Abstract
Background: Renal transplant recipients (RTRs) are often Vitamin D (VitD) depleted as a result of both chronic kidney disease and mandated sun avoidance behaviours. Repleting VitD may be warranted, but how, and for how long, is unknown, as is the impact of seasonality on the success of repletion. We investigated the impact of seasonality on VitD status following VitD repletion in a large cohort of stable, long-term RTRs.
Methods: Serum 25-hydroxyvitamin D [25(OH)D] concentrations and bone biochemistry parameters were analysed from 102 VitD repletion courses in 98 RTRs that had undergone VitD repletion. Repletion was delivered over 6 months with either 240 000 IU colecalciferol if pre-repletion serum VitD was between 20 and 50 nmol/L, or with 360 000 IU if VitD was <20 nmol/L. Twelve months post-repletion 25(OH)D and parathyroid hormone (PTH) were available for 75 patients.
Results: At baseline, 25(OH)D was 20.1 ± 1.0 nmol/L, increasing to 65.4 ± 1.8 nmol/L following repletion (+7.55 nmol/L/month, P < 0.0001). Twelve months post-repletion and after no further VitD administration, 25(OH)D fell to 35.4 ± 1.8 nmol/L (14.2 ± 0.7 ng/mL; −2.50 nmol/L/month, P < 0.0001). PTH followed the opposite trend with baseline, repletion-end and post-repletion values being 144.2 ± 12.0, 109.6 ± 7.5 and 129.2 ± 11.4 ng/L, respectively. VitD repletion during the summer was associated with significantly higher at repletion-end 25(OH)D compared with any other time of year [summer 80.9 ± 4.0, autumn 64.1 ± 3.0 (P = 0.002), winter 48.9 ± 3.0 (P <0.001), spring 63.8 ± 2.5 nmol/L (P <0.001)]. There was no hypercalcaemia during repletion and renal transplant function remained stable without any evidence of allograft rejection.
Conclusions: VitD repletion can safely and effectively be achieved in the majority of chronic stable RTRs using a 6-month bolus intermediate-dose schedule. Winter repletion is associated with an inadequate response in 25(OH)D; however, all patients experience a post-repletion fall towards deficiency in the absence of maintenance supplementation, irrespective of the season of repletion.
Keywords: bone mineral disease, chronic kidney disease, renal transplantation, vitamin D
Introduction
Vitamin D (VitD) therapy is currently under intense investigation in cardiovascular (CV), autoimmune and allergic conditions, chronic kidney disease (CKD), infections and cancer [1]. Many observational studies have demonstrated adverse outcomes strongly associated with VitD deficiency [2]. Skeletal, renal and gastro-intestinal effects of VitD on calcium and phosphate homeostasis are well known, with VitD deficiency linked most closely to increased risk of bone mineral loss and fractures [3], both of which are common post renal-transplantation challenges [4]. Renal transplant recipients (RTRs) have a high prevalence of VitD deficiency, and some residual CKD, and so also often display raised parathyroid hormone (PTH) values [5]. The reasons for this include (i) renal functional impairment with loss of renal tubular CYP27B1 (1-alpha-hydoxylase), (ii) raised serum fibroblast growth factor 23 [6], (iii) immunosuppressive drugs inducing VitD catabolism and (iv) medically advised sun-avoidance behaviour to mitigate the risk of UV-induced skin malignancy [7, 8]. VitD has also been demonstrated to influence both the innate and adaptive immune systems; thus, VitD status in RTRs could potentially impact upon immunologically driven post-transplantation outcomes, notably allograft rejection, transplant function and development of de novo post-transplant malignancies [9]. In three prospective observational studies in RTRs, 25-hydroxyvitamin D [25(OH)D] deficiency was directly linked to poor allograft outcomes, including delayed graft function and an increased risk of acute rejection [10–12].
