Abstract
Nutrients excess is one of the leading causes of metabolic syndrome globally. Protein post-translational O-GlcNAc modification has been recognized as an essential nutrient sensor of the cell. Emerging studies suggest that O-GlcNAcylation lies at the core linking nutritional stress to insulin resistance. Mitochondria are the major site for ATP production in most eukaryotes. Mitochondrial dysfunction and oxidative stress have long been considered as an important mechanism underlying insulin resistance. The metabolic process is under the influence of environmental and nutritional factors, thus sensing and transducing nutritional signals sit at the pivot of metabolism control. For a long time little was known about O-GlcNAcylation within mitochondria since mitochondrial O-GlcNAcylation was regarded rare. Recent findings have demonstrated that O-GlcNAcylation is widely spread among mitochondrial proteins, and that mitochondrial function and oxidative stress both can be regulated by O-GlcNAcylation, particularly under diabetic circumstances.
Keywords: O-GlcNAcylation, mitochondria, oxidative stress, insulin resistance, nutrient sensing
Metabolic syndrome is a series of complex metabolic disorders centering on insulin resistance, including obesity, hyperglycemia, hypertension, dyslipidemia, fatty liver and hyperinsulinemia, which underlie heart or brain vascular disease and diabetic pathology. The prevalence of metabolic syndrome keeps increasing worldwide at present, while its etiology is still yet unclear. Generally it is attributed to the interaction of multiple genetic and environmental factors. Among them, modern life style with the feature of high fat and high carbohydrate diet is a key risk factor, which usually leads to excessive nutrients, increased incidence of obesity, especially central obesity, and the development of insulin resistance, and eventually the occurrence of metabolic syndrome. In the light of lacking effective remedy in clinics, there is an urgent need to further explore the molecular mechanism of insulin resistance in order to find innovative strategies and key targets for the prevention and treatment of metabolic syndrome. The sensing of the external nutrient conditions is essential for regulation of metabolism. Therefore, it's of important significance to explore the biological mechanism of insulin resistance and metabolic syndrome from the perspective of nutrient sensing.
1. O-GlcNAcylation is a cellular sensor of nutrient and stress factors
O-GlcNAcylation is a highly dynamic and reversible protein post-translational modification (PTM) occuring on serine/threonine residues of cytosolic and nuclear proteins by a monosaccharide, O-linked N-acetylglucosamine (Figure 1A). This modification is catalyzed by a pair of opposing enzymes, O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA), mediating the additon and removel of the sugar resudue to or from target proteins, respectively, also called O-GlcNAc cycling [1], sort of resembling phosphorylation. O-GlcNAcylation can regulate key cellular activities including transcription, translation and fundamental protein behaviors like stability, degradation or function under physiological or pathophysiological conditions.
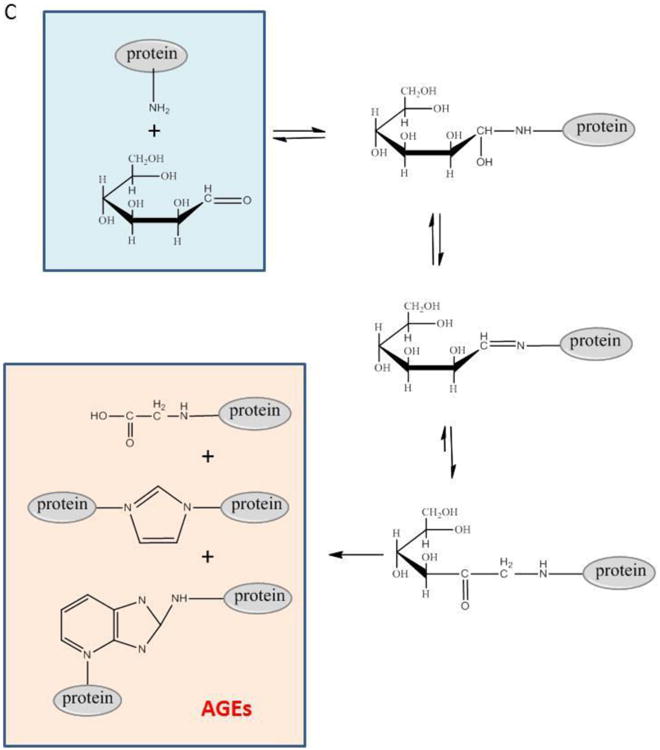
Figure 1. Chemical Structures of O-GlcNAcylation, glycation, and polysaccharide chain.
