Abstract
Purpose
The suitability of younger patients with prostate cancer (PCa) for initial active surveillance (AS) has been questioned on the basis of eventual treatment necessity and concerns of safety; however, the role of age on surveillance outcomes has not been well defined.
Patients and Methods
We identified men managed with AS at our institution with a minimum follow-up of 6 months. The primary study objective was to examine the association of age with risk of biopsy-based Gleason score upgrade during AS. We also examined the association of age with related end points, including overall biopsy-determined progression, definitive treatment, and pathologic and biochemical outcomes after delayed radical prostatectomy (RP), using descriptive statistics, the Kaplan-Meier method, and multivariable Cox proportional hazards regression.
Results
A total of 1,433 patients were followed for a median of 49 months; 74% underwent initial biopsy at a referring institution. Median age at diagnosis was 63 years, including 599 patients (42%) ≤ 60 years old and 834 (58%) > 60 years old. The 3- and 5-year biopsy-based Gleason score upgrade-free rates were 73% and 55%, respectively, for men ≤ 60 years old compared with 64% and 48%, respectively, for men older than 60 years (P < .01). On Cox regression analysis, younger age was independently associated with lower risk of biopsy-based Gleason score upgrade (hazard ratio per 1-year decrease, 0.969 [95% CI, 0.956 to 0.983]; P < .01), and persisted upon restriction to men meeting strict AS inclusion criteria. There was no significant association between younger age and risk of definitive treatment or risk of biochemical recurrence after delayed RP.
Conclusion
Younger patient age was associated with decreased risk of biopsy-based Gleason score upgrade during AS but not with risk of definitive treatment in the intermediate term. AS represents a strategy to mitigate overtreatment in young patients with low-risk PCa in the early term.
INTRODUCTION
Active surveillance (AS), a management strategy consisting of close, periodic disease observation with the opportunity for delayed definitive therapy with curative intent, has been advanced as a means to reduce overtreatment in men with low-risk prostate cancer (PCa) without compromising oncologic safety.1 The feasibility of AS has been demonstrated in several prospectively followed cohorts with extended follow-up in which treatment-free rates and preservation of health-related quality of life (HRQOL) remain high, with favorable cancer-specific survival.2-5 Despite historical patterns indicating high rates of initial primary treatment of patients with low-risk disease, recent longitudinal trends in the United States show increasing use of AS after diagnosis to nearly 40%.6,7
The suitability of younger patients for initial management with AS has received limited study and has been questioned because of concerns for patient safety, a longer burden of follow-up, and the perception that definitive treatment will eventually be needed. Although not always used as a firm criterion for enrollment in large, prospective AS cohort studies or in practice guidelines, patient age empirically serves as a major driver in the decision to pursue surveillance or immediate definitive therapy, favoring early treatment in younger individuals.8,9 Yet, population-level analyses indicate that increasing age at diagnosis is an independent risk factor for PCa-specific mortality.10 Moreover, younger patients have higher baseline sexual and urinary function scores at the time of diagnosis and, therefore, stand to lose the most by immediate treatment.11
From this context, we aimed to investigate the role of patient age on outcome by examining rates of disease reclassification over time, selection of definitive treatment, and long-term biochemical-free survival outcomes among individuals receiving delayed prostatectomy in a large cohort of men managed with AS at a tertiary referral center.
MATERIALS AND METHODS
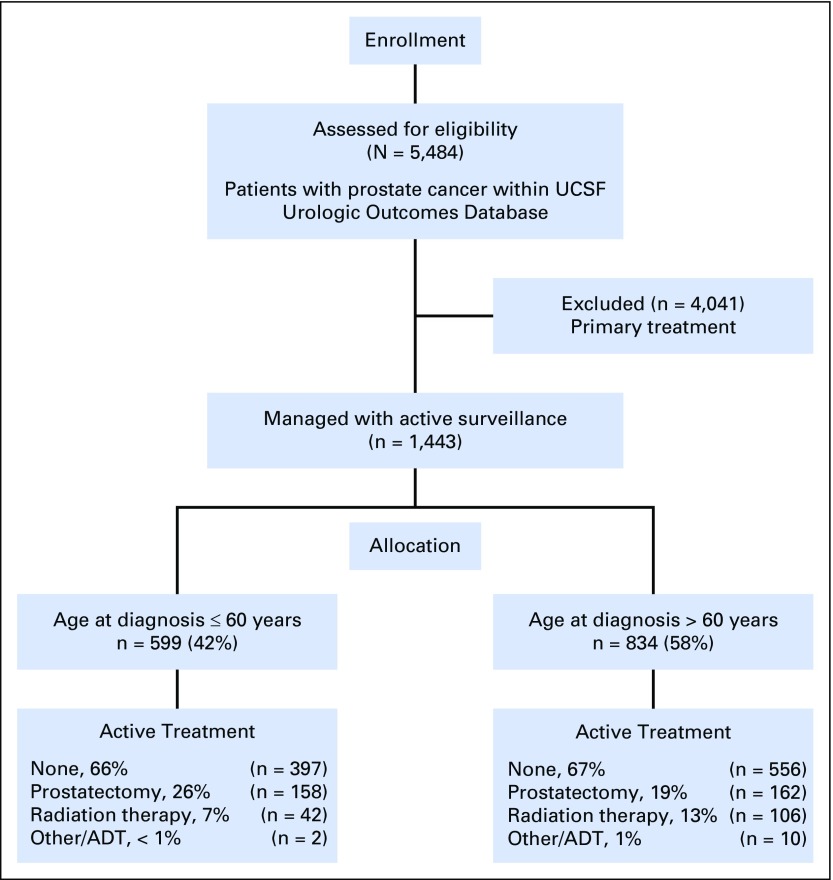
Under institutional review board approval, we identified patients within the Urological Oncology Database at the University of California San Francisco (UCSF), a prospectively accrued registry, including a large cohort of men managed with surveillance for PCa. We retrospectively identified all consenting individuals electing AS at our institution, beginning in 1992, with a minimum follow-up of 6 months from the time of initial diagnostic biopsy (Appendix Fig A1, online only). Most men (66%) elected AS in the context of strict institutional criteria, including Gleason score ≤ 3+3, prostate-specific antigen (PSA) concentration at diagnosis ≤ 10 ng/mL, clinical stage ≤ T2, ≤ 33% biopsy-specimen cores involved with cancer, and no single core with > 50% cancer. In addition, men with otherwise favorable low- and intermediate-risk disease were selectively enrolled in AS on the basis of their preferences. Age has not been an explicit criterion for AS eligibility.
