Abstract
Non-local or crossover (contralateral and non-stretched muscles) increases in range-of-motion (ROM) and balance have been reported following rolling of quadriceps, hamstrings and plantar flexors. Since there is limited information regarding plantar sole (foot) rolling effects, the objectives of this study were to determine if unilateral foot rolling would affect ipsilateral and contralateral measures of ROM and balance in young healthy adults. A randomized within-subject design was used to examine non-local effects of unilateral foot rolling on ipsilateral and contralateral limb ankle dorsiflexion ROM and a modified sit-and-reach-test (SRT). Static balance was also tested during a 30 s single leg stance test. Twelve participants performed three bouts of 60 s unilateral plantar sole rolling using a roller on the dominant foot with 60 s rest intervals between sets. ROM and balance measures were assessed in separate sessions at pre-intervention, immediately and 10 minutes post-intervention. To evaluate repeated measures effects, two SRT pre-tests were implemented. Results demonstrated that the second pre-test SRT was 6.6% higher than the first pre-test (p = 0.009, d = 1.91). There were no statistically significant effects of foot rolling on any measures immediately or 10 min post-test. To conclude, unilateral foot rolling did not produce statistically significant increases in ipsilateral or contralateral dorsiflexion or SRT ROM nor did it affect postural sway. Our statistically non-significant findings might be attributed to a lower degree of roller-induced afferent stimulation due to the smaller volume of myofascia and muscle compared to prior studies. Furthermore, ROM results from studies utilizing a single pre-test without a sufficient warm-up should be viewed critically.
Key points.
Unilateral foot rolling did not improve ROM of the ankle or hamstring and lower back ROM (SRT) in either limb.
Unilateral foot rolling did not affect postural sway (balance).
Studies examining changes in ROM should use multiple pre-tests or a comprehensive stretching warm-up to prevent a repeated order effect.
Key words: Crossover, flexibility, postural sway, myofascial, self massage
Introduction
Neuromuscular rolling, which has also been described as self-myofascial release or self-massage therapy has become a popular and legitimate technique for enhancing range of motion (ROM) by massaging muscles and connective tissue with a tool instead of a clinician’s manual therapy (Beardsley and Škarabot, 2015; Paolini, 2009). The device can for instance be a foam roller (MacDonald et al., 2013; 2014; Peacock et al., 2015; Škarabot et al., 2015), a roller massage stick (Halperin et al., 2014; Jay et al., 2014; Mikesky et al., 2002), or a tennis ball (Grieve et al., 2015). Prior studies have demonstrated improved ROM with rolling of the quadriceps (Bradbury-Squires et al., 2015; MacDonald et al., 2013; 2014; Pearcey et al., 2015), hamstrings (Mohr et al., 2014; Sullivan et al. 2013), plantar flexors (Aboodarda et al. 2015; Halperin et al., 2014; Kelly and Beardsley, 2016), and plantar soles (Grieve et al., 2015). Increased local ROM in the massaged muscles has been reported immediately after (Bradbury-Squires et al., 2015; Grieve et al., 2015; Halperin et al., 2014; Jay et al., 2014; MacDonald et al., 2013; Škarabot et al., 2015; Sullivan et al., 2013) and up to 20 minutes following the rolling intervention (Junker and Stoggl, 2015; Kelly and Beardsley, 2016; Mohr et al., 2014). While some studies used the contralateral limb for control (Lanigan and Harrison, 2012; Murray et al. 2016; Sullivan et al., 2013), only a few studies have examined non-local effects (i.e., effects in the non-treated area) on ROM (Behm et al., 2016).
Grieve et al. (2015) observed that rolling the foot with a tennis ball ameliorated hamstring and lower back ROM as a non-local effect. Moreover, Kelly and Beardsley (2016) reported that plantar flexor rolling increased ankle dorsiflexion ROM with the ipsilateral and contralateral limbs. These effects lasted for 20 and 10 minutes, respectively. The authors suggested neurophysiological mechanisms to be responsible for ROM effects due to neuromuscular rolling. While there is a void in the literature with regards to the global effects of neuromuscular rolling, recent research has indicated non-local (Behm et al., 2016) and cross-over (Behm et al., 2016; Chaouachi et al., 2015) stretching effects. Static stretching effects in these studies have also been attributed to neural responses. Of note, knowledge that is concerned with non-local and cross-over effects following neuromuscular rolling is of high clinical relevance. For instance, non-local effects may be useful when a patient or client is in need of greater ROM, but cannot tolerate neuromuscular rolling applied directly to the injured or painful limb or area. Hence, there is a need to expand the currently available foot rolling studies (Grieve et al., 2015; Kelly & Beardsley, 2016) by investigating global ROM effects of foot rolling including ipsilateral and contralateral limb responses.
Manual plantar sole (foot) stimulation is suggested to improve postural awareness (Chatchawan et al., 2015; Hlavackova and Vuillerme, 2012; Kavounoudias et al., 1998; 2001; Lanigan and Harrison, 2012; Maki et al., 1999; Maurer et al., 2001; McKeon et al., 2015; Meyer et al., 2004; Roll et al., 2002; Watanabe and Okubo, 1981). Kavounoudias et al. (1998) showed that the amplitude of whole body tilts were dependent upon the frequency of vibration stimulation to the sole. Changes in cutaneous afferent information were most likely responsible for the observed findings. Both supraspinal and spinal output could be affected by neuromuscular rolling. For example, Aboodarda et al. (2015) suggested that heavy roller massage stimulates both superficial cutaneous and deep tissue nociceptive receptors, which traverse both short (spinal) and long (supraspinal) loop reflex pathways. Other roller massage studies have suggested central modulation with the rolling responses attributed to pain inhibition theories such as gate control or diffuse noxious inhibitory control (DNIC) (Cavanaugh et al., 2016, Sullivan et al., 2013). H-reflexes (afferent excitation of the spinal motoneurons) are reduced with massage illustrating spinal motoneuron modulation (Behm et al., 2013). To the authors’ knowledge, the impact of foot rolling on static balance performance is unresolved. Whether neuromuscular rolling can be used as an alternative means to manually stimulate the foot and to ultimately improve static balance is of clinical importance.
Considering the aforementioned gaps in the literature, the objectives of the present study were to examine immediate and short-term effects of rolling the foot on local and non-local ROM and static balance of the ipsilateral and contralateral limbs in young healthy adults. With reference to the relevant literature (Grieve et al., 2015; Halperin et al., 2014; 2015; Kavounoudias et al., 2001), it was hypothesized that foot rolling enhances ROM along the posterior musculoskeletal chain on the ipsilateral and contralateral limb and improves static balance immediately and 10 min after the intervention. A secondary purpose was to examine the effect of repeated measures upon the consistency of the pre-test evaluation. It was hypothesized that a second ROM pre-test would result in greater flexibility scores.
Methods
Participants
We used the freeware tool G*Power (http://www.gpower.hhu.de/) to calculate an a priori power analysis based on a related study that examined the effects of foot rolling on active and passive ROM (Grabow et al., 2017). The power analysis was computed with an assumed Type I error of 0.01, a Type II error rate of 0.05 (95% statistical power), and aneffect size of 0.8for active ROM. The analysis revealed that 12 individuals would be sufficient to observe large main effects of time.
Hence, twelve healthy and physically active kinesiology students (Table 1) were recruited via posters to participate in this study. All participants were resistance and/or aerobically trained (minimum of 3 sessions with 20 min duration each per week) and did not roll the foot on a regular basis (less than once a month). Individuals with any history of neurological or musculoskeletal injuries in the past year were excluded from this study. Participants were asked to refrain from vigorous physical activity, abstain from alcoholic beverages for 24 hours and any caffeinated beverages two hours prior to testing sessions to reduce bias in the testing battery. After a brief explanation of the study, all individuals signed a written letter of consent and completed the Physical Activity Readiness Questionnaire form (PAR-Q; Canadian Society for Exercise Physiology 2011). The study was approved by the Memorial University of Newfoundland Human Research Ethics Board (reference # 2016.168). All procedures were in accordance with the Declaration of Helsinki (2013).
Table 1.
Participants' characteristics. Data are means (±SD).
| Participants | Males (n=6) |
Females (n=6) |
Overall (n=12) |
|---|---|---|---|
| Age, years | 27.7 (4.6) | 26.7 (1.5) | 27.2 (3.9) |
| Body height, m | 1.82 (.05) | 1.68 (.04) | 1.75 (.09) |
| Body mass, kg | 87.8 (6.5) | 68.3 (7.9) | 78.0 (13.1) |
| Body mass index, kg·m-2 | 26.4 (1.0) | 24.3 (4.0) | 25.4 (3.0) |
Experimental study design
A randomized within-subject design was used to examine non-local effects of foot rolling on ankle dorsiflexion and hamstring and lower back ROM as well as static balance performance on the ipsilateral and contralateral limb, respectively. On each of the three visits, which were separated by at least 24 hours, subjects either completed a control, balance or ROM protocol. All protocols are summarized in Figure 1. The order of testing sessions was randomized by an online randomizer (https://www.randomizer.org/). Testing was done at similar times during the day to reduce diurnal variations. All measures were performed barefoot and in a randomized order, on the dominant and non-dominant leg as identified by the lateral preference inventory (Coren, 1993). Both legs were assessed to control for ipsilateral and contralateral changes, respectively.
Figure 1.
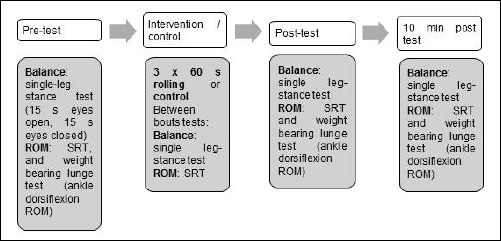
Experimental Design. SRT = modified sit-and-reach test, ROM: range-of-motion.
Tests: Weight bearing lunge test
Ankle dorsiflexion ROM was assessed using the weight bearing lunge test in accordance with previous studies (Halperin et al., 2014; Kelly & Beardsley, 2016). Participants were instructed to place the treated foot a short distance from and perpendicular to the wall and bend the knee until touching the wall against a vertical marker in line with the tibia bone. To ensure the heel did not elevate (Bennell et al., 1998), a Theraband© was placed 2 cm under the participant’s heel and pulled back with the same approximate tension by the same researcher during the trials. If the Theraband could be moved, heel elevation was deemed to have occurred. Depending on the success or failure of the trials (i.e. heel remained in contact with floor or not), the foot was moved 0.5 cm back or forward. The dependent variable was the distance of the great toe in centimeters from the wall.
Sit-and-reach test
To measure hamstring and lower back ROM on the ipsilateral and contralateral limb separately, a variation of the sit-and-reach-test (SRT) was used, as conducted previously in this laboratory (Behm and Chaouachi, 2011; Sullivan et al., 2013). Participants were instructed to extend both limbs with the soles of the feet placed against a flexometer (Acuflex 1, Novel Products Inc., USA) at each testing time to ensure an identical hip position. The contralateral limb was then bent and the longitudinal arch placed against the fully extended knee. A marker was placed on the fully extended knee to ensure matching positioning of the contralateral limb at each testing time. With both hands placed on top of each other, participants moved the flexometer clip until they reached their maximum point of discomfort (Figure 2). Both trials were held for 2 s and displacement measurements were taken to the closest half centimeter. The highest out of two measurements were used for analysis.
Figure 2.

Variation of sit-and-reach test.
Single-leg stance test
Depending on the leg being tested, participants stood on a force platform (AMTI, 400 x 600 x 83 mm, model BP400600 HF-2000 - Watertown, MA02472-4800 122 USA) with either their dominant or non-dominant limb. During all tests participants were instructed to focus on a visual target placed at a distance of approximately 4 m and a height of 170 cm on the opposite wall. Foot position and visual target location were consistent among all tests and participants as both factors are reported to influence posture (Watanabe and Okubo, 1981). Hands were held on the hips with the elbow turned outward (akimbo) during testing. With eyes open, participants were instructed to lift the opposite foot off the ground and to keep the limb in an extended knee position (Figure 3). After 15 s of standing with eyes opened, participants were asked to close their eyes and continue to maintain the posture for another 15 s. A trial was considered to fail when participants’ hands did not remain akimbo, the opposite limb swerved posteriorly or if individuals did not maintain full contact with the force plate.
Figure 3.

Single-leg stance position.
Force plate data was sampled at a rate of 2000 Hz (gain 4000) for the full 30 s trial (15 s eyes opened and 15 s eyes closed). Force data was then exported to an Excel worksheet where CoP was calculated using standard methods. The CoP measures were then examined to determine total CoP path length. In accordance with Prieto et al. (1996), mean CoP speed (m/s) was then assessed for further data analysis. CoP speed was determined for the 15 s eyes open and 15 s eyes closed trials.
Intervention
The Theraband© foot roller (The Hygenic Corporation, Akron, OH, USA) is composed of dense foam made of natural latex and a hollow core. Due to its rigid design, Theraband claims that it allows participants to increase pressure on the device (Figure 4) (http://www.thera-band.com/store/products.php?ProductID=58). Therefore, participants were instructed to reach a 7/10 ratio of perceived pain (RPP) on a visual analog scale on each aspect of the foot. The visual analog scale ranged from 0 (no pain or discomfort) to 10 (maximum tolerable pain). As suggested by Myers (2015), participants were asked to concentrate rolling on the medial, central, and lateral aspect of the dominant foot in each cycle, respectively. One cycle (distal to proximal and return) was completed in 2 s. The rolling pace was controlled by a metronome. Foot rolling was performed from a standing position.
Figure 4.
Plantar sole (foot) neuromuscular rolling procedure.
