol 2012; 167: 298–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Touyz RM, Endemann D, He G, et al. Role of AT2 receptors in angiotensin II-stimulated contraction of small mesenteric arteries in young SHR. Hypertension 1999; 33: 366–372. [DOI] [PubMed] [Google Scholar]
- 35. Higuchi S, Ohtsu H, Suzuki H, et al. Angiotensin II signal transduction through the AT1 receptor: Novel insights into mechanisms and pathophysiology. Clin Sci (Lond) 2007; 112: 417–428. [DOI] [PubMed] [Google Scholar]
- 36. Carey RM, Jin XH, Siragy HM. Role of the angiotensin AT2 receptor in blood pressure regulation and therapeutic implications. Am J Hypertens 2001; 14: 98S–102S. [DOI] [PubMed] [Google Scholar]
- 37. Tsutsumi Y, Matsubara H, Masaki H, et al. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J Clin Invest 1999; 104: 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moltzer E, Verkuil AV, van VR, et al. Effects of angiotensin metabolites in the coronary vascular bed of the spontaneously hypertensive rat: Loss of angiotensin II type 2 receptor-mediated vasodilation. Hypertension 2010; 55: 516–522. [DOI] [PubMed] [Google Scholar]
- 39. You D, Loufrani L, Baron C, et al. High blood pressure reduction reverses angiotensin II type 2 receptor-mediated vasoconstriction into vasodilation in spontaneously hypertensive rats. Circulation 2005; 111: 1006–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Phoon S, Howes LG. Forearm vasodilator response to angiotensin II in elderly women receiving candesartan: Role of AT(2)- receptors. J Renin Angiotensin Aldosterone Syst 2002; 3: 36–39. [DOI] [PubMed] [Google Scholar]
- 41. Hilliard LM, Jones ES, Steckelings UM, et al. Sex-specific influence of angiotensin type 2 receptor stimulation on renal function: A novel therapeutic target for hypertension. Hypertension 2012; 59: 409–414. [DOI] [PubMed] [Google Scholar]
- 42. Hilliard LM, Sampson AK, Brown RD, et al. The “his and hers” of the renin-angiotensin system. Curr Hypertens Rep 2013; 15: 71–79. [DOI] [PubMed] [Google Scholar]
- 43. Zimmerman MA, Sullivan JC. Hypertension: What’s sex got to do with it? Physiology (Bethesda) 2013; 28: 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Khalil RA. Sex hormones as potential modulators of vascular function in hypertension. Hypertension 2005; 46: 249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gallagher PE, Li P, Lenhart JR, et al. Estrogen regulation of angiotensin-converting enzyme mRNA. Hypertension 1999; 33: 323–328. [DOI] [PubMed] [Google Scholar]
- 46. Nickenig G, Baumer AT, Grohe C, et al. Estrogen modulates AT1 receptor gene expression in vitro and in vivo. Circulation 1998; 97: 2197–2201. [DOI] [PubMed] [Google Scholar]
- 47. Ji H, Menini S, Zheng W, et al. Role of angiotensin-converting enzyme 2 and angiotensin(1–7) in 17beta-oestradiol regulation of renal pathology in renal wrap hypertension in rats. Exp Physiol 2008; 93: 648–657. [DOI] [PubMed] [Google Scholar]
- 48. Lim YK, Retnam L, Bhagavath B, et al. Gonadal effects on plasma ACE activity in mice. Atherosclerosis 2002; 160: 311–316. [DOI] [PubMed] [Google Scholar]
- 49. Laughlin GA, Barrett-Connor E, Kritz-Silverstein D, et al. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: The Rancho Bernardo Study. J Clin Endocrinol Metab 2000; 85: 645–651. [DOI] [PubMed] [Google Scholar]
- 50. Rohrmann S, Sutcliffe CG, Bienstock JL, et al. Racial variation in sex steroid hormones and the insulin-like growth factor axis in umbilical cord blood of male neonates. Cancer Epidemiol Biomarkers Prev 2009; 18: 1484–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Potischman N, Troisi R, Thadhani R, et al. Pregnancy hormone concentrations across ethnic groups: Implications for later cancer risk. Cancer Epidemiol Biomarkers Prev 2005; 14: 1514–1520. [DOI] [PubMed] [Google Scholar]
- 52. Joham AE, Boyle JA, Zoungas S, et al. Hypertension in reproductive-aged women with polycystic ovary syndrome and association with obesity. Am J Hypertens 2015; 28: 847–851. [DOI] [PubMed] [Google Scholar]
- 53. Zhou Y, Zhou X, Guo X, et al. Prevalence and risk factors of hypertension among pre- and post-menopausal women: A cross-sectional study in a rural area of northeast China. Maturitas 2015; 80: 282–287. [DOI] [PubMed] [Google Scholar]
- 54. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2013 update: A report from the American Heart Association. Circulation 2013; 127: e6–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nora EH, Munzenmaier DH, Hansen-Smith FM, et al. Localization of the ANG II type 2 receptor in the microcirculation of skeletal muscle. Am J Physiol 1998; 275: H1395–H1403. [DOI] [PubMed] [Google Scholar]
- 56. Matrougui K, Loufrani L, Heymes C, et al. Activation of AT(2) receptors by endogenous angiotensin II is involved in flow-induced dilation in rat resistance arteries. Hypertension 1999; 34: 659–665. [DOI] [PubMed] [Google Scholar]
- 57. Wang M, Tsai BM, Kher A, et al. Role of endogenous testosterone in myocardial proinflammatory and proapoptotic signaling after acute ischemia-reperfusion. Am J Physiol Heart Circ Physiol 2005; 288: H221–H226. [DOI] [PubMed] [Google Scholar]
- 58. Williams MR, Ling S, Dawood T, et al. Dehydroe-piandrosterone inhibits human vascular smooth muscle cell proliferation independent of ARs and ERs. J Clin Endocrinol Metab 2002; 87: 176–181. [DOI] [PubMed] [Google Scholar]
- 59. Osuga Y, Liang SG, Dallas JS, et al. Soluble ecto-domain mutant of thyrotropin (TSH) receptor incapable of binding TSH neutralizes the action of thyroid-stimulating antibodies from Graves’ patients. Endocrinology 1998; 139: 671–676. [DOI] [PubMed] [Google Scholar]
- 60. Lushsinger PC, Sachs M, Patel DJ. Pressure-radius relationship in large blood vessels of man. Circ Res 1962; 11: 885–888. [DOI] [PubMed] [Google Scholar]