Abstract
Objectives
To compare plasma biomarkers of coagulation between HIV-infected individuals and HIV-uninfected controls and to assess the impact of disturbances in coagulation on neurocognitive functioning in HIV.
Design
Cross-sectional study of 66 antiretroviral therapy-treated virally suppressed HIV-infected and 34 HIV-uninfected older (≥50 years of age) adults.
Methods
Participants completed standardized neurobehavioral and neuromedical assessments. Neurocognitive functioning was evaluated using a well-validated comprehensive neuropsychological battery. Plasma biomarkers associated with procoagulation (fibrinogen, p-selectin, tissue factor, and von Willebrand factor), anticoagulation (antithrombin, protein C, and thrombomodulin), fibrinolysis (d-dimer, plasminogen activator inhibitor-1, and plasminogen) were collected. Multivariable linear regression was used to test the interaction of HIV and coagulation on neurocognitive functioning.
Results
Most participants were male (78.0%) and non-Hispanic white (73.0%) with a mean age of 57.8 years. Among HIV-infected participants, mean estimated duration of HIV infection was 19.4 years and median current CD4+ cell count was 654 cells/mm3. Levels of soluble biomarkers of procoagulation, anticoagulation, and fibrinolysis were comparable between the HIV serostatus groups. Coagulation and HIV had an interacting effect on neurocognitive functioning, such that greater coagulation imbalance was associated with poorer neurocognitive functioning among the HIV-infected participants. The moderating effect of coagulation on neurocognition was driven by procoagulant but not anticoagulant or fibrinolytic biomarkers.
Conclusions
Elevated levels of procoagulants may exert a particularly detrimental effect on neurocognitive functioning among older HIV-infected persons. A better understanding of the specific role of coagulation in the etiology of HIV-associated neurocognitive disorders may lead to treatments aimed at reducing coagulopathy, thereby improving neurocognitive outcomes.
Keywords: aging, coagulopathy, cognition, endothelial dysfunction, hemostasis, HIV/AIDS, inflammation
Introduction
HIV-associated neurocognitive impairment (NCI) is highly prevalent in the modern era of combination antiretroviral therapy (ART) [1], and older adults appear to be particularly vulnerable to HIV-associated NCI [2-5]. Persons living with HIV (HIV+) who are untreated for cerebrovascular risk demonstrate poorer performance in the neurocognitive domains of processing speed, learning/memory, and executive functioning compared to HIV+ persons who are pharmacologically treated for cerebrovascular risk [6].
HIV+ persons may experience imbalance in coagulation given impaired endothelial function and immune activation [7-9]. In a subset of participants in the Strategies for Management of Antiretroviral Therapy (SMART) trial, HIV replication was associated with complex changes in the extrinsic pathway, such as short-term increases in some procoagulants and decreases in anticoagulants [e.g., lower antithrombin (AT) and lower protein C] [10]. Some studies have found that biomarkers indicative of coagulation decrease with initiation of ART [11]; however, relative to HIV-uninfected (HIV-) persons, ART-treated persons appear to be vulnerable to coagulation imbalance [12]. Although untreated patients may have elevated levels of d-dimer (i.e., a fibrin degradation product frequently used as a marker of coagulation disturbances) relative to treated patients on ART, both untreated and treated patients on ART are observed to have impairments in platelet aggregation and clot initiation [13]. Furthermore, older HIV+ persons may be particularly vulnerable to coagulation imbalance, given that aging further exerts a strong influence on hemostatic biomarkers, such as d-dimer [14].
The clinical consequences of HIV disease on coagulation in the context of suppressive ART remains unclear. We aimed to assess the impact of disturbances in coagulation (i.e., coagulation imbalance) on neurocognitive functioning in HIV. We hypothesized that greater coagulation imbalance would have a detrimental effect on neurocognitive functioning in HIV.
Methods
Participants and Procedure
The present study examined 100 community-dwelling older (i.e., aged 50 and above) HIV+ (n = 66) and HIV- adults (n = 34) who participated in the California HIV/AIDS Research Program Successfully Aging Seniors with HIV study at the UCSD HIV Neurobehavioral Research Program. Specific inclusion criteria for HIV+ participants were being on ART and having an undetectable plasma HIV viral load (<48 copies/ml). General exclusion criteria included use of an anticoagulant medication, history of non-HIV related neurologic disorders or other known conditions that may be associated with impaired neurocognitive performance (e.g., seizure disorder). The study protocol was approved by the UCSD Institutional Review Board. After providing written, informed consent, each participant underwent a standardized neuropsychological, neuromedical, and psychiatric evaluation.
Global Neurocognitive Functioning
Participants completed a standardized neurocognitive test battery (administration time: 2 to 2.5 hours) that assesses seven neurocognitive domains commonly affected by HIV, including speed of information processing, learning, memory, executive functions, verbal fluency, working memory, and fine motor function (Heaton et al., 2010). Raw scores from the neurocognitive tasks were converted to demographically-adjusted T-scores (M = 50, SD = 10 in neurological normal subjects) using the best available normative standards [15-17]. The demographically-adjusted T-scores were then averaged to derive neurocognitive domain T-scores and a global T-score, the latter of which was used in statistical analyses as the primary dependent variable. Global T-scores were chosen over other approaches (e.g., deficit scores) given their normal distribution that matches the assumptions of parametric statistical analyses and their wider use across the neuropsychological literature [18]. Higher global T-scores represent better neurocognitive functioning with scores between 35 and 40 reflecting mild impairment.
Plasma Biomarkers of Coagulation
Biomarker assays were measured by immunoassay in duplicate in ethylenediaminetetraacetic acid (EDTA)-treated plasma derived from peripheral blood samples collected by routine phlebotomy. Commercial immunoassay suppliers were Millipore (fibrinogen, tissue factor, thrombomodulin; Darmstadt, Germany), R&D Systems [AT, plasminogen activator-inhibitor-1 (PAI-1), protein C, p-selectin, von Willebrand factor (vWF); Minneapolis, MN], SEKISUI (d-dimer; Lexington, MA), and Cell Biolabs (plasminogen; San Diego, CA). Measurements were repeated if the coefficient of variation was greater than 20% or if the measurement was greater than four standard deviations from the mean.