The recommended AS protocol included PSA testing at 3-month intervals and confirmatory biopsy within 12 months, followed by periodic transrectal ultrasound-guided surveillance biopsy procedures every 12 to 24 months based on clinical risk and observed disease trajectory. Specimens obtained during diagnostic biopsies performed at referring institutions were reviewed by experienced genitourinary pathologists, and surveillance biopsies consisted of extended octant sampling including a minimum of 12 cores. Indications for recommending definitive treatment included clinical progression, defined on the basis of increasing Gleason grade, tumor volume, PSA level, clinical stage, patient anxiety, or patient preference. The date of diagnosis was recorded as the date of first positive biopsy specimen at any institution, which was recorded as the time of enrollment. Duration of follow-up was calculated from the date of diagnosis to the date of last contact.
The primary study objective was to evaluate the association of younger patient age with biopsy-based Gleason score upgrade, defined as any increase in Gleason score to 3+4 or higher. Secondary study end points included any biopsy-determined progression (upgrade or tumor volume to > 33% positive cores), rates of definitive treatment, and biochemical recurrence (BCR) after delayed radical prostatectomy (RP), defined as two consecutive PSA values > 0.2 ng/mL or receipt of salvage therapy in the setting of a detectable PSA level. Age at diagnosis was examined as a continuous variable (per decreasing 1 year), and dichotomized as ≤ 60 years versus older than 60 years based on a putative threshold for clinical practice near the median age of the study population.
We described age, race/ethnicity, relationship status, PSA concentration, clinical T stage, number of biopsy-specimen cores sampled, and number of biopsy-specimen cores positive for cancer, prostate volume, and Gleason score at enrollment, using frequency tables, means, and medians. To account for anticipated differences in sampling that may occur due to age-related differences in prostate volume, log-transformed PSA density was included a priori in multivariable analyses. Pearson χ2 and analysis of variance comparisons were used for categorical and continuous variables, respectively. Clinical risk was calculated using the Cancer of the Prostate Risk Assessment (CAPRA) score and grouped as low (0-2), intermediate (3-5), or high (6-10), as previously described.12 Pathologic characteristics at delayed prostatectomy were compared using the postsurgical CAPRA instrument, CAPRA-S.13