Clinical safety data for VitD repletion have been analysed in two separate comprehensive Cochrane reviews, indicating that it is generally a well-tolerated and safe therapy in CKD in general [3, 13]. However, when assessing the efficacy in terms of clinically relevant outcomes, and safety, in the specific context of RTRs, VitD supplementation has yielded conflicting data. This is most likely attributable to differences in patient selection and study cohort time elapsed since transplantation, combined with variable VitD formulations and repletion regimens. Although post-transplant calcitriol supplementation in three studies was associated with reduced acute rejection [14], better transplant function [15] and improved graft survival [16], a smaller interventional study using cholecalciferol in the first year post-transplantation revealed conflicting results [17]. Currently, there are no fully reported long-term data or randomized controlled trials with hard clinical endpoints studying VitD repletion in RTRs.
The aims of this study were to investigate the impact of a modest monthly supplemental dose of ‘natural’ VitD (cholecalciferol) on serum PTH (the primary skeletal biomarker of VitD deficiency) in stable long-term RTRs with significant VitD deficiency. We investigated the influence of season on the response of VitD concentrations to VitD repletion and the impact of VitD repletion on total serum alkaline phosphatase (ALP) (a proxy for bone turnover [18]), while in terms of safety markers we carefully tracked serum calcium, phosphate, renal function [creatinine, estimated glomerular filtration rate (eGFR)] and biopsy-proven rejection episodes. Uniquely, in 75% of patients we were able to re-analyse these same parameters a year after the end of the VitD repletion period in the absence of on-going supplementation, to see just how many patients returned to a state of insufficiency or deficiency.
Materials and methods
Study design and participants
This retrospective follow-up study was performed at the kidney transplantation clinic at Guys’ Hospital. Since June 2010, a dedicated 3-monthly clinic for long-term transplant survivors has been undertaken with the focus on screening and prevention of CV, bone and skeletal health measures, and malignancy-related health issues. All patients who were > 8 years from engraftment and under continued follow-up in the transplant unit (now 3500 transplants from 1967 to 2015) were included in a long-term programme. In all such patients, VitD and PTH were measured quarterly. Nearly 800 patients have attended this long-term health management clinic (to June 2016). Patients in the main (>90%) were living and working between London and the southern coast of Kent and Sussex (latitudes 50.8–51.5°). All patients had received significant steroid exposure, particularly patients transplanted prior to 1977, though only 65% of those studied were currently continuing to take steroids as with the introduction of cyclosporin then tacrolimus many patients were able to have their steroids weaned away.
Routine biochemistry data, along with serum 25(OH)D and PTH measurements were collected from all patients presenting between January 2011 and June 2016 at their routine clinic visits. Patients underwent 6 months of cholecalciferol repletion if they had hypovitaminosis D [serum 25(OH)D <50 nmol/L; <20 ng/mL] with evidence of a biological response, such as associated elevated PTH concentration (>65 pg/mL). Careful note was made of both dietary and pharmaceutical VitD supplement use after specific enquiry at each clinical interview. No patients were receiving any other form of nutritional or synthetic VitD receptor agonist, any oral phosphate binders or any bisphosphates. Fifty percent of the patients were on long-term steroids, with no dose adjustments during the VitD supplementation phases. Solar UV radiation avoidance was routinely and regularly reinforced with both oral and written information at each clinic visit. Dietary VitD intake was not formally assessed.