Coagulation Imbalance
Coagulation imbalance was operationalized in a similar fashion to other composite indices representing physiological systems [19]. The selected biomarkers represent three physiological systems: procoagulation (fibrinogen, p-selectin, tissue factor, vWF), anticoagulation (AT, protein C, thrombomodulin), and fibrinolysis (d-dimer, PAI-1, and plasminogen). Coagulation imbalance was constructed first by dichotomizing the ten individual biomarkers based on median splits of the entire sample (i.e., assigning a score of “1” to values in the upper 50th percentile and a score of “0” to values in the lower 50th percentile). The individual binary variables were summed to create summary scores for each of the three physiological systems: procoagulation (values ranging from 0 to 4), anticoagulation (values ranging from 0 to 3), and fibrinolysis (values ranging from 0 to 3). A coagulation imbalance score (values ranging from 0 to 10) was calculated based on summation of the ten individual biomarker binary variables, with a higher coagulation imbalance score representing more activation/turnover of hemostatic factors.
Plasma Biomarkers of Inflammation
Biomarkers representing inflammatory processes [i.e., soluble CD163 (sCD163), soluble CD14 (sCD14), and complement C3[ [20] were available for a subset of participants (n = 93). Biomarker assays were measured by immunoassay in duplicate in EDTA-treated plasma. The commercial immunoassay suppliers were R&D Systems (sCD163 and sCD14; Minneapolis, MN) and Assaypro (complement C3; St. Charles, MO). As with the biomarkers of coagulation, measurements were repeated if the coefficient of variation was greater than 20% or the measurement was greater than four standard deviations from the mean.
Covariates
Neuromedical Assessment
Medical characterization of study participants included medical co-morbidities (e.g., dyslipidemia, diabetes mellitus, and hypertension) and current prescription medications [i.e., lipid-lowering drug, nonsteroidal anti-inflammatory drug (NSAID), antihypertensive, and anti-depressant drugs]. Medical comorbidities were defined by the presence of self-reported diagnosis and/or specific drug treatment for the condition (e.g., metformin for diabetes mellitus).
HIV Disease Characteristics
All HIV+ participants were on suppressive ART. The following information was obtained for our HIV+ sample: estimated duration of HIV infection, AIDS diagnosis, current and nadir CD4+ T-cell counts, and whether or not the participant was on a protease inhibitor (PI)-based regimen.
Psychiatric Assessment
All study participants were administered the Composite International Diagnostic Interview [21] to assess for the presence of lifetime and current affective and substance use disorders using diagnostic criteria based on the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders [22]. The Beck Depression Inventory-II (BDI-II) [23] was administered to assess current depressive symptoms.
Statistical Analyses
The distributions of residuals were visually inspected when performing statistical tests to determine whether transforming individual variables was appropriate to meet statistical assumptions of parametric tests. When appropriate, variables were log10 transformed. Comparison of demographic, neuromedical, and psychiatric data between the HIV+ and HIV– groups was performed with two-tailed t-test or Pearson's chi-squared test, as appropriate. Two-tailed t-tests were conducted to compare individual biomarkers and coagulation composite scores by HIV serostatus. When the assumption of equal variances was not met, Welch's t-test was used. Hedge's g statistic for continuous variables and odds ratios for binary variables were used to generate effect sizes for group comparisons. Next, correlates of coagulation imbalance were explored to identify both personal and inflammatory factors related to coagulation.
To test whether coagulation and HIV have an interacting effect on neurocognitive functioning, four multivariable linear regression models were conducted. Each multivariable model included an HIV by coagulation (i.e., coagulation imbalance, procoagulation, anticoagulation, or fibrinolysis) interaction term. Models were run with and without statistical adjustment for relevant covariates. Covariates were selected based on which variables in Table 1 demonstrated univariable associations (i.e., Pearson's correlations for continuous variables and t-tests for categorical variables) with the primary dependent variable (neurocognitive functioning, i.e., global T-score) at a critical α = .10. The following covariates were identified as having met our criterion for inclusion in the multivariable analyses: body mass index (BMI), diabetes mellitus, use of an antidepressant, current major depressive disorder (MDD), lifetime MDD, and self-reported depression scores. For analyses only involving the HIV+ participants, the following HIV disease specific variables met our criterion for inclusion in the multivariable analyses: current CD4 and AIDS status. The variable inflation factor was used to identify multicollinearity among predictor variables prior to final model selection. Final models included only one of the correlated variables. All statistical tests were performed with JMP 11.0.0 (SAS, 2013).
Table 1. Demographic and clinical characteristics of sample (N = 100).
