Abstract
The biosorbent was obtained from municipal solid waste (MSW) of the Mostaganem city. Before use the MSW was dried in air for three days and washed several times. The sorption of yellow procion reactive dye MX-3R onto biomass from aqueous solution was investigated as function of pH, contact time and temperature. The adsorption capacity of MX-3R was 45.84 mg/g at pH 2–3 and room temperature. MX-3R adsorption decreases with increasing temperature. The Langmuir, Freundlich and Langmuir–Freundlich adsorption models were applied to describe the related isotherms. Langmuir–Freundlich equation has shown the best fitting with the experimental data. The pseudo first-order, pseudo second-order and intra-particle diffusion kinetic models were used to describe the kinetic sorption. The results clearly showed that the adsorption of MX-3R onto biosorbent followed the pseudo second-order model. The enthalpy (ΔH°), entropy (ΔS°) and Gibbs free energy (ΔG°) changes of adsorption were calculated. The results indicated that the adsorption of MX-3R occurs spontaneously as an exothermic process.
Keywords: Municipal solid waste, Yellow procion MX-3R, Kinetic biosorption, Thermodynamic study
1. Introduction
The management of municipal solid waste is one of the major concerns of the governments on the future of these wastes. Among the existing solutions to solve this problem, we find the centers of terrestrial landfill [1], incineration [2], recycling [3] and others. But these methods are insufficient due to their average yield or their high cost.
Currently the scientists and the researchers focus on adsorbents for low cost and do not harm the environment, like those of the biomass. Indeed, it would be very interesting if we can use these household wastes as a source of depollution of surface waters. In literature, many biosorbents were used to removal different types of pollutants, such as, heavy metals [4], dyes [5], pesticides [6], [7] and pharmaceuticals [8]. Among the biomass used to eliminate the dyes in aqueous medium, we cite fungal biomass [9], fly ash of plant [10], activated sludge biomass [11] and Nostoc linckia biomass [12]. Generally the biosorbents resulting from household wastes are formed essentially by a single compound. Among of these biosorbents are banana peel [13], [14], olive pits [15], orange peel [16] and tea waste [17]. So the particularity of our bio sorbent it includes all these various compounds and others simultaneously.
In this context, we applied in the present work a biosorbent from municipal solid waste (MSW) of the Mostaganem city (Algeria) to eliminate a textile dye from aqueous solution. These household wastes were used without the presence of the plastic, paper, metals and glass, i.e., that these wastes contain only the biodegradable matter. After treatment and preparation of the biomass, we have tried to remove from aqueous solution, the yellow procion MX-3R reactive dye, which is a rejection of the textile industry by adsorption. The influence of pH, contact time and temperature were studied. The Freundlich, Langmuir and Langmuir–Freundlich models were applied to describe the adsorption isotherms. The order of the adsorption reaction was evaluated by kinetic study and at the end the thermodynamic parameters were calculated to determine the heats of adsorption of the MX-3R by MSW biomass.
2. Methods and materials
2.1. Preparation of adsorbent
The adsorbent is original of the municipal solid wastes (MSW), specially the biodegradable matter. These wastes are recuperated from household of the Mostaganem city (Algeria). The preparation steps of the adsorbent were as follows: MSW were dried in air for three days, washed several times with distilled water, dried at 100 °C, overnight, crushed and sieved at 200 μm. The resulting material was used without any further treatment in the adsorption experience.
2.2. Characteristics and characterization of the biomass
The biomass characteristics measured are acidity, pH(PZC), the total nitrogen, the volatile matter, the ash rate and the conductivity. The acidity was measured as follow: 1 g of the sample was added to 10 mL of calcium chloride (CaCl2) solution at 0.01 M under agitation for 10 min and allowing the solution stand for two hours before taking the pH value. The total nitrogen was determined according to the Kjeldahl method [18].
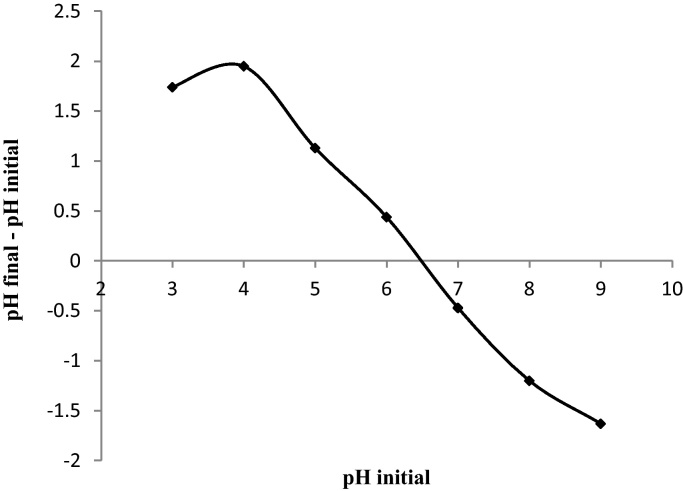
The zero charge point was measured as follow: a series of 20 mL of 0.01 M KNO3 solution was placed in a closed Erlenmeyer flask. The pH was adjusted in range between 2 and 10 by addition of 1 M HCl or 1 M NaOH solutions. Then 0.1 g of each biomass sample was added and agitated for 24 h under atmospheric conditions. When the final pH was determined, we plotted ΔpH (final pH–initial pH) versus the initial pH. The pH(PZC) is the point where the curve ΔpH versus pH initial crosses the line zero [19] (Fig. 1). The volatile matter and the amount of ash were calculated according to the calcinations method [20]. This method is based on heating of the biomass at 823 K for 2 h. The sample was weighed before and after calcination, the mass difference is the percentage of the volatile matter (VM). The ash content was calculated according to this relation: % Ash = 100-% VM. The conductivity was measured after addition of 1 g of the sample with 20 mL of distilled water under agitation for 10 min. The results of analyses are resumed in Table 1.