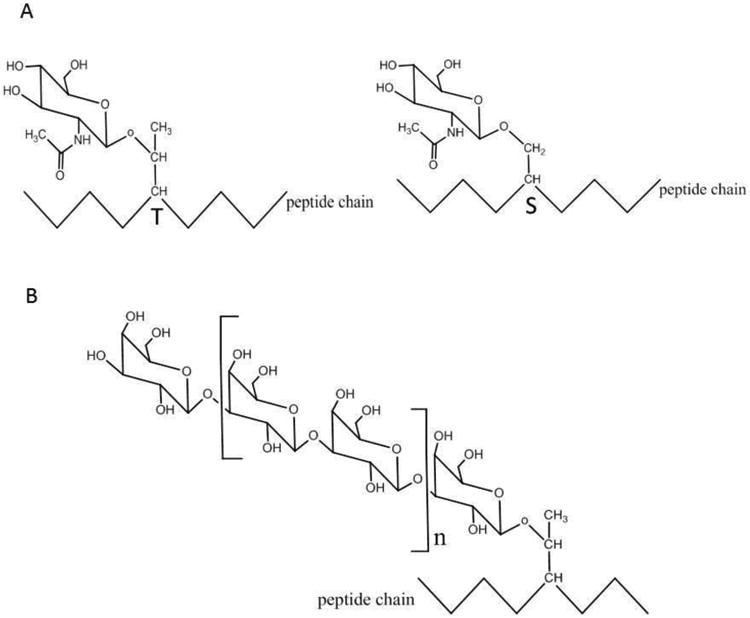
A: O-GlcNAcylation,on Threonine (T) or Serine (S) of a peptide chain
B: polysaccharide chain composed of glucose on a peptide chain
C: glycation process and the advanced glycation end products (AGEs)
O-GlcNAcylation is different from polysaccharide chain attached to surface proteins of plasma membrane (Figure 1B), or protein glycation which eventually forms accumulative advanced glycation end products (AGEs) (Figure 1C). The latter is associated with diabetes and aging, just like O-GlcNAcylation. However it has completely different chemical nature: glycation doesn't involve glycoside bond, in contrast it's formed by the reaction of amino group at the end of the protein and the aldehyde group in the sugar molecule. While the former dose form by glycoside bond, but it's much rather a structural existence in the membrane surface than be a dynamic and reversible functional regulator. The chemical structures of those three kinds of glycoproteins are shown in Figure 1.
O-GlcNAcylation was first reported in 1984 [2], since then it has been well acknowledged that numerous cytoplasmic and nuclear proteins are O-GlcNAcylated, though there seems to be no universal rules regarding which serine or threonine are O-GlcNAcylated. The biological roles and the regulating mechanisms of O-GlcNAcylation have not been elucidated until recently. Now emerging studies even begin to tackle how O-GlcNAcylation of specific sites on specific proteins modulates their function [3].
O-GlcNAc signal is intimately coupled to nutrient metabolism [4]. About 2 to 3 percent of the glucose entering cell fluxes through hexosamine biosynthesis pathway (HBP) and produces the donor substrate of O-GlcNAcylation, uridine diphosphate N-acetylglucosamine (UPD-GlcNAc). HBP integrates the metabolic information of nutrients including carbohydrate, amino acid, fatty acid and nucleic acid in the process of UPD-GlcNAc synthesis; consequently the availability of UPD-GlcNAc and the level of O-GlcNAcylation are both regulated by nutrient metabolism [5]. Nutrient factors hence can regulate complex cellular physiological processes like signal transduction, transcription, etc. via O-GlcNAcylation, which serves as the key linkage between nutrient sensing, energy matabolism and signal transduction [6]. It helps maintain the metabolic homeostasis under fluctuant environmental nutrient conditions by modulating key molecules or orchestrating intra-cellular signal cascades including insulin-AKT pathway, MAPK pathway, mTOR pathway, AMPK pathway, etc. [7]. Therefore, O-GlcNAcylation is widely recognized as an important cellular nutrient sensor integrating nutrient and metabolic signals; it regulates various biological processes through modifying comprehensive proteins localized at various cellular compartments.
So far, besides its role as a nutrient sensor, O-GlcNAcylation also responds to other environmental signals, especially stress stimuli. It's reported that overall O-GlcNAcylation is closely associated with stress tolerance including heat tolerance, the expression of which is regulated by overall O-GlcNAcylation [8]. O-GlcNAcylation of cytoplasmic and nucleic proteins is also regarded as a protective mechanism by which the cell detects and responds to various stress stimuli encompassing environmental stress, physiological stress, chemical stress, etc. to enhance cell survival and to avoid cell death [9]. To name a few examples, elevated O-GlcNAcylation in cardiovascular system is interpreted as a potential auto-protective alarm signal or a stress response; enhancing O-GlcNAcylation indeed can increase cell survival under acute stress conditions including hypoxia, ischemia, oxidative stress, etc. [10] Increased O-GlcNAcylation is evidenced to alleviate calcium overload induced by hypoxia or oxidative stress and the formation of mitochondrial permeability transition pore induced by hydrogen poroxide [11]. In our previous study, we found artificially boosting overall O-GlcNAcylation could enhance mitochondrial oxygen consumption and reduce reactive oxygen species (ROS) level in ARPE-19 cells (a human retinal epithelial cell line), and the increased O-GlcNAcylation in aged SD rat retina might be a strategy to combat the increased oxidative stress during ageing [12]. How dose O-GlcNAcylation mediate the response of the cell to various stresses has become a question under the spotlight in the field. Hart group [13] recently reported that O-GlcNAcylation might enhance cell survival against a series of stresses by regulating DNA damage signals and repair system.
