Graphical abstract
Abstract
Mixed cryoprotectants have been developed for the solubilization of ligands for crystallization of protein–ligand complexes and for crystal soaking. Low affinity lead compounds with poor solubility are problematic for structural studies. Complete ligand solubilization is required for co-crystallization and crystal soaking experiments to obtain interpretable electron density maps for the ligand. Mixed cryo-preserving compounds are needed prior to X-ray data collection to reduce radiation damage at synchrotron sources. Here we present dual-use mixes that act as cryoprotectants and also promote the aqueous solubility of hydrophobic ligands. Unlike glycerol that increases protein solubility and can cause crystal melting the mixed solutions of cryo-preserving compounds that include precipitants and solubilizers, allow for worry-free crystal preservation while simultaneously solubilizing relatively hydrophobic ligands, typical of ligands obtained in high-throughput screening. The effectiveness of these mixture has been confirmed on a human transthyretin crystals both during crystallization and in flash freezing of crystals.
1. Introduction
High-throughput screening is used to select among libraries of millions of compounds those that bind to a target protein, inhibit a particular enzymatic reaction or block a cellular transport mechanism. Typically the chemical compounds are dissolved in dimethyl sulfoxide (DMSO) [1] to produce an aqueous solution. This solution is diluted during the screening to ensure that the concentration of DMSO does not exceed 10%; as higher concentrations can be damaging to the target protein [2]. The discovered ‘hits’ are unlikely to be suitable for clinical use, but are good building blocks from which drugs may evolve. Structural studies are often essential to transform these hits into leads and eventually into potential drugs that can undergo clinical trials. The poor solubility of ligands is a problem for the crystallization of protein-complexes, more so when the ligand or fragment has a low affinity for its target. For co-crystallization, the ligand must be soluble in the crystallization precipitant so as to be in excess compared to the protein. The protein concentration needed for crystal growth starts from around 50 μM into the millimolar range. The crystallization conditions, including additives, must be chosen in such a manner as to ensure that the ligand remains in solution throughout the crystallization process.
In a previous study, by mixing cryo-preserving compounds that act as precipitants with compounds that have the opposite effect we developed a set of multicomponent mixtures that could be combined with a precipitant and a buffer so as to be able to prepare crystals for X-ray data collection at high intensity synchrotron facilities without tribulation [3]. These mixtures can stabilize crystals for periods long enough for ligand soaking experiments. The presence of DMSO, not higher than 10%, in addition to the other cryoprotectant molecules helps ligand solubilization while simultaneously providing an environment that ensures stabilization of the protein and the interactions it makes within the crystal lattice.
Here we report on an extended set of multicomponent solutions for crystal cryoprotection which includes additional components, namely dioxane and butanediol and analyze the contribution of dioxane toward ligand solubilization, alone and in conjunction with other cryoprotectant components and its compatibility for protein crystallization and crystal soaking. Dioxane has been extensively used as an additive in macromolecular crystallization for its ability to mediate lattice interactions [4]. Concentrations of 3–10% are typical for its use as an additive, but at around 20–35% it becomes an effective precipitant [5]. At similar concentrations, DMSO can be problematic. High concentrations of DMSO affect protein secondary structure and can lead to disordered proteins [2].
Ligand insolubility is also a common problem in chemistry and many compounds are insoluble in organic solvents. There is no general rule on how to dissolve chemical molecules [6], and the well-know phrase “similia similibus solvuntur” (polar solvents are best to dissolve polar solutes and non-polar solvents for non-polar solutes) is helpful only as a general guide. The strategy of using a mixture of solvents to solubilize ligands is well known. Two or more solvents together enhance the solubility of insoluble compounds [7].
Here we report on an extended set of multicomponent solutions for crystal cryoprotection (SM1-6) which includes two additional components, dioxane and 2,3-butanediol. These solubilizing cryo-preserving mixtures (mixes) have been designed for protein–ligand co-crystallization experiments, soaking of hydrophobic ligands into pre-formed crystals and for crystal cryoprotection before flash-cooling. The approach used to create cryoprotectant solutions [3] with mixed compounds that inhibit ice formation, has been extended by changing the composition, so as to increase their ability to solubilize ligands. The mixes have been tested on human transthyretin (TTR) [8] and on four matrix metalloproteinases (MMP-8, 9, 12 and 13) with diffraction comparable to that obtained with the use of CryoProtXTM (Molecular Dimensions, U.K. Ltd.)[3]. The term “mixes” will be used to refer to the mixture of cryoprotectant compounds without buffer or precipitant; the term “cryoprotectant solution” refers to the final solution in which crystals are soaked before vitrification at cryogenic temperatures.