Fig. 1.
Determination of PZC point.
Table 1.
Characteristics of biomass.
| Sample | N (%) | Acidity | pH PZC | Conductivity (μS/cm) | V.M. (%) | Ash (%) |
|---|---|---|---|---|---|---|
| MSW | 0.602 | 4.70 | 6.5 | 2.07 | 91.24 | 8.76 |
N: nitrogen; V.M: volatile matter.
The scanning electron microscopy (SEM) images were taken on a JEOL JSM 6360 with acceleration voltage of 20 kV. The spectrum of FT-IR was performed with Spectrometer Agilent Cary 630 in range of 4000–500 cm−1.
2.3. Adsorbate
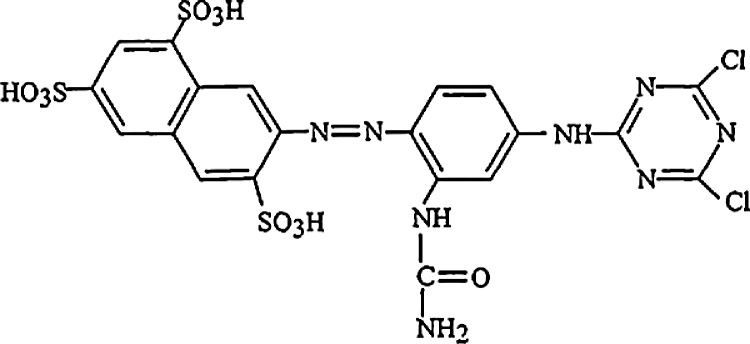
The dye yellow procion MX-3R was obtained from the SOITEX textile Society, Tlemcen, Algeria. The dye synonym is C.I. Reactive Orange 86 has a chemical formula of C20H16N8S3Cl2O10. Its molecular weight is 695 g/mol and has greater solubility in water. The chemical structure of the dye molecule is shown in Fig. 2. The MX-3R stock solution (1000 mg/L) was prepared by dissolving accurately weighed amount of the dye in distilled water.
Fig. 2.
Molecular structure of MX-3R.
2.4. Adsorption experiment
MX-3R solution was prepared in the range of initial concentrations 50–150 mg/L. For each experiment, 20 mL of dye solution was added to 50 mg of the biomass. The suspensions were shaken at room temperature (20 ± 2 °C) for 3 h. The pH was adjusted in range of 2–3 by addition of 1 M HCl solution. When adsorption procedure completed, the mixture was centrifuged at 4000 rpm to get supernatant liquid. Residual concentrations of MX-3R were detected using VIS spectrophotometer (VIS 7220 G, Biotech., Engineering Management) at the λmax = 410 nm. The dye concentration retained by the adsorbent phase was calculated using the following equation:
| (1) |
where qe is the equilibrium adsorption capacity (mg/g), C0 and Ce the initial and the equilibrium dye concentration (mg/L), respectively, V is the volume (L) of solution containing adsorbate and m is the mass (g) of the adsorbent.
3. Results and discussion
3.1. Results of biomass characteristics
The results have shown that the biomass contains a significant amount of carbon compared to that of Ash because the amount of volatile matter is great in MSW sample. This means that the biomass contains very low amounts of mineral matter. We note also a weak percentage of nitrogen in our household. These results are in accordance with the results published by Liu et al. [21] and San Miguel et al. [22].
3.2. Characterization results
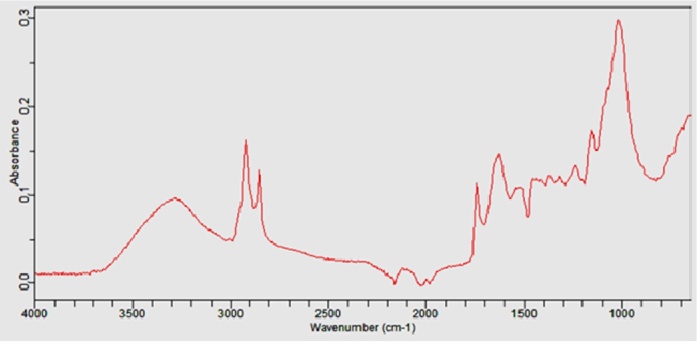
The FT-IR analysis shows the functional groups existing in the biomass (Fig. 3). The peak that appeared at 3290 cm−1 is assigned to O—H stretching vibration of hydroxyl function. The band at 2850 cm−1 is attributed to C—H stretching, the peak at 2100 cm−1 indicates the presence of C O stretching, at 1610 cm−1 indicates stretching of C C or aromatic-oxygen bond and the peak around 1500 cm−1 is attributed to the presence of highly conjugated C—O in quinone/carbonyl structure [23], [24], [25]. The peak at 1300 cm−1 may be due to the OH bending vibration indicating the presence of phenolic group. The bands at 1150 and 1010 cm−1 are may be attributed to the plane bending of aromatic C—H bond and to C—O structures, respectively [26].