Life tables, Kaplan-Meier curves, and the log-rank test were used to examine the unadjusted effect of older age on the outcomes of biopsy-based Gleason score upgrade, biopsy-determined progression, definitive treatment, and BCR after delayed RP. Multivariate Cox proportional hazards regression models were used to examine the association of age, adjusted for relevant clinical and demographic characteristics on the outcomes of biopsy-based Gleason score upgrade and biopsy-determined progression. Multivariate model fit based on the exclusion of (null model) or inclusion (final model) of age as a covariate was assessed using Bayesian information criteria. To adjust for referral to our institution, site of diagnosis (UCSF v referring provider) was used as a covariate in the multivariable models. The end points of biopsy-based Gleason score upgrade, biopsy-determined progression, and definitive treatment were evaluated from the date of first diagnosis until event occurrence or last follow-up. Subjects were censored at the time of treatment for the outcomes of biopsy-based Gleason score upgrade and biopsy-determined progression. Among those patients ultimately treated with RP, time to BCR was calculated from date of prostatectomy to event or last follow-up and censored at the time of salvage treatment (in response to or in the absence of progression) or death as the result of any cause. To account for differences in baseline clinical-risk profiles among younger and older patients, sensitivity analyses were performed within a restricted population of patients meeting strict criteria for AS, as defined previously in this section, to examine clinical outcomes. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
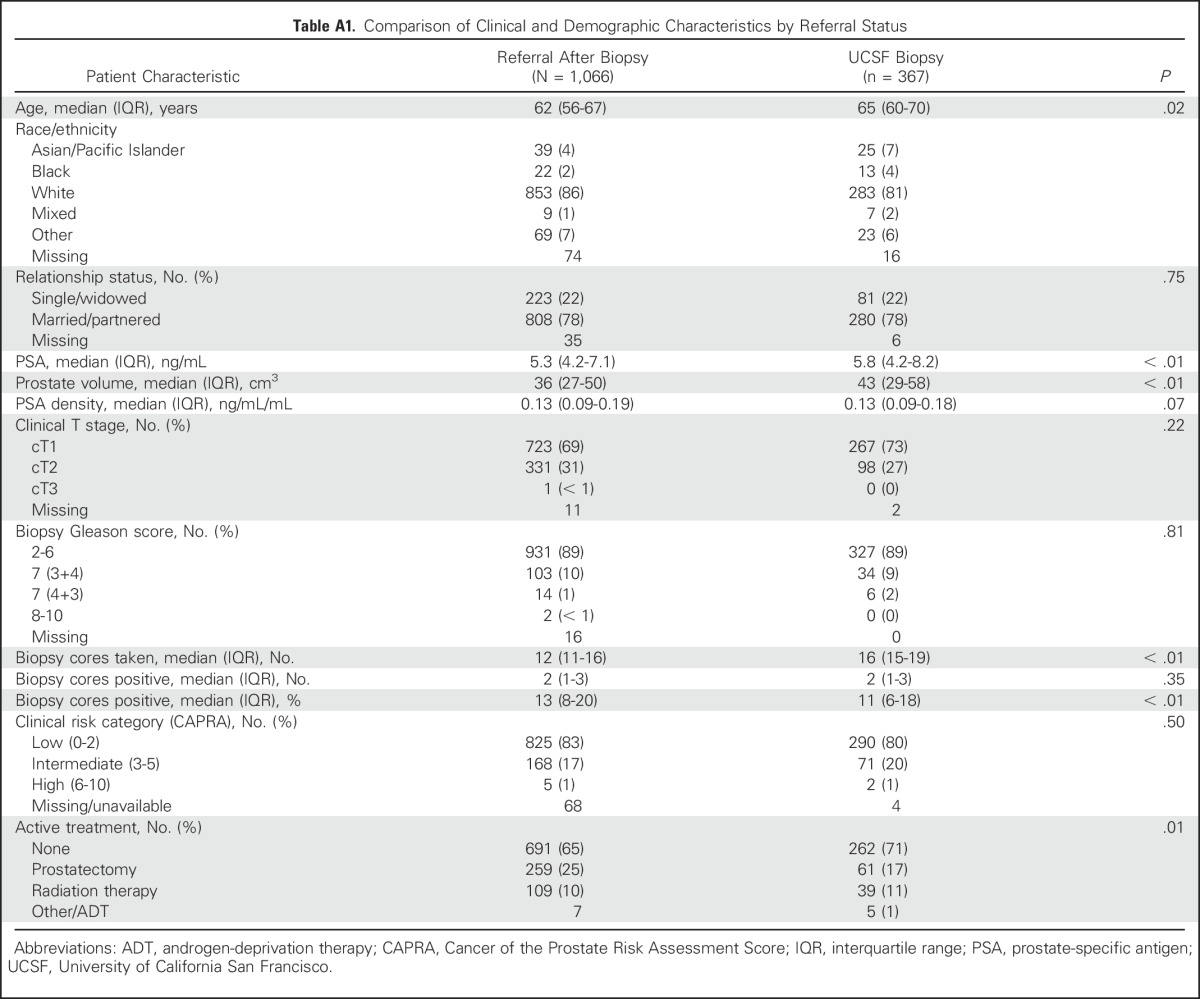
We identified 1,433 patients managed with AS; they were followed for a median of 49 months (interquartile range [IQR], 26 to 80 months). The median age at enrollment was 63 years (IQR, 57 to 68 years), including 599 patients (42%) ≤ 60 years old and 834 (58%) > 60 years old. Gleason score at diagnosis was ≤ 3+3 in 1,258 patients (89%) and 3+4 in 137 (10%). Compared with men > 60 years old, younger patients had lower PSA level at diagnosis (median, 5.0 ng/mL v 5.6 ng/mL; P < .01), a tumor that was more frequently of Gleason pattern 3+3 (93% v 85%; P < .01), and more frequently a low CAPRA risk classification (87% v 78%; P < .01). Younger patients underwent more surveillance biopsy procedures (median, 3 v 2; P < .01) and had similar numbers of PSA tests (median, 10 v 9; P = .27). Most patients (74%) were referred by an external institute to UCSF after a diagnosis of PCa, and 26% were diagnosed internally (Appendix Table A1, online only). Clinical and demographic characteristics at diagnosis are listed in Table 1 and participant allocation is explained in Appendix Figure A1.
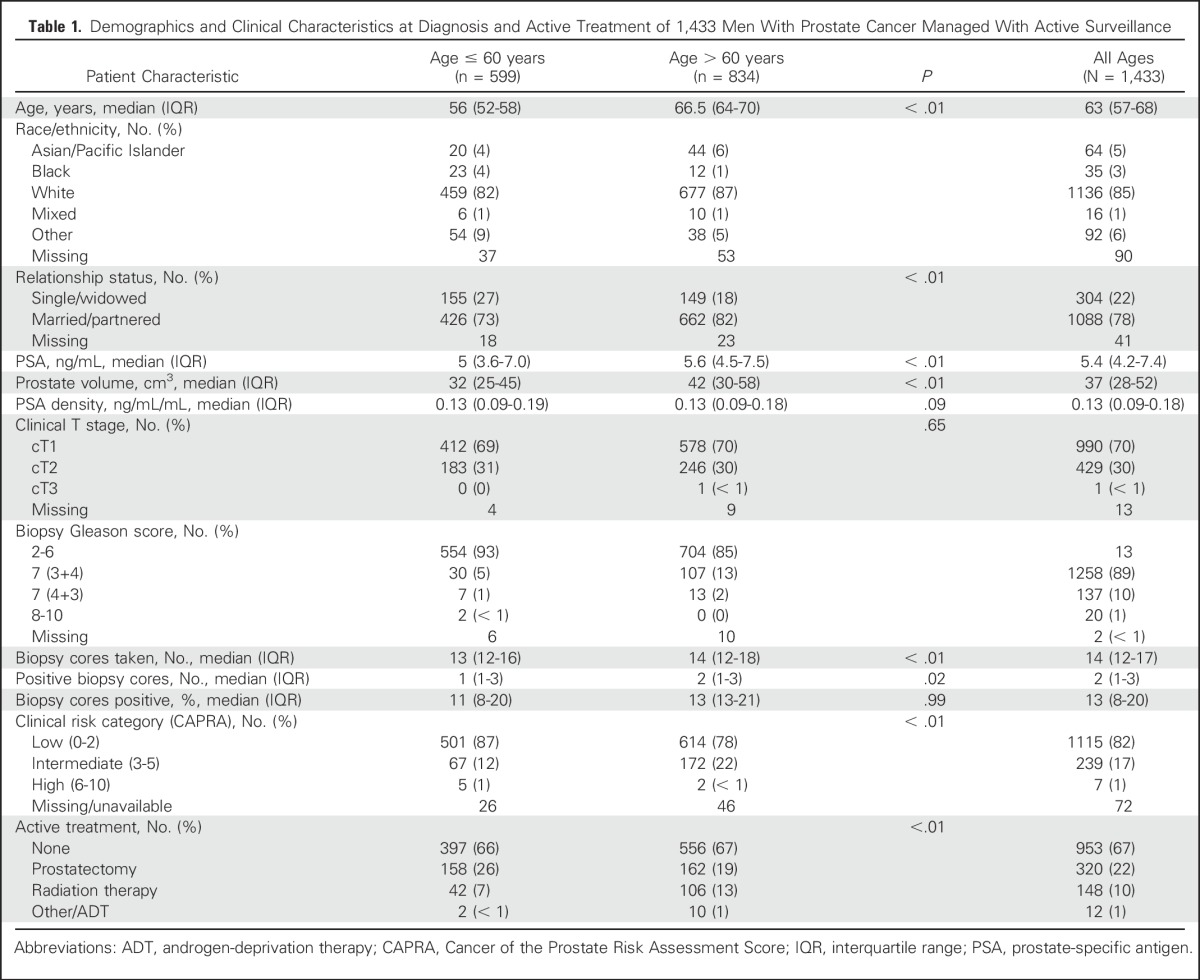
Table 1.