2. Experimental
2.1. Composition of solubilizing cryoprotectant solutions
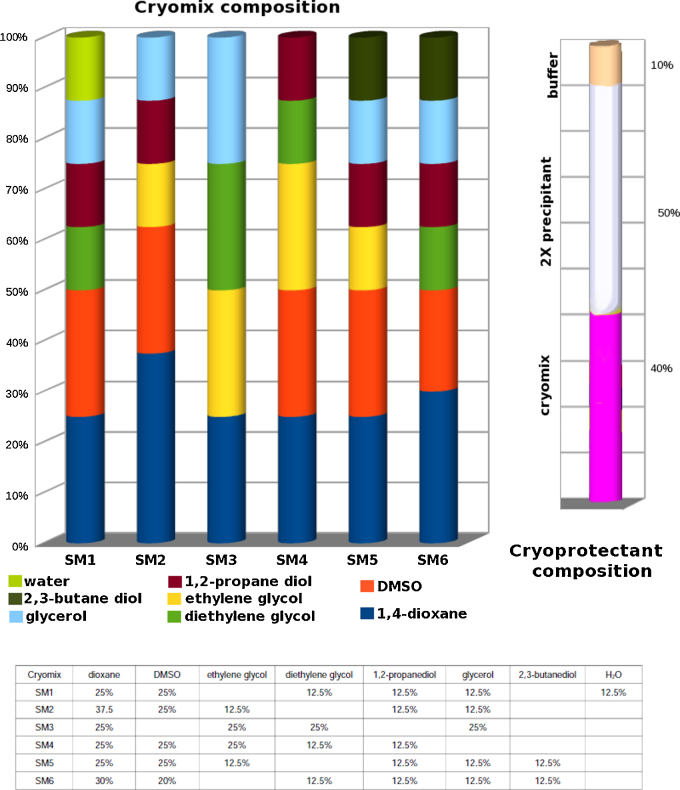
The composition of the solubilizing cryoprotectant solutions has been chosen on the same basis as those developed for the cryoprotection of crystals [3]. Compounds like ethanol and methanol, that are excellent for ligand solubilization and to reduce ice nucleation, have been excluded because their volatility makes their use in vapor diffusion experiments and for the cryoprotection of crystals impractical. This stems from the difficulty to maintain a known solvent concentration during manipulations because of evaporation. Additionally, evaporation causes vortex-stirring so that it becomes difficult to fish crystals out of the swirling cryoprotectant solution into the cryoloop. Compounds like ethylene glycol and propylene glycol are a better choice since they have been used as cryoprotectant compounds and in water co-solvent mixtures for pharmaceutical compounds [7]. Dioxane, a non-volatile solvent that has been used as a crystallization additive, not present in the previous design has been added to the new “mixes” (Fig. 1). Another addition is 2,3-butanediol, which is compatible with enzymatic activity [9].
Fig. 1.
Composition of the six ligand solubilizing mixed cryosolutions (cryomixes) SM1-6 shown graphically as cylinders.
When used to formulate a cryoprotectant solution for crystal soaking, the cryomixes represent 40% of the volume, 10% is assigned to the buffer and 50% to the precipitant-water mixture that is 2× of the crystallization precipitant. For the solubilization of ligands, the cryomix is used directly to dissolve the ligand.
The new solubilizing mixes have been formulated to maintain consistency with the previous design [3]. Solutions for crystal soaking are prepared in the same manner as the cryoprotectant solutions. A 100 μL solution consists of 40 μL from one of the ligand-solubilizing mixes, 10 μL (10×) buffer and 50 μL (2×) precipitant (2×: double the concentration used in the crystallization to grow the crystals) (Fig. 1). Experiments have been carried on crystals of TTR, MMP12, MMP9, MMP8 and MMP13, cryoprotectant solutions obtained with a variety of different precipitants to ensure that crystals do not dissolve or crack when kept for 20 min or overnight in the solutions, at the same temperature used for crystallization.
2.2. Ligand solubilization options
For inhibitors poorly soluble or insoluble in DMSO, the mixed solutions with DMSO/dioxane/ethylene glycol mixes of different ratios were selected to improve ligand solubilization. DMSO, ethylene glycol and dioxane belong to different selectivity classes: III, IV and VI, respectively [10] and combination of these compounds should cover a relatively wide range of selectivity values to render water soluble a large variety of organic compounds.
The target ligand concentration for soaking experiments was set at 1–10 mM and three different options were considered and tested. In the first trial the ligand was solubilized at a concentration of 10–30 mM in any of the six mixes SM1-6 (Fig. 1). The final ligand-solubilization/cryo-solution for crystal soaking is composed of the ligand-mix solution, 40% of the volume, the precipitant (at 2×), essential for crystal stability, represents 50% of the volume and the remaining 10% is given by the buffer (at 10×). The concentration of the solvents is brought within the limits that avoid protein denaturation and the ligand is within the target range of 1–10 mM. The second option is to solubilize the ligand at 100–300 mM in DMSO. The DMSO/ligand solution is then diluted 1:4 in SM3 (Fig. 1), the cryomix without DMSO. The resultant solution (40%), is then mixed with the buffer (10%) and the precipitant (50%) so that in the final soaking solution the ligand is at 10–30 mM and the DMSO at 10%. Solubilization of the ligand in dioxane is the third option. The same procedure as for DMSO can be used for all SM1-6, since a final 20% dioxane is tolerated [11].