In brief, O-GlcNAcylation has been widely appreciated as a cellular nutrient and stress sensor. However, the key molecules or even the specific sites in particular involved in the nutrient or stress sensing process are yet to be elucidated by further studies.
2. O-GlcNAcylation is involved in metabolism regulation and the pathogenesis of metabolic disorders including insulin resistance
How does the dynamic O-GlcNAcylation respond to nutrient condition and play the pivotal role of regulating fundamental metabolism, and how dose aberrant O-GlcNAcylation contribute to the pathogenesis of metabolic disorders and related diseases? Those questions need to be addressed urgently. Recent research demonstrates that O-GlcNAcylation altered in development [14], epigenetics [5], ageing [15], etc., and abnormal O-GlcNAcylation is involved in the pathogenesis of many age-related diseases including type 2 diabetes [16-18], cancer [19, 20], neurodegeneration [21], etc.. It's even been pointed that aberrant O-GlcNAcylation is actually the underlying mechanism linking type 2 diabetes as the shared potential risk factor of several major diseases that modern industrialized world faces including Alzheimer's disease, cardiovascular diseases and cancer [22, 23]. Epidemiological studies have shown that nutrient excess is intimately related to obesity, diabetes, cardiovascular risks and cancer [24, 25]. O-GlcNAcylation is believed to be the bridge from metabolism to chronic diseases [26]. A study shows long term western diet could increase the O-GlcNAcylation level in rat heart [27]. According to those evidences, it's inferrable that the effect of nutrition overload might be transferred to protein function, cellular activities and cellular physiological process through the way of modulating the O-GlcNAcylation level and sites of a series of target proteins.
O-GlcNAcylation is tightly coupled to insulin resistance. Hypergycemia or hyperlipidemia induced insulin resistance is closely related to increased flux of HBP and increased UDP-GlcNAc level; the overall O-GlcNAcylation level increases in multiple tissues of animals and human under diabetic conditions; artificially up-regulating overall O-GlcNAcylation level can lead to insulin resistance in multiple tissues of animals [28]. Research has revealed some approaches by which O-GlcNAcylation is involved in insulin resistance, from modulating the function of proteins in insulin signaling pathway to modulating glucose uptake, gluconeogenesis, glycogen synthesis, fatty acid synthesis, cell function, etc. [28]. Therefore, O-GlcNAcylation lies at the core linking nutrition overload and insulin resistance.
Mitochondrial dysfunction or impairment and the associated oxidative stress have been recognized as an important biological contributing mechanism to insulin resistance as well as diabetic complications [29, 30]. Besides being the energy engine of the cell, mitochondria are also playing vital roles in cellular signal transduction and other physiological activities. O-GlcNAcylation is known to regulate energy metabolism and the production of metabolic intermediates. However, whether the involvement of O-GlcNAcylation in insulin resistance has anything to do with the alteration of mitochondrial function and homeostasis as well as oxidative stress still warrants further study.
3. O-GlcNAcylation of mitochondrial proteins and the regulation of mitochondrial function by O-GlcNAcylation
OGT gene has three isoforms of products due to multiple transcription initiation sites and post transcriptional regulation of alternative splicing. One isoform contains a unique N terminus including a leading sequence targeting mitochondrial inner membrane and a transmembrane helix, called mOGT, the majority of the C terminus sequence of which are the same with the other two isoforms localized in both cytosol and nucleus [31]. Moreover, it's been reported in 2015 that the donor substrate of O-GlcNAcylation, UDP-GlcNAc, can be transported into mitochondrial matrix through mitochondrial inner membrane transporter, and OGA activity was detected in isolated mitochondria [32]. The existence of OGT and OGA specifically in mitochondria and the capability of mitochondria transporting UDP-GlcNAc synthesized in cytosol suggest that O-GlcNAc cycling can occur directly whthin mitochondria, as shown in Figure 2. Though the specific molecular or enzymatic mechanisms of O-GlcNAcylation in the mitochondria are by far still unclear, O-GlcNAcylation within mitochondria must have certain important roles to play. The fact that overexpression of mOGT results in high cytotoxicity and induces apoptosis or programmed cell death has impeded the progress of studying O-GlcNAcylation and its function in mitochondria [33], still the involvement of mOGT in the molecular system of mitochondria-related apoptosis is suggested to be highly possible. A recent proteomic analysis found association of large amount of mitochondrial proteins and OGT [34], further supporting the great importance of OGT to mitochondria.
Figure 2. O-GlcNAc cycling within mitochondria.