Demographics and Clinical Characteristics at Diagnosis and Active Treatment of 1,433 Men With Prostate Cancer Managed With Active Surveillance
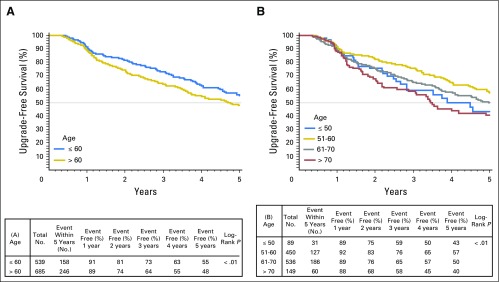
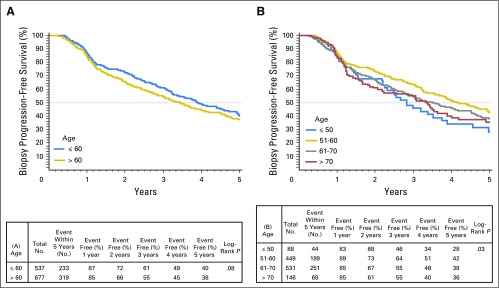
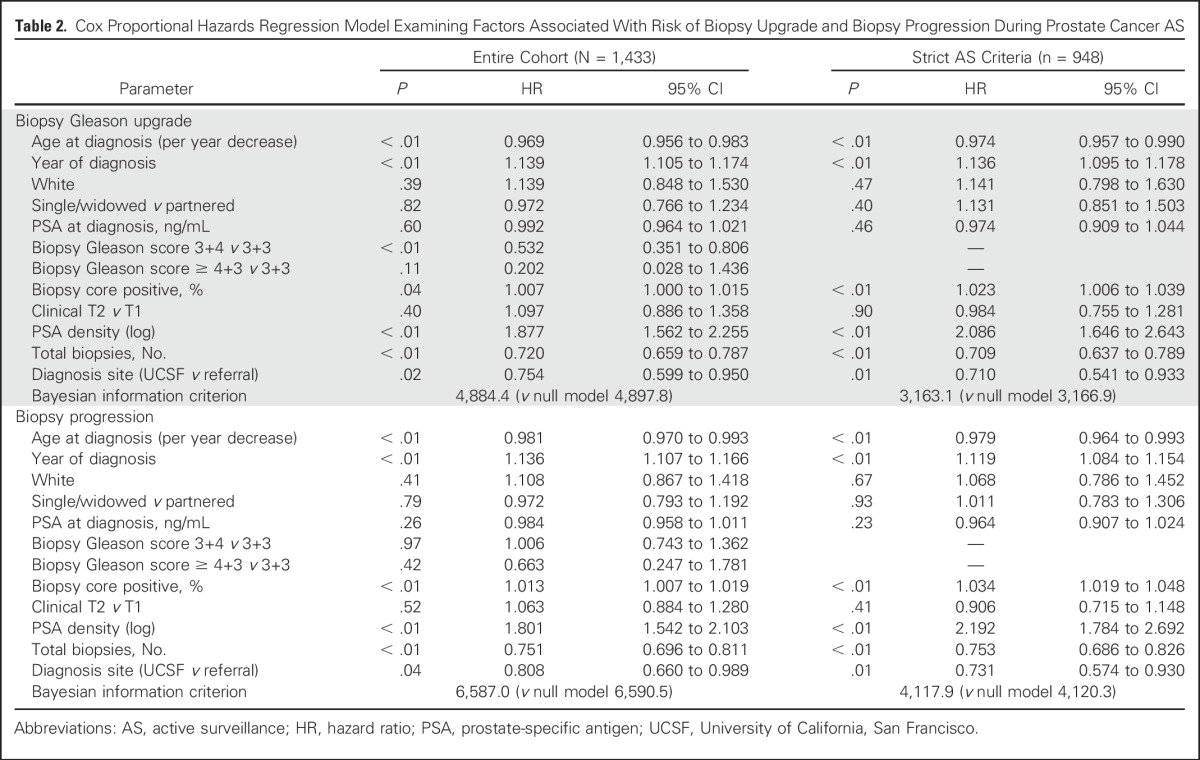
Unadjusted Gleason score upgrade-free rates for men ≤ 60 years old compared with men older than 60 years were 73% versus 64%, respectively, at 3 years of follow-up and 55% versus 48%, respectively, at 5 years (P < .01). When examined by decade at diagnosis, younger patients also had lower unadjusted rates of biopsy-based Gleason score upgrade and tumor progression (Appendix Figs A2 and A3, online only). On multivariate Cox regression analysis, younger age was independently associated with risk of biopsy-based Gleason score upgrade (hazard ratio [HR], 0.969 [95% CI, 0.956 to 0.983]; P < .001) and biopsy-determined progression (HR per 1-year decrease, 0.974 [95% CI, 0.957 to 0.990]; P < .001). Year of diagnosis, biopsy-specimen Gleason score, percentage of positive biopsy-specimen cores, PSA density, referral status, and number of total biopsy procedures were also significantly associated with time to biopsy-based Gleason score upgrade and progression (Table 2). Moreover, age group (≤ 60 years v older than 60 years) was independently associated with decreased risk of biopsy-based Gleason score upgrade (HR, 0.67 [95% CI, 0.55 to 0.83]; P < .01) and biopsy-determined progression (HR, 0.78 [95% CI, 0.65 to 0.92]; P < .01; data not shown). Multivariate models incorporating age demonstrated marginally better fit for the outcomes of biopsy-based Gleason score upgrade and biopsy-determined progression.