2.3. Preparation for soaking experiments
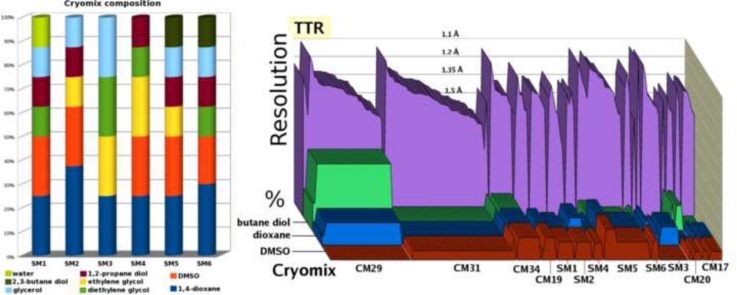
The SM1-6 solutions have been developed for a dual use, as cryoprotectants and for ligand solubilization. The preparation of the solutions for the soaking of ligands into crystals involves several steps. While complete solubilization of the ligand is not always necessary to achieve binding in the crystal. Success with incompletely solubilized ligands has been achieved using long soak periods [12], [13]. This practise is not advised for short soaks. Initially a series of mixtures are prepared with varying ratios of DMSO and dioxane to evaluate the ability of these two solvents to solubilize various ligands. Various DMSO/dioxane solvent mixtures are prepared with the dioxane concentration increasing from 10% to 90%. After adding the ligand, each sample is analyzed under the microscope to check for ligand crystals or insoluble residue so as to identify which DMSO/dioxane ratio is best at solubilizing the compound. After this initial step, one or two of the cryo-solubilizing mixes which contained ethylene glycol, diethylene glycol, 1,2-propanediol, glycerol and 2,3-butanediol is selected. The next step is to determine how long crystals are stable in the solutions (prepared as suggested in Fig. 1). The third step is to ensure that cryoprotectant solutions prepared with the selected mixes perform in diffraction experiments in a manner comparable to those in CryoProtXTM [3]. Compared to solubilization in just dioxane or DMSO alone, the multicomponent mixes are more likely to be compatible with protein structural integrity because the most potentially denaturing components are present at lower concentrations. The presence of glycerol and similar compounds are better known for their ability to stabilize rather than to denature proteins provide additional protection against partial unfolding. In a mix of dioxane and DMSO, in the absence of synergistic effects whereby DMSO and dioxane would be better at denaturing proteins than each component separately, the DMSO/dioxane mix, where each of the two components help solubilize the ligand, might be better tolerated by proteins. This assumption has been subjected to experimental verification in soaking and protein crystallization tests. The solutions preserve TTR crystal integrity for at least 20 min in ligand soaking experiments. In our tests, the cryomixes SM1-6 were evaluated at different pH (acid-neutral-basic) using several buffers for TTR [8] with different ligands. The diffraction limit obtained with SM1-6 have been compared with the mixes used in CryoProtXTM [3] (CM1-9) and with mixes CM10-34 that also include dioxane and butanediol. The CM solutions contain amounts of DMSO and dioxane that are well below the concentrations that might cause protein denaturation. Although MMP inhibitors do not require the SM mixes for ligand solubilization, these solutions were used in order to evaluate whether the extent of diffraction from MMP-inhibitor complex crystals is reduced when the SM mixes are used. SM1-6 gave results comparable to those obtained with the CM mixes (Fig. 2). Equally outstanding diffraction was obtained for TTR crystals with both the SM or CM mixes (Fig. 2). However, since susceptibility to denaturation is likely to vary from one protein to another as some proteins might be more prone to denaturation.
Fig. 2.
Resolution limitsǂ obtained from crystals of TTR, MMP-8, 9, 12 and 13 using various cryomixes containing varying lamounts of DMSO, dioxane and butanediol. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Each data point corresponds to a data set, so that the width of the cryomix along the X-axis corresponds to the number of co-crystals tested. The resolution is shown in purple and the vertical axis also shows the amounts of DMSO, dioxane and butanediol in the final cryoprotectant (% v/v): in blue (dioxane), red (DMSO) and green (butanediol). The composition of the cryomixes (except water) is given in the table below the figure (except SM1-6 mixes: Fig. 1).
2.4. Co-crystallization experiments with TTR
The SM1-6 mixes were tested in co-crystallization experiments with TTR [8] in the presence of curcumin and 16α-bromo-estradiol solubilized DMSO/dioxane mixture. For co-crystallization, glycerol and other diols are kept low. Even at low concentrations, glycerol reduces the number of crystal nuclei formed and above 10% it hinders crystal growth. In some experiments, SM3 was used as an additive to the crystallization precipitant, in others, to increase the strength of the precipitant, dioxane was used instead. Dioxane has been used in co-crystallization experiments where, instead of PEG, ammonium sulfate is the main precipitant (example: 12% 1,4-dioxane, 1.6 M ammonium sulfate in the crystallization of P450 with camphor—affinity 9.1 μM: PDB id 3LXI [12]). This approach was tested with TTR, but high molecular weight polyethylene glycol (PEG) was preferred and used more often. The dioxane/PEG600 combination was also found effective for certain inhibitors: (36–25% PEG 600, 15–40% dioxane, 0.2 M imidazole malate, pH 5.5). In preparation for X-ray data collection, the SM1-6 solutions were used in cryoprotectant solution formulation (Fig. 1). A 6 μL drop of the cryosolution is placed on a micro-bridge in an XRL plate with water in the reservoir to maintain moisture. For low affinity ligands, as is the case for most TTR amyloidogenesis inhibitors and for the example given above, it may be prudent to include ligand in the cryoprotectant. The ligand solubilization procedure followed is as stated above. After ensuring that the ligand does not precipitate or crystallize out, the protein–ligand co-crystal is added, flash-cooled in liquid nitrogen, or soaked for 15–20 min beforehand at room temperature. The tests were carried out on 152 ligands, most of which were co-crystallized. Full data sets were collected for all these ligands to create an extensive crystallographic database (see Supplementary Data) from which the effect of DMSO, dioxane and 2,3-butanediol on the resolution to which TTR and MMP co-crystals diffract can be evaluated (Fig. 2). Of the TTR structures solved to date, all active ligands have interpretable, even if weak electron density and some ligands that offer less than 40% inhibition in turbidimetric assays also appear bound to TTR. MMP inhibitors are more water soluble and have better affinities, so MMP co-crystals have been used only to evaluate diffraction.