Hypovitaminosis D was defined as either VitD deficiency [25(OH)D <20 nmol/L; <8.0 ng/mL] or insufficiency [25(OH)D 20–50 nmol/L; 8.0–20.0 ng/mL]. VitD sufficiency, defined as 25(OH)D >50 nmol/L (>20.0 ng/mL), was regarded as optimal VitD status and the point below which skeletal consequences of VitD deficiency may start to be seen [19]. We collected data from each patient at three data points; (i) baseline (prior to any nutritional or pharmaceutical VitD preparations); (ii) repletion-end [at the end of 6-months of VitD repletion with colecalciferol (vitamin D3)] and (iii) post-repletion (12 months following the end of repletion). Repletion courses were 6-months in duration, with either 240 000 IU colecalciferol [40 000 IU/month, if 25(OH)D was between 20 and 50 nmol/L] or 360 000 IU (60 000 IU/month, if <20 nmol/L). Subjects had multiple 25(OH)D values available over a prolonged period of clinic attendance; the data-points chosen for analysis were the sample closest to the desired time point (i.e. after 6 months of VitD repletion and 12 months following repletion end). Data from RTRs were collated into seasonal grouping according to timing of repletion: winter (December–February); spring (March–May); summer (June–August); autumn (September–November). Repletion courses lasted 6 months and spanned across two or three different seasons, for example, repletion from January to June spanned the end of winter, whole of spring and the start of summer. The season that was fully completed during the repletion period was arbitrarily designated as that season of repletion, that is, spring in this example. When repletion spanned only two seasons, with both seasons being fully completed, for example, March–August, then the 2nd season was taken as the season of repletion for consistency, that is, summer in this example.
Markers of bone turnover
Serum calcium, phosphate and creatinine were measured using standard laboratory methods using Roche Modular analysers (Roche Diagnostics Limited, West Sussex, UK). eGFR was calculated using the MDRD study equation. Serum PTH and ALP were measured by an electrochemiluminescence immunoassay on the Roche Elecsys 2010 analyzer (Roche Diagnostics, Indianapolis, IN, USA) and serum 25(OH)D using a Diasorin Liaison platform Chemiluminescence CLIA assay for which the coefficient of variation was 11.7% at concentration 43.0 nmol/L and 9.6% at 66.8 nmol/L. All markers were measured at three time points (baseline, repletion-end and post-repletion) except for ALP and phosphate, which were only available at baseline and repletion-end.
Statistical analysis
Statistical analyses were performed using the standard statistical software package, SPSS 23.0 for Mac (IBM, Charlotte, NC, USA). Mean, standard deviation and standard error were calculated for all variables. Rate of change in serum biomarkers was calculated by estimating the linear gradient between time points (baseline, repletion-end and post-repletion) and expressed as change in IU/month. A repeated measures ANOVA analysis was used to compare the equality of the means at the three different time points. Each time point was also compared directly with each other (baseline versus repletion-end; repletion-end versus post-repletion; baseline versus post-repletion) using a paired t-test. Correlations between variables were calculated using Pearson’s correlation coefficient and stepwise linear regression analysis. A P-value of <0.05 was considered statistically significant.
Ethical approval
The project involved anonymized use of routine clinical/laboratory parameters gathered in routine clinical practice. Ethical permission was not therefore sought.
Results
At baseline, the mean age of RTRs was 53.8 ± 1.4 years, 64% were male and 82% were Caucasian (Table 1). Median time post-transplantation was 194 (range 149–277) months. Mean eGFR was 50.7 ± 2.0 mL/min/1.73 m2. No studied patients were prescribed or were taking VitD supplements prior to repletion or after completion of repletion. Bone biochemistry parameters were analysed from 102 repletion courses in 98 RTRs (all >10 years post-engraftment). Twelve months post-repletion data were available for 75 repletion courses in 72 patients. All repleted patients had stable renal function at baseline and declared total compliance with their therapy at each follow-up interview.
Table 1.
Demographics and bone biochemistry concentrations at baseline, repletion-end and post-repletion
| Characteristic | Baseline n = 102 | Repletion end n = 102 | Percentage change | Post-repletion n = 75 | Percentage change |
|---|---|---|---|---|---|
| Time point (months) | 0 | 6 | 18 | ||
| Age (years) | 53.8 ± 1.4 | 56.9 ± 1.4 | |||
| Male gender | 64.1% | 63.2% | |||
| 25(OH)D | 20.1 ± 1.0 | 65.4 ± 1.8 | +225.6% | 37.4 ± 2.7 | −45.8% |
| nmol/L | P < 0.0001 | P < 0.0001 | |||
| PTH | 144.2 ± | 109.6 ± 7.5 | −24.0% | 129.2 ± 11.4 | +17.8% |
| ng/L | 12.0 | P < 0.0001 | P = 0.046 | ||
| Calcium | 2.40 ± 0.01 | 2.43 ± 0.01 | +1.33% | 2.42 ± 0.02 | 0% |
| mmol/L | P = 0.092 | P = 0.822 |
Means ± SEM.