Fig. 3.
FTIR spectra of biomass.
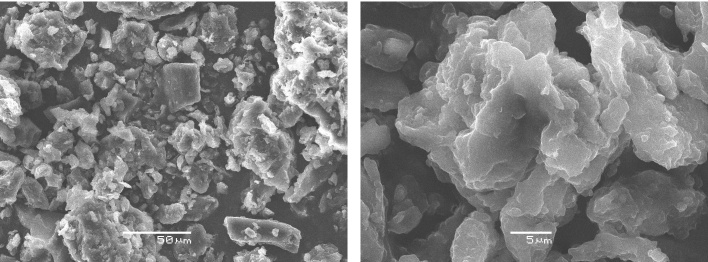
The SEM images observed in Fig. 4 show, that the particles of the biomass have the irregular forms and different sizes. We see also that the surface morphology is not homogenous with existence of some pores. These micrographs are in agreement with those of Rajamma et al. [27] and Gautam et al. [28], who worked on alkali activation of biomass fly ash and activated ficus fruit, respectively.
Fig. 4.
SEM micrographs of biomass.
3.3. Adsorption of MX-3R by biosorbent
3.3.1. Effect of pH
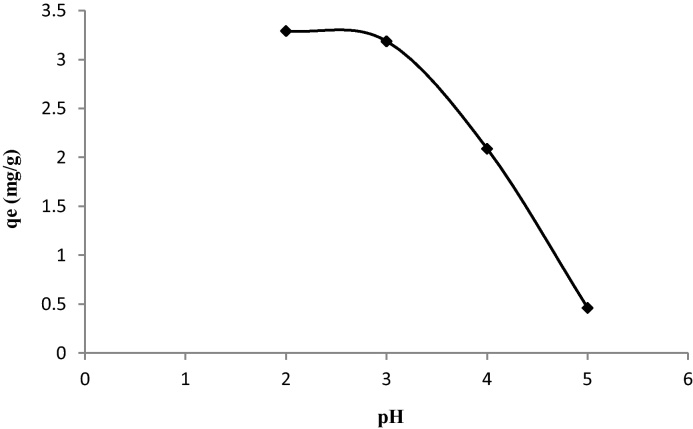
In this study we follow the evolution of pH with the adsorption of dye by the prepared biomass samples, for initial concentration about 50 mg/L. The Fig. 5 shows that the adsorption of MX-3R onto biomass is most important in acid medium. There is a significant decrease in the amount adsorbed of dye in range pH of 3–5. The maximum adsorbed amount Qadsmax is between pH 2 and 3. According to the theory, the surface of the adsorbent will be negatively charged above pH(PZC) and positively charged below pH(PZC). The zero point charge of the MSW biomass was found to occur at the pH of 6.5. According to the chemical structure (Fig. 2) of MX-3R, the dye is anionic, thus it tends to be adsorbed at the surface solid which is positively charged in the acid medium. So in the range of the pH 2–3 they are more attractive forces between the dye and our sample. For the rest of adsorption experiments we work at the pH between 2 and 3.
Fig. 5.
Effect of pH.
3.3.2. Effect of contact time
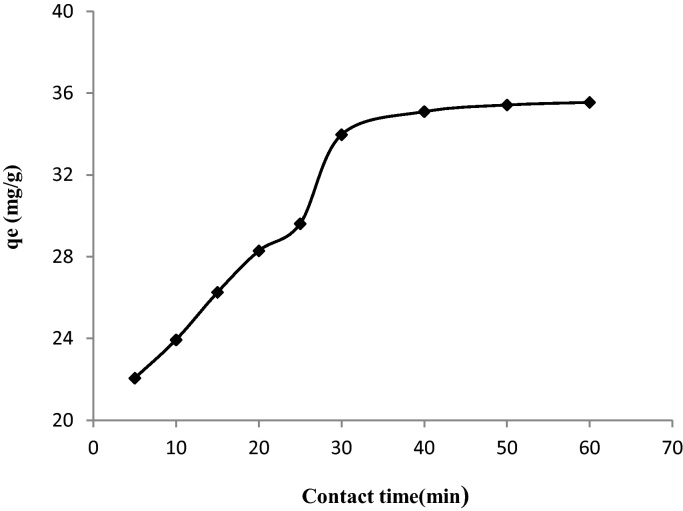
To determine the optimum contact time and to study the adsorption kinetic of MX-3R on biosorbent, we prepared a series of samples in the time range 5–60 min. The concentration of dye was fixed at 100 mg/L in 20 mL volume of solution, into which 0.05 g of material was added. Fig. 6 shows that the dye adsorption increased with the evolution of the contact time, and over 85% of removal MX-3R was achieved by the biomass in 30 min. The removal rate of MX-3R was very rapid during early stage of the adsorption process. The MX-3R uptake rate became slower over time and reached equilibrium value at about 40 min.