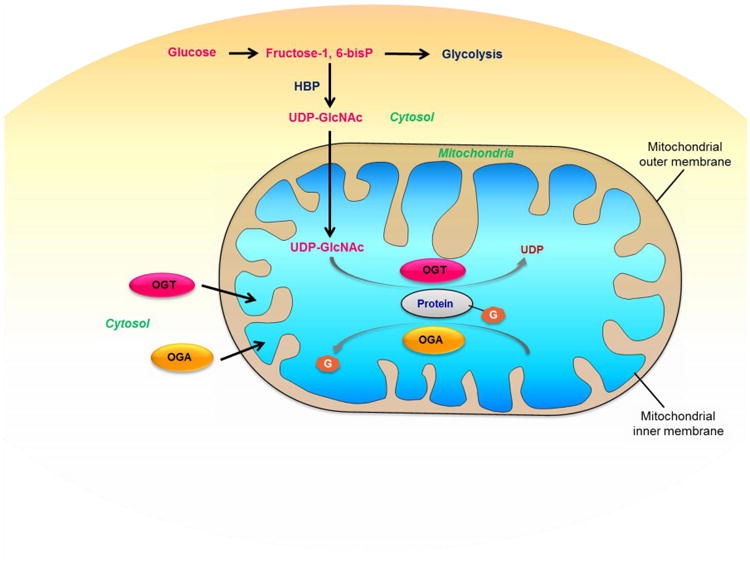
O-GlcNAc modification donor substrate, UDP-GlcNAc is synthetized via HBP in cytosol and then transported into mitochondria; O-GlcNAc cycling enzymes including OGT and OGA are also synthetized in cytosol and then transported into mitochondria. So O-GlcNAc cycling can occur directly within mitochondria.
In fact, the O-GlcNAcylation of mitochondrial proteins and its function have just started being unraveled lately. O-GlcNAcylation was thought to be rare within mitochondria [35, 36]; however, recent study has revealed more and more O-GlcNAcylated mitochondrial proteins. Now it's recognized that O-GlcNAcylation is extensively and dynamically existed in mitochondria, and this modification may protect or impair mitochondrial function.
O-GlcNAcylation might be able to control mitochondrial function by directly modulating mitochondrial proteome or through modulating proteins that can regulate mitochondria. It should be pointed out that the O-GlcNAcylation of mitochondrial proteome can occur in two manners: O-GlcNAcylation of nuclear DNA encoded proteins in the cytoplasm which targets mitochondria and would be transported into mitochondria later, and O-GlcNAcylation of mitochondrial DNA encoded proteins within mitochondria, as shown in Figure 3. Mitochondrial proteins especially those participating in oxidative phosphorylation system were found mostly to be the targets of O-GlcNAcylation in a comparative analysis of O-GlcNAcylated mitochondrial sub-proteome from Thiamet-G (TMG, a potent OGA inhibitor) treated and untreated rat heart, and TMG treatment could increase the overall O-GlcNAcylation level of mitochondrial proteome, mitochondrial oxygen consumption and ATP production, and lift the threshold of the opening of permeability transition pore induced by calcium [37]. Latest research involving quantitative proteomic screening work indicates that overexpression of OGT or OGA can both lead to down-regulation of proteins involved in mitochondrial respiratory chain and TCA cycle, and alterations of mitochondrial morphology and cell respiration, manifesting the importance of both OGT and OGA as regulatory molecules of mitochondrial metabolism and function [38, 39]. Alterations of the components of electron transport chain were found to be mediating the effects of O-GlcNAcylation on mitochondrial metabolism [40].
Figure 3. O-GlcNAcylation of mitochondrial proteome and its functions.
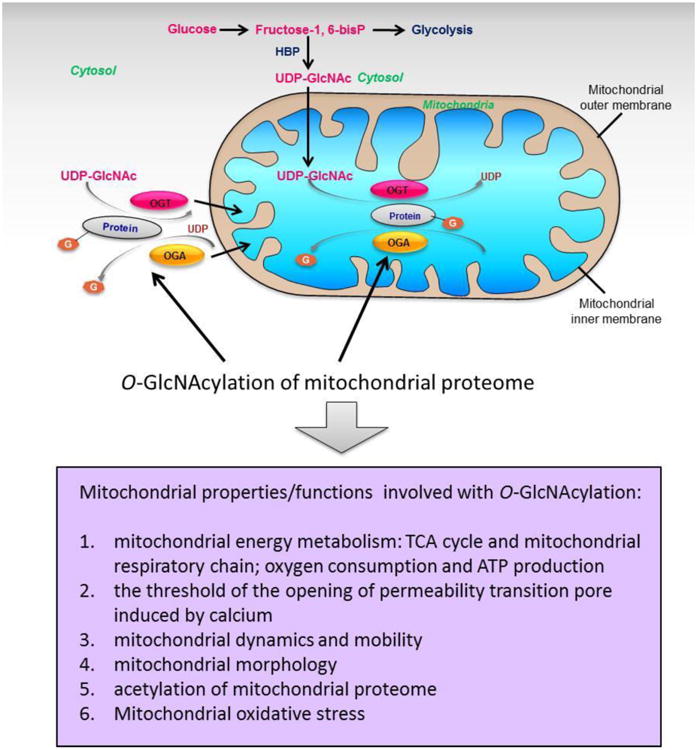
O-GlcNAcylation of mitochondrial proteins can either occur within mitochondria through mitochondrial O-GlcNAc cycling, or occur in cytosol prior to their translocation or transportation to mitochondria. O-GlcNAcylation of mitochondrial proteome might be able to regulate mitochondrial energy metabolism through modulating components of TCA cycle and mitochondrial respiratory chain, thus could steer oxygen consumption and ATP production. O-GlcNAcylation of mitochondrial proteome is also involved with the threshold of the opening of permeability transition pore induced by calcium, mitochondrial dynamics and mobility, mitochondrial morphology, acetylation of mitochondrial proteome as well as mitochondrial oxidative stress.