Serum VitD and repletion
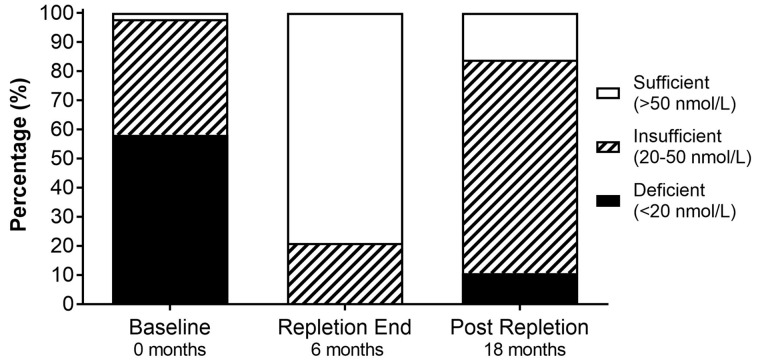
In 98 patients in the RTR cohort studied, at baseline 58% were VitD deficient (<20 nmol/L), 40% were VitD insufficient (20–50 nmol/L) and just 2% were VitD sufficient (>50 nmol/L). At baseline, 25(OH)D concentrations were higher in Caucasians than in non-Caucasians [mean 25(OH)D 50 ± 29 versus 30 ± 24 nmol/L, respectively, P < 0.0001].
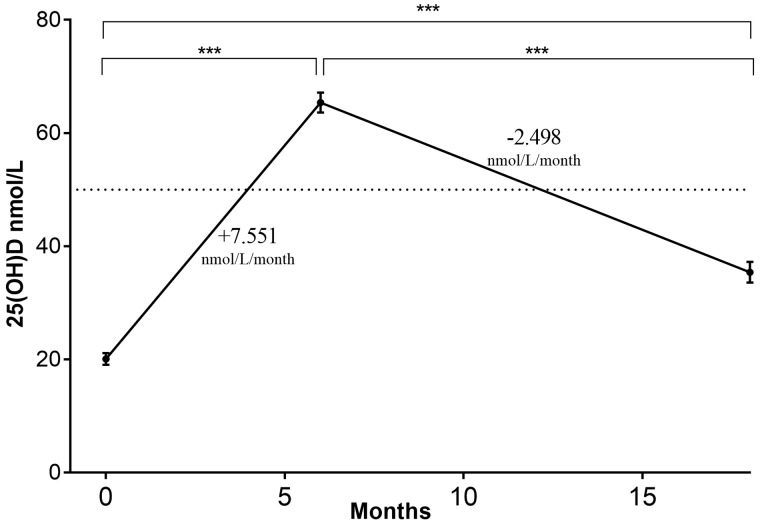
Six months of VitD repletion significantly increased 25(OH)D compared with baseline (P < 0.0001). At repletion-end 0% were VitD deficient, 21% were VitD insufficient and 79% were VitD sufficient. Twelve months post-VitD repletion there were significant reductions in 25(OH)D compared with repletion-end (P < 0.0001) but these values were still significantly greater than baseline pre-repletion (P < 0.0001; Figure 1). Twelve months post-repletion, 8% of RTRs were VitD deficient, 55% were VitD insufficient and 12% were VitD sufficient (Figure 2). The mean rate of increase in 25(OH)D during the 6 months repletion was +7.55 ± 0.31 nmol/L/month (+3.0 ± 0.1 ng/mL/month) and the mean rate of reduction of 25(OH)D post-repletion was −2.50 ± 0.23 nmol/L/month (−1.00 ± 0.1 ng/mL/month), relative difference 3.02, P < 0.0001.
Fig. 1.
