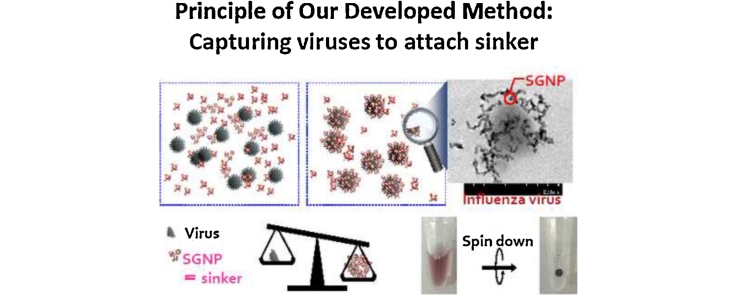
Graphical abstract
Abbreviations: RT-qPCR-SYBR, reverse transcription real-time quantitative PCR SYBR Green method; SC, Sugar Chip; SGNP, sugar chain-immobilized gold nanoparticles; SPR, surface plasmon resonance; HA, hemagglutinin
Keywords: Detecting influenza virus, Sugar chain-immobilized gold-nanoparticle, Lactobacillus acidophilus L-92
Abstract
A highly sensitive and convenient method for detecting influenza virus was developed using modified end-point melt curve analysis of a RT-qPCR SYBR Green method and influenza virus-binding sugar chain-immobilized gold-nanoparticles (SGNP). Because SGNPs capture influenza viruses, the virus-SGNP complex was separated easily by centrifugation. Viral RNA was detected at very low concentrations, suggesting that SGNP increased sensitivity compared with standard methods. This method was applied to clinical studies. Influenza viruses were detected in saliva of patients or inpatients who had been considered influenza-free by a rapid diagnostic assay of nasal swabs. Furthermore, the method was applied to a human trial of prophylactic anti-influenza properties of yogurt containing Lactobacillus acidophilus L-92. The incidence of influenza viruses in saliva of the L-92 group was found to be significantly lower compared to the control group. Thus, this method was useful for monitoring the course of anti-influenza treatment or preventive measures against nosocomial infection.
1. Introduction
Influenza virus infection is a common disease during Winter and can causes pandemics such as the Spanish influenza pandemic of 1918. Influenza virus infects the respiratory tract mucosa, sometimes leading to severe illness and high mortality accompanied by complications such as pneumonia [22]. Influenza virus transmission among humans occurs every year, and is also thought to be mediated by carriers who are infected but have no serious symptoms [10]. Virulence is thought to involve a number of factors. In the last century, four human influenza A virus pandemics have emerged, at least three of which resulted from re-assortment of human and animal influenza A viruses [14]. Presently, it is difficult to predict which influenza virus subtype will cause a pandemic. To detect and prevent a new pandemic, it is important to detect the virulent strain as soon as possible and suppress the risk of infection to a minimum. There are many relevant anti-viral drugs, such as Oseltamivir, Zanamivir, and Peramivir. All of these are prescribed after infection onset, which might be due to the lack of an efficient and convenient way to detect the low levels of the virus before the onset. Furthermore, prescription of anti-virals to uninfected individuals may lead to resistance. Therefore, easy, non-invasive, and accurate diagnostic methods for testing of asymptomatic patients with influenza are needed to reduce viral transmission.
At present, commercially available methods used for rapid influenza diagnosis are antigen-based immunochromatographic methods [23], which can detect the presence of virus using antibodies for viral nuclear or structural proteins of each influenza A or B. By these methods, the assay for the detection of influenza viruses is completed within 30 min or less (duration of the procedure), but their low sensitivity is a problem. Drexler et al. reported that a common rapid antigen-based test detected virus in only 11% of 144 clinical samples which were all confirmed to be influenza virus positive by means of PCR [2]. Uyeki and colleagues reported that sensitivity of rapid diagnostic tests is low [19]. Although the sensitivity of the antigen-based immunochromatographic methods is improving, these methods still cannot detect virus even in severely ill patients, who are likely to be heavily infected. Polymerase chain reaction (PCR) methods have been proposed to improve sensitivity. The PCR methods, however, also have shortcomings because they require special equipment, highly skilled personnel, a lengthy procedure, and tedious extraction of RNA, compared to antigen-based immunochromatographic methods. Recently, Kawai and co-workers applied the RT-SmartAmp assay to rapidly detect influenza virus in patients’ nasal swabs in 40 min without any cross-reaction with other relevant viruses [6]. In addition, some groups have published results on influenza virus detection using non-PCR-based amplification technologies, such as RT-LAMP, which can be completed in less than 60 min [25]. Thus, in principle, influenza virus detection methods based on cDNA have nearly reached the status of rapid detection, with higher sensitivity compared to antigen-based methods. Nevertheless, there are still difficulties with easy and non-invasive collection of samples from asymptomatic patients, which may be the key to prevent influenza epidemics/pandemics. In addition, infants, pregnant women, the elderly, and patients with diseases such as diabetes, cancer, or immunodeficiency, are especially prone to severe influenza illness, and therefore methods for pre-morbid detection are urgently needed.
A sugar chain-immobilized chip, named Sugar Chip (SC), and sugar chain-immobilized gold nanoparticles (SGNPs) were previously developed [21], [9]. In brief, sugar chains obtained from a natural source or via chemical synthesis were conjugated with an original linker molecule to prepare a linker-containing sugar chain (defined as ‘sugar chain’ ligand-conjugate), and then the sugar chains were immobilized on either the gold chip (to prepare SC) or on gold nanoparticles (to prepare SGNP). SC can serve as a sensor chip in a surface plasmon resonance (SPR) device for high-throughput analysis of proteins because the binding interaction with SPR can be evaluated without any labeling of proteins. SGNPs are used for visual detection because SGNPs lose the plasmon absorption ability when the interaction occurs between sugar chains on SGNP and analytes in a solution. SGNPs are also useful for capture and concentration of viruses [26].