| Variable | All (N = 100) | HIV+ (n = 66) | HIV- (n = 34) | p-value | Effect size |
|---|---|---|---|---|---|
| Descriptive demographics | |||||
| Age, mean (SD) | 57.8 (6.0) | 57.3 (6.1) | 58.8 (5.9) | .26 | -.25 |
| Education, mean (SD) | 14.3 (2.7) | 14.3 (2.7) | 14.2 (2.7) | .80 | .04 |
| Male, n (%) | 78 (78.0%) | 56 (84.8%) | 22 (64.7%) | .02 | 3.05 |
| Non-Hispanic White, n (%) | 73 (73.0%) | 52 (78.8%) | 21 (61.8%) | .07 | .43 |
| Medical comorbidities and anthropometric measurement | |||||
| Hyperlipidemia, n (%) | 43 (44.3%) | 33 (52.4%) | 10 (29.4%) | .03 | 2.64 |
| Ever smoker, n (%) | 40 (41.2%) | 26 (41.3%) | 14 (41.2%) | .99 | 1.00 |
| Hypertension, n (%) | 37 (38.1%) | 24 (38.1%) | 13 (38.2%) | .99 | .99 |
| Current smoker, n (%) | 32 (33.0%) | 23 (36.5%) | 9 (26.5%) | .32 | 1.60 |
| Diabetes, n (%) | 19 (19.6%) | 13 (20.6%) | 6 (17.6%) | .72 | 1.21 |
| Hepatitis C Virus, n (%) | 18 (18.6%) | 12 (19.0%) | 6 (17.6%) | .87 | 1.10 |
| BMI, mean (SD) | 27.2 (5.3) | 26.9 (5.4) | 27.7 (5.1) | .45 | -.15 |
| Current Medications | |||||
| Lipid-lowering drug, n (%) | 30 (30.0%) | 23 (34.8%) | 7 (20.6%) | .14 | 2.06 |
| NSAID, n (%) | 28 (28.0%) | 20 (30.3%) | 8 (23.5%) | .47 | 1.41 |
| Antihypertensive, n (%) | 27 (27.0%) | 19 (28.8%) | 8 (23.5%) | .57 | 1.31 |
| Antidepressant, n (%) | 32 (32.0%) | 28 (42.4%) | 4 (11.8%) | <.01 | 5.52 |
| Psychiatric characteristics/diagnoses | |||||
| BDI-II total, median [IQR] | 6.0 [0.3-14.8] | 9.5 [2.8-16.3] | 1.5 [0.0-5.3] | <.01 | 0.86 |
| Current MDD, n (%) | 11 (11.1%) | 10 (15.4%) | 1 (2.9%) | .06 | 6.00 |
| LT MDD, n (%) | 48 (48.0%) | 38 (57.6%) | 10 (29.4%) | <.01 | 3.26 |
| LT alcohol use disorder, n (%) | 50 (50.0%) | 33 (50.0%) | 17 (51.5%) | .89 | .94 |
| LT cannabis use disorder, n (%) | 25 (25.3%) | 18 (27.3%) | 7 (21.2%) | .51 | 1.39 |
| LT meth use disorder, n (%) | 28 (28.3%) | 20 (30.3%) | 8 (24.2%) | .53 | 1.36 |
| HIV disease characteristics | |||||
| Years of infection, median [IQR] a | -- | 19.4 [11.3-25.5] | -- | -- | -- |
| AIDS, n (%) | -- | 41 (62.1%) | -- | -- | -- |
| Current CD4, median [IQR]b | -- | 654 [476-865] | -- | -- | -- |
| Nadir CD4, median [IQR] a | -- | 180 [41-300] | -- | -- | -- |
| Current PI use, n (%) c | -- | 31 (47.7%) | -- | -- | -- |
Note: BDI-II = Beck Depression Inventory – II; BMI = body mass index; LT = lifetime; MDD = major depressive disorder; NSAID = nonsteroidal anti-inflammatory drug; PI = protease inhibitor. Effect sizes are based on Hedge's g statistic for continuous variables and odds ratios for binary variables.
n = 64
n = 62
n = 65
Results
Participants
The sample consisted of 100 participants who were predominantly middle-aged [mean 57.8 (SD 6.0) years], non-Hispanic white (73.0%) men (78.0%) with some college education [mean 14.3 (SD 2.7) years]. Demographic, medical, and psychiatric characteristics of the sample are presented in Table 1.
In general, HIV+ and HIV- groups were largely comparable (i.e., p values for group differences > .05) across many of the characteristics, including age, education, medical comorbidities (i.e., hypertension, tobacco smoking, diabetes mellitus, and hepatitis C), medical prescriptions (i.e., NSAID, antihypertensive and lipid-lowering drug use), and proportion of current MDD and lifetime substance use disorders. The HIV+ group had more men (84.8% vs. 64.7%, p = .02), cases of hyperlipidemia (52.4% vs. 29.4%, p = .03), cases of lifetime MDD (57.6% vs 29.4%, p < .01), and a higher proportion on an antidepressant medication (42.4% vs 11.8%, p < .01). The HIV+ group also had higher scores on a self-report measure of depression symptoms (9.5 vs. 1.5, p < .01).
Among the HIV+ participants (n = 66), the mean estimated duration of HIV infection was 19.4 years, the median current CD4+ T cell count was 654 cells/mm3, and the median nadir CD4+ T cell count was 180 cells/mm3.
Comparison of Coagulation Biomarkers between HIV serostatus groups
Of the ten individual coagulation biomarkers, the HIV serostatus groups only differed on fibrinogen, which was higher in the HIV- group (p = .04, hedges g = -.45; Table 2). No significant differences were found between the HIV- and HIV+ groups for the coagulation imbalance composite score or the three individual coagulation indices (i.e., procoagulation, anticoagulation, and fibrinolysis; p-values > .05). The procoagulant, anticoagulant, and fibrinolytic factor indices were not univariably associated with each other (p-values > .05; Table 3).
Table 2. Plasma biomarkers related to coagulation and inflammation by HIV serostatus.
| Biomarker | HIV+ (n = 66) Median (IQR) | HIV- (n = 34) Median (IQR) | p-value | Effect size |
|---|---|---|---|---|
| Procoagulants | ||||
| Fibrinogen (mg/dL) | 180.5 (131.9 – 214.8) | 195.6 (164.1 – 231.0) | .04 | -.45 |
| P-Selectin (ng/mL) | 37.2 (29.9 – 46.7) | 38.2 (29.4 – 48.4) | .74 | -.12 |
| Tissue Factor (pg/mL) | 1150 (490 – 1723) | 1175 (680 – 1583) | .87 | .09 |
| vWF (pg/mL) | 97.1 (97.1 – 152.5) | 97.1 (97.1 – 112.5) | .06 | .36 |
| Anticoagulants | ||||
| Antithrombin (μg/mL) | .67 (.50 – 1.36) | .81 (.57 – 1.73) | .99 | .22 |
| Protein-C (μg/mL) | .05 (.03 – .06) | .05 (.04 – .06) | .97 | .01 |
| Thrombomodulin (ng/mL) | 4.00 (2.69 – 5.24) | 3.77 (3.41 – 4.84) | .34 | .16 |
| Fibrinolytic factors | ||||
| D-dimer (μg/mL) | .41 (.31 – .64) | .50 (.33 – .70) | .46 | -.16 |
| PAI-1 (μg/mL) | .03 (.02 – .05) | .02 (.02 – .04) | .11 | .42 |
| Plasminogen (mg/dL) | 11.55 (8.92 – 13.71) | 12.54 (9.12 – 16.68) | .30 | -.24 |
| Inflammation | ||||
| Soluble CD163 (ng/ml) | 1225 (888 – 1798)a | 832 (594 – 1341) | .03 | .41 |
| Soluble CD14 (pg/ml) | 2106 (1842 – 2338) a | 1639 (1484 – 2020) | <.001 | .97 |
| Complement C3 (mg/dl) | 1010 (760 – 1476) a | 998 (693 – 1356) | .87 | .03 |
Note: PAI-1 = plasminogen activator inhibitor-1; vWF = Von Willebrand factor. Effect size based on Hedge's g statistic.