Impact of VitD repletion on serum 25(OH)D concentration at repletion-end and post-repletion. There is marked variation in VitD status with repletion and following repletion. Mean serum 25(OH)D was 20.1 nmol/L at baseline, 65.4 nmol/L after 6 months of VitD repletion and 35.4 nmol/L 12 months post-repletion. Repletion gradient = 7.551 nmol/L/month and post-repletion gradient = −2.498 nmol/L/month. ***P < 0.0001.
Fig. 2.
Impact of VitD repletion on serum 25(OH)D status (sufficient, insufficient and deficient) at baseline compared with repletion-end and post-repletion. At baseline 58% were VitD deficient, 40% were insufficient and 2% were sufficient. After 6 months of repletion (repletion-end) 0% were VitD deficient, 21% were insufficient and 79% were sufficient. Six to 24 months post-repletion, 11% were VitD deficient, 73% were insufficient and 16% were sufficient.
Bone turnover parameters
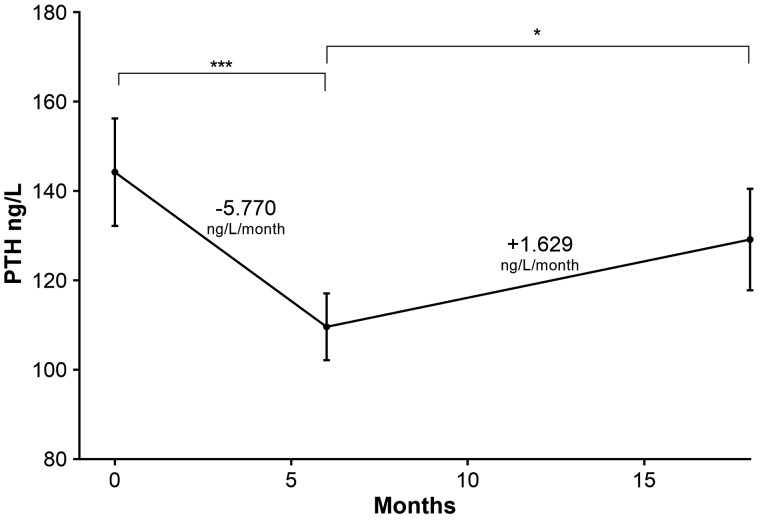
Serum PTH was abnormally raised at baseline (144.2 ± 12.0 ng/L), repletion-end (109.6 ± 7.5 ng/L) and post-repletion (129.2 ± 11.4 ng/L; Figure 3). However, repletion-end PTH was significantly lower than baseline PTH (P < 0.0001). Twelve months post-repletion PTH was significantly greater than repletion-end (P = 0.046) but was not different from baseline PTH (P = 0.191). There was an inverse correlation between 25(OH)D and PTH concentrations at baseline (r2 = 0.08; P < 0.05), repletion-end (r2 = 0.03; P < 0.05) and post-repletion (r2 = 0.09; P < 0.05).
Fig. 3.
PTH status in RTRs at baseline compared with repletion-end and post-repletion. PTH varied markedly with VitD repletion. End of repletion PTH (mean 109.6 ng/L) is significantly reduced compared with baseline PTH (mean 144.2 ng/L, P < 0.001). Post-repletion PTH (mean 129.2 ng/L) is significantly greater than repletion end PTH (P = 0.046), but not different from baseline (P = 0.191). Repletion gradient = 5.770 ng/L/month and post-repletion = 1.629 ng/L/month. *P < 0.05, ***P < 0.0001.
Serum calcium concentration was 2.40 ± 0.01 mmol/L at baseline, with one patient having a value <2.1 mmol/L and 11 patients having >2.55 mmol/L, but with none >2.65. Mean repletion-end calcium was 2.43 ± 0.01 mmol/L, with one patient <2.1 mmol/L and 14 patients >2.55 mmol/L, but with none >2.65 nmol/L. Twelve months post-repletion, mean calcium was 2.42 ± 0.02 mmol/L, with one patient <2.1 mmol/L and 12 patients >2.55 mmol/L, with none >2.65 nmol/L. RTRs mean phosphate concentration was 0.99 ± 0.02 mmol/L at baseline and 1.01 ± 0.02 mmol/L at repletion-end (P = 0.36), while total ALP fell from 81.6 ± 7.1 IU/L at baseline to 67.5 ± 28.4 IU/L (P = 0.004) at the 6 months repletion time point.