In this study, a new highly sensitive test for influenza virus in saliva was developed. The method involves capture and concentration of the viral particles with SGNP in combination with the inexpensive and convenient RT-qPCR SYBR Green method (RT-qPCR-SYBR). Sugar chains can serve as a receptor molecule for the virus [1], [12], [18]. For example, influenza viruses bind to neuraminic acid-containing oligosaccharides on the cell surface at the first stage of infection using hemagglutinin (HA) proteins on the surface of the virus. We previously analyzed the affinity of binding of 242 strains of influenza virus to oligosaccharides, using surface plasmon resonance (SPR) imaging and our SC immobilized with various sugar chains containing either anionic or neutral moieties. From the 242 strains of influenza virus, 1976 data sets were obtained, a database was created, and an algorithm was developed, based on the nearest neighbor with Euclidean distance approach to identify the strains most similar in their relative affinity for sugar chains. Based on the database and the algorithm, an influenza virus strain was identified that was most similar to the 2009 pandemic virus (H1N1pdm2009) obtained from clinical isolates. In addition, based on the binding data, heparin, a sulfated polysaccharide, was shown to bind to every influenza virus, which allowed preparation of heparin- or dextran sulfated oligosaccharide-immobilized gold nanoparticles. After that, a novel method for sensitive detection of influenza viruses was established using the nanoparticles in combination with the reverse transcription real-time quantitative PCR (RT-qPCR) SYBR Green method [17].
In the present work, the new method was applied to 2 clinical studies involving saliva samples from patients who visited the hospital or children’s clinic and from inpatients. The utility of the assay in comparison with the conventional immunochromatographic method (rapid kit) was evaluated using nasal swabs. The utility of our method was tested in a human trial in healthy Japanese adults to evaluate the prophylactic effect of yogurt containing Lactobacillus acidophilus strain L-92 against influenza virus infection and influenza-like symptoms during the winter season.
2. Materials and methods
2.1. Synthesis of SGNP
Two kinds of SGNP were prepared: {1} immobilized with heparin (Hep-GNP) or {2} immobilized with low molecular weight (MW = 2500) dextran sulfate (DS25-GNP) according to previous reports [15], [16], [21], [9] with slight modifications. In brief, to synthesize Hep-GNP, 1 mL of aqueous solution of sodium citrate (16.2 mM) was added to 9 mL of aqueous NaAuCl4 (1.1 mM) with stirring at 100 °C. Then, the heparin-linker conjugate (‘heparin’ ligand-conjugate, heparin-mono, 12 mg) was added to the solution with gentle stirring, and the mixture was further stirred for 1 h at 25 °C in the dark. The solution was centrifuged at 12,000 g for 40 min to obtain a precipitate. After several washes with double distilled water (ddH2O), the precipitate was re-suspended in ddH2O, and adjusted to A530 = 5.0 (a 10-mm equivalent of absorbance) to obtain Hep-GNP.
To synthesize DS25-GNP, 50 mM of sodium tetrahydroborate (10 mL) was added to 1.3 mM NaAuCl4 (38 mL) with stirring at 4 °C. Then, the linker-dextran sulfate conjugate (‘low molecular weight dextran sulfate's ligand conjugate, DS25-mono, 22.5 mg) was added to the solution, and incubated with gentle stirring for 1 h at 4 °C in the dark. The solution was dialyzed against ddH2O using a dialysis membrane with MW 3500 cutoff and then lyophilized. The lyophilized powder was re-suspended in ddH2O and adjusted to 10 mg/mL. Then, sequential centrifugation was performed at 12,000 × g for 15 min to obtain the precipitate. The precipitate was re-suspended in ddH2O, and adjusted to 1 mg/mL with ddH2O to obtain DS25-GNP. The content of heparin or low molecular weight dextran sulfate was evaluated using the ABEE method [24]; heparin content was 2% in Hep-GNP, and the content of low molecular weight dextran sulfate was 9% in DS25-GNP. The size of nanoparticles in ddH2O was measured using DLS (Zeta-Sizer Nano-ZS, Melbane, Kobe, Japan); Hep-GNP particles had a diameter of 62.0 ± 3.4 nm, whereas DS25-GNP’s diameter was 62.4 ± 16.4 nm, on average.
2.2. Virus preparation and construction of plasmids
As a quantitative control for real-time PCR, control plasmid DNAs were prepared. The RT-PCR products derived from clinical isolates of influenza viruses (type A: A/Okuda/57, or A/H1N1pdm09; type B: B/Osaka/304/2007) were synthesized using the primer sets described below using PrimeScript One Step RT-PCR Kit Version 2 (Takara Bio Inc., Otsu, Japan). The RT-PCR products were purified using NucleoSpin Gel and PCR Clean-up (Macherey-Nagel GmbH & Co., Germany) and ligated into the pGEM-T Easy Vector (Promega, Germany). The plasmid vectors were introduced into DH5α Escherichia coli cells, which were grown in a culture medium, and plasmid DNA was purified using the NucleoSpin Plasmid kit (Macherey-Nagel GmbH & Co.). The control plasmid DNA was quantified using OD260 measurement, and a series of 10-fold dilutions was prepared (from 108 to 100 copies/μL) and tested by means of the RT-qPCR SYBR Green assay.
2.3. RT-qPCR conditions and evaluation criteria
The RT-qPCR SYBR Green assay was carried out using a Thermal Cycler Dice Real Time System (Takara Bio Inc.) with a commercial reagent (One-Step SYBR Prime Script RT-PCR Kit 2 (Perfect Real Time), Takara Bio Inc.) according to the manufacturer’s instructions. Primers for these reactions were designed for the gene encoding the M protein of influenza virus type A and the NS protein of type B influenza virus as follows: type A forward 5′ GGA CTG CAG CGT AGA CGC TT, reverse 5′ CAT YCT GTT GTA TAT GAG GCC CAT; type B forward 5′ AAA TAC GGT GGA TTA AAT AAA AGC AA, reverse 5′ CCA GCA ATA GCT CCG AAG AAA [20]. The RT-qPCR protocol consisted of 45 °C for 5 min, 95 °C for 10 s, and 40 cycles of 95 °C for 5 s and 60 °C for 30 s. The identity of the amplified product was verified using the melting curve analysis (denaturation at 95 °C for 15 s followed by stepwise elevation of the temperature from 60 °C to 95 °C by 0.5 °C in 30-s increments). Ct (threshold cycle) corresponded to the cycle number at which the fluorescence significantly exceeded the background.