Table 2.
Cox Proportional Hazards Regression Model Examining Factors Associated With Risk of Biopsy Upgrade and Biopsy Progression During Prostate Cancer AS
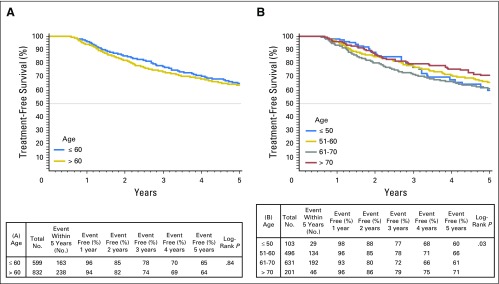
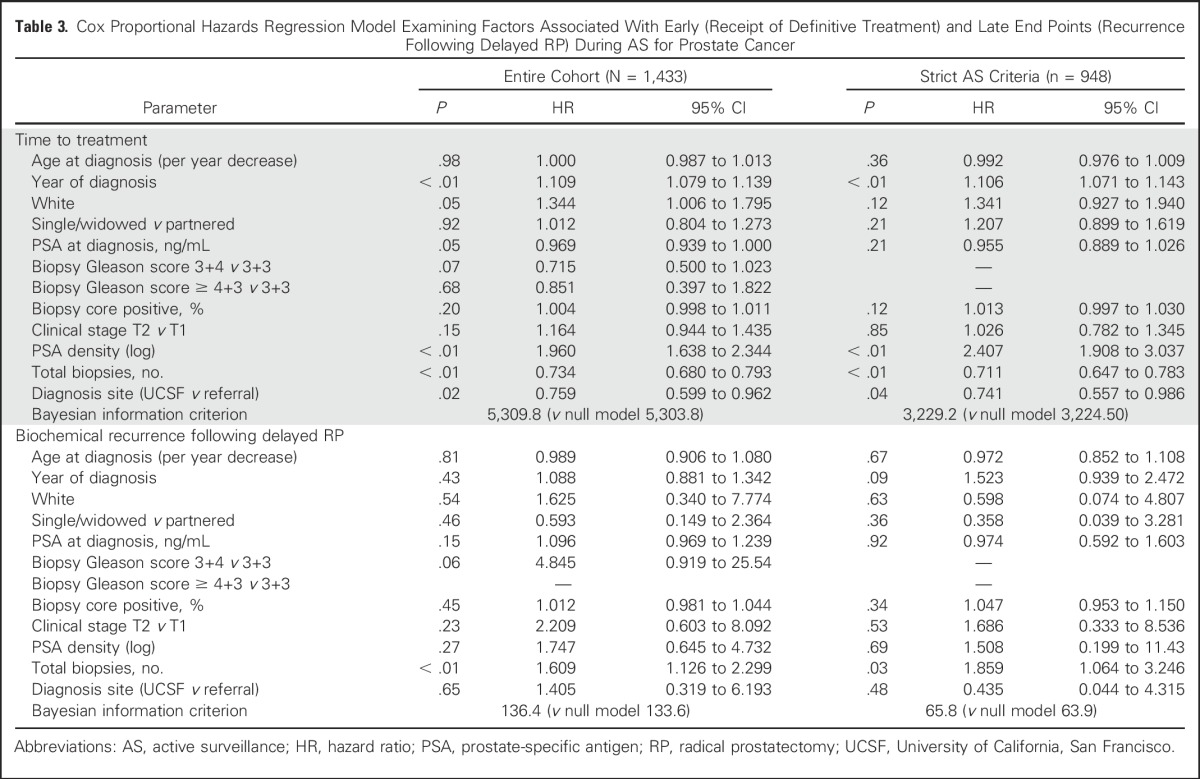
Rates of definitive treatment were similar among age groups. For men ≤ 60 years versus those older than 60 years, treatment-free survival rates were 78% versus 74%, respectively at 3 years and 65% versus 64%, respectively at 5 years (log-rank P = .84). Among individuals ultimately undergoing definitive treatment, a larger proportion of older patients received radiation therapy than younger patients (38% v 21%) and fewer received RP (58% v 78%; P < .01 for both. At the extremes of age, the 5-year treatment-free survival was 60% for men ≤ 50 years old at diagnosis compared with 71% for those ≥ 70 years (Appendix Fig A4, online only). In Cox proportional hazards regression models adjusted for clinical and pathologic characteristics, age at diagnosis was not associated with receipt of definitive treatment (HR per 1-year decrease, 1.000 [95% CI, 0.987 to 1.013]; P = 0.98; Table 3).
Table 3.
Cox Proportional Hazards Regression Model Examining Factors Associated With Early (Receipt of Definitive Treatment) and Late End Points (Recurrence Following Delayed RP) During AS for Prostate Cancer
A total of 320 men (22%) underwent delayed treatment with RP. A total of 206 mean (64%) were treated after progression as determined from evaluation of biopsy specimen, 10 (3%) with a PSA doubling time < 36 months, and 20 (6%) who were categorized as at intermediate clinical risk at diagnosis. Eighty-four men (26%) were treated in the absence of PSA or biopsy-determined progression, including 20 who did not undergo a second biopsy before RP. Median follow-up among men treated with prostatectomy was 41 months (IQR, 24-81 months). On unadjusted analysis, pathologic T stage (P = .42), Gleason score (P = .10), lymph node status (P = .40), and CAPRA-S score (P = .42) were similar between men ≤ 60 years old and those older than 60 years. In Cox proportional hazards regression models, younger age at diagnosis was not significantly associated with time to PSA recurrence (HR, 0.989 [95% CI, 0.906 to 1.08]; P = .81).
Within the AS cohort, 948 men (66%) met strict UCSF eligibility criteria at diagnosis. Within this subset, patient age was independently associated with time to biopsy-based Gleason score upgrade (HR, 0.974 [95% CI, 0.957 to 0.990]; P < .01) and biopsy-determined progression (HR, 0.970 [95% CI, 0.964 to 0.993]; P < .01; Table 3). These relationships also remained significant at a cutoff age of 60 years. Age group was not significantly associated with time to treatment (HR, 0.992 [95% CI, 0.976 to 1.009]; P = .36) or BCR after RP (HR, 0.972 [95% CI, 0.852 to 1.018]; P = .67). Furthermore, the addition of age as a variable did not improve model performance for the outcomes of time to treatment or recurrence.