O-GlcNAcylation can also regulate the dynamics and mobility of mitochondria. O-GlcNAcylation of Milton mediates the changes in mitochondrial mobility of neurons in response to availability of the major nutrient, glucose [41]. Increased O-GlcNAcylation of DRP1 can decrease its phosphorylation and lead to the recruitment of DRP1 from cytosol to mitochondria, and then increase mitochondrial fragmentation as well as lower mitochondrial membrane potential [42]. In addition, The change of O-GlcNAcylation can affect the acetylation level of mitochondrial proteins and expression of SIRT3 [39]. Therefore, modulating other PTMs is also among the diversified repertoire of approaches that O-GlcNAcylation regulates mitochondrial function.
All in all, it's well accepted now that the O-GlcNAcylation of mitochondrial proteins is quite extensive, as listed in Table 1. It can regulate mitochondrial function and homeostasis encompassing the metabolism, respiration, dynamics, mobility and apoptosis of mitochondria, etc. through various approaches, as described in Figure 3. Furthermore, considering that merely 13 subunits of respiratory chain complexes are encoded by mitochondrial DNA to date, while the rest of the mitochondrial proteome are encoded by nuclear DNA, a coordinating system must be required to fine orchestrate the expression of both sets. In mammalian cells, OGT interacts with histone de-acetylation complex (HDAC), SIN3A in particular, which plays a key role in the mitochondria-nuclear coordinating mechanism, and the binding sites of OGT and SIN3A are different from where SIN3A binds to histone deacetylase as a corepressor [43]. Reduced SIN3A in drosophila could down-regulate both nuclear encoded and mitochondria encoded mitochondrial proteins [43]. Moreover, OGT deficiency is reported to be associated with changes in mitochondrial gene expression [43]. Whether the involvement of OGT in orchestrating nuclear encoded and mitochondrial encoded mitochondrial protein expressions is mediated by O-GlcNAcylation is yet to be studied.
Table 1. Potential O-GlcNAcylated mitochondrial proteins.
4. O-GlcNAcylation is intimately involved with mitochondrial dysfunction and oxidative stress under metabolic syndrome circumstance
Augmented or prolonged O-GlcNAcylation signal is suggested to be closely related to diabetic complications and the abnormality of mitochondrial function. Hyperglycemia under diabetic conditions is regarded to cause increased O-GlcNAcylation in mitochondria, which further leads to mitochondrial dysfunction, and eventually leading to diabetic cardiomyopathy. It has been reported that there's an increase in OGT and decrease in OGA, a disordered localization of OGT in mitochondria, and a reduction of the interaction between OGT and complex IV in myocardial mitochondria of diabetic rats [32]. O-GlcNAcylation of respiratory chain complex I, III and IV under high glucose conditions can inhibit the enzymatic activities of these complexes and reduce the generation of ATP and calcium reserve [44]. Artificially screened rat models with low or high running capacity provides two distinct genetic backgrounds, which is useful for assessing the influence of innate capacity of aerobic exercise on a range of risk factors for diseases. At present, it is believed that low aerobic capacity is a strong predictor of metabolic disease, since rats with low running capacity are more likely to be obese and to develop insulin resistance and cardiovascular dysfunction. Enhanced O-GlcNAcylation is suggested to be associated with degeneration of cardiovascular function in rats with low running capacity; specifically, mitochondrial complex I and complex IV, VDAC and SERCA of heart are all significantly more O-GlcNAcylated in rats with low running capacity than in rats with high running capacity, which might contribute to the development of mitochondrial dysfunction and insulin resistance phenotype in rats with high running capacity [45]. Gerald Hart's group [46] recently identified many O-GlcNAcylated proteins from heart mitochondrial samples using an analytical method involving chemical treatment of samples and reversed-phase liquid chromatography coupled with tandem mass spectrometry, and those proteins are involved in energy metabolism (oxidative phosphorylation, TCA cycle, beta-oxidation), amino acid metabolism, and many other biological processes. Also, they further found that heart mitochondrial respiratory chain proteins were differently O-GlcNAcylated under diabetic condition, and those changes in O-GlcNAcylation seemed to be closely related to changes in mitochondrial function, in particular respiration and ROS generation. However, there're also studies concluding that change of myocardial mitochondrial respiration induced by hyperglycemia is not related to O-GlcNAcylation [47].