n=59
Table 3. Correlation matrix among coagulation factors and neurocognitive function.
| Coagulation Imbalance Score | Procoagulants | Anticoagulants | Fibrinolytic factors | |
|---|---|---|---|---|
| Procoagulants | ||||
| All (N=100) | .73*** | |||
| HIV+ (n=66) | .71*** | |||
| HIV- (n=34) | .76*** | |||
| Anticoagulants | ||||
| All (N = 100) | .62*** | .12 | ||
| HIV+ (n = 66) | .58*** | .05 | ||
| HIV- (n = 34) | .68*** | .34 | ||
| Fibrinolytic factors | ||||
| All (N = 100) | .53*** | .13 | -.01 | |
| HIV+ (n = 66) | .57*** | .19 | -.01 | |
| HIV- (n = 34) | .48** | .04 | -.02 | |
| Global t score | ||||
| All (N = 100) | -0.10 | -.14 | -.06 | .03 |
| HIV+ (n = 66) | -.30* | -.31* | .17 | .05 |
| HIV- (n = 34) | .22 | .18 | .05 | .18 |
Note:
p < .05,
p < .01,
p < .001
Personal Factors and Inflammatory Biomarkers Are Associated with Coagulation
Higher coagulation imbalance scores were univariably associated with higher BMI (r = .23, p = .02), being on lipid-lowering drug, having a dyslipidemia diagnosis, and having a current MDD diagnosis (p-values < .05). Higher coagulation imbalance scores were univariably associated with higher levels of inflammatory biomarkers complement C3 (r = .25, p = .01), sCD163 (r = .24, p = .02), and sCD14 (r = .22, p = .03). Higher procoagulant scores were univariably associated with higher BDI-II scores and both current and lifetime MDD diagnoses (p-values < .05). Higher anticoagulant score was only univariably associated with being on a lipid-lowering drug (p = .01), whereas higher fibrinolytic score was univariably associated with higher BMI (r = .21, p = .03) and complement C3 (r = .25, p = .02). None of the HIV disease characteristics were univariably associated with any of the coagulation indices within the HIV+ group (p-values > .05).
Coagulation Moderates the Association between HIV Serostatus and Neurocognitive Functioning
HIV+ participants had worse neurocognitive functioning [mean global T-score = 46.2 (SD 6.9)] than HIV- participants [mean global T-score = 49.5 (SD 6.6)] (p = .02; hedge's g = -.48). Four separate multivariable linear regression models were used to test whether HIV and the coagulation imbalance composite scores have an interacting effect on neurocognitive functioning. The first model of neurocognitive functioning (Model adjusted R2 = .10, p = .005; Figure 1) identified a statistically significant interaction between coagulation imbalance composite score and HIV (β = -.39, p = 0.01). Follow-up analyses indicated that coagulation imbalance composite score had a statistically significant positive association with neurocognitive functioning among the HIV+ group (r = -.30, p = .01) but not the HIV- group (r = .22, p = .22). The second model of neurocognitive functioning (Model adjusted R2 = .10, p = .005) identified a statistically significant interaction between the procoagulant index score and HIV (β = -.38, p = 0.02). Similar to the coagulation imbalance composite score, the procoagulant index score had a statistically significant positive association with neurocognitive functioning among the HIV+ group (r = -.31, p = .01) but not the HIV- group (r = .18, p = .30). Models 1 and 2 and their respective interaction terms remained statistically significant (p-values < .05) after adjusting for relevant covariates (i.e., BMI, diabetes, antidepressant use, and current MDD diagnosis). In post-hoc analyses, variables associated with coagulation imbalance (i.e., BMI, lipid-lowering drug use, dyslipidemia diagnosis, current MDD diagnosis, and inflammatory biomarkers C3, sCD163, and sCD14) were added to Models 1 and 2 to examine whether inflammation, rather than coagulation imbalance, was uniquely associated with neurocognitive functioning. Both models 1 and 2 and their respective interaction terms remained statistically significant (p-values < .05). The inflammatory biomarkers were not statistically significant predictors of neurocognitive functioning in the multivariable models (p-values > .05).
Figure 1. HIV serostatus and coagulation imbalance interact on neurocognitive functioning.
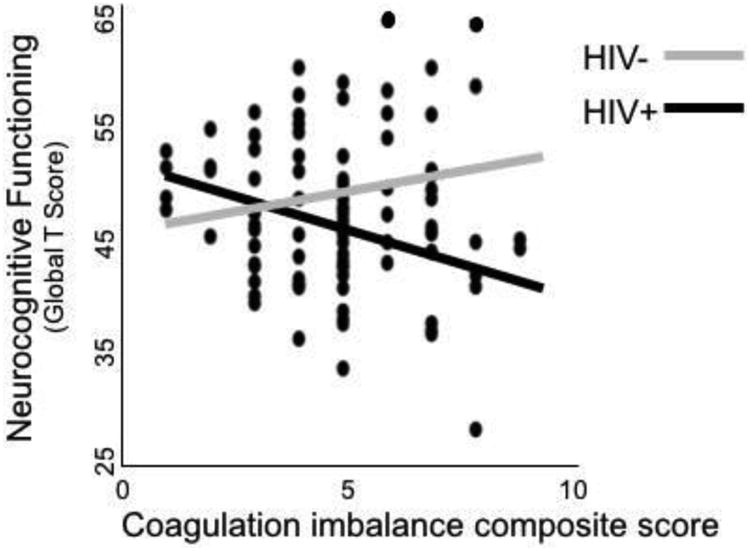
Models 3 and 4 separately tested the interaction between the anticoagulant index score and HIV and the interaction between the fibrinolytic factor score and HIV. Neither model 3 nor 4 was statistically significant with or without adjustment of relevant covariates (overall model p-values > .05).