Fig. 6.
Effect of contact time.
3.3.3. Adsorption isotherms
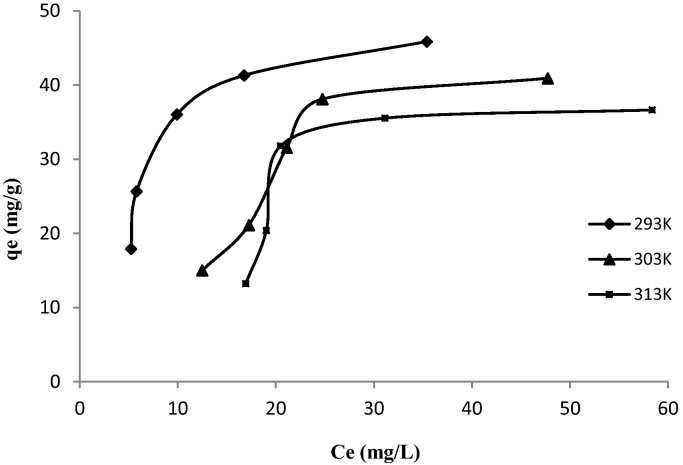
The adsorption isotherms were obtained at different initial concentrations during 1 h at three different temperatures. The isotherms are formed by plot adsorbed amounts of the dye versus equilibrium concentrations. Fig. 7 shows the adsorption of MX-3R by the MSW adsorbent at different temperatures.
Fig. 7.
Isotherm adsorption.
This figure indicates that the adsorbed amount of MX-3R onto MSW material at room temperature increases in parallel with the equilibrium concentrations. Using the classification of Giles et al. [29], the experimental isotherm obtained is of type L (Langmuir isotherm). This type of isotherm suggests a gradual saturation of the solid. Langmuir isotherms are used to explain the chemical interactions and/or physical (or both) between the adsorbate and the adsorbent. The maximum of reactive dye adsorbed amount is registered to 45.84 mg/g.
The adsorption of MX-3R by biomass at temperature 303 and 313 K was examined. The adsorption capacities slightly decrease with increased temperature. The amounts adsorbed of dye at 303 and 313 K are 40.9 and 36.64 mg/g, respectively. For comparison, the adsorption capacity of the dyes by the minerals adsorbents increases according to the temperature, but it decreases in the case of the biomass [30].
3.3.4. Models fitting
In this study, the adsorption equilibrium data were fitted by three isotherm equations including, Langmuir and Freundlich as two-parameter isotherms and Langmuir–Freundlich as three-parameter isotherm. The correlation coefficient R2, is used as criterion for fitting isotherm equations.
3.3.4.1. Langmuir and Freundlich
The Langmuir sorption isotherm has been widely used to characterize the adsorption phenomena from solution. The form of Langmuir isotherm can be represented by the following equation [31], [32]:
| (2) |
Eq. (2) can be represented by linear form:
| (3) |
where Q0 is the maximum adsorption capacity (mg/g), and KL (L/mg) is a constant relating to the heat of adsorption.
Freundlich isotherm can be represents properly the sorption data at low and intermediate concentrations on heterogeneous surfaces [33]. The model is expressed as the form bellow:
| (4) |
Eq. (4) can be expressed in the linear form:
| (5) |
where KF and n are the Freundlich constants, indicating the capacity and intensity of adsorption, respectively.
We applied the Langmuir and Freundlich models to fitting adsorption equilibrium data at different temperatures, and we found that the results which were not mathematically consistent with samples at 303 and 313 K, were considered to be insignificant in terms of adsorption. Therefore, they have not been presented here. However, the Langmuir model represented the adsorption data at room temperature better than the Feundlich model, and the obtained parameters of linearization are given in Table 2. The values of correlation coefficient R2, are close to unit, proving good fitting of the experimental results by this model. According to the Langmuir theory, the multilayer formation on the adsorbent surface cannot be possible and the sites are homogeneous with same fixation energies. The maximal adsorbed amount of the dye was found by Langmuir equation equal to 54.47 mg/g, it is higher than the value of adsorbed equilibrium amount. Russo et al. [9] were had the same conclusions about the adsorption of acid blue 62 and acid red 266 by the fungal biomass.
Table 2.