Several studies have demonstrated that increased O-GlcNAcylation can protect cardiomyocytes against oxidative stress. High dose of oxidative stress can lead to gradual loss of overall O-GlcNAcylation over time as well as mitochondrial membrane potential in cardiomyocytes; enhancing O-GlcNAcylation by drug treatment can improve the survival rate of cardiomyocytes under oxidative stress while protecting mitochondrial membrane potential [48]. The protective effect of O-GlcNAcylation may be mediated by regulating mitochondrial autophagy. Besides, the O-GlcNAcylation of mitochondrial protein VDAC could interfere with the occurrence of oxidative stress and the formation of mitochondrial permeability transition pore induced by calcium overload, thereby protecting the integrity of the mitochondria [48]. Therefore, it has been shown that O-GlcNAcylation on one hand is intimately involved with mitochondrial function, and on the other hand may be playing vital roles in the regulation of or the response to oxidative stress.
Superoxide Dismutase 2, one of the most important antioxidative enzymes within mitochondria, is frequently involved in metabolic syndromes like insulin resistance, obesity and diabetic complications [50]. It has been recently shown that under oxidative stress not only the expression of SOD2 could be up-regulated, but also the enzymatic activity of SOD2 can be modulated through PTMs, like phosphorylation[51] and acetylation [52]. Most importantly, α-lipoic acid, a supplement that can improve diabetes, has been reported to be able to reduce both overall O-GlcNAcylation and the O-GlcNAcylation of SOD2 in diabetic rat liver [53], and can increase the protein content of SOD2 as well as the total enzymatic activity of SOD2 in diabetic rat kidney [54].
5. Summary and perspectives
In a nutshell, existing research suggests: first, O-GlcNAcylation is playing key roles in the development of insulin resistance and metabolic syndrome as an important mechanism in nutrient sensing and metabolic regulation; second, O-GlcNAcylation is intimately related to mitochondrial dysfunction and oxidative stress, which are key contributing factors of insulin resistance and metabolic syndrome. It might bring breakthroughs for the field to explore the O-GlcNAcylation of nuclear encoded and mitochondria encoded mitochondrial constituent proteins and its regulatory roles in mitochondrial function and oxidative stress under insulin resistance or metabolic syndrome context. Key molecules or even key O-GlcNAcylation sites and the detailed mechanisms involved in the process of sensing overloading nutrients and transducing the information to mitochondria-related metabolic regulation and modulation of oxidative stress are urgently to be identified and clarified, in view of the relatively scarce experimental discoveries currently reported.
The ultimate answer shall reveal novel clues to etiology of insulin resistance or metabolic syndrome and conceive the potential of practical applications. Manipulation of O-GlcNAcylation might be able to modulate the homeostasis of mitochondria as well as help resist oxidative stress and restore redox balance, thus shed new light on the quest for strategy of prevention or therapeutics for insulin resistance and metabolic syndrome.
Acknowledgments
We'd like to acknowledge Zhanwu Hou's assistance in drawing chemical structure formulas for Figure 1. This work was supported by National Natural Science Foundation of China (31600682) and the 973 program (2015CB553602).
References
- 1.Hart GW, Akimoto Y. In: The O-GlcNAc Modification, in Essentials of Glycobiology. Varki A, et al., editors. Cold Spring Harbor; NY: 2009. [Google Scholar]
- 2.Torres CR, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. Journal of Biological Chemistry. 1984;259(5):3308–3317. [PubMed] [Google Scholar]
- 3.Vaidyanathan K, Durning S, Wells L. Functional O-GlcNAc modifications: implications in molecular regulation and pathophysiology. Crit Rev Biochem Mol Biol. 2014;49(2):140–63. doi: 10.3109/10409238.2014.884535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeidan Q, Hart GW. The intersections between O-GlcNAcylation and phosphorylation: implications for multiple signaling pathways. J Cell Sci. 2010;123(Pt 1):13–22. doi: 10.1242/jcs.053678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanover JA, Krause MW, Love DC. Bittersweet memories: linking metabolism to epigenetics through O-GlcNAcylation. Nat Rev Mol Cell Biol. 2012;13(5):312–21. doi: 10.1038/nrm3334. [DOI] [PubMed] [Google Scholar]
- 6.Hardiville S, Hart GW. Nutrient regulation of signaling, transcription, and cell physiology by O-GlcNAcylation. Cell Metab. 2014;20(2):208–13. doi: 10.1016/j.cmet.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanover JA, Krause MW, Love DC. The hexosamine signaling pathway: O-GlcNAc cycling in feast or famine. Biochim Biophys Acta. 2010;1800(2):80–95. doi: 10.1016/j.bbagen.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zachara NE, et al. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem. 2004;279(29):30133–42. doi: 10.1074/jbc.M403773200. [DOI] [PubMed] [Google Scholar]