Procoagulant Biomarkers, and Not HIV-disease Characteristics, Are Uniquely Associated with Neurocognitive Functioning in Older HIV+ Adults on Suppressive ART
A series of multivariable models were run that included only the HIV+ cases in order to adjust for HIV-disease related variables associated with neurocognitive functioning (i.e., AIDS status and current CD4). The procoagulant index remained a statistically significant predictor of neurocognition (β = -.27, p = .03) in a multivariable model adjusting for the effects of BMI, diabetes, antidepressant use, current MDD diagnosis, AIDS status, and current CD4. None of the covariates included in this multivariable model were statistically significant (p-values > .05). The coagulation imbalance composite score (β = -.19, p = .18) did not remain statistically significant in the multivariable model adjusting for these same covariates. In addition, neither the anticoagulant (β = -.04, p = .76) or fibrinolytic factor (β = .01, p = .93) indices achieved statistical significance in analogous multivariable models involving only the HIV+ sample.
Discussion
In our cross-sectional study, levels of soluble biomarkers of procoagulation, anticoagulation, and fibrinolysis were comparable between ART-treated virally suppressed HIV+ older adults and the HIV- comparison group. Elevated markers of coagulation were associated with traditional cardiovascular risk factors (e.g., higher BMI and diagnosis and treatment of dyslipidemia) and depressed mood. HIV and coagulation imbalance had an interacting effect on neurocognitive functioning, such that greater coagulation imbalance was associated with poorer neurocognitive functioning among the HIV+, but not the HIV-, group. The moderating effect of coagulation imbalance appeared to be driven by procoagulant factors, rather than anticoagulant or fibrinolytic markers. These findings indicate that elevated levels of procoagulant markers may exert a particularly detrimental effect on neurocognitive functioning among ART-treated virally suppressed HIV+ older adults.
HIV was not found to confer risk for coagulation imbalance in our study sample consisting of ART-treated virally suppressed HIV+ older adults and an HIV- comparison group. Although ART-naïve HIV+ persons show elevated levels of various coagulation-related biomarkers (e.g., fibrinogen, d-dimer, and vWF) [24, 25], it remains unclear whether initiation and long-term use of ART produces a normal coagulation profile. With the initiation of ART, biomarkers of coagulation may decrease (e.g., fibrinogen, d-dimer, vWF and P-selectin) or increase (e.g., protein C and AT), such that levels may be comparable to those among HIV- comparison groups [24-27]. Not all biomarkers, however, may travel with ART status, as was the case of fibrinogen in the SMART study [10]. When comparing HIV+ adults on versus off ART, ART does appear to alter coagulation biomarkers, resulting in lower levels of d-dimer [28-31], vWF [10, 28, 30, 32], fibrinogen [33], and PAI-1 [31], and higher levels of AT [10] and protein C [10, 30]. Comparison between ART-treated HIV+ persons and HIV- comparison groups has yielded mixed results, with some studies reporting differences in levels of coagulation biomarkers (e.g., elevations of d-dimer, thrombomodulin, AT, fibrinogen, and PAI-1 among HIV+ adults) [34-38] and others finding no differences among the same biomarkers (e.g., comparable levels of thrombomodulin and d-dimer between HIV serostatus groups) [39, 40].
The profile of coagulation in HIV disease is clearly complex. Discrepant findings in the existing literature are likely an artifact of unique features of individual study cohorts. For example, studies differ on whether participants are in the early and acute infection period versus long-term survivors of HIV disease. Our study, in particular, enrolled an HIV+ cohort who were on suppressive ART, and our HIV- cohort was recruited to be medically and behaviorally similar to our HIV+ cohort (e.g., similar prevalence of substance use disorders, diabetes mellitus, hepatitis C). Thus, the results of this study should be interpreted with consideration of our cohort's unique demographic and clinical characteristics.
Specific HIV disease characteristics, such as current CD4+ T cell count, do not appear to be associated with coagulation indices among older HIV+ persons. In older HIV+ persons with virologic suppression on ART, the effect of HIV disease parameters on coagulation may be eclipsed by other factors exerting an influence on hemostasis factors, such as BMI, presence and treatment of dyslipidemia, and inflammatory biomarkers. Our failure to detect an association between the coagulation indices and HIV disease characteristics may reflect that this association is weak or absent in the context of ART, viral suppression, chronic HIV disease, older age, and/or presence of medical comorbidity burden.
In our study, coagulation biomarkers were related to several inflammatory biomarkers. Specifically, higher coagulation imbalance scores had positive associations with sCD163, sCD14 and complement C3. Monocyte/macrophage activation is hypothesized to be a source of inflammatory cells in the central nervous system (CNS) and a key mechanism for CNS pathogenesis [41]. Persistent monocyte/macrophage activation, measured by plasma sCD163, has been previously observed in neurophysiologically impaired HIV+ individuals on virally suppressive ART [42]. Among chronically-infected HIV+ adults, sCD163 levels appear to decrease with decreasing HIV RNA levels but may not return to seronegative levels, indicating residual monocyte/macrophage activation [43]. Similarly, sCD14, a marker of systemic immune activation, appears to interact with a marker of abdominal obesity on neurocognitive impairment [44]. Complement C3, a primary mechanism of innate immunity, may also be a marker of cardiometabolic risk among persons aging with HIV [20]. Likely, the relationship between coagulation imbalance and inflammation is bidirectional, with coagulation imbalance being both a consequence of inflammation and an amplifier of the inflammatory response [8].