Isotherms constants.
| Model | Constants |
|---|---|
| Langmuir | |
| Q0 (mg/g) | 54.47 ± 0.0020 |
| KL (L/mg) | 0.124 ± 0.0351 |
| R2 | 0.967 ± 0.0022 |
| Freundlich | |
| n | 2.30 ± 0.127 |
| KF (mg/g(L/mg) 1/n) | 11.06 ± 1.376 |
| R2 | 0.795 ± 0.041 |
| Langmuir–Freundlich | |
| Qm (mg/g) | 45.82 ± 3.0286 |
| KLF (L/mg) 1/n | 0.180 ± 0.0148 |
| n | 2.429 ± 0.7712 |
| R2 | 0.988 ± 0.0272 |
3.3.4.2. Langmuir–Freundlich
The Langmuir–Freundlich model was used for the mathematical description of the adsorption equilibrium data of MX-3R onto MSW sorbent. This isotherm equation includes three adjustable empirical parameters and it is necessary to apply nonlinear least-squares (NLLS) method to fit the experimental data. The Langmuir–Freundlich isotherm is given in the following equation [34]:
| (6) |
where KLF ((L/mg)1/n) is the equilibrium constant, Qm is the maximal adsorbed amount (mg/g) and n is heterogeneity factor.
It is obvious that the regression coefficient of fitting by the Langmuir–Freundlich equation, listed in Table 2, are higher than the individual Langmuir equation. The adsorption capacity of the biomass adsorbent at saturation from Langmuir–Freundlich isotherm was 45.82 mg/g, it is almost equal than the adsorbed equilibrium amount. As seen the Langmuir–Freundlich equation provides a very satisfactory description of dye on the biomass.
3.3.5. Adsorption kinetic
To evaluate the adsorption rate, the adsorption kinetic was examined by the models pseudo first-order, pseudo second-order and intra-particle diffusion. The results obtained are listed in Table 3. The linear form of pseudo-first order rate equation is given by [35], [36]:
| (7) |
where qt is the amount adsorbed (mg/g) at time t, k1 is the rate constant (min−1). The value of k1 was calculated by plots ln (qe–qt) versus t from different concentrations.
Table 3.
Constants rate of the adsorption of MX-3R by biomass.
| Model | Constants |
|---|---|
| Pseudo-first order | |
| qe (mg/g) | 41.85 ± 1.387 |
| K1 (min−1) | 0.108 ± 0.012 |
| R2 | 0.936 ± 0.218 |
| Pseudo-second order | |
| qe (mg/g) | 39.52 ± 0.0077 |
| K2 (g/mg min) | 0.004 ± 0.0001 |
| R2 | 0.993 ± 0.0016 |
| Intra-particle | |
| Kint (mg/g min0.5) | 2.729 ± 0.204 |
| R2 | 0.968 ± 0.089 |
The pseudo second-order kinetic model equation is expressed as follows [7], [37]:
| (8) |
where k2 is the rate constant of the pseudo second-order model for the adsorption process (g/mg min). The rate parameters were calculated by plots of t/qt versus t for the adsorption of MX-3R onto biomass.
We see that the correlation coefficient for pseudo second-order is almost equal to unit (0.993). So, for the both models, the constant rate of the adsorption of MX-3R on biosorbent is better presented by pseudo second-order.
3.3.5.1. Intra-particle diffusion model
Several steps are involved in the sorption of adsorbate by a sorbent. These involve transport of the solute molecules from the aqueous phase to the surface of the solid articulates and diffusion of the solute molecules into the interior of the pores, which is usually a slow process. The intra-particle diffusion rate constant (kint) is given by the following equation [38]:
| (9) |
where Kint is intra-particle rate constant (mg/g min1/2) and C is the boundary layer thickness.
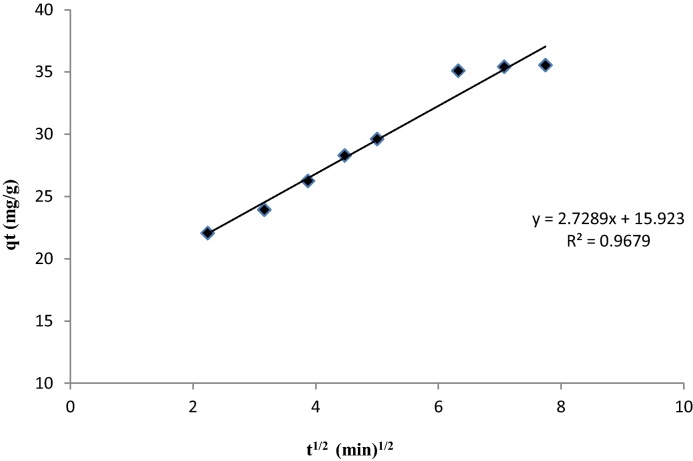
If the rate limiting step is intra-particle diffusion, a plot of solute sorbed against square root of contact time should yield a straight line passing through the origin [38]. Numerous researchers have found that this equation described the kinetic of sorption of dye in biosorbents [39]. In Fig. 8, it is clear that the curve is a straight line with high regression coefficient equal to 0.968, but the line does not passing through the origin because the value of the constant C which is the intercept point is different to zero and equal to 15.92. That means the pore diffusion was not the only controlling step. The correlation coefficient for the pseudo second-order kinetic model are higher than those for the intra-particle diffusion model (Table 3), suggesting a chemical reaction mechanism [40], [41]. Similar results have been reported in the literature [42], [43].