- 9.Butkinaree C, Park K, Hart GW. O-linked beta-N-acetylglucosamine (O-GlcNAc): Extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress. Biochim Biophys Acta. 2010;1800(2):96–106. doi: 10.1016/j.bbagen.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ngoh GA, et al. O-GlcNAc signaling in the cardiovascular system. Circ Res. 2010;107(2):171–85. doi: 10.1161/CIRCRESAHA.110.224675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ngoh GA, et al. Augmented O-GlcNAc signaling attenuates oxidative stress and calcium overload in cardiomyocytes. Amino Acids. 2011;40(3):895–911. doi: 10.1007/s00726-010-0728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao L, et al. Aging leads to elevation of O-GlcNAcylation and disruption of mitochondrial homeostasis in retina. Oxid Med Cell Longev. 2014;2014:425705. doi: 10.1155/2014/425705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zachara NE, et al. The dynamic stress-induced 201C;O-GlcNAc-ome” highlights functions for O-GlcNAc in regulating DNA damage/repair and other cellular pathways. Amino Acids. 2011;40(3):793–808. doi: 10.1007/s00726-010-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gurel Z, et al. Retinal O-linked N-acetylglucosamine protein modifications: implications for postnatal retinal vascularization and the pathogenesis of diabetic retinopathy. Molecular vision. 2013;19:1047. [PMC free article] [PubMed] [Google Scholar]
- 15.Yang YR, et al. O-GlcNAcase is essential for embryonic development and maintenance of genomic stability. Aging Cell. 2012;11(3):439–448. doi: 10.1111/j.1474-9726.2012.00801.x. [DOI] [PubMed] [Google Scholar]
- 16.Vaidyanathan K, Wells L. Multiple tissue-specific roles for the O-GlcNAc post-translational modification in the induction of and complications arising from type II diabetes. J Biol Chem. 2014;289(50):34466–71. doi: 10.1074/jbc.R114.591560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang K, Yin R, Yang X. O-GlcNAc: A Bittersweet Switch in Liver. Front Endocrinol (Lausanne) 2014;5:221. doi: 10.3389/fendo.2014.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang X, et al. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008;451(7181):964–9. doi: 10.1038/nature06668. [DOI] [PubMed] [Google Scholar]
- 19.Ma Z, Vosseller K. O-GlcNAc in cancer biology. Amino Acids. 2013 doi: 10.1007/s00726-013-1543-8. [DOI] [PubMed] [Google Scholar]
- 20.Singh JP, et al. O-GlcNAc signaling in cancer metabolism and epigenetics. Cancer Lett. 2015;356(2 Pt A):244–50. doi: 10.1016/j.canlet.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong CX, Liu F, Iqbal K. O-GlcNAc cycling modulates neurodegeneration. Proc Natl Acad Sci U S A. 2012;109(43):17319–20. doi: 10.1073/pnas.1215395109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slawson C, Copeland RJ, Hart GW. O-GlcNAc signaling: a metabolic link between diabetes and cancer? Trends in Biochemical Sciences. 2010;35(10):547–555. doi: 10.1016/j.tibs.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bond MR, Hanover JA. O-GlcNAc cycling: a link between metabolism and chronic disease. Annual review of nutrition. 2013;33:205–229. doi: 10.1146/annurev-nutr-071812-161240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makarem N, et al. Trends in dietary carbohydrate consumption from 1991 to 2008 in the Framingham Heart Study Offspring Cohort. Br J Nutr. 2014:1–14. doi: 10.1017/S0007114513004443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Connor T, et al. Metabolic remodelling in obesity and type 2 diabetes: pathological or protective mechanisms in response to nutrient excess? Clin Exp Pharmacol Physiol. 2015;42(1):109–15. doi: 10.1111/1440-1681.12315. [DOI] [PubMed] [Google Scholar]
- 26.Bond MR, Hanover JA. O-GlcNAc cycling: a link between metabolism and chronic disease. Annu Rev Nutr. 2013;33:205–29. doi: 10.1146/annurev-nutr-071812-161240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medford HM, Chatham JC, Marsh SA. Chronic ingestion of a Western diet increases O-linked-beta-N-acetylglucosamine (O-GlcNAc) protein modification in the rat heart. Life Sci. 2012;90(23-24):883–8. doi: 10.1016/j.lfs.2012.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruan HB, et al. Cracking the O-GlcNAc code in metabolism. Trends in Endocrinology & Metabolism. 2013;24(6):301–309. doi: 10.1016/j.tem.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102(4):401–14. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–70. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanover JA, et al. Mitochondrial and nucleocytoplasmic isoforms of O-linked GlcNAc transferase encoded by a single mammalian gene. Arch Biochem Biophys. 2003;409(2):287–97. doi: 10.1016/s0003-9861(02)00578-7. [DOI] [PubMed] [Google Scholar]