2.5. Use of dioxane in MMP-12 crystallization
Crystallization experiments were performed in CrysChem sitting drop vapor diffusion plates with MMP-12 prepared as previously described [14]. Drops consisting of 1 μL protein and 1 μL precipitant solutions were equilibrated by vapor diffusion and stored in a cooled incubator at 20 °C. Initial screening was carried out with MMP-12 at 366 μM in a systematic manner following the principles of reverse screening [15]. Single drop screening was carried out with pre-prepared working solutions used for the crystallization of other MMP-12 ligands to evaluate the change in solubility of the glycoconjugated inhibitors [16] (Fig. 3). The inhibitors (10 mM in 100% DMSO) were added with a 1.2-fold stoichiometric excess. Four working solutions were tested: (A: 45% PEG 4,000, 0.2 M imidazole piperidine, pH 8.5; B: 17% PEG 20,000, 200 mM imidazole malate, pH 8.5, 250 mM NaCl; C: 27% PEG 10,000, 150 mM imidazole piperidine, pH 8.5; D: 17% PEG 20,000, 250 mM NaCl, 100 mM Tris–HCl, pH 10.0). No protein precipitation was observed in any of the trials. To stimulate the crystal formation all drops were seeded with various polymorphs and 50 μL of a booster solution consisting of 5 M NaCl was added to each reservoir without success. Further trials with higher PEG concentrations and different pH also failed to precipitate the MMP-12 complexed to the glycoconjugated inhibitors. Seeding and a long series of NaCl boosts (dehydration of the protein-precipitant drop by adding 5 M NaCl to the reservoir) were ineffective.
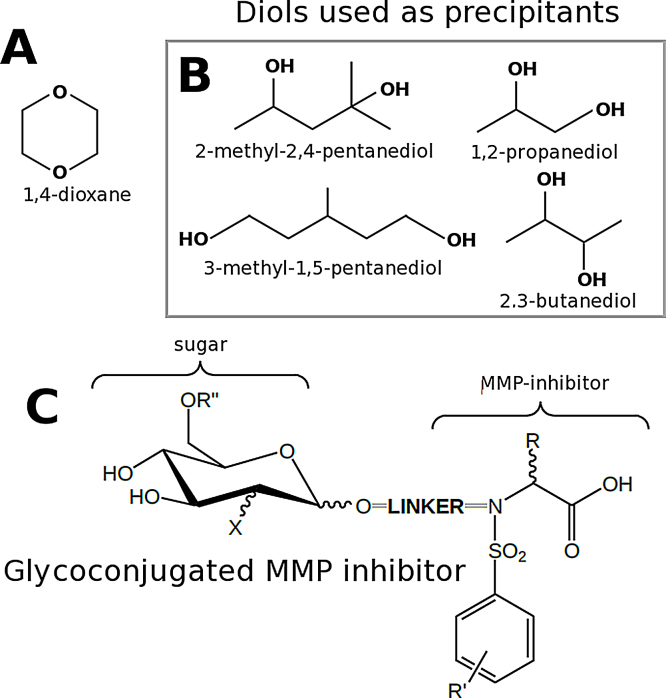
Fig. 3.
Co-precipitants for protein crystallization. (a) Dioxane is used to supplement the precipitating power of both ammonium sulfate and polyethylene glycol. (b) 2-methyl-2,4-pentanediol (MPD) is commonly used as a precipitant, but similar molecules are starting to used for similar applications. (c) In the crystallization of glycoconjugated MMP-inhibitors, dioxane was added to the precipitant since the precipitant power of polyethylene glycol was insufficient to achieve supersaturation of MMP-12.
To obtain crystals the following modifications were made: 1,4 dioxane (5–20%) was added to the precipitant and, if this was not sufficient, a higher protein concentration was used. Wild-type protein was concentrated to 631 μM (with 10 mM acetohydroxamic acid (AHA)—to prevent self-degradation of the proteinase) as well as the less active MMP-12E219Q catalytic site mutants at 465 μM with 20 mM AHA. To further prevent proteolytic activity, the inhibitors at 2 mM or 5 mM (in 100% DMSO) were added to the protein solution in a volumetric ratio of 1:10. Crystals were obtained by screening with working solution C and dioxane in the range from 5 to 20%. Showers of small crystals appeared spontaneously with MMP-12E219Q overnight in the drops containing 10–20% dioxane. Several changes were needed to improve crystal size and to reduce the nucleation. Additions of ethylene glycol or 30–50 mM NaCl in the precipitant effectively reduced nucleation. Larger crystals were obtained within a week by reducing the PEG and dioxane concentrations and reintroducing seeding.