At baseline creatinine was 138.8 ± 4.8 umol/L (eGFR 50.7 ± 2.0 mL/min/1.73 m2) increasing to 143.6 ± 5.3 umol/L (eGFR 48.6 ± 1.9 mL/min/1.73 m2) at repletion-end (baseline versus repletion-end +0.82 umol/L/month, P = 0.496). Renal function in this cohort prior to VitD repletion has been reported previously [20].
Seasonality
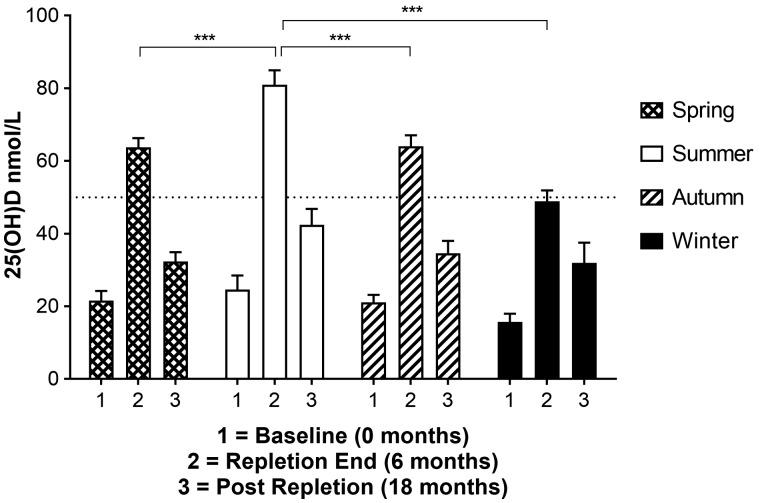
VitD status for RTRs repleted in the different seasons of the year is shown in Figure 4. Baseline serum 25(OH)D were not significantly different; however, there was a trend towards higher values in summer (24.6 ± 3.9 nmol/L) and lower values in winter (15.7 ± 2.3 nmol/L). Patients who underwent VitD repletion during the summer months had significantly higher repletion-end 25(OH)D concentrations compared with patients repleted at any other time of year (summer 80.9 ± 4.0, autumn 64.1 ± 3.0, winter 48.9 ± 3.0, spring 63.8 ± 2.5; Table 2). The increment in 25(OH)D from baseline to repletion-end was significantly greater with summer repletion compared with the other months (summer + 9.4 ± 0.9 nmol/L/month; autumn +7.2 ± 0.6; winter +5.5 ± 0.4; spring +7.0 ± 0.6). Post-repletion 25(OH)D concentrations were not significantly different between seasons of repletion. Moreover, the fall from repletion-end to post-repletion was not significantly different between summer and spring or autumn repletion courses; however, winter repletion was associated with a lower rate of change [summer −3.2 ± 0.3 nmol/L/month; autumn −2.5 ± 0.4 (P = 0.18); winter −1.4 ± 0.5 (P = 0.006); spring −2.6 ± 0.2 (P = 0.15)].
Fig. 4.
Impact of season of VitD repletion on serum 25(OH)D concentration at baseline, repletion-end and post repletion. Winter VitD repletion is associated with significantly lower end of repletion serum 25(OH)D concentrations compared with repletion during the other seasons. Baseline and post-repletion serum 25(OH)D are not dependent on the season of repletion. ***P < 0.0001.
Table 2.