DISCUSSION
Younger men who are diagnosed with PCa report higher baseline urinary and sexual quality of life and, in light of favorable anticipated longevity, may endure a longer detriment to HRQOL with definitive treatment.11,14,15 Although younger patients may fare reasonably well, given their high baseline level of functioning if they undergo well-performed primary treatment, some decline in function is not uncommon, and at least a transient decline is nearly universal. AS represents a potential strategy to avoid or delay treatment in younger patients with otherwise favorable-risk disease. Yet uncertainty remains about the appropriateness of surveillance in younger patients because of potential risks of missed opportunities for cure.16 With the present duration of follow-up, this study is, to our knowledge, the largest and most comprehensive study to explicitly address the role of patient age at diagnosis with outcome during AS. Among 1,433 men, younger patient age was associated with decreased risk of biopsy-based Gleason score upgrade and biopsy-determined progression, yet with similar rates of definitive treatment during follow-up.
PCa incidence and lethality are age-related phenomena.10 Although older patients possess comparatively higher burdens of advanced or metastatic disease at presentation, our study population represented a cohort in which inclusion was restricted to those with favorable disease characteristics. Indeed, 66% of our cohort elected to pursue AS in the setting of strict clinical criteria and otherwise possessed low-risk and low-intermediate–risk disease. When restricted to patients meeting more restrictive eligibility criteria, the associations with age remained similar; age remained a significant independent predictor of biopsy-based Gleason score upgrade and progression but was not associated with time to treatment or subsequent clinical recurrence after delayed RP. In this analysis, our findings are also consistent with a broader body of literature that has identified clinical parameters associated with disease reclassification over time, including age, PSA density, and tumor characteristics at diagnosis.17-19
The associations of age and AS outcomes in our series are in agreement with other surveillance cohorts.2,19-21 Anderson et al22 examined the role of patient age on Gleason score upgrading at confirmatory biopsy within a cohort of men with initial biopsy Gleason score ≤ 3+3 and clinical stage ≤ T2a, where older patient age was associated with increased risk of Gleason score upgrading. Notably, however, in our analysis, the risk of biopsy-determined progression persisted over time, suggesting that this may not be limited to a phenomenon of misclassification on evaluation of diagnostic biopsy specimens alone. Refined biopsy strategies, including magnetic resonance-fusion guidance, may improve the accuracy of initial sampling and may affect the rates of disease reclassification over time. A large prospective study would be required to answer this question definitively.23,24
Despite attempts to standardize AS protocols, considerable variation exists in the practice of, and compliance with, surveillance.25 Perhaps reflecting greater concern for disease control and the possibility of biopsy undersampling, younger patients have been shown to undergo greater degrees of AS intensity.26 Indeed, in our cohort, men ≤ 60 years old underwent more surveillance biopsy procedures than men older than 60 years within the same interval. From this perspective, the lower risks of progression despite greater scrutiny (ie, an approximately 30% lower risk of upgrading over time) should be informative to counsel younger patients with low-risk PCa who are considering initial management with surveillance. Furthermore, we anticipate that the dynamics of biopsy-determined progression may vary as our contemporary surveillance population ages. For example, detection of disease reclassification via biopsy specimen may decline in older patients in later decades of life because of competing medical comorbidities and decreasing intensity of surveillance. Moreover, reclassification after multiple biopsy procedures does occur; however, it is less common than at earlier periods of surveillance.27 Accordingly, for men diagnosed at younger ages, a relatively longer horizon of surveillance may offer a greater opportunity for disease progression.
There are limitations in this study that merit discussion. First, although a formal surveillance protocol is offered at our institution, strict adherence has not been enforced, owing to patient preference as well as an evolving, risk-adapted approach with the intention of limiting the biopsy burden for those with low risk of progression.28 As a consequence, it is conceivable that missing data and limited follow-up did not occur randomly and might favor inclusion of individuals with higher-risk features or those whose disease has progressed, as determined by evaluation of biopsy specimens. In addition, most of the study cohort underwent a diagnostic biopsy procedure at an outside institution, where younger patients more likely to be referred. Although we attempted to adjust for referral status, the influence of unmeasured biases not fully accounted for in these methods may limit the generalizability of the study results outside of academic referral institutions. Furthermore, we used biopsy-based Gleason score upgrade and biopsy-determined progression, defined by a change in tumor volume, as study end points for surveillance. Although the detection of higher Gleason-pattern disease in subsequent biopsy specimens is often a triggering event for definitive treatment, a small proportion of patients within our cohort have opted for continued AS.29 We also noted that younger patients exhibited relatively lower-risk disease profiles compared with older men, and were more likely to have an initial diagnosis outside of our institution—both factors that may introduce unmeasured biases that may impact the perceived risks of progression over time. Last, because patients were identified from a departmental database, it is possible that a few patients receiving PCa care through other disciplines may not have been captured in this analysis.
The relative contributions of biopsy undersampling versus disease progression during surveillance remain unclear.30 This may be particularly relevant to the issue of age, because older patients tend to have a larger prostate volume that may lead to sampling biases on systematic biopsy.4 However, the observation that observed age-related differences persisted after adjustment for PSA density suggests that prostate volume did not greatly influence our results. Other limitations include the length of follow-up after prostatectomy that may underestimate the rates of recurrence. Furthermore, our analysis lacks complete longitudinal HRQOL data, which prevented a direct comparison of urinary, sexual function, and psychologic outcomes by age. In light of multicenter studies indicating better preservation of urinary and sexual function among men managed with AS, as compared with those receiving definitive treatment, an opportunity also exists to examine these outcomes by age.31
Overtreatment of low-risk prostate cancer remains a major driver of the controversy surrounding PSA-based early detection efforts, and the use of AS as the preferred initial management strategy for most men with low-risk disease is a critical component of screening practices.32 Although there is no consensus on the optimal screening approach, a growing body of literature supports obtaining an initial baseline screening at a young age—as young as 45 years old—and basing subsequent screens on this initial result.33 Such an approach is supported by robust biologic and epidemiologic rationales and has been endorsed by the National Comprehensive Cancer Network.34 However, men in their 40s and 50s cannot reasonably be offered PSA testing unless they also will be offered AS if low-risk cancer is identified. Treatment decisions should be consistently driven by disease characteristics, overall life expectancy, and patient preferences rather than by chronological age.