For data collection crystals were picked up with a cryoloop and soaked for short period in selected mixtures of cryoprotectant compounds before rapidly plunging in liquid nitrogen for subsequent data collection at the Soleil synchrotron facility (Saint Aubin, France) on beamline Proxima 2 and at the European Synchrotron Radiation Facility (ESRF) in Grenoble (France) on beamlines ID23-1 and ID23-2. The larger crystals diffracted to 1.6 Å resolution, while data to 2.2 Å resolution was obtained for the smaller ones (Fig. 2).
3. Results
3.1. Co-crystallization experiments
The SM1-6 mixes tested in co-crystallization experiments with TTR in the presence of curcumin and 16α-bromo-estradiol solubilized DMSO/dioxane mix were shown to be effective [8]. In these two cases, these hydrophobic compounds were solubilized in a DMSO/dioxane mix and co-crystallized with a precipitant consisting of high/low molecular weight PEG which helps to keep the inhibitor soluble in the drop during crystal growth. This is preferable to co-crystallization in high ionic strength which can result in phase separation or in the ligand crystallizing out. Both of these problems were encountered with TTR when crystallization with these compounds was attempted with either ammonium sulfate or sodium citrate. TTR crystallization requires high concentration of these precipitants. We observed that the ligand was segregated predominately the organic rich/salt poor phase while the protein tended to concentrate in the salt rich phase. As the vapor diffusion experiment progressed, the ligand concentrated and crystallized in it own phase while the protein crystallized in the other phase. In high ionic strength crystallization experiments, ligand insolubility is a problem that can be solved with long co-crystallization periods or by adding solid ligand to the mother liquor during the last crystallization phase: “Camphor saturates the crystal solution after several days and the soaking time was varied from 1 week to 1 month [12].” Alternatively, long soak times can achieve similar results: “TTR–ligand complexes were prepared from crystals soaked with a fivefold molar excess of ligand for 3–4 weeks to ensure saturation of both binding sites... [13]”. In our experiments, the use of PEG in TTR co-crystallization experiments has prevented ligand crystallization or precipitation in the drops, avoided phase separation or the use of long incubation times in soaking experiments.
However, when setting up the vapor diffusion experiments with PEG under low salt conditions with ligand solubilized in a cryomix we added an appropriate amount of cryomix to the reservoir to avoid disequilibrium between the drop and the reservoir [17]. This precaution is needed because the cryomixes contain glycerol and other hygroscopic compounds that affect vapor diffusion equilibration [17]. Without this precaution the drop might increase in volume rather than decrease. To remedy, 50 μL of booster solution consisting of 5 M NaCl and acetic acid can be added to the reservoir (500 μL), as soon as the problem is noted. Repeat additions may be needed to restore equilibrium, to induce nucleation or to promote crystal growth. Deliberately omitting to add the cryomix to the reservoir can be used to combat excessive nucleation that can occur during rapidly during protein and reservoir mixing. The abundant nuclei dissolve during the phase the drop grows in size and new crystals form later during the reversal when the booster solution is added to the reservoir.
3.2. Solubilizing cryomix formulations
Even for those inhibitors that are highly soluble in DMSO, it may still be preferable to use the mixed solutions for ligand solubilization. Although in the mixed solutions the DMSO concentration is only 25% and the extra components may not contribute much to the solubilization, the advantage is gained in the next dilution step. When preparing the ligand solution for crystal soaking, 40% of solution with the dissolved ligand is mixed with the buffer and precipitant. The DMSO concentration drops down to 10%, but the additional components will contribute to maintain the ligand in solution. When preparing the protein-ligand complex for co-crystallization, the option of using the ligand solubilized in DMSO, in DMSO/dioxane or in the SM1-6 depends on the ligand and on the protein. The extra components will help achieve a higher final ligand concentration which could improve homogeneity, but the diols will reduce crystal nucleation, which may require seeding or other intervention to restore nucleation. In a situation of excessive nucleation, as we encountered in the crystallization of bi-functional ligands [18], a reduction in the nucleation rate is welcome. In the TTR co-crystallization with curcumin or 16α-bromo-estradiol all options were tested [8]. The cryomix compositions were formulated to maximize ligand solubility. The mixes were shown to be compatible with cryoprotection of crystals. No ice rings were observed in the X-ray data images. In cases where ice deposited on the crystals stored in liquid nitrogen, because of excessive ambient moisture, the annealing procedure [19] allowed ice to be cleared from the crystal and diffraction observed was comparable to that of similar crystals.
3.3. Cryoprotectant and Ligand Soaking of Crystals
For all compounds tested so far, solubilization in the SM1-6 solutions, achieved concentrations of 1 mM in the soaking solution. The solutions remained totally clear without the formation of ligand crystals. The co-crystallization with curcumin was challenging as this ligand degraded during co-crystallization to yield TTR-cocrystals with ferulic acid. With a replenishment step, consisting of a 20 min soak in 1.5 mM curcumin, the ferulic acid was exchanged for the intact ligand. To confirm that such an exchange is possible, the experiment was repeated with crystals grown in the presence of 16α-bromo-estradiol that were soaked in a cryoprotectant solution containing curcumin. Again the ligands exchanged during the soaking step [8]. The ability of the SM1-6 solutions to act as cryo-preserving agents for the preparation of crystals for X-ray data collection was tested on crystals of catalytic domains of matrix metalloproteinases (MMP). MMP inhibitors are relatively soluble and only exceptionally require special precautions for their solubilization. The results of the cryoprotection tests are shown diagrammatically in Fig. 2. Overall the SM1-6 solutions performed in a comparable manner to other cryomix formulations (CM mixes). The variations in diffraction quality between one crystal and another was attributable mainly to crystal size and not due to the cryoprotectant used. No crystal was lost in any of the manipulations if the transfer from the crystallization mother liquor was done with a large cryoloop. Some crystals were lost when the transfer was carried out with loops 0.1 mm or smaller because the surrounding liquid dried out. Thus crystals were placed in the cryo-solution with a large loop and retrieved with a loop more appropriate to the crystal size before flash cooling. The components of the cryomixes are hygroscopic and dry out at a much slower rate compared to the mother liquor, facilitating all subsequent manipulations. Some of the tests on MMP-12 were carried out with concentrations of DMSO and dioxane higher than recommended (Fig. 2) because dioxane was needed in the crystallization, as explained below. The reduced diffraction might not be due to the high levels of these components, but to the difficulties in growing the crystals and their relative small size compared to other crystals in the same graph.
3.4. Cryoprotectants as precipitants.
The SM1-6 mixes are formulated with balanced components that neither precipitate nor solubilize protein but their use as additives to the precipitant is unlikely to be neutral and their effect needs to be understood in a case by case manner. In our laboratory, small amounts of these mixes are effective in eliminating denatured protein skins that can be found at times in vapor diffusion experiments. The results obtained from the use of the mixes need to be understood by varying the concentrations of each component. Components like MPD, DMSO and propanediol can act as precipitants as previously discussed [3]. Dioxane is well known as a precipitant and as an additive [4] and butanediol like propanediol, can also act as a precipitant [20]. The use of dioxane to increment the precipitating power of polyethylene glycol has been essential for obtaining crystals of the MMP-12 glycoconjugated complexes.
4. Discussion
Increasing aqueous ligand solubility for co-crystallization and ligand soaking is more complex that solving the same problem in chemistry since the number of organic solvents that can be used is smaller. The solvents need to be miscible in water and preferably compatible with precipitants used in crystallization. Low ligand affinity for their target requires higher ligand concentrations compared to biological tests. The poor water solubility of initial hits poses greater difficulties for co-crystallization since sufficient ligand concentrations are hard to achieve without high organic solvent concentrations that can lead to denaturation of the target protein. The use of mixed solvents achieves better ligand solubilization because of an overall higher concentration of organic solvents without leading to protein denaturation. For co-crystallization and ligand soaking, the DMSO concentration can in some cases be raised above the 10% level, but in others it disrupts the integrity of the protein fold [2].
For ligand solubilization, dioxane complements DMSO, but does not substitute for DMSO. Curcumin is completely soluble in DMSO and not in dioxane, but the combination of the two solvents has allowed the preparation of protein–ligand solutions suitable for crystallization. The mixes when used as suggested in Fig. 1 achieve high concentrations of DMSO and dioxane. Since denaturation will vary from protein to protein, higher concentrations might be tolerated by certain enzymes [11] but less by others. For difficult ligand solubilization cases, acetonitrile [11] or other suitable volatile solvent can be tried. Unless their use is essential, volatile solvents should be avoided since their use can complicate crystal manipulation when the crystals are retrieved from the mother liquor and transferred to the cryoprotectant and then to the liquid nitrogen. The same problems arise when ethanol and propanol are used as crystallization additives in vapor diffusion experiments. Harvesting becomes difficult as evaporation of the alcohols makes the crystals swirl around. Getting crystals in perpetual movement rapidly into the cryoloop is a challenge. Volatile compounds can still be valuable for cryoprotection, but it may be more practical to use alternative procedures, for example introducing alcohols into crystals pre-mounted in cryoloops by vapor diffusion [21].
In ligand exchange experiments, like the one described for the in situ steroid-curcumin exchange [8], the extra solubilizing effect that come from all the components that belong to a different selectivity classes comes into play. The ligands may require different solubilization methods, and both ligands need to be soluble simultaneously in the same soaking solution for the exchange to take place rapidly. The ability of the mixes to solubilize a wider set of ligands is realized when trying to exchange ligands in preformed crystals rather than soaking a ligand into an empty binding site. This strategy could be useful when crystals grow easily with one ligand but not with another one. Curcumin was soaked into the larger crystals of 16α-bromo-estradiol rather than the other way round to ensure that even when large crystals are used, exchange still takes place readily. To improve on the exchange procedure, a two step procedure could be employed to obtain a more complete exchange of the two ligands. After the first 20 min exchange-soak with the new ligand, the crystal should be transferred to a new drop and the soak repeated, so as to wash out the old ligand more thoroughly. Considering that the exchange might be slower for high affinity ligands, adding more exchange steps, rather than longer exchange periods, should make the exchange more efficient in such cases.
Co-crystallization allows for protein conformational changes in response to ligand binding. For this reason it should be preferred over soaking. For co-crystallization it is important to separate the solvents based on their ability to solubilize or precipitate the target protein. Dioxane was used as a co-precipitant both in TTR [8] and MMP-12 crystallization experiments to supplement the action of polyethylene glycol. As needed, its effect can be counterbalanced by glycerol and other diols that tend to increase protein solubility. The use of the mixed solutions as part of the crystallization precipitant, aids the solubilization of the ligands but also affects protein supersaturation in the drop. The crystallization precipitant for the TTR-ligand complexes was selected to work effectively for the non-ligated protein with the belief that such crystallization conditions would give crystals more predictably and translate into more complexes with less material. Some failures with TTR were noted when acidic ligands were co-crystallized under acidic conditions. These compounds will be further studied using a higher pH. Over all experiments on TTR and the four MMPs, the pH range covered by the tests was 4.5–10, imposed by the crystallization conditions of the proteins.
Dioxane was essential in the crystallization of glycoconjugated MMP-inhibitor complexes (Fig. 3) to increase the precipitating power of polyethylene glycol. The MMP-12 complex with these ligands could not be crystallized with PEG alone. The glycosylated ligand–protein complex was more difficult to precipitate than similar complexes without the sugar moiety. Without dioxane, to achieve the same effect, the protein should have been concentrated further. The strategy of adding dioxane is effective in extending the use of a successful precipitant to crystallize more soluble ligand complexes, without having to resort to higher protein concentrations. MPD, DMSO and propanediol might have been used instead, as these compounds can also act as precipitants as previously discussed [3]. Dioxane was chosen because it had been beneficial in previous work [4].
Butanediol like propanediol can act as a precipitant when used in the 20–40% range [22]. Unfortunately, without more trials with propanediol and butanediol to better understand the pros and cons of their use as co-precipitants, the preference for proven additives like dioxane and MPD will remain privileged. Propanediol is becoming more popular as a precipitant (Bacillus subtilis manganese transport regulator [23]; Listeria monocytogenes post-transcriptional regulator Hfq [24]), but butanediol is seldom used. As robotic screening expands, a greater variety of diols will be added to crystallization screens. MPD in the form 2-methyl-2,4-pentanediol has been extensively used for the crystallization of proteins [25] while other isomers of the compound had been ignored. Recently, 3-methyl-1,5-pentanediol (Fig. 3) has been used to obtain large crystals of glycoside hydrolase [26]. Cryoprotection cum ligand-solubilization mixes are likely to follow the trend and incorporate a wider spectrum of molecules to achieve higher ligand concentrations of poorly water soluble ligands of pharmaceutical interest.
The addition of 2,3-butanediol in the mixes contributes to ligand solubilization and to cryoprotection. This compound was considered as an antifreeze agent more than 60 years ago [27]. It is used in cryobiology because of important advantages in supercooling and vitrification at lower concentrations [22] compared to other cryoprotecting agents. For flash-cooling of protein crystals this compound has performed well in the solubilizing mixtures reported here and in other experiments as a complement to the CryoProtX solutions [3].
To conclude, the use of biologically compatible mixed solvents is likely to expand the range of projects that can be tackled by X-ray crystallography, or, in some cases, only simplify procedures that used to span weeks or months, with uncertain results. A similar approach, using mixed solvents instead of DMSO alone, could be beneficial to a wider range of biological applications.
Acknowledgments
We are grateful to the ESRF and SOLEIL and to their staff for allocation of beam time and assistance during data collection. The help of the local contacts is acknowledged with gratitude. This work was partially supported by the Italian Ministero dell’Istruzione, dell’Università e della Ricerca (PRIN 20109MXHMR_007).
Footnotes
Available online 23 June 2015
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.btre.2015.05.008.
Contributor Information
Lidia Ciccone, Email: lidia.ciccone@cea.fr.
Laura Vera, Email: laura.vera@cea.fr.
Livia Tepshi, Email: livia.tepshi@cea.fr.
Enrico A. Stura, Email: estura@cea.fr.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Fedarovich A., Djordjevic K.A., Swanson S.M., Peterson Y.K., Nicholas R.A., Davies C. High-throughput screening for novel inhibitors of Neisseria gonorrhoeae penicillin-binding protein 2. PLoS One. 2012;7:e44918. doi: 10.1371/journal.pone.0044918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson M., Mantsch H.H. Beware of proteins in DMSO. Biochim. Biophys. Acta. 1991;1078:231–235. doi: 10.1016/0167-4838(91)90563-f. [DOI] [PubMed] [Google Scholar]
- 3.Vera L., Stura E.A. Strategies for protein cryocrystallography. Cryst. Growth Des. 2014;14:427–435. [Google Scholar]
- 4.Ménétrey J., Perderiset M., Cicolari J., Houdusse A., Stura E.A. Improving diffraction from 3 to 2Å for a complex between a small GTPase and its effector by analysis of crystal contacts and use of reverse screening. Cryst. Growth Des. 2007;7:2140–2146. [Google Scholar]
- 5.Oren D.A., Li Y., Volovik Y., Morris T.S., Dharia C., Das K. Structural basis of BLyS receptor recognition. Nat. Struct. Biol. 2002;9:288–292. doi: 10.1038/nsb769. [DOI] [PubMed] [Google Scholar]
- 6.Barton A.F.M. Solubility parameters. Chem. Rev. 1975;75:733. [Google Scholar]
- 7.Jouyban A. Review of the cosolvency models for predicting solubility of drugs in water-cosolvent mixtures. J. Pharm. Pharm. Sci. 2008;11:32–58. doi: 10.18433/j3pp4k. [DOI] [PubMed] [Google Scholar]
- 8.Ciccone L., Tepshi L., Nencetti S., Stura E.A. Transthyretin complexes with curcumin and bromo-estradiol: evaluation of solubilizing multicomponent mixtures. New Biotechnol. 2014;32:54–64. doi: 10.1016/j.nbt.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Mozhaev V.V., Khmelnitsky Y.L., Sergeeva M.V., Belova A.B., Klyachko N.L., Levashov A.V. Catalytic activity and denaturation of enzymes in water/organic cosolvent mixtures. Alpha-chymotrypsin and laccase in mixed water/alcohol, water/glycol and water/formamide solvents. Eur. J. Biochem. 1989;184:597–602. doi: 10.1111/j.1432-1033.1989.tb15055.x. [DOI] [PubMed] [Google Scholar]
- 10.Snyder L.R. Classification of the solvent properties of common liquids. J. Chromatogr. A. 1974;92:223–230. [Google Scholar]
- 11.Schmitke J.L., Stern L.J., Klibanov A.M. Organic solvent binding to crystalline subtilisin1 in mostly aqueous media and in the neat solvents. Biochem. Biophys. Res. Commun. 1998;248:273–275. doi: 10.1006/bbrc.1998.8937. [DOI] [PubMed] [Google Scholar]
- 12.Yang W., Bell S.G., Wang H., Zhou W., Hoskins N., Dale A. Molecular characterization of a class I P450 electron transfer system from Novosphingobium aromaticivorans DSM12444. J. Biol. Chem. 2010;285:27372–27384. doi: 10.1074/jbc.M110.118349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palaninathan S.K., Mohamedmohaideen N.N., Orlandini E., Ortore G., Nencetti S., Lapucci A. Novel transthyretin amyloid fibril formation inhibitors: synthesis, biological evaluation, and X-ray structural analysis. PLoS One. 2009;4:e6290. doi: 10.1371/journal.pone.0006290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vera L., Antoni C., Devel L., Czarny B., Cassar-Lajeunesse E., Rossello A. Screening using polymorphs for the crystallization of protein–ligand complexes. Cryst. Growth Des. 2013;13:1878–1888. [Google Scholar]
- 15.Stura E.A., Satterthwait A.C., Calvo J.C., Kaslow D.C., Wilson I.A. Reverse screening. Acta Crystallogr. D. Biol Crystallogr. 1994;50:448–455. doi: 10.1107/S0907444994001794. [DOI] [PubMed] [Google Scholar]
- 16.Rosalia L., Cuffaro D., Nuti E., D’Andrea F., Rossello A., Stura E.A. Potent glycoconjugate inhibitors of MMP-12 belonging to the family of selective sulfonamide based carboxylates. J. Med. Chem. 2015 (manuscript submitted) [Google Scholar]
- 17.Vera L., Czarny B., Georgiadis D., Dive V., Stura E.A. Practical use of glycerol in protein crystallization. Cryst. Growth Des. 2011;11:2755–2762. [Google Scholar]
- 18.Antoni C., Vera L., Devel L., Catalani M.P., Czarny B., Cassar-Lajeunesse E. Crystallization of bi-functional ligand protein complexes. J. Struct. Biol. 2013;182:246–254. doi: 10.1016/j.jsb.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Giraud T., Dobias F., Gabadinho J., Rey-Bakaikoa V., Nurizzo D., Leonard G.A. An inexpensive automatically operated device for the flash annealing of crystals of macromolecules. J. Appl. Crystallogr. 2008;42:125–128. [Google Scholar]
- 20.Gallop J.L., Jao C.C., Kent H.M., Butler P.J.G., Evans P.R., Langen R. Mechanism of endophilin N-BAR domain-mediated membrane curvature. EMBO J. 2006;25:2898–2910. doi: 10.1038/sj.emboj.7601174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farley C., Juers D.H. Efficient cryoprotection of macromolecular crystals using vapor diffusion of volatile alcohols. J. Struct. Biol. 2014;188:102–106. doi: 10.1016/j.jsb.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boutron P. Levo- and dextro-2,3-Butanediol and their racemic mixture: very efficient solutes for vitrification. Cryobiology. 1990;27:55–69. [Google Scholar]
- 23.McGuire A.M., Cuthbert B.J., Ma Z., Grauer-Gray K.D., Brunjes Brophy M., Spear K.A. Roles of the A and C sites in the manganese-specific activation of MntR. Biochemistry. 2013;52:701–713. doi: 10.1021/bi301550t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovach A.R., Hoff K.E., Canty J.T., Orans J., Brennan R.G. Recognition of U-rich RNA by Hfq from the gram-positive pathogen Listeria monocytogenes. RNA. 2014;20:1548–1559. doi: 10.1261/rna.044032.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anand K., Pal D., Hilgenfeld R. An overview on 2-methyl-2,4-pentanediol in crystallization and in crystals of biological macromolecules. Acta Crystallogr. Sect. D Biol. Crystallogr. 2002;58:1722–1728. doi: 10.1107/S0907444902014610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura A., Ishida T., Fushinobu S., Kusaka K., Tanaka I., Inaka K. Phase-diagram-guided method for growth of a large crystal of glycoside hydrolase family 45 inverting cellulase suitable for neutron structural analysis. J. Synchrotron Radiat. 2013;20:859–863. doi: 10.1107/S0909049513020943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clendenning K.A. Production and properties of 2,3-butanediol XI: evaluation of levo-2,3-butanediol as a non-volatile antifreeze compound. Can. J. Res. Sect. F. 1946;24:249–271. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.