2.4. Virus detection in saliva (clinical samples)
Two clinical studies were designed at Kagoshima University Hospital, Murakami Clinic for Children, and Hyogo College of Medicine Hospital. At the Kagoshima University Hospital and Murakami Clinic for Children in 2011–2012, saliva samples were collected from patients (total number 183; under the age of 16:109, over the age of 16:74) who visited the hospital or clinic because of a fever or flu-like symptoms. This study was reviewed and approved by the Kagoshima University Hospital Ethical Committee (No. 23–140); written informed consent was obtained from each subject’s parents/guardians before study entry. All patients were also tested by means of a conventional immunochromatographic method (rapid kit, Clearline-Influenza-A/B/(H1N1) 2009, Meiji Seika Pharma Co., Ltd., Tokyo, Japan, or QuickNavi™-Flu Otsuka Pharmaceutical Co., Ltd., Tokushima, Japan) using their nasal swabs. At the Hyogo Medical College Hospital in 2012–2013, saliva samples were collected from inpatients (total number was 55) who either had or did not have flu-like symptoms; these patients had been treated for other diseases or received surgical care in the hospital. The study was reviewed and approved by the ethics committee of Hyogo Medical College Hospital (No. 783). All subjects read and signed informed consent forms before study entry. Nasal swab samples of 24 inpatients were also tested using the rapid kit (ESPLINE® Influenza A and B–N, Fujirebio Inc., Tokyo, Japan).
Approximately 0.5 mL of saliva was collected from symptomatic or asymptomatic patients/inpatients using a dropper and transferred to the collection tube by patients themselves when the patients/inpatients were in the hospital. The collected saliva samples were stored at 4 °C after being mixed with 0.5 mL of MEM medium containing 1% penicillin–streptomycin solution (abbreviated as PS, NacalaiTesque, Japan) or PBS containing 1% PS without divalent cations. Within 6 h, after centrifugation at 10,000 × g for 2 min at 4 °C to remove debris in saliva samples, 500 μL of the supernatant was mixed with 10 μL of SGNP (Hep-GNP or DS25-GNP) and incubated for 30 min at room temperature in the dark with gentle agitation. After that, the reaction mixture was centrifuged at 10,000 × g for 10 min at 4 °C to obtain a precipitate containing SGNP and viruses. The precipitate was washed with 500 μL of PBS and centrifuged again at 10,000 × g for 10 min at 4 °C. The resulting precipitates were re-suspended in 10 μL of RNase-free water, and heated at 100 °C for 10 min to disrupt viral particles and release viral RNA that was used for RT-qPCR.
2.5. Virus detection in saliva in a human trial
The study protocol was approved by the Ethical Committee of Calpis Co., Ltd., registered under number 5 and Ethical Committees of Shiba Palace Clinic in Tokyo, Japan, registered under KRPS-10887, and this trial was performed according to the Declaration of Helsinki on Human Rights.
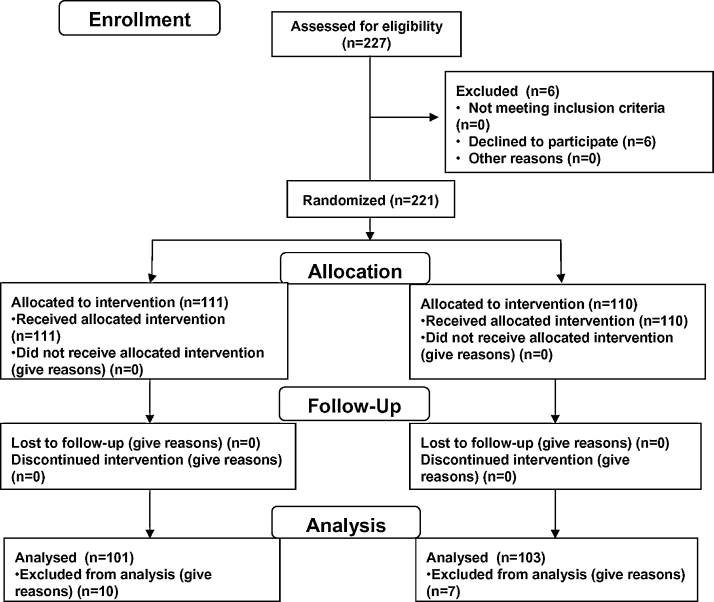
This study was conducted during the winter season, between December 2010 and February 2011. 227 Japanese healthy volunteers agreed to participate in this study by the SOUKEN Co., Ltd., according to previous trial [8]. Exclusion criteria were as follows: (a) those believed to be ingesting a drug that may influence test results (b) those who were vaccinated one year previously (c) those who were diagnosed with influenza one year previously (d) those believed to be routinely ingesting health food that may influence test results e.g., food containing lactic acid bacteria (e) those judged as being disqualified to participate in this test by a leading physician au fait with the test. In regard to characteristics of subjects, all volunteers enrolled in this trial had no cold-like symptoms at the beginning of the trial and lived in the same area throughout the experiment. Six volunteers were excluded because they declined to participate. All volunteers read and signed informed consent forms. 111 subjects were assigned to the test group who, once a day for 8 weeks, consumed 100 g of the commercial product Nyusankin-Seikatsu Yogurt containing Lactobacillus acidophilus L-92 (at least 1010 cells) provided by Calpis Co., Ltd. (Tokyo, Japan). One hundred and ten subjects who did not take yogurt for 8 weeks were assigned to the control group. Randomization was performed by the study statistician using a computer generated code. Study design and a flow diagram is shown in Fig. 1. All participants were asked to avoid an irregular lifestyle (lack of sleep, over-eating, and over-drinking alcohol) during the whole study period. In addition, intake of products with components known to have an immunostimulatory effects, such as Lactobacillus, was prohibited during the test period. The subjects reported relevant signs and symptoms, such as body temperature, and a score for 13 questions about flu-like symptoms and preventive measures, every morning after awakening.
Fig. 1.
Subject recruitment and retention.
Subject recruitment and retention is described.
Approximately 0.5 mL of saliva specimens were collected from the volunteers using a dropper in the morning, every 7 days. The saliva samples were stored at 4 °C after being mixed with 0.5 mL of MEM medium containing 1% PS. Within 2 days, the sample solution was tested as described above. If a subject was judged to be unsuitable for data analysis for any valid reason (e.g., non-compliance with the prescribed dose, failure to follow instructions and virus testing before the trial), the subject was excluded from data analysis. Statistical significance was determined using the log rank test or Mann–Whitney U-test and accepted as p < 0.05. Statistical analysis was performed using SPSS Version 20.0 (SPSS Inc., Chicago, IL, USA).