Impact of the season of VitD repletion on serum 25(OH)D concentrations at baseline, repletion-end and post-repletion
| Repletion season | n | Baseline | Repletion-end | Increment/month | Post-repletion | Fall/month |
|---|---|---|---|---|---|---|
| Summer | 19 | 24.6 ± 3.9 | 80.9 ± 4.0 | +9.4 ± 0.9 | 42.4 ± 4.4 | −3.2 ± 0.3 |
| Autumn | 19 | 21.1 ± 1.8 | 64.1 ± 3.0 | +7.2 ± 0.6 | 34.6 ± 3.4 | −2.5 ± 0.4 |
| P = 0.40 | P = 0.002 | P = 0.03 | P = 0.16 | P = 0.18 | ||
| Winter | 17 | 15.7 ± 2.3 | 48.9 ± 3.0 | +5.5 ± 0.4 | 31.9 ± 5.6 | −1.4 ± 0.5 |
| P = 0.09 | P < 0.001 | P = 0.001 | P = 0.15 | P = 0.006 | ||
| Spring | 20 | 21.6 ± 2.7 | 63.75 ± 2.5 | +7.0 ± 0.6 | 32.3 ± 2.6 | −2.6 ± 0.2 |
| P = 0.52 | P < 0.001 | P = 0.03 | P = 0.05 | P = 0.15 |
All values units are nmol/L and represented as means ± SEM. P-values represent unpaired Student’s t-test comparing season with summer repletion.
Transplant rejection
Three patients required a renal transplant biopsy during or following VitD repletion (in each case done to investigate a more abrupt decline in kidney function). Histology demonstrated acute cellular rejection in only one of these three cases, which occurred 1-year post the end of the VitD repletion period, and this was judged to be due to poor compliance with immunosuppressive medication. There were no other cases of acute rejection in the entire follow-up period during repletion or post-repletion.
Discussion
In this study, we explored the impact of 6 months of VitD repletion on 25(OH)D and key markers of bone turnover in a large, stable group of long-term RTRs. 25(OH)D status improved significantly with VitD repletion, however these salutary effects were relatively short-lived with a significant but incomplete fall in VitD in around 12 months without on-going VitD supplementation. This supports the use of long-term maintenance supplementation, following an initial repletion strategy, to avoid a trend back towards deficiency. Interestingly, the linearized rate of 25(OH)D increase with VitD repletion was 3-fold faster than the fall post-repletion.
Our major finding and studied here for the first time was that the season of repletion independently determined the 25(OH)D concentration increment and also final 25(OH)D concentration, which likely reflected the additional impact of sunlight hours. These seasonal cyclical VitD results suggest there may be poor compliance with sun avoidance behaviours despite repeated reminders to avoid sunlight, sunbathing and to use sun protection (hats, gloves, creams) [20]. This asymmetrical seasonality response to VitD repletion, with winter repletion associated with inadequate restoration of 25(OH)D, indicates that structured dose-adjustments are probably required to optimize the repletion regimen. We also report that VitD repletion in RTRs using modest bolus doses of cholecalciferol was not associated with any appreciable increase in hypercalcaemia episodes or acute rejection episodes, and was safe in respect of stability of renal transplant function over a more extended period.
As with other observational studies of RTRs, we identified that 25(OH)D concentrations were deranged in 98% of RTRs studied, with deficiency and insufficiency recorded as 58% and 40%, respectively [12, 21–23]. This is concerning since deficiency is independently associated with all-cause mortality, acute rejection and poorer graft function among RTRs [12, 23–25]. We identified a reciprocal relationship between circulating 25(OH)D and PTH concentrations at all stages of the repletion protocol, which acts to confirm the strong links between skeletal health and VitD status in RTRs. The consistent reciprocal relationship between VitD and PTH concentrations (pre-, at end- and post-supplementation) clearly indicates the strong physiological links between these parameters. Mean repletion-end PTH (109.6 ± 7.5 ng/L) was reduced relative to baseline (144.2 ± 12.0 ng/L), however it still remained significantly elevated. Thus, a repletion-end mean 25(OH)D value of 65.4 nmol/L may still be inadequate and supports a higher therapeutic threshold of 75 mmol/L [26, 27]. Despite this, the significant suppression of the elevated PTH levels following VitD repletion supports the notion that supplementation could be part of an effective therapy to prevent chronic bone density loss. The drop in total ALP—approximately 50% of which is bone-specific ALP and reflects bone turnover—after VitD repletion further supports this [18, 28].