- 32.Banerjee PS, Ma J, Hart GW. Diabetes-associated dysregulation of O-GlcNAcylation in rat cardiac mitochondria. Proc Natl Acad Sci U S A. 2015;112(19):6050–5. doi: 10.1073/pnas.1424017112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin SH, Love DC, Hanover JA. Elevated O-GlcNAc-dependent signaling through inducible mOGT expression selectively triggers apoptosis. Amino Acids. 2011;40(3):885–93. doi: 10.1007/s00726-010-0719-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruan HB, et al. O-GlcNAc transferase/host cell factor C1 complex regulates gluconeogenesis by modulating PGC-1alpha stability. Cell Metab. 2012;16(2):226–37. doi: 10.1016/j.cmet.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lubas WA, Hanover JA. Functional expression of O-linked GlcNAc transferase. Domain structure and substrate specificity. J Biol Chem. 2000;275(15):10983–8. doi: 10.1074/jbc.275.15.10983. [DOI] [PubMed] [Google Scholar]
- 36.Love DC, et al. Mitochondrial and nucleocytoplasmic targeting of O-linked GlcNAc transferase. J Cell Sci. 2003;116(Pt 4):647–54. doi: 10.1242/jcs.00246. [DOI] [PubMed] [Google Scholar]
- 37.Ma J, et al. O-GlcNAcomic Profiling Identifies Widespread O-Linked beta-N-Acetylglucosamine Modification (O-GlcNAcylation) in Oxidative Phosphorylation System Regulating Cardiac Mitochondrial Function. J Biol Chem. 2015;290(49):29141–53. doi: 10.1074/jbc.M115.691741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan EP, et al. Altering O-linked beta-N-acetylglucosamine cycling disrupts mitochondrial function. J Biol Chem. 2014;289(21):14719–30. doi: 10.1074/jbc.M113.525790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan EP, et al. Interplay between O-GlcNAc and acetylation regulates mitochondrial function (756.7) The FASEB Journal. 2014;28(1 Supplement):756.7. [Google Scholar]
- 40.Lozano L, et al. The mitochondrial O-linked N-acetylglucosamine transferase (mOGT) in the diabetic patient could be the initial trigger to develop Alzheimer disease. Exp Gerontol. 2014;58:198–202. doi: 10.1016/j.exger.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 41.Pekkurnaz G, et al. Glucose regulates mitochondrial motility via Milton modification by O-GlcNAc transferase. Cell. 2014;158(1):54–68. doi: 10.1016/j.cell.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gawlowski T, et al. Modulation of dynamin-related protein 1 (DRP1) function by increased O-linked-beta-N-acetylglucosamine modification (O-GlcNAc) in cardiac myocytes. J Biol Chem. 2012;287(35):30024–34. doi: 10.1074/jbc.M112.390682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bond MR, Hanover JA. A little sugar goes a long way: the cell biology of O-GlcNAc. J Cell Biol. 2015;208(7):869–80. doi: 10.1083/jcb.201501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu Y, et al. Increased enzymatic O-GlcNAcylation of mitochondrial proteins impairs mitochondrial function in cardiac myocytes exposed to high glucose. J Biol Chem. 2009;284(1):547–55. doi: 10.1074/jbc.M808518200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnsen VL, et al. Enhanced cardiac protein glycosylation (O-GlcNAc) of selected mitochondrial proteins in rats artificially selected for low running capacity. Physiological genomics. 2013;45(1):17–25. doi: 10.1152/physiolgenomics.00111.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma J, et al. O-GlcNAcomic profiling reveals altered O-GlcNAcylation of mitochondrial proteins in diabetes (608.4) The FASEB Journal. 2014;28(1 Supplement):608.4. [Google Scholar]
- 47.Dassanayaka S, et al. High glucose induces mitochondrial dysfunction independently of protein O-GlcNAcylation. Biochem J. 2015;467(1):115–26. doi: 10.1042/BJ20141018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones SP, et al. Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation. 2008;117(9):1172–82. doi: 10.1161/CIRCULATIONAHA.107.730515. [DOI] [PubMed] [Google Scholar]
- 49.Wright J, et al. Role of O-GlcNAcylation in Regulating Mitophagy in Cardiomyocytes. The FASEB Journal. 2015;29(1 Supplement):954.5. [Google Scholar]
- 50.Styskal J, et al. Oxidative stress and diabetes: what can we learn about insulin resistance from antioxidant mutant mouse models? Free Radic Biol Med. 2012;52(1):46–58. doi: 10.1016/j.freeradbiomed.2011.10.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Candas D, Li JJ. MnSOD in oxidative stress response-potential regulation via mitochondrial protein influx. Antioxid Redox Signal. 2014;20(10):1599–617. doi: 10.1089/ars.2013.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ozden O, et al. Acetylation of MnSOD directs enzymatic activity responding to cellular nutrient status or oxidative stress. Aging (Albany NY) 2011;3(2):102–7. doi: 10.18632/aging.100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dinic S, et al. Decreased O-GlcNAcylation of the key proteins in kinase and redox signalling pathways is a novel mechanism of the beneficial effect of alpha-lipoic acid in diabetic liver. Br J Nutr. 2013;110(3):401–12. doi: 10.1017/S0007114512005429. [DOI] [PubMed] [Google Scholar]
- 54.Arambasic J, et al. Alpha-lipoic acid upregulates antioxidant enzyme gene expression and enzymatic activity in diabetic rat kidneys through an O-GlcNAc-dependent mechanism. Eur J Nutr. 2013;52(5):1461–73. doi: 10.1007/s00394-012-0452-z. [DOI] [PubMed] [Google Scholar]