3. Results and discussion
3.1. Development of the sensitive and accurate semi-quantitative evaluation method for the RT-qPCR SYBR Green method
To semi-quantitatively evaluate the results of the RT-qPCR SYBR Green method with the low copy numbers of viral RNA, a convenient and accurate method was developed. In the standard RT-qPCR analysis, the presence of the target RNA is quantified using the threshold cycle number (Ct value). On the other hand, when the quantity of target RNA is low, the SYBR Green method needs careful comparison of the Tm (melting temperature) curve with that of a positive control. This approach means that the quantification based on the Tm curve is a more conventional and accurate evaluation technique for the results of qPCR of the SYBR Green method than the Ct value, especially at low RNA copy numbers. In our method, after 40 cycles of PCR, the temperature increased from 60 to 95 °C stepwise by 0.5 °C every 30 s to monitor how the dsDNA (PCR product) melted into ssDNA with a drop in fluorescence as the dye (SYBR Green I) becomes inactive. The melting curve is converted to a distinct melting peak by plotting the first negative derivative of the fluorescence (−dF/dT) as a function of the temperature. First, using a positive control, which was the PCR product of 10,000 copies of plasmid cDNA with the forward/reverse primers in a PCR reaction, melting point, Tm (the temperature at the greatest absolute value of −dF/dT) was determined. Then, the temperature points Tm ± 0.5 °C, and Tm + 1.0 °C or −1.0 °C (with greater absolute value of −dF/dT) were determined. The absolute values of −dF/dT of the 4 temperature points (Tm, Tm + 0.5 °C, Tm–0.5 °C, Tm + 1.0 °C or −1.0 °C), were summed up to obtain the total value A. Using the negative control (ddH2O), the absolute values of −dF/dT of the 4 temperature points determined in the positive control were also calculated to obtain the total value B. Similarly, the absolute values of −dF/dT of the same 4 temperature points measured in the sample were calculated to obtain the total value of C. Using A, B, and C, the relative intensity (RI) of a sample was determined as follows:
It is crucial that the temperature of the greatest absolute value of −dF/dT of the sample is within ±0.5 °C of the Tm for the positive control.
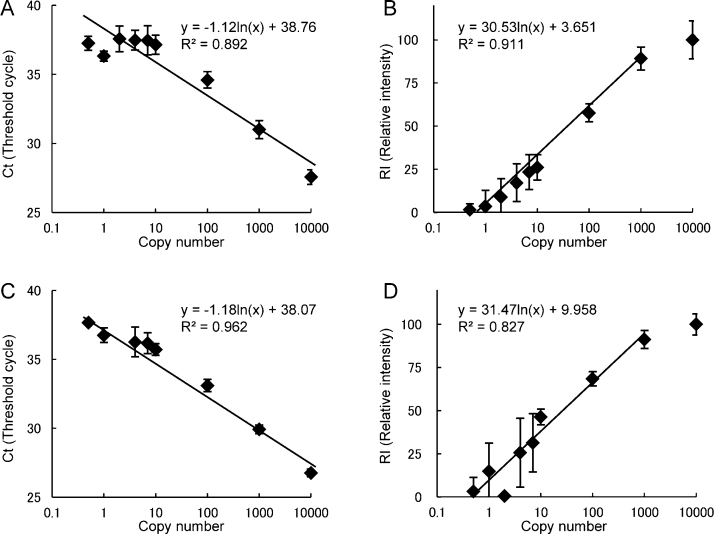
In Fig. 2, the Ct values and RI values of control samples datasets, (culture supernatant of influenza virus A/Okuda/57 and B/Osaka/304/2007), were plotted against the virus copy number. The copy number of the culture supernatant of each virus was determined by comparing the Ct value at the high concentration with that of the target cDNA containing a plasmid. We found that the detection limit of RI for type A influenza virus was 1% of RI, and calibration was possible (reproducible) over 4% of RI. On the contrary, the threshold of type B influenza virus was 3%, and the calibration was possible over 10% of RI. However, the primers for type B may not be adequate, according to these data. Thus, we established 3 semi-quantitative categories for each type of influenza virus as follows: type A: plus RI ≥ 4%, plus-minus 1% ≤ RI < 4%, minus RI < 1%; type B: plus RI ≥ 10%, plus-minus 3% ≤ RI < 10%, minus RI < 3%. The PCR products of the control and clinical samples after concentration with SGNP as follows were also analyzed in part by agarose gel (Fig. S1).
Fig. 2.
Ct and RI values of influenza virus in the RT-qPCR SYBR Green method.
The standard curve of the threshold cycle (Ct) and relative intensity values (RI) of type A influenza (panels A, B) and type B influenza (panels C, D). The culture supernatant of influenza virus (type A: A/Okuda/57, type B: B/Osaka/304/2007) was diluted to the 0.5, 1, 2, 4, 7, 10, 100, 1000, and 10,000 copies per sample. Three independent experiments were performed in triplicate.
4.1. An increase of sensitivity in buffer and in saliva
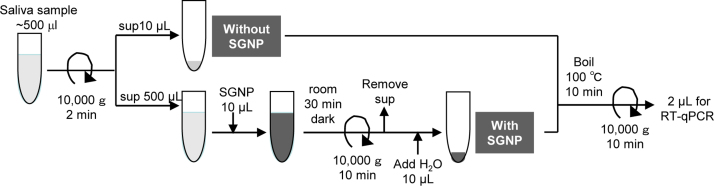
An outline of the assay using SGNP and saliva samples is shown in Fig. 3, where 500 μL of a sample solution was concentrated to 10 μL, suggesting that a 50-fold increase in sensitivity was achieved, in theory. The Ct (threshold cycle) values of RT-qPCR-SYBR were compared between the conditions with and without SGNP treatment of the culture supernatant.
Fig. 3.
The schematic of our concentration method using SGNP and saliva samples.
Approximately 0.5 mL of saliva is collected from symptomatic or asymptomatic patients/inpatients using a dropper and mixed with 0.5 mL of MEM medium or PBS containing 1% penicillin-streptomycin mixed solution. After centrifugation at 10,000 × g for 2 min at 4 °C to remove debris from the saliva sample, 500 μL of the supernatant is mixed with 10 μL of SGNP and incubated for 30 min at room temperature in the dark with gentle agitation. Then, the reaction mixture is centrifuged at 10,000 × g for 10 min at 4 °C to obtain a precipitate containing SGNP and influenza virus. The precipitate was washed with 500 μL of PBS and centrifuged again at 10,000 × g for 10 min at 4 °C. The resulting precipitate was re-suspended in 10 μL of RNase-free water, and heated at 100 °C for 10 min to destroy viral particles and release viral RNA that is used for RT-qPCR.