Other published studies of VitD supplementation in RTRs are limited, with large heterogeneity in VitD repletion regimens, and few studies reporting on >100 repletion courses like ours and with longer-term follow-up. We utilized a structured use of two repletion dose-adjusted regimens, always delivered over 6 months, 360 000 IU for those <20 nmol/L and 240 000 IU for those 20–50 nmol/L. This was based on findings from the general population that for every 100 units (2.5 µg) of vitamin D3 (cholecalciferol), 25(OH)D concentrations increase by approximately 1.75–2.5 nmol/L, with greater increases in those with lower baseline 25(OH)D levels [29–31]. There is unresolved debate as to which preparation of VitD should be used for repletion along with dose, dosing frequency and treatment time periods; however, a meta-analysis of seven randomized trials evaluating colecalciferol (vitamin D3) versus ergocalciferol (vitamin D2) has indicated that cholecalciferol (vitamin D3) is more efficacious at raising 25(OH)D concentration [32].
Current studies of VitD repletion in RTRs demonstrate improvements in PTH and calcium; however, the effects on bone density remain controversial with no published data available on long-term post-repletion data (Table 3). In a retrospective cohort study of 64 RTRs, cholecalciferol supplementation (50 000 IU/week for 2 months followed by 10 months maintenance) failed to prevent progression of eGFR, interstitial fibrosis, tubular atrophy or proteinuria [17]. Another retrospective analysis of 110 adult RTRs undergoing VitD repletion observed 96.3% of the cohort was VitD deficient or insufficient at baseline. Of 63 patients who were followed up after repletion with 6 months of oral calcidiol (mean dose 8044 IU/week), the 61.3% who were deficient at baseline decreased to 2.1% (mean baseline VitD 34.7 ± 12.5; repletion-end 85.9 ± 30.0 nmol/L). This equates to a mean increment of +8.5 nmol/L/month, which is similar to +7.55 nmol/L/month that we reported. This study also followed up patients for a further 6 months, with patients continuing to receive oral calcidiol (mean dose 5600 IU/week), and demonstrated a significant fall in 25(OH)D from 85.9 ± 30.0 to 76.9 ± 25.0 nmol/L (mean fall −0.75 nmol/L/month) [33]. Although shorter in follow-up than our analysis, this study demonstrates that the dramatic fall in 25(OH)D following repletion but without ongoing supplementation that we observed can only be attenuated, but not abrogated, by long-term supplementation.
Table 3.
Studies of VitD repletion in renal transplantation recipients
| Author, year | N | Study design | VitD agent | Dose (IU) | Follow-up (months) | Outcome |
|---|---|---|---|---|---|---|
| Torres, 2004 [40] | 86 | RCT: calcium + calcitriol versus calcium + placebo | Calcitriol | 0.5 µg/48 h for 3 months | 12 | PTH significantly lowers with calcitriol at 3 and 12 months. Total hip BMD preserved better with calcitriol |
| Wissing, 2005 [41] | 90 | RCT: calcium versus calcium + cholecalciferol. | Cholecalciferol | 25 000/month | 12 | BMD loss trend towards higher with cholecalciferol (P = NS). Negative correlation between VitD and PTH. |
| Sahin, 2008 [42] | 58 | RTR <6 months versus RTR >6 months | Cholecalciferol + calcium | 400/day | 12 | BMD improved in both groups (no difference between groups). PTH reduced with repletion (192–82 pg/mL). |
| Courbebaisse, 2009 [43] | 94 | Cholecalciferol versus no treatment | Cholecalciferol | 100 000 4 doses in 2 months then maintenance | 12 | Repletion: 25(OH)D normalized with repletion and PTH decreased. Calcium increased. No adverse effects. Maintenance: 25(OH)D fell (P = NS). |
| