Abstract
Background
We investigated the influence of Resolvin D1 (RvD1) on the inflammatory response in PC12 cells (a cell model of Parkinson disease, PD).
Material/Methods
4 mmol/L 1-methyl-4-phenylpyridinium ion (Mpp+) was used in PC12 cells for an in vitro PD model. 3-(4,5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2-H-tetrazolium bromide (MTT) assay was used to explore PC12 cell viability. Western blot (WB) experiments were used to identify nuclear factor-κB (NF-κB), phosphorylated extracellular signal-regulated kinase (p-ERK)/p-Jun N-terminal kinase (JNK)/p-P38 mitogen-activated protein kinase (MAPK), tumor necrosis factor (TNF)-α, and interleukin (IL)-6 protein levels. Transcription levels of inflammatory factors, for instance, TNF-α and IL-6, were explored by real-time quantitative polymerase chain reaction (RT-QPCR). Lactic dehydrogenase (LDH) level was detected by enzyme-linked immunosorbent (ELISA). Cell apoptosis was assessed by Annexin-V Fluorescein (FITC) kit.
Results
RvD1 dose-dependently inhibited MPP+ induced upregulation of PC12 cell apoptosis/cellular damage/TNF-α and p-P38/p-ERK/NF-κB as well as downregulation of PC12 cell viability.
Conclusions
We can draw the conclusion that RvD1 attenuates PD via inhibiting Mpp+-induced inflammation in PC12 cells.
MeSH Keywords: Inflammation, Mitogen-Activated Protein Kinase Kinases, Parkinsonian Disorders
Background
Parkinson disease (PD) affects the motor system of about several million patients globally [1,2], and is much more common in the elderly population [2]. In 1990, PD led to 44,000 deaths, which increased to 103,000 deaths in 2013, worldwide [3].
The primary pathology of PD is dopaminergic (DA) neuron loss in the area of the substantia nigra (SN) [4,5]. Studies have demonstrated that inflammation participates in the process of DA neuron degeneration [6,7].
TNF-α, IL-1β, and nitric oxide (NO), which are from either neuronal nitric oxide synthase (nNOS) or inducible nitric oxide synthase (iNOS), represent three pivotal harmful inflammatory mediators [8,9]. Furthermore, TNF-α and IL-1β were found to deteriorate injury of the DA neuron, which was generated by NO [10], thus generating chronic inflammation. This maintained inflammation may further deteriorate PD. Phosphatidyl inositol 3-kinase (PI3K)/protein kinase B (Akt)/NF-κB signaling pathways were found to be activated in PD, thus upregulating levels of TNF-α/IL-β [11]. Nuclear factor (erythroid-derived 2)-like 2 (Nrf2)/antioxidant responsive element (ARE) signaling pathway has also been demonstrated to be inactivated to provide harmful effects on experimental models of neurological diseases [12].
Resolvins are compounds synthesized by the human body from omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [13]. There are three kinds of resolvins, i.e., RvE that comes from EPA, RvD which is derived from DHA, and aspirin-triggered RvD (AT-RvD) [14]. RvD1 has been reported to play a therapeutic role in reduction of inflammatory pain [15]. Meanwhile, RvD1 attenuates lipopolysaccharide (LPS)-induced lung inflammation through peroxisome proliferator-activated receptor gamma (PPARγ)/nuclear factor-kappa B (NF-κB) pathway [16]. Whereas, it is unclear whether RvD1 inhibits PD progression via suppressing inflammation.
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is an environmental toxin that specifically damages DA neurons, brings about symptoms which are similar to PD in humans, clinically [17,18]. Therefore, MPTP and MPP+ (metabolite of MPTP) are commonly applied to obtain PD models [18,19]. Our current study aimed to investigate whether RvD1 protects PC12 cells from MPP+-induced PD in vitro, for providing a neuroprotective agents for PD.
Material and Methods
Cell culture
PC12 cells were incubated in RPMI-1640 (Invitrogen, USA), inactivated in 10% horse serum (Invitrogen, USA) and 5% fetal calf serum (Invitrogen, USA) in the incubator with 5% CO2 at the temperature of 37°C. PC12 cells were prompted to differentiate on collagen-treated plates via 100 ng/mL nerve growth factor (NGF) for about nine days. At 24 hours before the experiments, PC12 cells were rinsed with RPMI-1640 that contained 1% fetal bovine serum (FBS).
The in vitro PD model was established by treatment of PC12 cells with 250 μM MPP+ for approximately 24 hours. Different concentrations of RvD1 (50, 100, 200 nM) were administrated two hours prior to MPP+ treatment, afterwards, PC12 cells were used for following experiments.
Flow cytometry assay
Cell apoptosis was assessed by FITC apoptosis detection kit (Oncogene Research Products, San Diego, CA, USA) in accordance with manufacturer’s instructions. Samples were analyzed by a flow cytometry apparatus (Becton Dickinson FACSVantage SE, San Jose, CA, USA). Dual analysis was adopted while necrotic cells were PI-positive, early apoptotic cells were annexin V-FITC-positive, and cells at late apoptosis stage were positive for annexin V-FITC/PI. Cells that stained with neither annexin V-FITC nor PI were classified as live cells. The majority of live cells fell into the FITC/PI negative area which indicated the gating strategy was correct in current study. Cell numbers in each category are presented.
MTT assay
Cell viability was assessed by MTT; 200 μL cells at the concentration of 1×104/mL were incubated in 96-well plates. After incubation for about 24 hours, 20 μL MTT solution (at the concentration of 5 mg/mL) was added into each well and the plate was further incubated at 37°C for four hours. Then the wells were washed with PBS, and 150 μL DMSO was added. The microtiter plate was placed on a shaker to thoroughly dissolve the dye. Absorbance at 450 nm was read using a Bio-Rad iMark plate reader.
ELISA
Supernatant culture medium in different groups were collected and applied to evaluate the degree of PC12 cellular damage via LDH detection kit (Promega) according to manufacturer’s instruction. Absorbance at 492 nm was read.
RT-QPCR
RNA was transcribed into cDNA by reverse transcriptase according to manufacturer’s instructions (Takara). RT-QPCR reactions were: 2 μL cDNA, 5 μL mixture (Bio-Rad, Hercules, CA), 0.5 μL forward/reverse primer, and 2 μL nanopure water. Conditions for amplification were: denaturation at 95 °C for 10 seconds, extension at the 60 °C for 60 seconds, the process lasted for 40 cycles. Data were conducted in replicates. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) acted as the corresponding control. The primers were:
TNF-α,
forward: 5′GACCCTCACACTCAGATCATCT-T-3′,
reverse, 5′-CCACTTGGTGGTTTGCTACGA-3′; IL-6,
forward: 5′-GAGGATACCACTCCCAACAGACC-3′,
reverse, 5′-AAGTGCATCATCGTTGTTCATACA-3′;
GAPDH,
forward: 5′-ATGTGTCCGTCGTGGATCTGA-3′,
reverse: 5′-ATGCCTGCTTCACCACCTTCT-3′.
Western blot (WB)
To identify protein levels of GAPDH (37kD), ERK (42/44kD)/p-ERK (42/44kD)/JNK (46/54kD)/p-JNK (46/54kD)/P38 (38kD)/p-P38 (43kD) MAPK, NF-κB p50 (50kD), and TNF-α (17kD)/IL-6 (18.5kD), cell lysates were analyzed. Briefly, samples were washed with PBS and treated with RIPA lysis buffer (Roche, China), separated with sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and electro-transferred onto polyvinylidene fluoride (PVDF) membranes. Membranes were blocked with 5% bovine serum albumin (BSA) at room temperature for about one hour, and incubated with primary antibody at 4°C for about 10 hours. Thereafter, PVDF membranes were incubated with the secondary antibody at room temperature for approximately one hour. GAPDH performed as a control.
Statistical analysis
Differences among groups were tested with two-way analysis of variance (ANOVA) with the following Bonferroni post hoc tests; p<0.05 indicates significant difference.
Results
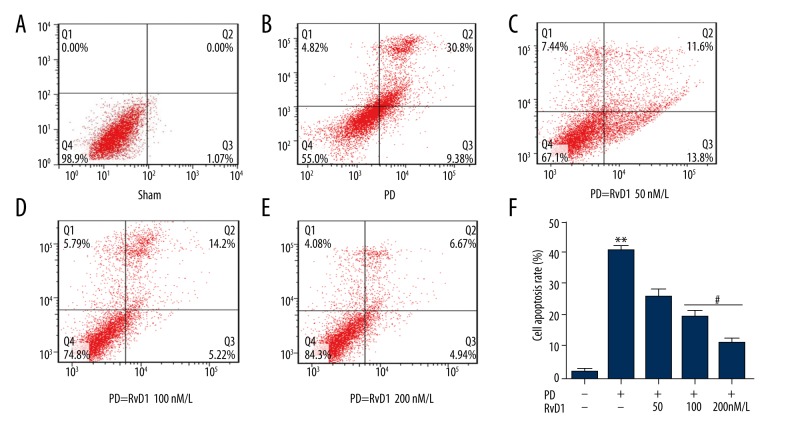
RvD1 attenuates MPP+ induced PC12 cell apoptosis
FITC was applied to detect cell apoptosis in different groups, results demonstrated that compared with the control group (Figure 1A), MPP+ significantly induced PC12 cell apoptosis (Figure 1B), which was rescued by RvD1 (50, 100, and 200 nM) (Figure 1C–1E). Only RvD1 (100 and 200 nM) showed remarkable effects as shown in Figure 1F.
Figure 1.
Effects of RvD1 on MPP+ induced upregulation of PC12 cell apoptosis (A–F). ** p<0.01, PD vs. sham group; # p<0.05 PD + RvD1 vs. PD group.
RvD1 rescues MPP+ induced downregulation of PC12 cell viability
MTT assay was carried out to identify cell viability in different groups. Results showed that MPP+ seriously downregulated PC12 cell viability when compared with the control group, which was significantly rescued by (50, 100, and 200 nM). Only RvD1 (100 and 200 nM) showed remarkable effects (Figure 2).
Figure 2.
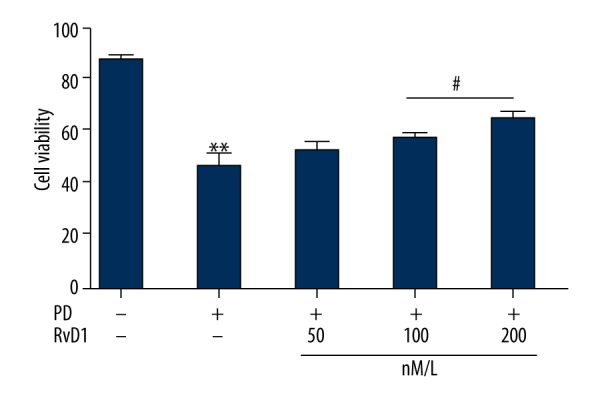
Effects of RvD1 on MPP+ induced downregulation of PC12 cell viability. ** p<0.01, PD vs. sham group; # p<0.05, PD + RvD1 vs. PD group.
RvD1 attenuates MPP+ induced PC12 cellular damage
LDH level in the supernatant culture medium was assessed by ELISA to reflect the degree of PC12 cellular damage in different groups. Results showed that there was a higher LDH level in the MPP+ treated group than the control group, moreover, when PC12 cells were co-administrated with MPP+ and RvD1, results revealed that RvD1 (50, 100, and 200 nM) dose-dependently lowered MPP+ induced upregulation of LDH (Figure 3).
Figure 3.
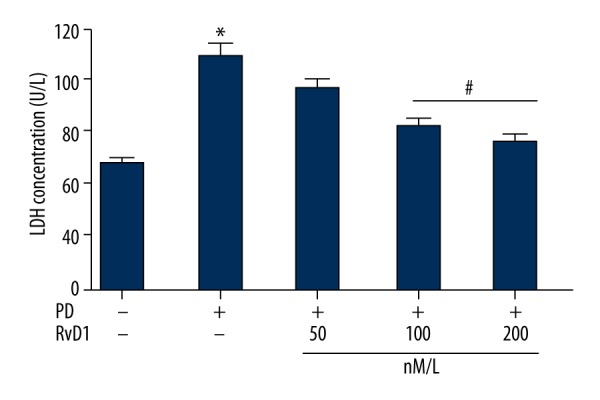
Effects of RvD1 on MPP+ induced elevation of LDH level. * p<0.05, PD vs. sham group; # p<0.05, PD + RvD1 vs. PD group.
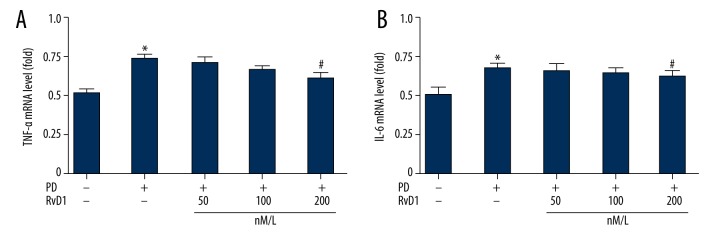
RvD1 attenuates MPP+ induced upregulation of mRNA levels of TNF-α in PC12 cells
Transcription levels of inflammatory factors including TNF-α and IL-6 were tested by RT-QPCR. Results showed that, in comparison with the control, PC12 cells that were treated with MPP+ synthesized more inflammatory cytokines, including TNF-α and IL-6; and RvD1 (200 nM) inhibited elevation of TNF-α (Figure 4A) but not IL-6 (Figure 4B).
Figure 4.
Effects of RvD1 on MPP+ induced elevation of mRNA levels of TNF-α (A) and IL-6 (B). * p<0.05, PD vs. sham group; # p<0.05, PD + RvD1 vs. PD group.
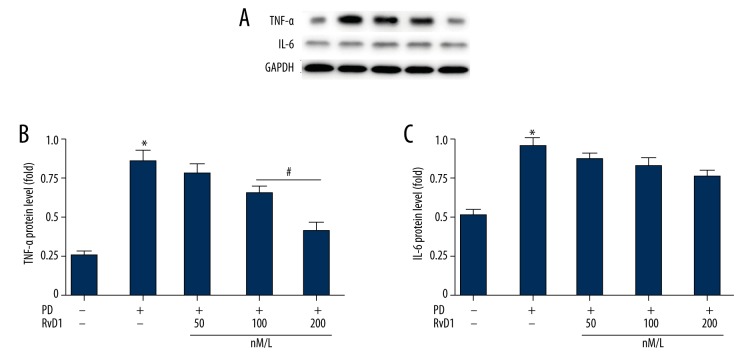
RvD1 attenuates MPP+ induced upregulation of protein levels of TNF-α in PC12 cells
Meanwhile, we tested the protein levels of TNF-α and IL-6 by WB. Results were consistent with that of RT-QPCR (Figure 5). Expression levels of TNF-α and IL-6 are shown in Figure 5A. RvD1 (100 and 200 nM) significantly inhibited MPP+ induced elevation of TNF-α (Figure 5B), but RvD1 showed no significant influence effect on IL-6 levels (Figure 5C).
Figure 5.
Effects of RvD1 on MPP+ induced elevation of protein levels of TNF-α (A, B) and IL-6 (A, C). * p<0.05, PD vs. sham group; # p<0.05, PD + RvD1 vs. PD group.
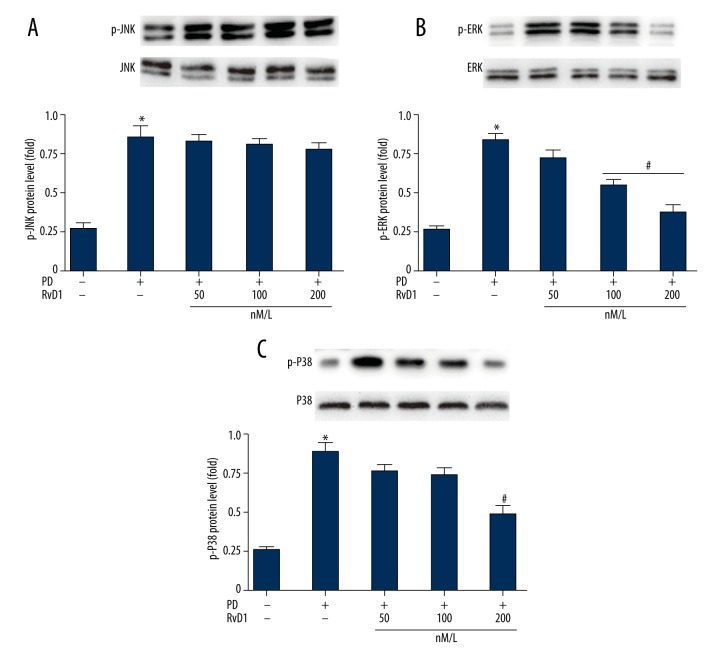
RvD1 attenuates MPP+ induced upregulation of p-P38/p-ERK in PC12 cells
Protein levels of p-JNK/ERK/P38 in different groups were evaluated by WB (Figure 6). Results revealed that in the MPP+ groups, all of them were higher than the control group (Figure 6A). And RvD1 inhibited elevation of p-P38 and p-ERK (Figure 6C, 6D) but not p-JNK (Figure 6B).
Figure 6.
Effects of RvD1 on MPP+ induced elevation of protein levels of p-JNK (A, B), p-ERK (A, C) and p-P38 (A, D). * p0.05, PD vs. sham group; # p<0.05, PD + RvD1 vs. PD group.
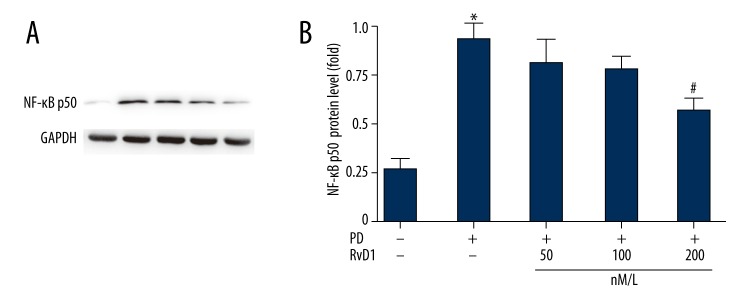
RvD1 attenuates MPP+ induced upregulation of NF-κB p50 in PC12 cells
Protein levels of NF-κB p50 in the different groups were evaluated by WB (Figure 7). Results showed that in the MPP+ groups, NF-κB p50 was higher than in the control group (Figure 7A) which was inhibited by RvD1 administration. The statistical data are presented in Figure 7B.
Figure 7.
Effects of RvD1 on MPP+ induced elevation of protein levels of NF-κB p50 (A). The statistical data were shown in B. * p<0.05, PD vs. sham group; # p<0.05, PD + RvD1 vs. PD group.
Discussion
In the present study, RvD1 was discovered to dose-dependently inhibit MPP+ induced upregulation of PC12 cell apoptosis/cellular damage/TNF-α/IL-6 and p-P38/p-ERK as well as downregulation of PC12 cell viability. We can draw the conclusion that RvD1 ameliorated PD by repressing inflammation, and provided new possible therapeutic targets for PD.
PC12 cells exhibited physiological properties and similar characteristics to DA neurons, consequently, they were adopted to establish MPP+-induced cellular model of PD [20,21].
Cell apoptosis in different groups was detected by flow cytometry assay. In MPP+ treated group, there was about 50% PC12 cell apoptosis, which was higher than the control group. This result is in agreement with previous studies [19,22]. Moreover, RvD1 (50, 100, and 200 nM) rescued MPP+-induced upregulation of PC12 cell apoptosis; and only higher doses of RvD1 (100 and 200 nM) showed remarkable effects. Meanwhile, we carried out MTT assays to identify the effects of RvD1 on cell viability in different groups. Compared with the control group, MPP+ seriously downregulated PC12 cell viability, which was notably rescued by RvD1 (100 and 200 nM). These results were consistent with a previous study [23]. Taken together, we could hypothesize that RvD1 played a protective role on MPP+-induced damage to PC12 cells. It was unknown whether RvD1 showed effective effects depending on molecules that were correlated with the aforementioned results.
LDH is an enzyme found in nearly all living cells, including animals, plants and prokaryotes. Comparison of LDH value with normal range helps guide diagnosis. Broken tissue releases LDH, which can be measured as a surrogate for tissue breakdown. Released LDH by PC12 cells was collected from the supernatant culture medium and assessed by ELISA to assess cell damage. Higher LDH level was detected in the MPP+ group compared to the control group. Furthermore, when PC12 cells were co-treated with MPP+ and RvD1, RvD1 (50, 100, and 200 nM) dose-dependently lower MPP+ induced upregulation of LDH.
Dying neurons can trigger elevated expression/release of immunological participants [24]. These include IL-1β, IL-6, and TNF-α [24,25]. RT-QPCR results showed that, in comparison with the control, PC12 cells that were treated with MPP+ synthesized more inflammatory cytokines, including TNF-α and IL-6. RvD1 (100 and 200 nM) inhibited elevation of TNF-α but not IL-6. Meanwhile, their protein level changes were consistent with that of mRNA.
Major regulators of this response involve tyrosine kinase, PI3K/Akt, and MAPK signaling pathways such as JNK, ERK1/2, and p38 MAPK [26,27]. Moreover, MAPKs could be stimulated by cytokines or inflammatory factors, and induced gene transcription via phosphorylating NF-κB [28]. There are multiple forms of NF-κB, and the most common form is heterodimer p50/p65, which is tightly correlated with the transcription of TNF-α and IL-6 [29]. Therefore, protein levels of p-JNK/ERK/P38/NF-κB p50 in different groups were evaluated by WB in our current study to identify whether MAPKs were correlated with the protective effects of RvD1. Results revealed that in the MPP+ groups, all were higher than the control group. And RvD1 (100 and 200 nM) inhibited elevation of p-P38/p-ERK/NF-κB p50 protein levels.
Taken together, our current study demonstrated that RvD1 attenuated PD via inhibiting activation of p-P38/p-ERK1/2/NF-κB signaling pathway, and the synthesis of TNF-α and IL-6.
There remain two questions unresolved. 1) As acknowledged, p-ERK1/2 is required for cell survival, growth, and proliferation. In our current study, we observed increased p-ERK1/2 in PC12 cells following MPP treatment; however, this is contrary to the results from the cell viability assay. Thus, there might be other unknown signaling pathways that took part in the process. RvD1 has been reported to attenuate LPS-induced acute lung injury by interacting with PPARγ pathway in mice [16]. RvD1 was also found to inhibit TGF-β1-induced epithelial mesenchymal transition in A549 cells by virtue of lipoxin A4 receptor/formyl peptide receptor 2 and GPR32 [30]. It is unknown whether the aforementioned molecules were regulated by RvD1 in PC12 cells. 2) It is unknown whether RvD1 can rescue MPP induced PC12 cell death in the presence of TNF-α. We will test this in future work.
Conclusions
RvD1 attenuates PD via inhibiting inflammation; and provides a possible therapeutic method for PD.
Acknowledgement
The authors thank Dr Xiang Gao, Professor of Medicine, for his help in interpreting the significance of the results in the current study. We also thank biological laboratory staff in the Medical School of Ningbo University for their advice on experimental design.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Yao SC, Hart AD, Terzella MJ. An evidence-based osteopathic approach to Parkinson disease. Osteopathic Family Physician. 2013;5:96–101. [Google Scholar]
- 2.de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525–35. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2013 Mortality and Causes of Death, Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;385:117–71. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes AJ, Daniel SE, Kilford L, Leea AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–84. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergman H, Deuschl G. Pathophysiology of Parkinson’s disease: From clinical neurology to basic neuroscience and back. Mov Disord. 2002;17(Suppl 3):28–40. doi: 10.1002/mds.10140. [DOI] [PubMed] [Google Scholar]
- 6.Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG. Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener. 2009;4:47. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGeer PL, McGeer EG. Glial reactions in Parkinson’s disease. Mov Disord. 2008;23:474–83. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- 8.Sawada M, Imamura K, Nagatsu T. Role of cytokines in inflammatory process in Parkinson’s disease. J Neural Transm. 2006;(Suppl):373–81. doi: 10.1007/978-3-211-45295-0_57. [DOI] [PubMed] [Google Scholar]
- 9.Nagatsu T, Sawada M. Inflammatory process in Parkinson’s disease: Role for cytokines. Curr Pharm Des. 2005;11:999–1016. doi: 10.2174/1381612053381620. [DOI] [PubMed] [Google Scholar]
- 10.Stoll G, Jander S, Schroeter M. Cytokines in CNS disorders: Neurotoxicity versus neuroprotection. J Neural Transm Suppl. 2000;59:81–89. doi: 10.1007/978-3-7091-6781-6_11. [DOI] [PubMed] [Google Scholar]
- 11.Han MH, Lee WS, Nagappan A, et al. Flavonoids isolated from flowers of lonicera japonica thunb. Inhibit inflammatory responses in BV2 microglial cells by suppressing TNF-α and IL-β through PI3K/Akt/NF-kb signaling pathways. Phytother Res. 2016;30:1824–32. doi: 10.1002/ptr.5688. [DOI] [PubMed] [Google Scholar]
- 12.Kumar H, Kim IS, More SV, et al. Natural product derived pharmacological modulators of Nrf2/ARE pathway for chronic diseases. Nat Prod Rep. 2014;31:109–39. doi: 10.1039/c3np70065h. [DOI] [PubMed] [Google Scholar]
- 13.Serhan CN, Hong S, Gronert K, et al. Resolvins: A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–37. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy BD. Resolvins and protectins: Natural pharmacophores for resolution biology. Prostaglandins Leukotrienes Essential Fatty Acids. 2010;82:327–32. doi: 10.1016/j.plefa.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu ZZ, Zhang L, Liu T, et al. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nature Medicine. 2010;16:592–97. doi: 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao ZL, Dong J, Wu W, et al. Resolvin D1 attenuates inflammation in lipopolysaccharide-induced acute lung injury through a process involving the PPAR gamma/NF-kappa B pathway. Respir Res. 2012;13(1):110. doi: 10.1186/1465-9921-13-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–80. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 18.Tetrud JW, Langston JW. MPTP-induced parkinsonism as a model for Parkinson’s disease. Acta Neurol Scand Suppl. 1989;126:35–40. doi: 10.1111/j.1600-0404.1989.tb01780.x. [DOI] [PubMed] [Google Scholar]
- 19.Marongiu ME, Piccardi MP, Bernardi F, et al. Evaluation of the toxicity of the dopaminergic neurotoxins MPTP and MPP+ in PC12 pheochromocytoma cells: Binding and biological studies. Neurosci Lett. 1988;94:349–54. doi: 10.1016/0304-3940(88)90043-2. [DOI] [PubMed] [Google Scholar]
- 20.Presse F, Cardona B, Borsu L, Nahon JL. Lithium increases melanin-concentrating hormone mRNA stability and inhibits tyrosine hydroxylase gene expression in PC12 cells. Brain Res Mol Brain Res. 1997;52:270–83. doi: 10.1016/s0169-328x(97)00273-8. [DOI] [PubMed] [Google Scholar]
- 21.Zachor DA, Moore JF, Brezausek C, et al. Cocaine inhibits NGF-induced PC12 cells differentiation through D(1)-type dopamine receptors. Brain Res. 2000;869:85–97. doi: 10.1016/s0006-8993(00)02355-6. [DOI] [PubMed] [Google Scholar]
- 22.Lee CS. Modulation of 1-methyl-4-phenylpyridinium-induced mitochondrial dysfunction and cell death in PC12 cells by K (ATP) channel block. J Neural Transm. 2007;114:297–305. doi: 10.1007/s00702-006-0594-3. [DOI] [PubMed] [Google Scholar]
- 23.Lee HJ, Park MK, Lee EJ, Lee CH. Resolvin D1 inhibits TGF-beta 1-induced epithelial mesenchymal transition of A549 lung cancer cells via lipoxin A4 receptor/formyl peptide receptor 2 and GPR32. International Journal of Biochemistry & Cell Biology. 2013;45:2801–7. doi: 10.1016/j.biocel.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Hald A, van Beek J, Lotharius J. Inflammation in Parkinson’s disease: Causative or epiphenomenal? Subcell Biochem. 2007;42:249–79. [PubMed] [Google Scholar]
- 25.Tansey MG, Goldberg MS. Neuroinflammation in Parkinson’s disease: Its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis. 2010;37:510–18. doi: 10.1016/j.nbd.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SH, Kim J, Sharma RP. Inhibition of p38 and ERK MAP kinases blocks endotoxininduced nitric oxide production and differentially modulates cytokine expression. Pharmacol Res. 2004;49:433–39. doi: 10.1016/j.phrs.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Thomas T, Timmer M, Cesnulevicius K, et al. MAPKAP kinase 2-deficiency prevents neurons from cell death by reducing neuroinflammation-relevance in a mouse model of Parkinson’s disease. J Neurochem. 2008;105:2039–52. doi: 10.1111/j.1471-4159.2008.05310.x. [DOI] [PubMed] [Google Scholar]
- 28.Hua LL, Zhao ML, Cosenza M, et al. Role of mitogen-activated protein kinases in inducible nitric oxide synthase and TNF alpha expression in human fetal astrocytes. J Neuroimmunol. 2002;126:180–89. doi: 10.1016/s0165-5728(02)00055-3. [DOI] [PubMed] [Google Scholar]
- 29.Tilstra JS, Clauson CL, Niedernhofer LJ, Robbins PD. NF-kappaB in Aging and Disease. Aging Dis. 2011;2:449–65. [PMC free article] [PubMed] [Google Scholar]
- 30.Lee HJ. Resolvin D1 inhibits TGF-beta 1-induced epithelial mesenchymal transition of A549 lung cancer cells via lipoxin A4 receptor/formyl peptide receptor 2 and GPR32. International Journal of Biochemistry & Cell Biology. 2013;45:2801–7. doi: 10.1016/j.biocel.2013.09.018. [DOI] [PubMed] [Google Scholar]