Fig. 8.
Isotherms adsorption at 303 and 313 K.
3.3.6. Thermodynamic study
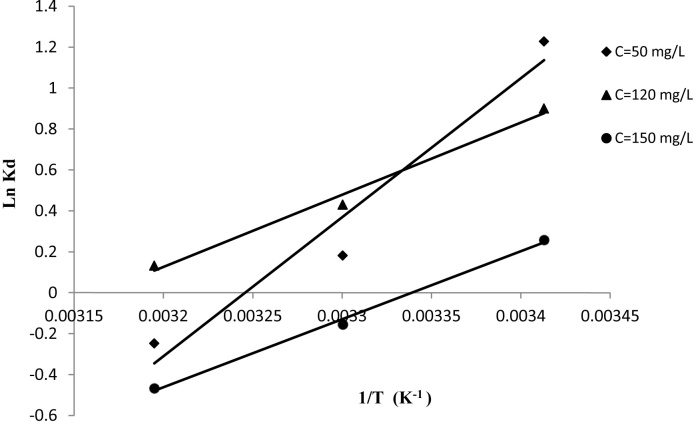
The objective of thermodynamic study is to calculate the heats adsorption of reactive dye MX-3R onto MSW biomass. For this we carried out the reaction of adsorption at 293, 303 and 313 K, by using the following equation [44], [45]:
| (10) |
| (11) |
where ΔH°, ΔS°, and T are the adsorption enthalpy, entropy and temperature in Kelvin, respectively, and R is the gas constant. The slope and intercept of the plot of ln Kd versus 1/T correspond to ΔH°/R and ΔS°/R, respectively.
The Gibbs free energy, ΔG°, of specific adsorption is represented by the following equation:
| (12) |
The thermodynamic parameters (ΔH°, ΔS°) were shown in Fig. 9, and the values of the heats adsorption (ΔH°, ΔS° and ΔG°) in the range of initial concentrations dye 50, 120 and 150 mg/L, were reported in Table 4. The values of the three thermodynamic parameters are negative, proving that we are in the case of physical adsorption reaction, exothermic and spontaneous. The spontaneity decreases with increasing of temperature, where the adsorption of MX-3R on biomass is favourable at room temperature. This fact was confirmed by the lower removal dye concentration from the aqueous phase at 303 and 313 K.
Fig. 9.
Curve of intra-particle model.
Table 4.
Thermodynamic parameters.
| C (mg/L) | ΔH (KJ/mol) | ΔS (KJ/mol K) | ΔG (KJ/mol) |
|||
|---|---|---|---|---|---|---|
| 293 | 303 | 313 | R2 | |||
| 50 | −56.493 | −0.183 | −2.769 | −0.935 | 0.897 | 0.953 ± 0.0541 |
| 120 | −29.342 | −0.928 | −2.135 | −1.207 | −0.278 | 0.988 ± 0.0036 |
| 150 | −27.640 | −0.922 | −0.599 | 0.323 | 1.246 | 0.996 ± 0.0009 |
Also notes that the disorder of the dye molecules decreases, approaching to the solid surface. These findings have been reported by the works of Karagoz et al. [46], which removes the methylene blue by activated carbon from waste biomass.
4. Conclusion
The biosorbent used in this study is obtained from municipal solid waste. After preparation, the biomass was applied successfully for removed the yellow procion MX-3R in aqueous solution by adsorption. The initial concentrations of the dye used are in the range of 50–150 mg/L with 50 mg of the biomass. The adsorption capacity of the dye on the biomass equal to 45.85 mg/g, it was obtained at 20 °C and pH between 2 and 3. The adsorption on biomass is favourable at room temperature. The adsorption isotherm was fitted by Langmuir equation and more better by Langmuir–Freundlich isotherm, where the theoretical adsorbed amount of MX-3R is the same as that adsorbed experimentally.
The pseudo second-order kinetic model can be successfully opted to describe the biosorption reaction of MX-3R. The intra-particle diffusion model was applied and the correlation coefficient was higher than 0.96, but the pore diffusion was not the only controlling step of the dye retention reaction.
The values of the enthalpy, entropy and Gibbs free energy changes indicate that the adsorption of MX-3R on MSW biomass is an exothermic and spontaneous process.
Footnotes
Available online 14 May 2015
References
- 1.Pastor J., Hernández A.J. Heavy metals,salts and organic residues in old solid urban waste landfills and surface waters in their discharge areas: determinants for restoring their impact. J. Environ. Manage. 2012;95:42–49. doi: 10.1016/j.jenvman.2011.06.048. [DOI] [PubMed] [Google Scholar]
- 2.Beylot A., Villeneuve J. Environmental impacts of residual Municipal Solid Waste incineration: a comparison of 110 French incinerators using a life cycle approach. Waste Manage. 2013;33:2781–2788. doi: 10.1016/j.wasman.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Ferreira da Cruz N., Simões P., Marques R.C. Costs and benefits of packaging waste recycling systems. Resour. Conserv. Recy. 2014;85:1–4. [Google Scholar]
- 4.Areco M.M., Saleh-Medina L., Trinelli M.A., Marco-Brown J.L., Afonso M.S. Adsorption of Cu(II), Zn(II): Cd(II) and Pb(II) by dead Avena fatuabiomass and the effect of these metals on their growth. Colloids Surf. B. 2013;10:305–312. doi: 10.1016/j.colsurfb.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 5.Nethaji S., Sivasamya A., Thennarasua G., Saravanan S. Adsorption of Malachite Green dye onto activated carbon derived from Borassus aethiopum flower biomass. J. Hazard. Mater. 2010;181:271–280. doi: 10.1016/j.jhazmat.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Khoshnood M., Azizian S. Adsorption of 2,4-dichlorophenoxyacetic acid pesticide by graphitic carbon nanostructures prepared from biomasses. J Ind. Eng. Chem. 2012;18:1796–1800. [Google Scholar]
- 7.Azizian S. Kinetic models of sorption: a theoretical analysis. J.Colloid Interface Sci. 2004;276:47–52. doi: 10.1016/j.jcis.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 8.Moussavi G., Alahabadi A., Yaghmaeian K., Eskandari M. Preparation, characterization and adsorption potential of the NH4Cl-induced activated carbon for the removal of amoxicillin antibiotic from water. Chem. Eng. J. 2013;217:119–128. [Google Scholar]
- 9.Russo M.E., Di Natale F., Prigione V., Tigini V., Marzocchella A., Varese G.C. Adsorption of acid dyes on fungal biomass: equilibrium and kinetics characterization. Chem. Eng. J. 2010;162:537–545. [Google Scholar]
- 10.Pengthamkeerati P., Satapanajaru T., Chatsatapattayakul N., Chairattanamanokorn P., Sananwai N. Alkaline treatment of biomass fly ash for reactive dye removal from aqueous solution. Desalination. 2010;261:34–40. [Google Scholar]
- 11.Chu H.C., Chen K.M. Reuse of activated sludge biomass: I. Removal of basic dyes from waste water by biomass. Process Biochem. 2002;37:595–600. [Google Scholar]
- 12.Mona S., Kaushik A., Kaushik C.P. Biosorption of reactive dye by waste biomass of Nostoc linckia. Ecol. Eng. 2011;37:1589–1594. [Google Scholar]
- 13.Namasivayam C., Kanchana N., Yamuna R.T. Waste banana pith as adsorbent for the removal of rhodamine-B from aqueous solution. Waste Manage. 1993;13:89–95. [Google Scholar]
- 14.Annadurai G., Juang R., Lee D. Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J. Hazard. Mater. 2002;92:263–274. doi: 10.1016/s0304-3894(02)00017-1. [DOI] [PubMed] [Google Scholar]
- 15.Baccar R., Blánquez P., Bouzid J., Feki M., Sarrà M. Equilibrium, thermodynamic and kinetic studies on adsorption of commercial dye by activated carbon derived from olive-waste cakes. Chem. Eng. J. 2010;165:457–464. [Google Scholar]
- 16.Khaled A., El Nemr A., El-Sikaily A., Abdelwahab O. Treatment of artificial textile dye effluent containing Direct Yellow 12 by orange peel carbon. Desalination. 2009;238:210–232. [Google Scholar]
- 17.Tamez Uddin M., Akhtarul Islam M., Mahmud S., Rukanuzzaman M. Adsorptive removal of methylene blue by tea waste. J. Hazard. Mater. 2009;164:53–60. doi: 10.1016/j.jhazmat.2008.07.131. [DOI] [PubMed] [Google Scholar]
- 18.Aubert G. CRDP Publisher; Marseille, France: 1978. Soils Analysis Methods. [Google Scholar]
- 19.Preethi S., Sivasamy A. Removal of safranin basic dye from aqueous solutions by adsorption onto corncob activated carbon. Ind. Eng. Chem. Res. 2006;45:7627–7632. [Google Scholar]
- 20.Zoes V., Dinel H., Pare T., Jaouich A. Growth substrates made from duck excreta enriched wood shavings and source separated municipal solid waste compost and separates: physical and chemical characteristics. Bioresour. Technol. 2001;78:21–30. doi: 10.1016/s0960-8524(00)00169-3. [DOI] [PubMed] [Google Scholar]
- 21.Liu W.J., Zeng F.X., Jiang H., Zhang X.S. Preparation of high adsorption capacity bio-chars from waste biomass. Bioresour. Technol. 2011;102:8247–8252. doi: 10.1016/j.biortech.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 22.San Miguel G., Dominguez M.P., Hernandez M., Sanz-Pérez F. Characterization and potential applications of solid particles produced at a biomass gasification plant. Biomass Bioenergy. 2012;47:134–144. [Google Scholar]