Chronic inflammation can lead to disruption of the endothelium, which has been reported in HIV disease [45-47]. Endothelial dysfunction may play a pivotal role in the pathogenesis of cerebral small vessel disease [48] via the breakdown of the blood-brain barrier [49] and impairment of cerebral reactivity and autoregulation [50]. Hemostatic changes are hypothesized to play a secondary role to endothelial activation, such that damaged endothelial cells can act as a substrate for the initiation of coagulation [51]. The procoagulant index employed in this study was based on values of fibrinogen, p-selectin, tissue factor, and vWF. Thus, the procoagulant index may represent both endothelial dysfunction and coagulation imbalance. When accompanied by endothelial dysfunction, elevation in plasma fibrinogen levels has been found to increase the risk of subclinical white matter lesions [52]. Elevation in procoagulant factors, such as fibrinogen, are hypothesized to influence cerebral hypoperfusion and the development of white matter lesions, thereby contributing to neurocognitive decline [52].
Several limitations of this study should be noted. This study used a cross-sectional, observational design and thus causality from the observed associations among HIV, coagulation imbalance, and neurocognitive functioning cannot be inferred. Furthermore, both HIV infection and ART likely exert an influence on neurocognitive functioning, and without an ART-naïve comparison sample, we cannot disentangle the effect of ART on neurocognitive functioning. Given that the parent study aimed to investigate “successful aging” with HIV, there is a high likelihood of selection bias such that we recruited a sample of HIV+ patients demonstrating good immunologic and virologic profiles, and these older HIV-infected individuals mostly represent a long-term “survivor” cohort. Lastly, we operationalized the coagulation biomarkers into three theorized physiological systems that have not been previously validated; however, a large panel of biomarkers as utilized in the present study has not been previously used in relation to both HIV and neurocognition. These three physiological systems are likely dynamic and complex, and future research is needed to determine the most optimal method for modeling biomarkers of coagulation.
Endothelial activation and coagulation imbalance likely play mechanistic roles leading to poorer neurocognitive functioning outcomes in HIV+ adults. Thus, biomarkers of endothelial activation and coagulation may provide valuable information regarding the prognosis and/or risk stratification of HIV+ adults. Although greater procoagulant values were found to have an association with neurocognitive functioning for ART-treated virally suppressed HIV+ older adults, the clinical utility of coagulation imbalance as a predictor of neurocognitive functioning depends on whether treatment-induced reductions in procoagulant levels translate to improved neurocognitive outcomes. A better understanding of the specific role of coagulation in the etiology of HIV-associated neurocognitive disorders may lead to specific treatments aimed at reducing coagulopathy, thereby improving neurocognitive outcomes.
Acknowledgments
J.L.M, J.I., H.A.O., P.J.M., R.J.E., I.G., S.L.L, and D.J.M. provided substantial contribution to the conception and design of the study. P.L.F. contributed to the acquisition of data for the work. M.P. performed biomarker assays. J.L.M. performed statistical analysis and wrote the first draft of the manuscript. All authors critically revised and provided final approval of the manuscript.
The HIV Neurobehavioral Research Center (HNRC) is supported by Center award P30MH062512 from NIMH.
Source of Funding: Ms. Montoya was supported by NIMH Diversity Supplement R01MH099987. The study was more broadly supported by the California HIV/AIDS Research Program IDEA Award ID10-SD-057 (PI: Moore, David J.); the HIV Neurobehavioral Research Center (HNRC) Award P30MH062512; and NIH K24 M097873 (PI: Letendre, Scott).
Footnotes
Conflicts of Interest: For the remaining authors, none were declared.
The San Diego HIV Neurobehavioral Research Center [HNRC] group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Robert K. Heaton, Ph.D., Co-Director: Igor Grant, M.D.; Associate Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and Scott Letendre, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), Scott Letendre, M.D., J. Allen McCutchan, M.D., Brookie Best, Pharm.D., Rachel Schrier, Ph.D., Debra Rosario, M.P.H.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Thomas D. Marcotte, Ph.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Monte S. Buchsbaum, M.D., John Hesselink, M.D., Sarah L. Archibald, M.A., Gregory Brown, Ph.D., Richard Buxton, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D.; Neurovirology Component: David M. Smith, M.D. (P.I.), Douglas Richman, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.), Mariana Cherner, Ph.D.; Developmental Component: Cristian Achim, M.D., Ph.D.; (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Jennifer Marquie-Beck, M.P.H.; Data Management and Information Systems Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D. (Co-PI), Reena Deutsch, Ph.D., Anya Umlauf, M.S.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
References
- 1.Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–96. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker JT, Lopez OL, Dew MA, Aizenstein HJ. Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS. 2004;18 Suppl 1:S11–8. [PubMed] [Google Scholar]
- 3.Cherner M, Ellis RJ, Lazzaretto D, Young C, Mindt MR, Atkinson JH, et al. Effects of HIV-1 infection and aging on neurobehavioral functioning: preliminary findings. AIDS. 2004;18 Suppl 1:S27–34. [PubMed] [Google Scholar]
- 4.Sacktor N, Skolasky R, Selnes OA, Watters M, Poff P, Shiramizu B, et al. Neuropsychological test profile differences between young and old human immunodeficiency virus-positive individuals. J Neurovirol. 2007;13:203–9. doi: 10.1080/13550280701258423. [DOI] [PubMed] [Google Scholar]