In conclusion, among 1,433 men with clinical low- and intermediate-risk PCa, younger patient age was independently associated with decreased risk of biopsy-based Gleason score upgrade and progression. Nevertheless, the rates of definitive treatment were similar among older and younger patients. Men ≤ 60 years old received subsequent RP more often than radiation therapy. Among men treated with delayed prostatectomy, age was not significantly associated with the risk of biochemical failure. At diagnosis, appropriately risk-stratified patients may be counseled that in the short to intermediate term, younger age was not associated with worse surveillance outcomes assessed by biopsy outcome and recurrence-free survival after delayed treatment. Longer follow-up is needed to assess more distant outcomes.
Appendix
Fig A1.
CONSORT diagram of patient selection within the prospective study of active surveillance at UCSF. ADT, androgen-deprivation therapy; UCSF, University of California San Francisco.
Fig A2.
Kaplan-Meier plots depicting rates of biopsy Gleason upgrade-free survival stratified by (A) age ≤ 60 years or older than 60 years and (B) decade of age a diagnosis.
Fig A3.
Kaplan-Meier plots depicting rates of overall biopsy progression-free survival stratified by (A) age ≤ 60 years or older than 60 years, and (B) decade of age a diagnosis.
Fig A4.
Kaplan-Meier plots depicting rates of active treatment-free survival stratified by (A) age ≤ 60 years or older than 60 years, and (B) decade of age at diagnosis (B).
Table A1.
Comparison of Clinical and Demographic Characteristics by Referral Status
Footnotes
Supported by US Department of Defense Transformative Impact Aware Prostate Cancer Research Program Grant No. W81XWH-13-2-0074 (M.R.C., P.R.C.); National Cancer Institute Grants No. P50 CA1809952, U01CA089600-10A1, and P30CA60553; and the Urological Research Foundation (W.J.C.).
See accompanying Editorial on page 1870
AUTHOR CONTRIBUTIONS
Conception and design: Michael S. Leapman, Janet E. Cowan, William J. Catalona, Peter R. Carroll
Provision of study materials or patients: Peter R. Carroll
Collection and assembly of data: Michael S. Leapman, Janet E. Cowan, Katsuto K. Shinohara, Nannette Perez, Matthew R. Cooperberg, Peter R. Carroll
Data analysis and interpretation: Michael S. Leapman, Janet E. Cowan, Hao G. Nguyen, Matthew R. Cooperberg, William J. Catalona
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Active Surveillance in Younger Men with Prostate Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Michael S. Leapman
No relationship to disclose
Janet E. Cowan
Stock or Other Ownership: OPKO Diagnostics (I), Glaxo (I)
Research Funding: Genomic Health
Hao G. Nguyen
No relationship to disclose
Katsuto K. Shinohara
Consulting or Advisory Role: Nihon Mediphysics, 3D Biopsy
Travel, Accommodations, Expenses: Nihon Mediphysics, 3D Biopsy
Nannette Perez
No relationship to disclose
Matthew R. Cooperberg
No relationship to disclose
William J. Catalona
Patents, Royalties, Other Intellectual Property: Co-inventor of a urine assay for prostate-specific enzyme activity
Peter R. Carroll
Research Funding: Genomic Health (Inst)
REFERENCES
- 1.Cooperberg MR, Carroll PR, Klotz L. Active surveillance for prostate cancer: Progress and promise. J Clin Oncol. 2011;29:3669–3676. doi: 10.1200/JCO.2011.34.9738. [DOI] [PubMed] [Google Scholar]
- 2.Bul M, Zhu X, Valdagni R, et al. Active surveillance for low-risk prostate cancer worldwide: The PRIAS study. Eur Urol. 2013;63:597–603. doi: 10.1016/j.eururo.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33:272–277. doi: 10.1200/JCO.2014.55.1192. [DOI] [PubMed] [Google Scholar]
- 4.Welty CJ, Cowan JE, Nguyen H, et al. Extended followup and risk factors for disease reclassification in a large active surveillance cohort for localized prostate cancer. J Urol. 2015;193:807–811. doi: 10.1016/j.juro.2014.09.094. [DOI] [PubMed] [Google Scholar]
- 5.Tosoian JJ, Trock BJ, Landis P, et al. Active surveillance program for prostate cancer: An update of the Johns Hopkins experience. J Clin Oncol. 2011;29:2185–2190. doi: 10.1200/JCO.2010.32.8112. [DOI] [PubMed] [Google Scholar]
- 6.Womble PR, Montie JE, Ye Z, et al. Contemporary use of initial active surveillance among men in Michigan with low-risk prostate cancer. Eur Urol. 2015;67:44–50. doi: 10.1016/j.eururo.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 7.Cooperberg MR, Carroll PR. Trends in management for patients with localized prostate cancer, 1990-2013. JAMA. 2015;314:80–82. doi: 10.1001/jama.2015.6036. [DOI] [PubMed] [Google Scholar]
- 8.Loeb S, Berglund A, Stattin P. Population based study of use and determinants of active surveillance and watchful waiting for low and intermediate risk prostate cancer. J Urol. 2013;190:1742–1749. doi: 10.1016/j.juro.2013.05.054. [DOI] [PubMed] [Google Scholar]
- 9. Nezolosky MD, Dinh KT, Muralidhar V, et al. Significant increase in prostatectomy and decrease in radiation for clinical T3 prostate cancer from 1998 to 2012. Urol Oncol.34:57.e15-22, 2016. [DOI] [PubMed]
- 10.Scosyrev E, Messing EM, Mohile S, et al. Prostate cancer in the elderly: Frequency of advanced disease at presentation and disease-specific mortality. Cancer. 2012;118:3062–3070. doi: 10.1002/cncr.26392. [DOI] [PubMed] [Google Scholar]
- 11.Brajtbord JS, Punnen S, Cowan JE, et al. Age and baseline quality of life at radical prostatectomy--who has the most to lose? J Urol. 2014;192:396–401. doi: 10.1016/j.juro.2014.02.045. [DOI] [PubMed] [Google Scholar]
- 12.Cooperberg MR, Pasta DJ, Elkin EP, et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: A straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. 2005;173:1938–1942. doi: 10.1097/01.ju.0000158155.33890.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooperberg MR, Hilton JF, Carroll PR. The CAPRA-S score: A straightforward tool for improved prediction of outcomes after radical prostatectomy. Cancer. 2011;117:5039–5046. doi: 10.1002/cncr.26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hampson LA, Cowan JE, Zhao S, et al. Impact of age on quality-of-life outcomes after treatment for localized prostate cancer. Eur Urol. 2015;68:480–486. doi: 10.1016/j.eururo.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Resnick MJ, Koyama T, Fan KH, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368:436–445. doi: 10.1056/NEJMoa1209978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godtman RA, Holmberg E, Khatami A, et al. Long-term results of active surveillance in the Göteborg randomized, population-based prostate cancer screening trial. Eur Urol. 2016;70:760–766. doi: 10.1016/j.eururo.2016.03.048. [DOI] [PubMed] [Google Scholar]
- 17.Bechis SK, Carroll PR, Cooperberg MR. Impact of age at diagnosis on prostate cancer treatment and survival. J Clin Oncol. 2011;29:235–241. doi: 10.1200/JCO.2010.30.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. doi: 10.1016/j.juro.2015.08.087. Newcomb LF, Thompson IM, Jr., Boyer HD, et al: Outcomes of active surveillance for clinically localized prostate cancer in the prospective, multi-institutional Canary PASS cohort. J Urol 195:313-320, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tosoian JJ, Mamawala M, Epstein JI, et al. Intermediate and longer-term outcomes from a prospective active-surveillance program for favorable-risk prostate cancer. J Clin Oncol. 2015;33:3379–3385. doi: 10.1200/JCO.2015.62.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain S, Loblaw A, Vesprini D, et al. Gleason upgrading with time in a large prostate cancer active surveillance cohort. J Urol. 2015;194:79–84. doi: 10.1016/j.juro.2015.01.102. [DOI] [PubMed] [Google Scholar]
- 21.Wong LM, Alibhai SM, Trottier G, et al. A negative confirmatory biopsy among men on active surveillance for prostate cancer does not protect them from histologic grade progression. Eur Urol. 2014;66:406–413. doi: 10.1016/j.eururo.2013.04.038. [DOI] [PubMed] [Google Scholar]
- 22.Anderson CB, Sternberg IA, Karen-Paz G, et al. Age is associated with upgrading at confirmatory biopsy among men with prostate cancer treated with active surveillance. J Urol. 2015;194:1607–1611. doi: 10.1016/j.juro.2015.06.084. [DOI] [PubMed] [Google Scholar]
- 23. Walton Diaz A, Shakir NA, George AK, et al. Use of serial multiparametric magnetic resonance imaging in the management of patients with prostate cancer on active surveillance. Urol Oncol;3:202.e201-207, 2015. [DOI] [PMC free article] [PubMed]
- 24.Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313:390–397. doi: 10.1001/jama.2014.17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bokhorst LP, Alberts AR, Rannikko A, et al. Compliance rates with the Prostate Cancer Research International Active Surveillance (PRIAS) protocol and disease reclassification in noncompliers. Eur Urol. 2015;68:814–821. doi: 10.1016/j.eururo.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Chamie K, Williams SB, Hershman DL, et al. Population-based assessment of determining predictors for quality of prostate cancer surveillance. Cancer. 2015;121:4150–4157. doi: 10.1002/cncr.29574. [DOI] [PubMed] [Google Scholar]
- 27.Porten SP, Whitson JM, Cowan JE, et al. Changes in prostate cancer grade on serial biopsy in men undergoing active surveillance. J Clin Oncol. 2011;29:2795–2800. doi: 10.1200/JCO.2010.33.0134. [DOI] [PubMed] [Google Scholar]
- 28.Cary KC, Cowan JE, Sanford M, et al. Predictors of pathologic progression on biopsy among men on active surveillance for localized prostate cancer: The value of the pattern of surveillance biopsies. Eur Urol. 2014;66:337–342. doi: 10.1016/j.eururo.2013.08.060. [DOI] [PubMed] [Google Scholar]
- 29.Hussein AA, Welty CJ, Ameli N, et al. Untreated Gleason grade progression on serial biopsies during prostate cancer active surveillance: Clinical course and pathological outcomes. J Urol. 2015;194:85–90. doi: 10.1016/j.juro.2015.01.077. [DOI] [PubMed] [Google Scholar]
- 30.Inoue LY, Trock BJ, Partin AW, et al. Modeling grade progression in an active surveillance study. Stat Med. 2014;33:930–939. doi: 10.1002/sim.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeldres C, Cullen J, Hurwitz LM, et al. Prospective quality-of-life outcomes for low-risk prostate cancer: Active surveillance versus radical prostatectomy. Cancer. 2015;121:2465–2473. doi: 10.1002/cncr.29370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vickers AJ, Edwards K, Cooperberg MR, et al. A simple schema for informed decision making about prostate cancer screening. Ann Intern Med. 2014;161:441–442. doi: 10.7326/M14-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vickers AJ, Ulmert D, Sjoberg DD, et al. Strategy for detection of prostate cancer based on relation between prostate specific antigen at age 40-55 and long term risk of metastasis: case-control study BMJ 346f2023, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carroll PR, Parsons JK, Andriole G, et al. NCCN Clinical Practice Guidelines prostate cancer early detection, version 2.2015. J Natl Compr Canc Netw. 2015;13:1534–1561. doi: 10.6004/jnccn.2015.0181. [DOI] [PubMed] [Google Scholar]