Procedures
After a brief description of the tests, anthropometrics were assessed. For the ROM protocol, the modified SRT and a weight bearing lunge test were conducted. A second modified SRT was performed prior to the intervention (INTpre) to reduce the effects of repeated measures on SRT (Atha and Wheatley, 1976). This INTpre test served as the baseline for comparison. Following the INTpre-tests, participants performed three bouts of 60 s plantar sole (foot) rolling using the Theraband© foot roller on the dominant foot. The rest interval was 60 s. To assess possible changes after each roll, participants repeated the SRT during each rest period. Consequently, the total intervention time was 6 min. Post-test measurements (weight bearing lunge test for ankle dorsiflexion ROM and SRT) were repeated immediately after the third 60 s bout of plantar sole rolling (INTpost) and 10 min after INTpost (INTpost10). The balance effects of rolling were assessed in another session during which participants performed one single-leg stance at each test time (INTpre, between the rolls, INTpost, and INTpost10). After the first and second roll, balance measures were completed in the 60 s recovery breaks between rolls. The control session consisted of the ROM and balance protocol performed in a sequenced order to prevent stretch-induced changes in the single-leg stance (Behm et al., 2004). Instead of performing foot rolling, participants stood quietly for the same duration as the intervention (3 x 60 s).
Statistical analysis
All data were analyzed using SPSS software (Version 22.0). Normality of data was verified by the Shapiro Wilk test. Accordingly, data were presented as means and standard deviations (SD). The dominant (foot rolled) limb was considered separately from the contralateral non-dominant limb. Intersession reliability from control session to intervention session was determined by calculating Cronbach’s alpha for each limb separately. To evaluate the effect of repeated measures, the SRT scores from the first pre-test and the INTpre-test were analyzed independently in a 2 (intervention, control) x 2 (first and second pre-test) repeated measures analysis of variance (ANOVA). A 2 (condition: intervention or control) x 3 (time: INTpre, INTpost, INTpost10) ANOVA with repeated measures was calculated for ankle dorsiflexion ROM. SRT and single-leg stance included two additional measures (between the sets of foot rolling) and thus a 2 (condition: intervention or control) x 5 (time: INTpre, post-roll 1, post-roll 2, INTpost, INTpost10) repeated measures ANOVA was calculated. Greenhouse-Geisser (GG) corrections were applied if sphericity was disturbed. Bonferroni corrected post-hoc analyses were used if statistically significant main effects of time were identified. In the case of statistically significant interaction effects of condition x time, paired t-tests were used to identify the specific location of differences between variables. Additionally, effect sizes (Cohen’s d) were calculated by assessing partial eta squared with the formula: Cohen’s d = (square root(partial eta square/ABS(1-partial eta partial eta square))*2). Effect sizes were considered small if d < 0.5, medium if 0.5 ≤ d < 0.8 and large with d ≥ 0.8 (Cohen, 1988). Pre- to post-test changes were reported in percentage changes. The significance level was set at p ≤ 0.05.
Results
In general, the determined test-retest reliability scores were classified as high for both limbs (Table 2).
Table 2.
Intersession reliability scores (Cronbach's alpha) for each limb separately.
| Test item | Dominant limb |
Non-dominant limb |
|---|---|---|
| SRT | .978 | .983 |
| Ankle dorsiflexion ROM | .964 | .993 |
| CoP speed | .991 | .993 |
SRT = sit-and-reach test, ROM = range-of-motion, CoP = center of pressure
Sit-and-Reach Test (SRT)
When comparing the two pre-tests, there were no statistically significant main effects of condition in SRT displacement scores differences (F(1,23) = 1.244, p = 0.417, d = 0.51). A significant main effect of time was present (F(1,23) = 18.480, p = 0.009, d = 1.91) (Figure 5). Bonferroni corrected post-hoc analyses revealed that the second SRT pre-test (INTpre) was 6.6% higher on the dominant limb and 5.3% higher on the non-dominant limb than the first pre-test ROM scores. There were no statistically significant differences with the interaction of condition x time (F(1,23) = 0.328, p = 0.502, d = 0.42)(Table 3). With the main effect of time, INTpre served as a baseline for subsequent measures of the dominant and non-dominant limb, respectively.
Figure 5.
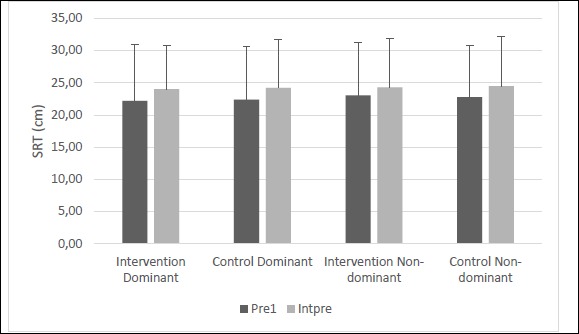
Figure illustrates means and standard deviations of the two sit-and-reach pre-tests (Pre1 and Intpre). Horizontal line indicates there was a main effect for time with an overall increase in sit and reach performance from pre1 to Intpre.
Table 3.
Illustrates the course of sit-and-reach test (SRT) changes on the dominant and non-dominant limb during the intervention session.
| Part | Dominant limb | Non-dominant limb | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| INTpre | Roll 1 | Roll 2 | INTpost | INTpost10 | Δ(%) pre-post |
Δ(%)pre- INTpost10 |
INTpre | Roll 1 | Roll 2 | INTpost | INTpost10 | Δ(%) pre-post |
Δ(%) pre- INTpost10 |
|
| 1 | 33.0 | 35.0 | 36.0 | 37.0 | 36.5 | 12.1 | 10.6 | 36.0 | 36.5 | 37.0 | 37.0 | 37.0 | 2.8 | 2.8 |
| 2 | 16.5 | 16.5 | 16.0 | 20.0 | 21.5 | 21.2 | 30.3 | 18.0 | 19.5 | 19.5 | 20.5 | 23.0 | 13.9 | 27.8 |
| 3 | 20.0 | 20.0 | 21.5 | 22.5 | 21.0 | 12.5 | 5.0 | 16.0 | 16.5 | 18.0 | 20.5 | 20.0 | 28.1 | 25.0 |
| 4 | 34.0 | 35.0 | 36.5 | 36.0 | 33.0 | 5.9 | -2.9 | 33.0 | 32.0 | 34.0 | 34.5 | 33.0 | 4.5 | 0.0 |
| 5 | 23.0 | 24.0 | 25.5 | 23.0 | 26.0 | 0.0 | 13.0 | 24.5 | 24.5 | 23.5 | 25.0 | 26.0 | 2.0 | 6.1 |
| 6 | 37.0 | 37.0 | 37.5 | 37.0 | 36.5 | 0.0 | -1.4 | 36.0 | 36.5 | 36.0 | 37.5 | 36.5 | 4.2 | 1.4 |
| 7 | 19.0 | 19.5 | 16.5 | 17.0 | 19.0 | -10.5 | 0.0 | 14.5 | 16.5 | 16.5 | 17.0 | 16.5 | 17.2 | 13.8 |
| 8 | 41.0 | 43.0 | 45.0 | 45.0 | 43.0 | 9.8 | 4.9 | 41.0 | 41.5 | 42.0 | 41.0 | 39.5 | 0.0 | -3.7 |
| 9 | 26.0 | 26.0 | 27.5 | 27.5 | 26.5 | 5.8 | 1.9 | 25.0 | 27.0 | 27.0 | 26.0 | 26.5 | 4.0 | 6.0 |
| 10 | 32.5 | 33.5 | 35.0 | 35.5 | 33.0 | 9.2 | 1.5 | 33.0 | 32.5 | 34.0 | 34.5 | 35.0 | 4.5 | 6.1 |
| 11 | 43.5 | 45.0 | 44.0 | 44.0 | 44.0 | 1.1 | 1.1 | 41.0 | 43.5 | 44.0 | 42.0 | 43.0 | 2.4 | 4.9 |
| 12 | 30.0 | 30.0 | 31.0 | 30.5 | 30.5 | 1.7 | 1.7 | 27.5 | 29.0 | 27.5 | 28.5 | 27.5 | 3.6 | 0.0 |
| Mean | 29.6 | 30.4 | 31.0 | 31.3 | 30.9 | 5.7 | 5.5 | 28.8 | 29.6 | 29.9 | 30.3 | 30.3 | 7.3 | 7.5 |
| SD | 8.8 | 9.3 | 9.8 | 9.3 | 8.3 | 8.1 | 9.1 | 9.3 | 9.2 | 9.3 | 8.5 | 8.3 | 8.3 | 9.8 |
Part = Participant, INTpre = pre-test prior to intervention, Roll 1 = after the first 60 s foot rolling, Roll 2 = after the second 60 s foot rolling, INTpost = post-test, INTpost10 = post-test 10 min after the intervention, SD = standard deviation
The dominant, foot rolled, limb showed a statistically non-significant main effect of condition, but large magnitude effect size for greater SRT lower back and hamstring ROM with the intervention versus control (Δ 4.3%, F(1,11) = 3.443, p = 0.090, d = 1.1). Additionally, there was a main effect of time (F(4,44) = 3.672, p = 0.012, d = 1.2). Bonferroni corrected post-hoc tests showed a small magnitude effect size for increased ROM from INTpre to INTpost measures (Δ 4.4%, p = 0.067, d = 0.49). There were no interaction effects of condition x time on the dominant limb (F(4,44) = 0.548, p = 0.702, d = 0.44).
There was no main effect of condition on the contralateral, non-dominant, limb (F(1,11) = 2.246, p = 0.162, d = 0.91) but a statistically significant main effect of time (F(4,44) = 4.788, p = 0.003, d = 1.32). According to Bonferroni corrected post hoc tests, INTpost scores were 4.5% higher than the baseline (INTpre) scores (p = 0.049, d = 0.49). There were no interaction effects of condition x time present on the non-dominant limb (F(4,44) = 1.946, p = 0.120, d = 0.84).
Ankle dorsiflexion Range-of-Motion (ROM)
The dominant (foot rolling) side revealed no statistically significant main effect of time (F(2,22) = 2.304, p = 0.123, d = 0.915), condition (F(1,11) = 0.143, p = 0.713, d = 0.23) or the interaction of condition x time (F(2,22) = 0.142, p = 0.869, d = 0.23). Even though our analysis did not detect any statistically significant main effects of condition (F(1,11) = 4.329, p = 0.062, d = 1.253) for the non-dominant (contralateral) limb, a large and thus practically relevant effect size was found for the magnitude of change between intervention and control measures. More specifically, the intervention scores were 2.6% higher than the control measures. There were no main effects of time (F(2,22) = 1.703, p = 0.205, d = 0.78) or interaction effects of condition x time (F(2,22) = 2.879, p = 0.078, d = 1.0). Individual results are presented in Table 4.
Table 4.
Illustrates the course of ankle dorsiflexion ROM changes on the dominant and non-dominant limb during the intervention session.
| Part | Dominant limb | Non-dominant limb | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| INTpre | Roll 1 | Roll 2 | INTpost | INTpost10 | Δ(%) pre-post |
Δ(%) pre-INTpost10 | INTpre | Roll 1 | Roll 2 | INTpost | INTpost10 | Δ(%) pre-post |
Δ(%) pre-INTpost10 | |
| 1 | 16.5 | 14.5 | 15.5 | -12.1 | -6.1 | 16.5 | 14.5 | 16.0 | 16.0 | 16.5 | 0.0 | 3.1 | 16.0 | 16.0 |
| 2 | 10.0 | 10.0 | 10.0 | 0.0 | 0.0 | 10.0 | 10.0 | 10.0 | 11.0 | 11.0 | 10.0 | 10.0 | 10.0 | 11.0 |
| 3 | 11.0 | 11.0 | 10.5 | 0.0 | -4.5 | 11.0 | 11.0 | 12.0 | 13.0 | 13.0 | 8.3 | 8.3 | 12.0 | 13.0 |
| 4 | 10.0 | 10.5 | 11.5 | 5.0 | 15.0 | 10.0 | 10.5 | 12.0 | 12.0 | 11.5 | 0.0 | -4.2 | 12.0 | 12.0 |
| 5 | 17.0 | 17.5 | 18.0 | 2.9 | 5.9 | 17.0 | 17.5 | 19.5 | 20.0 | 20.0 | 2.6 | 2.6 | 19.5 | 20.0 |
| 6 | 11.5 | 12.0 | 11.5 | 4.3 | 0.0 | 11.5 | 12.0 | 11.0 | 10.5 | 11.0 | -4.5 | 0.0 | 11.0 | 10.5 |
| 7 | 14.0 | 13.0 | 13.0 | -7.1 | -7.1 | 14.0 | 13.0 | 12.5 | 12.5 | 12.5 | 0.0 | 0.0 | 12.5 | 12.5 |
| 8 | 12.0 | 13.5 | 13.0 | 12.5 | 8.3 | 12.0 | 13.5 | 15.0 | 15.5 | 15.5 | 3.3 | 3.3 | 15.0 | 15.5 |
| 9 | 7.0 | 8.0 | 8.0 | 14.3 | 14.3 | 7.0 | 8.0 | 9.5 | 10.5 | 9.5 | 10.5 | 0.0 | 9.5 | 10.5 |
| 10 | 10.0 | 9.0 | 10.0 | -10.0 | 0.0 | 10.0 | 9.0 | 9.5 | 10.5 | 11.5 | 10.5 | 21.1 | 9.5 | 10.5 |
| 11 | 18.0 | 19.0 | 19.0 | 5.6 | 5.6 | 18.0 | 19.0 | 19.0 | 20.0 | 19.0 | 5.3 | 0.0 | 19.0 | 20.0 |
| 12 | 6.5 | 8.5 | 8.5 | 30.8 | 30.8 | 6.5 | 8.5 | 7.0 | 7.0 | 7.0 | 0.0 | 0.0 | 7.0 | 7.0 |
| Mean | 12.1 | 11.7 | 13.0 | 3.8 | 5.2 | 12.1 | 11.7 | 12.8 | 14.1 | 13.5 | 3.8 | 3.7 | 12.8 | 14.1 |
| SD | 3.6 | 3.6 | 4.2 | 11.7 | 10.9 | 3.6 | 3.6 | 3.8 | 4.8 | 5.1 | 5.1 | 6.7 | 3.8 | 4.8 |
Part = Participant, INTpre = pre-test prior to intervention, INTpost = post-test, INTpost10 = post-test 10 min after the intervention, SD = standard deviation
Static balance performance
During the first 15 s when participants had their eyes open, CoP speed had no statistically significant main effects or interactions for the condition (p = 0.57-0.72, d = 0.04 – 0.08) and time (p = 0.12-0.45, d = 0.13) on the dominant or non-dominant limb respectively. Considering the 15 s trials during which the participants balanced with their eyes closed, no statistically significant main effects of condition (p = 0.06-0.270, d = 0.41-0.61) and time (p = 0.197-0.456, d = 0.29-0.32) were observed for CoP speed. In addition, no significant condition x time interaction effects (p = 0.330-0.393, d = 0.60-0.64) were found on both sides (Table 6). Individual CoP speed results are presented in Table 5.
Table 6.
Effects of neuromuscular foot rolling on center of pressure speed. Data are means (±SD).
| CoP speed (m·s-1) | INTpre | Roll 1 | Roll 2 | INTpost | INTpost10 | Δ(%) pre-post |
Main and Interaction effects p-value (d), F-statistics |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Condition | Time | Condition x Time | ||||||||
| Eyes closed dominant limb | INT | .3218 (.0770) |
.3091 (.0630) |
.3309 (.0759) |
.3205 (.0653) |
.3380 (.0770) |
-4.0 | .060 (1.27) F(1,11)=4.407 |
.456 (.54) F(4,44)=.802 |
.393 (.60) F(4,44)=.985 |
| CON | .3211 (.0594) |
.3132 (.0567) |
.3083 (.0559) |
.3091 (.0583) |
.3135 (.0570) |
-3.7 | ||||
| Eyes closed non-dominant limb | INT | .3347 (.0753) |
.3191 (.0628) |
.3343 (.0800) |
.3158 (.0583) |
.3217 (.0473) |
-5.7 | .478 (.44) F(1,11)=.538 |
.189 (.81) F(4,44)=1.828 |
.330 (.644) F(4,44)=1.146 |
| CON | .3447 (.0835) |
.3166 (.0584) |
.3117 (.0585) |
.3120 (.0589) |
.3107 (.0562) |
-9.5 | ||||
| Eyes open dominant limb | INT | .2976 (.0594) |
.2974 (.0610) |
.2999 (.0602) |
.2986 (.0615) |
.3004 (.0580) |
0.4 | .728 (.21) F(1,11)=.128 |
.126 (.83) F(4,44)=1.906 |
.692 (.45) F(4,44)=.561 |
| CON | .2975 (.0585) |
.2990 (.0553) |
.2981 (.0586) |
.3006 (.0565) |
.3025 (.0582) |
1.0 | ||||
| Eyes open non-dominant limb | INT | .2972 (.0610) |
.2983 (.0608) |
.2995 (.0607) |
.3001 (.0600) |
.2989 (.0602) |
1.0 | .573 (.35) F(1,11)=.337 |
.452 (.55) F(4,44)=.842 |
.720 (.43) F(4,44)=.720 |
| CON | .2994 (.0645) |
.2986 (.0597) |
.2981 (.0582) |
.3010 (.0574) |
.3015 (.0580) |
0.5 | ||||
CoP = centre of pressure, INT = intervention, CON = control, SD = standard deviation. INTpre = pre-test, Roll 1 = after the first foot rolling, Roll 2 = after the second foot rolling, INTpost = post-test, INTpost10 = post-test 10 min after the intervention, d= Cohen's d.
Table 5.
Illustrates the course of centre of pressure (CoP) speed changes on the dominant and non-dominant limb during the intervention session when participants had their eyes closed.
| Part | Dominant limb | Non-dominant limb | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| INTpre | Roll 1 | Roll 2 | INTpost | INTpost10 | Δ(%) pre-post |
Δ(%) pre-INTpost10 | INTpre | Roll 1 | Roll 2 | INTpost | INTpost10 | Δ(%) pre-post |
Δ(%) pre-INTpost10 | |
| 1 | .295 | .277 | .281 | .284 | .298 | -3.7 | .9 | .294 | .322 | .285 | .350 | .303 | 18.7 | 3.0 |
| 2 | .237 | .224 | .234 | .226 | .245 | -4.9 | 3.4 | .241 | .239 | .499 | .239 | .331 | -.8 | 37.5 |
| 3 | .261 | .269 | .275 | .325 | .512 | 24.7 | 96.3 | .344 | .274 | .272 | .272 | .291 | -21.0 | -15.4 |
| 4 | .402 | .395 | .405 | .433 | .401 | 7.8 | -.1 | .400 | .405 | .408 | .405 | .397 | 1.2 | -.8 |
| 5 | .292 | .249 | .424 | .277 | .272 | -5.2 | -6.8 | .338 | .271 | .261 | .261 | .286 | -22.6 | -15.2 |
| 6 | .333 | .335 | .331 | .337 | .339 | 1.4 | 1.8 | .336 | .338 | .333 | .345 | .336 | 2.7 | 0.3 |
| 7 | .301 | .301 | .290 | .230 | .332 | -0.4 | 10.5 | .301 | .286 | .290 | .292 | .289 | -3.1 | -4.1 |
| 8 | .341 | .336 | .342 | .335 | .348 | -1.8 | 2.0 | .337 | .357 | .345 | .332 | .341 | -1.5 | 1.1 |
| 9 | .260 | .253 | .252 | .254 | .262 | -2.6 | .5 | .264 | .268 | .251 | .256 | .258 | -2.8 | -1.9 |
| 10 | .352 | .391 | .439 | .379 | .359 | 7.7 | 2.0 | .367 | .349 | .357 | .348 | .340 | -5.0 | -7.4 |
| 11 | .517 | .408 | .432 | .424 | .414 | -18.0 | -19.9 | .520 | .449 | .445 | .414 | .412 | -21.2 | -21.5 |
| 12 | .271 | .270 | .267 | .272 | .274 | 0.4 | 1.0 | .271 | .273 | .667 | .276 | .275 | 2.1 | 1.6 |
| Mean | .322 | .309 | .331 | .321 | .338 | .5 | 7.6 | .335 | .319 | .334 | .316 | .322 | -4.5 | -1.9 |
| SD | .077 | .063 | .076 | .065 | .077 | 10.1 | 28.9 | .075 | .063 | .080 | .058 | .047 | 12.0 | 14.7 |
PART = Participant, INTpre = pre-test prior to intervention, Roll 1 = after the first 60 s foot rolling, Roll 2 = after the second 60 s foot rolling, INTpost = post-test, INTpost10 = post-test 10 min after the intervention, SD = standard deviation.
Discussion
The most important findings of this study were that foot (plantar sole) rolling revealed no statistically significant effects on ankle dorsiflexion ROM during the weight bearing lunge test or hamstring and lower back ROM during SRT with the rolled or non-rolled (contralateral) limbs. Secondly, the first pre-intervention assessment of the SRT was significantly different than the second assessment (INTpre). This finding supports the methodological importance of implementing multiple SRT pre-tests. Finally, the intervention did not change static balance performance.
In this study, ankle dorsiflexion ROM and hamstring and lower back ROM of the ipsilateral and contralateral limbs were not statistically significant affected by foot rolling. All increases in ROM (intervention and control conditions) were time dependent, so ROM increases were substantially affected by the testing procedure. This finding contrasts with the results of Grieve et al. (2015). They found 23.4% greater hamstring and lower back ROM after rolling both feet twice for two minutes, respectively. Their total intervention time accounted for 8 min while the present study provided an approximately statistically non-significant 4% SRT increase with 3 min (3 x 60 s) of rolling a single foot (INTpre to INTpost). However, the present results compared the second pre-test with the post-test. There was a 6.6% increase in SRT between the first and second (INTpre) pre-test measures. A second pre-test in the study of Grieve et al. (2015) might have reduced the reported ROM increases from pre- to post-test measures. Comparing the post-intervention tests to the first pre-test rather than INTpre in the present study would have exhibited an approximate > 10% statistically significant increase in SRT. The statistically significant differences between the first SRT and the INTpre test support the methodological importance of implementing multiple SRT pre-tests as emphasized by Atha and Wheatley (1976). They emphasized the effects of repeated measures on increasing hip flexion measured with the SRT. Furthermore, they acknowledged higher increases in the first four trials. As the present study included two pre-tests and another two tests to monitor between set effects, there were a total of four SRTs prior to the INTpost measure. The total number of SRT seemed to affect ROM more than foot rolling, thus, leading to greater ROM in both the intervention and control sessions. Studies reporting increased ROM from a single pre-test or with an insufficient warm-up should be viewed critically.
Furthermore, the present study used a within-subject design, which was highly recommended by Vigotsky et al. (2015). Vigotsky et al. (2015) demonstrated highly inter-individual ROM responses to quadriceps rolling. For example, in the Vigotsky study some participants gained 3.9% length in the m. rectus femoris while others responded with a 3.1% decrease to foam rolling. Inter-individual changes on the dominant limb in the present study fluctuated between a 10.5% decrease and a 21.2% increase in SRT scores (see Table 3). These inter-individual variations may reflect the individual’s response to pain and discomfort and its relationship to stretch tolerance. For example, Behm and St.-Pierre (1997) reported a correlation of 0.1 between pain and the extent of muscle activation when examining individuals who had previously fractured their ankles. They reported highly divergent responses to pain, with some individuals able to withstand and overcome the discomfort of maximal contractions while others experienced substantial pain-induced inhibition. Hence, the effects of foot rolling may exhibit very individualized effects depending on the personal sensation and response to discomfort (Table 3-5).
Kelly and Beardsley (2016) showed cross-over effects after three 30 s bouts of plantar flexor foam rolling. The presence of global effects might be due to the greater volume of muscle group treated. Rolling a single foot only versus both feet (Grieve et al. (2015), might not provide sufficient afferent feedback to increase stretch tolerance as suggested by Kelly and Beardsley (2016). Chaouachi et al. (2015) found increased contralateral hip flexion ROM after ten 30 s unilateral hip stretches, advocating increased stretch tolerance. Behm et al. (2016) also reported an increased non-local joint ROM when applying the same stretching volume as Chaouachi et al. (2015) at an intensity of 70% to 90% of the maximum discomfort to shoulder and hip joints. Both studies stretched large muscle groups and caused substantial discomfort or pain. As increased stretch tolerance is a mechanism that operates when high discomfort or pain is present (Magnusson et al., 1996), it is possible that 3 min of foot rolling of a single foot did not provide sufficient discomfort to induce an accommodation of discomfort or increased stretch tolerance.
To the authors’ knowledge, this was the first study that investigated the impact of foot rolling on steady-state balance performance. Although the sole of the foot is reported to play an important role in proprioception (Roll et al., 2002) and equilibrium (Watanabe & Okubo, 1981), there were no statistically significant effects of foot rolling on static balance performance in the present study. Similarly, a single Thai foot massage application did not result in single-leg stance balance improvements (Chatchawan et al., 2015). Likewise, Halperin et al. (2014) reported that neither plantar flexor roller massage nor static stretching affected balance (single-leg stork test). Costa et al. (2009) also did not find any effects on balance of three bouts of 15 s or 45 s static stretching applied to the quadriceps, hamstrings and plantar flexors muscles on both limbs, respectively. However, Behm, et al. (2004) reported a statistically significant decrease in balance performance (-9.2%) following three 45 s stretches to the point of discomfort to the quadriceps, hamstrings, and plantar flexors on both limbs, respectively. Since large muscle groups were treated and the greatest point of discomfort was reached, Behm, et al. (2004) suggested that either stretch-induced changes to the the afferent limb muscle response or stretch-induced force impairments could have altered the ability of the peripheral neuromuscular system to cope with stability challenges.
Although the a priori power analysis indicated that a maximum of 12 participants would be sufficient to achieve medium effects with a Type I error of 0.05 and a Type II error rate of 0.20 (80% statistical power), a possible limitation of the present study is that it might be considered underpowered. However, a post data collection power analysis with G*Power indicated that 121-210 participants would be needed to achieve the desired statistical power dependent upon the measure utilized. Thus, the validity of the statistically non-significant findings would not be altered by doubling, tripling or more the number of participants. The discrepancy between the a priori and post data collection power analysis estimates may be related to the larger muscle groups that were rolled in the studies used to determine the a priori power analysis. Another possible limitation is that participants were asked to subjectively roll at an intensity equal to 7/10 on a pain scale. It is possible that some of the participants did not self-inflict this degree of discomfort on their foot and thus decreased the intensity of afferent stimulation. However, in prior studies from our laboratory (Aboodarda et al., 2015; Bradbury-Squires et al., 2015; Halperin et al., 2014; MacDonald et al., 2014; Pearcey et al., 2015), statistically significant non-local fatigue responses have been elicited with the participants using the same subjective 7/10 rolling level of discomfort. Furthermore, since our lab has demonstrated similar increases in ROM with rolling intensities of 5, 7, and 9/10 on a pain scale (Grabow et al., 2017), some deviation from the prescribed 7/10 pain perception should not have resulted in substantial differences.
Conclusion
Unilateral rolling of the foot did not provide statistically significant increases in ipsilateral or contralateral dorsiflexion or SRT ROM nor did it affect postural sway (CoP speed). Whereas, a number of prior studies that have rolled or stretched a target muscle have shown increases in ROM of non-local, non-exercised (i.e., stretched or rolled) muscle groups, most of these studies have targeted larger muscle groups (i.e., quadriceps, hamstrings, plantar flexors, shoulders) and thus the statistically non-significant findings with rolling of a single foot might be attributed to the lower volume of afferent stimulation. Furthermore, it is also possible that ROM results from some of the studies utilizing a single pre-test without a sufficient warm-up were affected by a repeated measures effect.
Acknowledgment
The authors declare that there is no conflict of interests regarding the publication of this article.
Biographies

Lena GRABOW
Employment
Graduate Student
Degree
Master of Education
Research interests
Exercise/Sport science
E-mail: grabowlena@gmail.com

James Douglas YOUNG
Employment
Graduate Student / Strength and Conditioning Coach
Degree
Master of Science (Kinesiology)
Research interests
Exercise/Sport science
E-mail: jdy665@mun.ca
Jeannette M. BYRNE
Employment
Associate Professor
Degree
PhD
Research interests
Factors influencing joint and muscle function in both healthy and injured populations, biomechanical modelling.
E-mail: jmbyrne@mun.ca

Urs GRANACHER
Employment
Full Professor in Training and Movement Sciences at University of Potsdam, Germany
Degree
PhD & Habilitation
Research interests
Biomechanics, exercise science, postural control, muscle strength
E-mail: urs.granacher@uni-potsdam.de

David G. BEHM
Employment
University Research Professor
Degree
PhD
Research interests
Applied Neuromuscular physiology and exercise/sport science
E-mail: dbehm@mun.ca
References
- Aboodarda S.J., Spence A.J., Button D.C. (2015) Pain pressure threshold of a muscle tender spot increases following local and non-local rolling massage. BMC Musculoskeletal Disorders 16, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atha J., Wheatley D.W. (1976) The mobilising effects of repeated measurement on hip flexion. British Journal of Sports Medicine 10(1), 22-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley C., Škarabot J. (2015) Effects of self-myofascial release: A systematic review. Journal of Bodywork and Movement Therapies 19(4), 747-758. [DOI] [PubMed] [Google Scholar]
- Behm D.G., Bambury A., Cahill F., Power K.E. (2004) Effect of Acute Static Stretching on Force, Balance, Reaction Time, and Movement Time. Medicine & Science in Sports & Exercise 36(8), 1397-1402. [DOI] [PubMed] [Google Scholar]
- Behm D.G., Chaouachi A. (2011) A review of the acute effects of static and dynamic stretching on performance. European Journal of Applied Physiology 111(11), 2633-2651. [DOI] [PubMed] [Google Scholar]
- Behm D.G., Peach A., Maddigan M., Aboodarda S.J., DiSanto M.C., Button D.C., Maffiuletti N.A. (2013) Massage and stretching reduce spinal reflex excitability without affecting twitch contractile properties. Journal of Electromyography and Kinesiology 23(5), 1215-1221. [DOI] [PubMed] [Google Scholar]
- Behm D.G., St.-Pierre D.M.M. (1997) Fatigue characteristics following ankle fractures. Medicine & Science in Sports & Exercise 29(9), 1115-1123. [DOI] [PubMed] [Google Scholar]
- Behm D.G., Cavanaugh T., Quigley P., Reid J.C., Nardi P.S.M., Marchetti P.H. (2016) Acute bouts of upper and lower body static and dynamic stretching increase non-local joint range of motion. European Journal of Applied Physiology 116(1), 241-249. [DOI] [PubMed] [Google Scholar]
- Bennell K., Talbot R., Wajswelner H., Techovanich W., Kelly D., Hall A.J. (1998) Intra-rater and inter-rater reliability of a weight-bearing lunge measure of ankle dorsiflexion. Australian Journal of Physiotherapy 44(3), 175-180. [DOI] [PubMed] [Google Scholar]
- Bradbury-Squires D.J., Noftall J.C., Sullivan K.M., Behm D.G., Power K.E., Button D.C. (2015) Roller-massager application to the quadriceps and knee-joint range of motion and neuromuscular efficiency during a lunge. Journal of Athletic Training 50(2), 133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh M.T., Döweling A., Young J.D., Quigley P.J., Hodgson D.D., Whitten J.H.D., Reid J.C., Aboodarda S.J., Behm D.G. (2016) An acute session of roller massage prolongs voluntary torque development and diminishes evoked pain. European Journal of Applied Physiology 117(1), 109-117. [DOI] [PubMed] [Google Scholar]
- Chaouachi A., Padulo J., Kasmi S., Othmen A.B., Chatra M., Behm D.G. (2015) Unilateral static and dynamic hamstrings stretching increases contralateral hip flexion range of motion. Clinical Physiology and Functional Imaging 37(1), 23-29. [DOI] [PubMed] [Google Scholar]
- Chatchawan U., Eungpinichpong W., Plandee P., Yamauchi J. (2015) Effects of thai foot massage on balance performance in diabetic patients with peripheral neuropathy: a randomized parallel-controlled trial. Medical Science Monitor Basic Research 21, 68-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. (1988) Statistical power analysis for the behavioral sciences. 2. edition Hillsdale, NJ: Erlbaum. [Google Scholar]
- Coren S. (1993) The lateral preference inventory for measurement of handedness, footedness, eyedness, and earedness: Norms for young adults. Bulletin of the Psychonomic Society 31(1), 1-3. [Google Scholar]
- Costa P.B., Graves B.S., Whitehurst M., Jacobs P.L. (2009) The acute effects of different durations of static stretching on dynamic balance performance. Journal of Strength and Conditioning Research 23(1), 141-147. [DOI] [PubMed] [Google Scholar]
- Grabow L., Young J.D., Alcock L.R., Quigley P.J., Byrne J.M., Granacher U., Škarabot J., Behm D.G. (2017) Higher quadriceps roller massage forces do not amplify range-of-motion increases or impair strength and jump performance. Journal of Strength and Conditioning Research, in press. [DOI] [PubMed] [Google Scholar]
- Grieve R., Goodwin F., Alfaki M., Bourton A.-J., Jeffries C., Scott H. (2015) The immediate effect of bilateral self myofascial release on the plantar surface of the feet on hamstring and lumbar spine flexibility: A pilot randomised controlled trial. Journal of Bodywork and Movement Therapies 19(3), 544-552. [DOI] [PubMed] [Google Scholar]
- Halperin I., Aboodarda S.J., Button D.C., Andersen L.L., Behm D.G. (2014) Roller massager improves range of motion of plantar flexor muscles without subsequent decreases in force parameters. International Journal of Sports Physical Therapy, 9(1), 92–102. [PMC free article] [PubMed] [Google Scholar]
- Halperin I., Chapman D.W., Behm D.G. (2015) Non-local muscle fatigue: effects and possible mechanisms. European Journal of Applied Physiology 115(10), 2031-2048. [DOI] [PubMed] [Google Scholar]
- Hlavackova P., Vuillerme N. (2012). Do somatosensory conditions from the foot and ankle affect postural responses to plantar-flexor muscles fatigue during bipedal quiet stance? Gait & Posture 36(1), 16-19. [DOI] [PubMed] [Google Scholar]
- Jay K., Sundstrup E., Sondergaard S.D., Behm D., Brandt M., Saervoll C.A., Jakobsen M.D., Andersen L.L. (2014) Specific and cross over effects of massage for muscle soreness: randomized controlled trial. International Journal of Sports Physical Therapy, 9(1), 82-91. [PMC free article] [PubMed] [Google Scholar]
- Junker D.H., Stoggl T.L. (2015) The Foam Roll as a Tool to Improve Hamstring Flexibility. Journal of Strength and Conditioning Research 29(12), 3480-3485. [DOI] [PubMed] [Google Scholar]
- Kavounoudias A., Roll R., Roll J.-P. (1998) The plantar sole is a ‘dynamometric map’ for human balance control. NeuroReport, 9(14), 3247–3252. [DOI] [PubMed] [Google Scholar]
- Kavounoudias A., Roll R., Roll J.-P. (2001). Foot sole and ankle muscle inputs contribute jointly to human erect posture regulation. The Journal of Physiology 532(3), 869-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S., Beardsley C. (2016) Specific and cross-over effects of foam rolling on ankle dorsiflexion range of motion. International Journal of Sports Physical Therapy 11(4), 544-551. [PMC free article] [PubMed] [Google Scholar]
- Lanigan C.S., Harrison A.J. (2012) The Effects of self myofascial release on the plantar surface of the foot during sledge rebound jumps. Journal of Bodywork and Movement Therapies 16(4), 521-522. [Google Scholar]
- MacDonald G.Z., Button D.C., Drinkwater E.J., Behm D.G. (2014) Foam rolling as a recovery tool after an intense bout of physical activity. Medicine and Science in Sports and Exercise 46(1), 131-142. [DOI] [PubMed] [Google Scholar]
- MacDonald G.Z., Penney M.D.H., Mullaley M.E., Cuconato A.L., Drake C.D.J., Behm D.G, Button D.C. (2013) An acute bout of self-myofascial release increases range of motion without a subsequent decrease in muscle activation or force. Journal of Strength and Conditioning Research 27(3), 812-821. [DOI] [PubMed] [Google Scholar]
- Magnusson S.P., Simonsen E.B., Aagaard P., Sørensen H., Kjaer M. (1996) A mechanism for altered flexibility in human skeletal muscle. The Journal of Physiology 497(1), 291-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki B.E., Perry S.D., Norrie R.G., McIlroy W.E. (1999) Effect of facilitation of sensation from plantar foot-surface boundaries on postural stabilization in young and older adults. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 54(6), M281-287. [DOI] [PubMed] [Google Scholar]
- Maurer C., Mergner T., Bolha B., Hlavacka F. (2001) Human balance control during cutaneous stimulation of the plantar soles. Neuroscience Letters 302(1), 45-48. [DOI] [PubMed] [Google Scholar]
- McKeon P.O., Hertel J., Bramble D., Davis I. (2015) The foot core system: a new paradigm for understanding intrinsic foot muscle function. British Journal of Sports Medicine 49(5), 290. [DOI] [PubMed] [Google Scholar]
- Meyer P.F., Oddsson L.I.E., de Luca C.J. (2004) The role of plantar cutaneous sensation in unperturbed stance. Experimental Brain Research 156(4), 505-512. [DOI] [PubMed] [Google Scholar]
- Mikesky A.E., Bahamonde R.E., Stanton K.A., Thurman Fitton. (2002) Acute Effects of The Stick on Strength, Power, and Flexibility. Journal of Strength and Conditioning Research 16(3), 446-450. [PubMed] [Google Scholar]
- Mohr A.R., Long B.C., Goad C.L. (2014). Effect of foam rolling and static stretching on passive hip-flexion range of motion. Journal of Sport Rehabilitation 23(4), 296-299. [DOI] [PubMed] [Google Scholar]
- Murray A.M., Jones T.W., Horobeanu C., Turner A.P., Sproule J. (2016). Sixty Seconds of foam rolling does not affect functional flexibility or change muscle temperature in adolescent athletes. International Journal of Sports Physical Therapy 11(5), 765-776. [PMC free article] [PubMed] [Google Scholar]
- Myers T.W. (2015) Anatomy Trains: Myofasziale Leitbahnen (für Manual- und Bewegungstherapeuten). 3rd edition Elsevier Health Sciences; Germany. [Google Scholar]
- Paolini J. (2009) Review of Myofascial Release as an Effective Massage Therapy Technique. Athletic Therapy Today 14(5), 30-34. [Google Scholar]
- Peacock C.A., Krein D.D., Antonio J., Sanders G.J., Silver T.A., Colas M. (2015) Comparing acute bouts of sagittal plane progression foam rolling vs. frontal plane progression foam rolling. Journal of Strength and Conditioning Research 29(8), 2310-2315. [DOI] [PubMed] [Google Scholar]
- Pearcey G.E.P., Bradbury-Squires D.J., Kawamoto J.-E., Drinkwater E.J., Behm D.G., Button D.C. (2015) Foam rolling for delayed-onset muscle soreness and recovery of dynamic performance measures. Journal of Athletic Training 50(1), 5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto T.E., Myklebust J.B., Hoffmann R.G., Lovett E.G., Myklebust B.M. (1996) Measures of postural steadiness: differences between healthy young and elderly adults. IEEE Transactions On Bio-Medical Engineering 43(9), 956-966. [DOI] [PubMed] [Google Scholar]
- Roll R., Kavounoudias A., Roll J.-P. (2002) Cutaneous afferents from human plantar sole contribute to body posture awareness. NeuroReport 13(15), 1957-1961. [DOI] [PubMed] [Google Scholar]
- Škarabot J., Beardsley C., Štirn I. (2015) Comparing the effects of self-myofascial release with static stretching on ankle range-of-motion in adolescent athletes. International Journal of Sports Physical Therapy 10(2), 203-212. [PMC free article] [PubMed] [Google Scholar]
- Sullivan K.M., Silvey D.B., Button D.C., Behm D.G. (2013) Roller-massager application to the hamstrings increases sit-and-reach range of motion within five to ten seconds without performance impairments. International Journal of Sports Physical Therapy 8(3), 228-236. [PMC free article] [PubMed] [Google Scholar]
- Vigotsky A.D., Lehman G.J., Contreras B., Beardsley C., Chung B., Feser E.H. (2015) Acute effects of anterior thigh foam rolling on hip angle, knee angle, and rectus femoris length in the modified Thomas test. PeerJ 3, e1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe I., Okubo J. (1981) The role of the plantar mechanoreceptor in equilibrium control. Annals of the New York Academy of Sciences 374(1 Vestibular an), 855-864. [DOI] [PubMed] [Google Scholar]