- 23.Bouchelta C., Medjram M.S., Bertrand O., Bellat J.-P. Preparation and characterization of activated carbon from date stones by physical activation with steam. J. Anal. Appl. Pyrolysis. 2008;82:70–77. [Google Scholar]
- 24.Ourania A., Ioannidou A.A., Zabaniotou G.G., Stavropoulos M.D., Azharul I., Triantafyllos A.A. Peparation of activated carbons from agricultural residues forpesticide adsorption. Chemosphere. 2010;80:1328–1336. doi: 10.1016/j.chemosphere.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 25.Namasivayam C., Kavitha D. IR, XRD and SEM studies on the mechanism of adsorption of dyes and phenols by coir pith carbon from aqueous phase. Microchem. J. 2006;82:43–48. [Google Scholar]
- 26.Duggan O., Allen S.J. Study of the physical and chemical characteristics of a range of chemically treated, lignite based carbons. Water Sci. Technol. 1997;35:21–27. [Google Scholar]
- 27.Rajamma R., Labrincha J.A., Ferreira V.M. Alkali activation of biomass fly ash–metakaolin blends. Fuel. 2012;98:265–271. [Google Scholar]
- 28.Gautam R.K., Mudhoo A., Lofrano G., Chattopadhyaya M.C. Biomass-derived biosorbents for metal ions sequestration: adsorbent modification and activation methods and adsorbent regeneration. J. Environ. Chem. Eng. 2014;2:239–259. [Google Scholar]
- 29.Giles C.H., Mac Ewan T.H., Nakhwa S.N., Smith D. Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. 1960:3973–3993. [Google Scholar]
- 30.Xu Y., Chen B. Investigation of thermodynamic parameters in the pyrolysis conversion of biomass and manure to biochars using thermogravimetric analysis. Bioresour. Technol. 2013;146:485–493. doi: 10.1016/j.biortech.2013.07.086. [DOI] [PubMed] [Google Scholar]
- 31.Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918;40:1361–1403. [Google Scholar]
- 32.Sawalha M.F., Peralta-Videa J.R., Romero-Gonzalez J., Gardea-Torresdey J.L. Biosorption of Cd(II), Cr(III), and Cr(VI) by saltbush (Atriplex canescens) biomass: thermodynamic and isotherm studies. J. Colloid Interface Sci. 2006;300:100–104. doi: 10.1016/j.jcis.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 33.Freundlich H.M.F. Über die adsorption in lösungen. Z. Phys. Chem. 1906;57:385–470. [Google Scholar]
- 34.Hamdaoui O., Naffrechoux E. Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon. Part II. Models with more than two parameters. J. Hazard. Mater. 2007;147:401–411. doi: 10.1016/j.jhazmat.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 35.Lagergren S., Svenska B.K. Zur theorie der sogenannten adsorption geloester stoffe. Veternskapsakad Handlingar. 1898;4:1–39. [Google Scholar]
- 36.Ho Y.S. Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics. 2004;59:171–177. [Google Scholar]
- 37.Ho Y.S., McKay G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998;70:115–124. [Google Scholar]
- 38.Weber W.J., Morris J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. ASCE. 1963;89:31–60. [Google Scholar]
- 39.Ho Y.S., Ng J.C.Y., McKay G. Kinetics of pollutant sorption by biosorbents: review. Sep. Purif. Methods. 2000;29:189–232. [Google Scholar]
- 40.Ho Y., McKay G. Sorption of dyes and copper ions onto biosorbents. Process Biochem. 2003;38:1047–1061. [Google Scholar]
- 41.Miretzky P., Muñoz C., Carrillo-Chavez A. Cd(II) removal from aqueous solution by Eleocharis acicularis biomass, equilibrium and kinetic studies. Bioresour. Technol. 2010;101:2637–2642. doi: 10.1016/j.biortech.2009.10.067. [DOI] [PubMed] [Google Scholar]
- 42.Ur Rehman M.S., Kim I., Han J.I. Adsorption of methylene blue dye from aqueous solution by sugar extracted spent rice biomass. Carbohydr. Polym. 2012;90:1314–1322. doi: 10.1016/j.carbpol.2012.06.078. [DOI] [PubMed] [Google Scholar]
- 43.Safa Y., Bhatti H.N. Kinetic and thermodynamic modeling for the removal of Direct Red-31 and Direct Orange-26 dyes from aqueous solutions by rice husk. Desalination. 2011;272:313–322. [Google Scholar]
- 44.Fan Q.H., Shao D.D., Hu J., Wu W.S., Wang X.K. Comparison of Ni2+ sorption to bare and ACT-graftattapulgites: effect of pH, temperature and foreign ions. Sur. Sci. 2008;602:778–785. [Google Scholar]
- 45.Wang X.K., Chen C.L., Hu W.P., Ding A.P., Xu D., Zhou X. Sorption of 243Am(III) to multiwall carbon nanotubes. Environ. Sci. Technol. 2005;39:2856–2860. doi: 10.1021/es048287d. [DOI] [PubMed] [Google Scholar]
- 46.Karagoz S., Tay T., Ucar S., Erdem M. Activated carbons from waste biomass by sulfuric acid activation and their use on methylene blue adsorption. Bioresour. Technol. 2008;99:6214–6222. doi: 10.1016/j.biortech.2007.12.019. [DOI] [PubMed] [Google Scholar]