- 5.Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes O, et al. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology. 2004;63:822–7. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foley J, Ettenhofer M, Wright MJ, Siddiqi I, Choi M, Thames AD, et al. Neurocognitive functioning in HIV-1 infection: effects of cerebrovascular risk factors and age. Clin Neuropsychol. 2010;24:265–85. doi: 10.1080/13854040903482830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Funderburg NT. Markers of coagulation and inflammation often remain elevated in ART-treated HIV-infected patients. Current opinion in HIV and AIDS. 2014;9:80–6. doi: 10.1097/COH.0000000000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Funderburg NT, Lederman MM. Coagulation and morbidity in treated HIV infection. Thromb Res. 2014;133 Suppl 1:S21–4. doi: 10.1016/j.thromres.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hileman CO, Longenecker CT, Carman TL, Milne GL, Labbato DE, Storer NJ, et al. Elevated D-dimer is independently associated with endothelial dysfunction: a cross-sectional study in HIV-infected adults on antiretroviral therapy. Antivir Ther. 2012;17:1345–9. doi: 10.3851/IMP2297. [DOI] [PubMed] [Google Scholar]
- 10.Baker JV, Brummel-Ziedins K, Neuhaus J, Duprez D, Cummins N, Dalmau D, et al. HIV replication alters the composition of extrinsic pathway coagulation factors and increases thrombin generation. J Am Heart Assoc. 2013;2:e000264. doi: 10.1161/JAHA.113.000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker JV, Neuhaus J, Duprez D, Kuller LH, Tracy R, Belloso WH, et al. Changes in inflammatory and coagulation biomarkers: a randomized comparison of immediate versus deferred antiretroviral therapy in patients with HIV infection. J Acquir Immune Defic Syndr. 2011;56:36–43. doi: 10.1097/QAI.0b013e3181f7f61a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neuhaus J, Jacobs DR, Jr, Baker JV, Calmy A, Duprez D, La Rosa A, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–95. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haugaard AK, Lund TT, Birch C, Ronsholt F, Troseid M, Ullum H, et al. Discrepant coagulation profile in HIV infection: elevated D-dimer but impaired platelet aggregation and clot initiation. AIDS. 2013;27:2749–58. doi: 10.1097/01.aids.0000432462.21723.ed. [DOI] [PubMed] [Google Scholar]
- 14.Deguchi K, Deguchi A, Wada H, Murashima S. Study of cardiovascular risk factors and hemostatic molecular markers in elderly persons. Semin Thromb Hemost. 2000;26:23–7. doi: 10.1055/s-2000-9798. [DOI] [PubMed] [Google Scholar]
- 15.Cherner M, Suarez P, Lazzaretto D, Fortuny LA, Mindt MR, Dawes S, et al. Demographically corrected norms for the Brief Visuospatial Memory Test-revised and Hopkins Verbal Learning Test-revised in monolingual Spanish speakers from the U.S.-Mexico border region. Arch Clin Neuropsychol. 2007;22:343–53. doi: 10.1016/j.acn.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10:317–31. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- 17.Heaton RK, Taylor MJ, Manly JJ, editors. Demograhic effects and use of demographically corrected norms with the WAIS-III and WMS-III. San Diego, CA: Academic Press; 2002. [Google Scholar]
- 18.Bowie CR, Leung WW, Reichenberg A, McClure MM, Patterson TL, Heaton RK, et al. Predicting schizophrenia patients' real-world behavior with specific neuropsychological and functional capacity measures. Biol Psychiatry. 2008;63:505–11. doi: 10.1016/j.biopsych.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seeman TE, Crimmins E, Huang MH, Singer B, Bucur A, Gruenewald T, et al. Cumulative biological risk and socio-economic differences in mortality: MacArthur studies of successful aging. Soc Sci Med. 2004;58:1985–97. doi: 10.1016/S0277-9536(03)00402-7. [DOI] [PubMed] [Google Scholar]
- 20.Bryant AK, Fazeli PL, Letendre SL, Ellis RJ, Potter M, Burdo TH, et al. Complement Component 3 Is Associated with Metabolic Comorbidities in Older HIV-Positive Adults. AIDS Res Hum Retroviruses. 2016;32:271–8. doi: 10.1089/aid.2015.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. Composite International Diagnostic Interview (CIDI, version 2.1) Geneva: World Health Organization; 1998. [Google Scholar]
- 22.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 23.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 24.Arildsen H, Sorensen KE, Ingerslev JM, Ostergaard LJ, Laursen AL. Endothelial dysfunction, increased inflammation, and activated coagulation in HIV-infected patients improve after initiation of highly active antiretroviral therapy. HIV Med. 2013;14:1–9. doi: 10.1111/j.1468-1293.2012.01027.x. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan RC, Landay AL, Hodis HN, Gange SJ, Norris PJ, Young M, et al. Potential cardiovascular disease risk markers among HIV-infected women initiating antiretroviral treatment. J Acquir Immune Defic Syndr. 2012;60:359–68. doi: 10.1097/QAI.0b013e31825b03be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamlyn E, Stöhr W, Cooper DA, Fisher M, Tambussi G, Schechter M, et al. The effect of short-course antiretroviral therapy initiated in primary HIV-1 infection on interleukin-6 and D-dimer levels. AIDS. 2015;29:1355–61. doi: 10.1097/QAD.0000000000000675. [DOI] [PubMed] [Google Scholar]
- 27.O'Halloran J, Dunne E, Gurwith M, Lambert J, Sheehan G, Feeney E, et al. The effect of initiation of antiretroviral therapy on monocyte, endothelial and platelet function in HIV-1 infection. HIV Med. 2015;16:608–19. doi: 10.1111/hiv.12270. [DOI] [PubMed] [Google Scholar]
- 28.Baker JV, Hullsiek KH, Bradford RL, Prosser R, Tracy RP, Key NS. Circulating levels of tissue factor microparticle procoagulant activity are reduced with antiretroviral therapy and are associated with persistent inflammation and coagulation activation among HIV positive patients. J Acquir Immune Defic Syndr. 2013;63:367. doi: 10.1097/QAI.0b013e3182910121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cioe PA, Baker J, Kojic EM, Onen N, Hammer J, Patel P, et al. Elevated soluble CD14 and lower d-dimer are associated with cigarette smoking and heavy episodic alcohol use in persons living with HIV. J Acquir Immune Defic Syndr. 2015;70:400–5. doi: 10.1097/QAI.0000000000000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jong E, Louw S, Meijers JC, de Kruif MD, ten Cate H, Büller HR, et al. The hemostatic balance in HIV-infected patients with and without antiretroviral therapy: partial restoration with antiretroviral therapy. AIDS Patient Care STDs. 2009;23:1001–7. doi: 10.1089/apc.2009.0173. [DOI] [PubMed] [Google Scholar]