As shown in Table 1, the Ct values for samples of culture supernatant diluted (×100, ×1000, ×10,000, and ×100,000) with PBS were 21.31, 25.41, 30.31, and 32.98 with SGNP (Hep-GNP) treatment and 26.25, 30.39, 33.63, and 36.62 without SGNP treatment, respectively. This observation means that the viral particles were concentrated 23–25 times (8- to 32-fold) by Hep-GNP. In addition, with DS25-GNP, the Ct values were 20.99, 26.10, 29.88, and 33.43 compared with 26.68, 30.70, 33.27, and 37.13, suggestive of concentration by a factor of 23.–26 times (10- to 54-fold). The Ct values of extracted RNA from the same diluted sample using an RNA extraction kit were almost identical to those obtained with SGNP concentration procedure. The above data suggest that SGNPs captured and concentrated viral particles as effectively as an RNA extraction method, and the difference in efficiency between Hep-GNP and DS25-GNP was negligible.
Table 1.
Ct values of influenza virus with or without the use of SGNPs and the extracted RNA.
|
Ct values of RT-qPCR SYBR Green method |
|||||
|---|---|---|---|---|---|
| DS25-GNP |
Hep-GNP |
Extracted RNA | |||
| Dilution of virus (-fold) | With | Without | With | Without | |
| 100 | 20.99 | 26.68 | 21.31 | 26.25 | 20.65 |
| 1000 | 26.10 | 30.70 | 25.41 | 30.39 | 26.16 |
| 10000 | 29.88 | 33.27 | 30.31 | 33.63 | 27.27 |
| 100000 | 33.43 | 37.13 | 32.98 | 36.62 | 33.06 |
After that, similar in vitro experiments were conducted, where the virus culture supernatant of the strain A/H1N1pdm09 was mixed with saliva from a healthy volunteer. In Table 2, the effects of SGNP seem to be similar to the ones described above. The Ct values decreased (sensitivity increased) when the sample mixture was treated with Hep-GNP, even though the Ct values in the saliva were greater than those in PBS. This difference was most likely due to the inhibitors of PCR in saliva, such as gp340 [7], which is a highly anionic polymer containing many neuraminic acid residues (mucin). The values of RI of SGNP-treated samples were all increased compared with those of the starting sample mixture (without SGNP), even if 50% saliva was present in the sample mixture, where viral RNA remained in the supernatant. This result suggests that mucin in saliva has an inhibitory effect on the binding of virus to SGNP. Although the inhibitory effect was appreciable, the capture and concentration with SGNP was sufficient for use with our method in clinical study or in a human trial. Thus, our method was used for further experimentation.
Table 2.
A concentration procedure for culture supernatant of H1N1pdm09 type A influenza virus containing human saliva by means of Hep-GNP.
| Ratio of saliva (%) | Dilution of virus (fold) | Hep-GNP |
|||
|---|---|---|---|---|---|
| With | Without | ||||
| Ct | RI (%) | Ct | RI (%) | ||
| 50 | 1000 | 28.33 | 76.80 | 31.44 | 59.87 |
| 10,000 | 31.36 | 63.15 | 36.04 | 32.74 | |
| – | |||||
| 25 | 1000 | 29.10 | 82.09 | 36.93 | 23.56 |
| 10,000 | 30.02 | 79.45 | 36.62 | 24.48 | |
| – | |||||
| 10 | 1000 | 26.21 | 98.58 | 35.03 | 47.64 |
| 10,000 | 29.66 | 90.43 | 36.83 | 26.10 | |
| – | |||||
| 0 | 1000 | 19.11 | 105.87 | 28.89 | 76.70 |
| 10,000 | 23.16 | 85.39 | 31.85 | 63.48 | |
4.2. Clinical study
Our sensitive detection method, including the analytical modification, was applied to a clinical study at Kagoshima University Hospital, Murakami Clinic for Children, and Hyogo College of Medicine Hospital.
In season 2011–2012, a total of 183 patients were enrolled in the study (<16 years old: 109; older than 16 years: 74) including medical doctors and nurses at Kagoshima University Hospital or outpatients who visited Murakami Clinic for Children because of flu- or cold-like symptoms. In addition to saliva, nasal swab samples were taken from all patients and tested using the antigen-based immunochromatographic assay (rapid kit). Because the purpose of this clinical research was to detect symptoms in patients, only the plus categories of RI in our method were analyzed and compared with the diagnostic results obtained with the nasal swabs and the rapid kit (Table 3). It was surprising that influenza viruses in 52% (24/46) of adult patients and 57% (26/46) of childhood patients were detected by our method using saliva and RT-qPCR, but were not detected in the 46 adult and 46 childhood patients by the rapid kit with the nasal swab. From the large difference in sensitivity between RT-qPCR and the rapid kit [2], the results described above might be reasonable if nasal swabs were used as samples for our method. However, since the concentration of influenza viral particles in saliva is much lower than in nasal swabs [11], the data using saliva samples suggested that our technology was both useful and effective.
Table 3.
Comparison between the rapid kit using nasal swabs and the SGNP-concentration/RT qPCR SYBR Green method in saliva.
| Patients | Rapid kit using nasal swab |
Our method using saliva |
|||
|---|---|---|---|---|---|
| A+ | B+ | – | |||
| Adults | A+ | 25 | 25 | 0 | 0 |
| B+ | 3 | 1 | 2 | 0 | |
| – | 46 | 24 | 0 | 22 | |
| – | |||||
| Children (under 16 years old) | A+ | 56 | 54 | 0 | 2 |
| B+ | 7 | 1 | 4 | 2 | |
| – | 46 | 23 | 3 | 20 | |
The second clinical study was conducted at Hyogo College of Medicine Hospital during the 2012–2013 season. An outbreak of influenza occurred during the winter of 2012–2013 where 16 inpatients received a diagnosis of influenza according to the rapid diagnostic kit in 2 independent wings of the hospital, within 4 days. Saliva samples were obtained from 55 inpatients who had or did not have flu-like or cold-like symptoms; these patients had been treated for other diseases or received surgical care in the hospital. Twenty four inpatients out of 55 agreed to provide a nasal swab, which was tested using the rapid kit. Because the purpose of this clinical research was to find symptoms in inpatients, only plus categories of RI in the developed method were analyzed.
As shown in Table 4, this method showed that 5 of 33 inpatients who did not have flu-like symptoms carried influenza virus RNA in their saliva, suggesting that they were asymptomatic patients with influenza virus. In addition, 5 of 9 inpatients who had been diagnosed as negative by the rapid kit were found to have influenza viral RNA in their saliva (Table 5) according to our method. These data suggest that the rapid kit generated some false-negative results in symptomatic influenza patients due to low sensitivity. Because those asymptomatic inpatients were in a controlled environment and received timely treatment, according to the results above, the method using saliva may contribute to the prevention of onward infection or terminate nosocomial outbreaks.
Table 4.
Diagnostic results of inpatients using our SGNP-concentration/RT qPCR SYBR Green method: comparison of symptoms.
| Symptoms |
Our method using saliva |
||
|---|---|---|---|
| Type A positive (%) | Negative (%) | ||
| Flu-like | 22 | 20 (90.9) | 2 (9.1) |
| No | 33 | 5 (15.2) | 28 (84.8) |
Table 5.
Diagnostic results of inpatients using our SGNP-concentration/RT qPCR SYBR Green method: comparison with the rapid kit.
| Rapid kit |
Our method using saliva |
||
|---|---|---|---|
| Type A positive (%) | Negative (%) | ||
| Type A positive | 15 | 13 (86.7) | 2 (13.3) |
| Negative | 9 | 5 (55.6) | 4 (44.4) |
The diagnosis of influenza is ascertained by culturing/isolation of virus, or serological tests, or PCR method. To corroborate the data, other virus detection methods such as virus isolation should be compared. However, at present, diagnosis needs to be more rapid to treat patients more promptly, and therefore, the rapid test is commonly used for influenza virus detection. Our new developed method was compared with a rapid test in this study.
For future analysis, a large-scale follow-up cohort study to clarify usability of our new method should be conducted. For example, it might be possible to investigate how many asymptomatic healthy subjects are found to have influenza viral RNA in saliva by our method and who develop the symptoms of influenza thereafter.
4.3. Detection of influenza viruses in the human trial
Using this newly developed method, a human trial of the anti-influenza effect of yogurt containing Lactobacillus acidophilus L-92 was conducted, which was previously shown to have an anti-viral effect in laboratory animals [3] and anti-allergy properties in both humans and animals [4], [5], [13]. In such a human trial, recruiting a significant sample size of subjects is problematic because the average percentage of symptomatic influenza patients is usually less than 10% of the general population. Therefore, it would be better to evaluate asymptomatic cases at high sensitivity. Testing the method for collecting samples is also important. Getting saliva samples every week was straightforward, but obtaining nasal swabs every week was difficult due to the uncomfortable procedure. Our method matches the above criteria. Because the purpose of the study was to make an accurate diagnosis in asymptomatic and symptomatic patients as far as possible, both plus and plus-minus categories of RI were analyzed in this experiment.
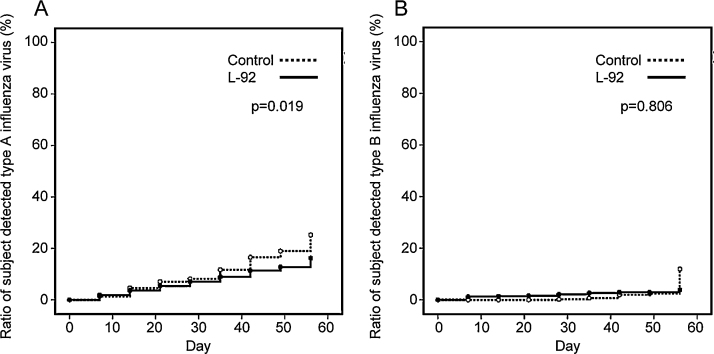
A total of 221 subjects who had a tendency to fall ill during the winter season were selected and assigned randomly to 2 groups with the following demographics: the L-92 group (n = 111; males: 42, females: 69; average age: 41.0 ± 7.7 [mean ± SD]) and the control group (n = 110; males: 36, females: 74; average age: 41.0 ± 7.0). This study was conducted during the winter season, between December 2010 and February 2011, and no adverse events specific to L-92 was observed throughout the whole test period. Type A or B influenza viruses were detected from 17 subject’s saliva despite the fact that they had no cold-like symptoms at the beginning of the trial. The analysis excluded these 17 subjects but revealed that the rate of the subjects who had type A influenza virus RNA in their saliva was increased in both groups during the test period after starting the trial in January (Fig. 4A). This suggests that type A influenza was circulating in Japan at epidemic levels. The following participants tested positive for the influenza virus RNA eight weeks after the onset of treatment: 25% of the control group and 16% of the L-92 group. The percentage of people of which influenza type A viruses were detected in the L-92 group was significantly lower than that in the control group (p < 0.05) (Fig. 4A). On the other hand, no difference in type B influenza virus infection was observed between the 2 groups (and the detection rate was low; Fig. 4B). The data suggested that there was no evidence of a type B influenza epidemic happening in Japan at the time. To evaluate the severity of flu-like symptoms, body temperature during initial awakening was measured every day in the morning. The number of subjects whose body temperature in the morning was above 38 °C (which indicates severe symptoms) was 2 of 111 subjects in the L-92 group and 10 of 110 subjects in the control group (p < 0.01, χ2 test). In addition, most of the subjective symptom scores, such as the degree of throat soreness, frequency of coughing, fatigue, chills, and hoarseness, were significantly lower in the L-92 group compared to the control group (Fig. S2).
Fig. 4.
Detection of influenza virus in the L-92 group and control group by means of SGNP-concentration/RT qPCR SYBR Green method.
Time course of the number of subjects in the L-92 group and the control group who tested positive for influenza virus type A (panel A) and type B (panel B) in saliva during 8 weeks. The subjects gave their saliva samples every 7 days and influenza virus was detected in saliva using our newly developed method. Regarding influenza virus type A, statistical significances between the 2 groups were calculated using the Kaplan–Maier log rank test.
No significant differences were found in 3 questionnaires relating to preventive measures other than the rest time throughout this test period in the L-92 group compared to the control group (data not shown). According to the above data, it seems that the developed method was applicable not only in detecting influenza virus at early stage, but could evaluate preventive effects on virus infection in the development of drugs or functional foods. As for L-92, it was recently demonstrated that continuous oral administration of L-92 for 2 weeks suppressed the viral titer and activated NK cells in lungs of mice infected with influenza virus [3]. Therefore, L-92 may have the potential to promote vigorous host immune responses. In this clinical study, supplementation of L-92 is considered useful for prevention of influenza, although a double blinded, placebo-controlled trial was not performed. In order to confirm the effect of L-92 on prevention of influenza virus infection, another study must be performed. L-92 is easily applicable in various food products, such as beverages and supplements. If the promotion of host immune response by L-92 is confirmed in well-designed human studies, daily intake of the food products containing L-92 might be one of the easy approaches to prevent viral infection.
In the present study, a highly sensitive, accurate, convenient, and non-painful method for influenza virus detection using saliva was proposed with a nano-biotechnology and inexpensive RT-qPCR CYBR Green method. According to this new method, false-negative diagnostic results of the antigen-based rapid kit were suggested in the patients; this finding helped to initiate appropriate and early treatment of the patients. In addition, the newly developed method might be helpful in preventing nosocomial infection with influenza virus by finding asymptomatic patients at an early stage. The method has also been applied to a human trial of L. acidophilus L-92 strain in protecting against influenza virus infection in approximately 200 volunteers.
In conclusion, because the new method was able to detect trace amounts of influenza virus, the method may be used in a variety of applications, such as testing for nosocomial infection or detection of bioterrorism efforts. This test may decrease the cost of medical care via early treatment of asymptomatic patients and may prevent the loss of labor productivity and the resultant losses to the economy.
Acknowledgment
This work was supported in parts by grants from Japan Science and Technology Agency (V07-05 and AS2416907P to YS), and Japan Ministry of Health, Labour, and Welfare (H21-Nano-Ippan-002, to YS).
Footnotes
Available online 19 May 2015
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.btre.2015.05.004.
Contributor Information
Yasuo Suda, Email: ysuda@eng.kagoshima-u.ac.jp.
Mami Nagatomo, Email: mnagatomo@sudxbiotec.jp.
Risa Yokoyama, Email: ryokoyama@sudxbiotec.jp.
Mami Ohzono, Email: mohzono@sudxbiotec.jp.
Kazue Aoyama, Email: kaoyama@sudxbiotec.jp.
Xu Zhang, Email: kchou@sudxbiotec.jp.
Kazuhiko Nakajima, Email: nakajima@hyo-med.ac.jp.
Naoki Murakami, Email: naoki-mu@zb3.so-net.ne.jp.
Tadashi Shinoda, Email: tadashi.shinoda@calpis.co.jp.
Tatsuhiko Hirota, Email: tatsuhiko.hirota@calpis.co.jp.
Sae Yanagihara, Email: sae.yanagihara@calpis.co.jp.
Jun-Ichiro Nishi, Email: nishi1@m2.kufm.kagoshima-u.ac.jp.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Compton T., Nowlin D.M., Cooper N.R. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology. 1993;193:834–841. doi: 10.1006/viro.1993.1192. http://dx.doi.org/10.1006/viro.1993.1192 [DOI] [PubMed] [Google Scholar]
- 2.Drexler J.F., Helmer A., Kirberg H., Reber U., Panning M. Poor clinical sensitivity of rapid antigen test for influenza A pandemic (H1N1) 2009 virus. Emerg. Infect. Dis. 2009;15(10):1662–1664. doi: 10.3201/eid1510.091186. http://dx.doi.org/10.3201/eid1510.091186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goto H., Sagitani A., Ashida N., Kato S., Hirota T. Anti-influenza virus effects of both live and non-live Lactobacillus acidophilus L-92 accompanied by the activation of innate immunity. Br. J. Nutr. 2013;110(10):1810–1818. doi: 10.1017/S0007114513001104. http://dx.doi.org/10.1017/S0007114513001104. [DOI] [PubMed] [Google Scholar]
- 4.Ishida Y., Bandou I., Kanzato H., Yamamoto N. Decrease in ovalbumin specific IgE of mice serum after oral uptake of lactic acid bacteria. Biosci. Biotechnol. Biochem. 2003;67:951–957. doi: 10.1271/bbb.67.951. http://dx.doi.org/10.1271/bbb.67.951 [DOI] [PubMed] [Google Scholar]
- 5.Ishida Y., Nakamura F., Kanzato H., Sawada D., Yamamoto N. Effect of milk fermented with Lactobacillus acidophilus strain L-92 on symptoms of Japanese cedar pollen allergy: a randomized placebo-controlled trial. Biosci. Biotechnol. Biochem. 2005;69:1652–1660. doi: 10.1271/bbb.69.1652. http://dx.doi.org/10.1271/bbb.69.1652 [DOI] [PubMed] [Google Scholar]
- 6.Kawai Y., Kimura Y., Lezhava A., Kanamori H., Usui K. One-step detection of the pandemic influenza A(H1N1) virus by the RT-SmartAmp assay and its clinical validation. PLoS One. 2009;7(1):e30236. doi: 10.1371/journal.pone.0030236. http://dx.doi.org/10.1371/journal.pone.0030236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kukita K., Kawada-Matsuo M., Oho T., Nagatomo M., Oogai Y. Staphylococcus aureus SasA is responsible for binding to the salivary agglutinin gp340, derived from human saliva. Infect. Immunol. 2013;81:1870–1879. doi: 10.1128/IAI.00011-13. http://dx.doi.org/10.1128/IAI 11–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makino S., Ikegami S., Kume A., Horiuchi H., Sasaki H. Reducing the risk of infection in the elderly by dietary intake of yoghurt fermented with Lactobacillus delbruekii ssp. bulgaricus OLL1073R-1. Br. J. Nutr. 2010;104:998–1006. doi: 10.1017/S000711451000173X. http://dx.doi.org/10.1017/S000711451000173X [DOI] [PubMed] [Google Scholar]
- 9.Nakamura-Tsuruta S., Kishimoto Y., Nishimura T., Suda Y. One-step purification of lectins from banana pulp using sugar-immobilized gold nanoparticles. J. Biochem. 2008;143:833–839. doi: 10.1093/jb/mvn038. http://dx.doi.org/10.1093/jb/mvn038 [DOI] [PubMed] [Google Scholar]
- 10.Papenburg J., Baz M., Hamelin M., Rhéaume C., Carbonneau J., Ouakki M., Rouleau I., Hardy I., Skowronski D., Roger M., Charest H., De Serres G., Boivin1 G. Household transmission of the 2009 pandemic A/H1N1 influenza virus: elevated laboratory-confirmed secondary attack rates and evidence of asymptomatic infections. Clin. Infect. Dis. 2010;9:1033–1041. doi: 10.1086/656582. http://dx.doi.org/10.1086/656582 [DOI] [PubMed] [Google Scholar]
- 11.Robinson J.L., Lee B.E., Kothapalli S., Craig W.R., Fox J.D. Use of throat swab or saliva specimens for detection of respiratory viruses in children. Clin. Infect. Dis. 2008;46:e61–64. doi: 10.1086/529386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rostand K.S., Esko J.D. Microbial adherence to and invasion through proteoglycans. Infect. Immunol. 1997;65:1–8. doi: 10.1128/iai.65.1.1-8.1997. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC174549/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah M.M., Miyamoto Y., Yamada Y., Yamashita H., Tanaka H. Orally supplemented Lactobacillus acidophilus strain L-92 inhibits passive and active cutaneous anaphylaxis as well as 2,4-dinitroflurobenzene and mite fecal antigen induced atopic dermatitis-like skin lesions in mice. Microbiol. Immunol. 2010;54:523–533. doi: 10.1111/j.1348-0421.2010.00251.x. [DOI] [PubMed] [Google Scholar]
- 14.Schrauwen E.J., de Graaf M., Herfst S., Rimmelzwaan G.F., Osterhaus A.D., Fouchier R.A. Determinants of virulence of influenza A virus. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33(4):479–490. doi: 10.1007/s10096-013-1984-8. http://dx.doi.org/10.1007/s10096-013-1984-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suda Y., Kishimoto Y., Nishimura T., Yamashita S., Hamamatsu M. Sugar-immobilized gold nanoparticles (SGNP): novel bioprobe for the on-site analysis of the oligosaccharide protein interactions. Polym. Prepr. 2006;47:156–157. [Google Scholar]
- 16.Suda Y., Arano A., Fukui Y., Koshida S., Wakao M. Immobilization and clustering of structurally defined oligosaccharides for sugar chips: an improved method for surface plasmon resonance analysis of protein carbohydrate interactions. Bioconjugate Chem. 2006;17:1125–1135. doi: 10.1021/bc0600620. [DOI] [PubMed] [Google Scholar]
- 17.Suda Y., Zhang W., Takahashi Y., Yokoyama R., Nagatomo M. Discrimination of influenza virus strains and super high sensitive detection of viruses using sugar chip and sugar chain immobilized goldnanoparticles. In: Scholz C., Kressler J., editors. Tailored Polymer Architectures for Pharmaceutical and Biomedical Applications. 2013. pp. 331–350. ACS Symposium Series 1135. [Google Scholar]
- 18.Suzuki Y., Ito T., Suzuki T., Holland R., Jr., Chambers T.M. Sialic acid species as a determinant of the host range of influenza A viruses. J. Virol. 2000:11825–11831. doi: 10.1128/jvi.74.24.11825-11831.2000. http://dx.doi.org/10.1128/JVI.74.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uyeki T.M., Prasad R., Vukotich C., Stebbins S., Rinaldo C.R., Ferng Y.H., Morse S.S., Larson E.L., Aiello A.E., Davis B., Monto A.S. Low sensitivity of rapid diagnostic test for influenza. Clin. Infect. Dis. 2009;9:e89–e92. doi: 10.1086/597828. http://dx.doi.org/10.1086/597828 [DOI] [PubMed] [Google Scholar]
- 20.van Elden L.J.R., Nijhuis M., Schipper P., Schuurman R., van Loon A.M. Simultaneous detection of influenza viruses A and B using real-time quantitative PCR. J. Clin. Microbiol. 2001;39(1):196–200. doi: 10.1128/JCM.39.1.196-200.2001. http://dx.doi.org/10.1128/JCM.39.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wakao M., Saito A., Ohishi K., Kishimoto Y., Nishimura T. Sugar Chips immobilized with synthetic sulfated disaccharides of heparin/heparan sulfate partial structure. Bioorg. Med. Chem. Lett. 2008;18:2499–2504. doi: 10.1016/j.bmcl.2008.01.069. http://dx.doi.org/10.1016/j.bmcl.2008.01.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiselka M. Influenza: diagnosis, management, and prophylaxis. BMJ. 1994;308:1341–1345. doi: 10.1136/bmj.308.6940.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamazaki M., Mitamura K., Kimura K. Clinical evaluation of an immunochromatography test for rapid diagnosis of influenza. Kansenshogaku Zasshi. 2001;75:1047–1053. doi: 10.11150/kansenshogakuzasshi1970.75.1047. [DOI] [PubMed] [Google Scholar]
- 24.Yasuno S., Murata T., Kokubo K., Yamaguchi T., Kamei M. Two-mode analysis by high-performance liquid chromatography of p-aminobenzoic ethyl ester-derivatized monosaccharides. Biosci. Biotechnol. Biochem. 1997;61(11):1944–1947. doi: 10.1271/bbb.61.1944. http://dx.doi.org/10.1271/bbb.61.1944 [DOI] [PubMed] [Google Scholar]
- 25.Ma X.J., Shu Y.L., Nie K., Qin M., Wang D.Y., Gao R.B., Wang M., Wen L.Y., Han F., Zhou S.M., Zhao X., Cheng Y.H., Li D.X., Dong X.P. Visual detection of pandemic influenza A H1N1 virus by reverse-transcription loop-mediated isothermal amplification with hydroxynaphthol blue dye. J. Virol. Methods. 2009;167(2):214–217. doi: 10.1016/j.jviromet.2010.03.027. http://dx.doi.org/10.1016/j.jviromet.2010.03.027 [DOI] [PubMed] [Google Scholar]
- 26.Zhang X., Nakamura-Tsuruta S., Haruyama M., Yokoyama R., Nagatomo M. Super high sensitive detection of viruses using sugar chain immobilized gold nanoparticles (SGNPs) Polym. Prepr. 2012;53(1):671–672. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.