- 31.Kiefer E, Hoover DR, Shi Q, Kuniholm MH, Augenbraun M, Cohen MH, et al. Association of markers of hemostasis with death in HIV-infected women. J Acquir Immune Defic Syndr. 2014;67:287–94. doi: 10.1097/QAI.0000000000000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross AC, Armentrout R, O'Riordan MA, Storer N, Rizk N, Harrill D, et al. Endothelial activation markers are linked to HIV status and are independent of antiretroviral therapy and lipoatrophy. J Acquir Immune Defic Syndr. 2008;49:499–506. doi: 10.1097/QAI.0b013e318189a794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eastburn A, Scherzer R, Zolopa AR, Benson C, Tracy R, Do T, et al. Association of low level viremia with inflammation and mortality in HIV-infected adults. PloS one. 2011;6:e26320. doi: 10.1371/journal.pone.0026320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bastard JP, Fellahi S, Couffignal C, Raffi F, Gras G, Hardel L, et al. Increased systemic immune activation and inflammatory profile of long-term HIV-infected ART-controlled patients is related to personal factors, but not to markers of HIV infection severity. J Antimicrob Chemother. 2015;70:1816–24. doi: 10.1093/jac/dkv036. [DOI] [PubMed] [Google Scholar]
- 35.de Larranaga GF, Petroni A, Deluchi G, Alonso BS, Benetucci JA. Viral load and disease progression as responsible for endothelial activation and/or injury in human immunodeficiency virus-1-infected patients. Blood Coagul Fibrinolysis. 2003;14:15–8. doi: 10.1097/00001721-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Hsue PY, Scherzer R, Grunfeld C, Nordstrom SM, Schnell A, Kohl LP, et al. HIV infection is associated with decreased thrombin generation. Clin Infect Dis. 2012;54:1196–203. doi: 10.1093/cid/cis014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madden E, Lee G, Kotler DP, Wanke C, Lewis CE, Tracy R, et al. Association of antiretroviral therapy with fibrinogen levels in HIV infection. AIDS. 2008;22:707–15. doi: 10.1097/QAD.0b013e3282f560d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pirs M, Jug B, Erzen B, Sabovic M, Karner P, Poljak M, et al. Relationship between markers of endothelial dysfunction and inflammation and subclinical atherosclerosis in HIV-infected male patients below 55 years of age. Acta dermatovenerologica Alpina, Pannonica, et Adriatica. 2014;23:49–52. doi: 10.15570/actaapa.2014.12. [DOI] [PubMed] [Google Scholar]
- 39.Rönsholt FF, Gerstoft J, Ullum H, Johansson PI, Katzenstein TL, Ostrowski SR. Thromboelastography on plasma reveals delayed clot formation and accelerated clot lyses in HIV-1 infected persons compared with healthy controls. BMC infectious diseases. 2015;15:1. doi: 10.1186/s12879-015-1124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wallet MA, Buford TW, Joseph AM, Sankuratri M, Leeuwenburgh C, Pahor M, et al. Increased inflammation but similar physical composition and function in older-aged, HIV-1 infected subjects. BMC immunology. 2015;16:1. doi: 10.1186/s12865-015-0106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burdo TH, Lackner A, Williams KC. Monocyte/macrophages and their role in HIV neuropathogenesis. Immunol Rev. 2013;254:102–13. doi: 10.1111/imr.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KC. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS. 2013;27:1387–95. doi: 10.1097/QAD.0b013e32836010bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burdo TH, Lentz MR, Autissier P, Krishnan A, Halpern E, Letendre S, et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis. 2011;204:154–63. doi: 10.1093/infdis/jir214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sattler FR, He J, Letendre S, Wilson C, Sanders C, Heaton R, et al. Abdominal obesity contributes to neurocognitive impairment in HIV-infected patients with increased inflammation and immune activation. J Acquir Immune Defic Syndr. 2015;68:281–8. doi: 10.1097/QAI.0000000000000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang AL, Vita JA. Effects of systemic inflammation on endothelium-dependent vasodilation. Trends Cardiovasc Med. 2006;16:15–20. doi: 10.1016/j.tcm.2005.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lopez M, San Roman J, Estrada V, Vispo E, Blanco F, Soriano V. Endothelial dysfunction in HIV infection--the role of circulating endothelial cells, microparticles, endothelial progenitor cells and macrophages. AIDS Rev. 2012;14:223–30. [PubMed] [Google Scholar]
- 47.Solages A, Vita JA, Thornton DJ, Murray J, Heeren T, Craven DE, et al. Endothelial function in HIV-infected persons. Clin Infect Dis. 2006;42:1325–32. doi: 10.1086/503261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hassan A, Hunt BJ, O'Sullivan M, Parmar K, Bamford JM, Briley D, et al. Markers of endothelial dysfunction in lacunar infarction and ischaemic leukoaraiosis. Brain. 2003;126:424–32. doi: 10.1093/brain/awg040. [DOI] [PubMed] [Google Scholar]
- 49.Tomimoto H, Akiguchi I, Suenaga T, Nishimura M, Wakita H, Nakamura S, et al. Alterations of the blood-brain barrier and glial cells in white-matter lesions in cerebrovascular and Alzheimer's disease patients. Stroke. 1996;27:2069–74. doi: 10.1161/01.str.27.11.2069. [DOI] [PubMed] [Google Scholar]
- 50.Bakker SL, de Leeuw FE, de Groot JC, Hofman A, Koudstaal PJ, Breteler MM. Cerebral vasomotor reactivity and cerebral white matter lesions in the elderly. Neurology. 1999;52:578–83. doi: 10.1212/wnl.52.3.578. [DOI] [PubMed] [Google Scholar]
- 51.Markus HS, Hunt B, Palmer K, Enzinger C, Schmidt H, Schmidt R. Markers of endothelial and hemostatic activation and progression of cerebral white matter hyperintensities: longitudinal results of the Austrian Stroke Prevention Study. Stroke. 2005;36:1410–4. doi: 10.1161/01.STR.0000169924.60783.d4. [DOI] [PubMed] [Google Scholar]
- 52.Wada M, Takahashi Y, Iseki C, Kawanami T, Daimon M, Kato T. Plasma fibrinogen, global cognitive function, and cerebral small vessel disease: results of a cross-sectional study in community-dwelling Japanese elderly. Intern Med. 2011;50:999–1007. doi: 10.2169/internalmedicine.50.4752. [DOI] [PubMed] [Google Scholar]


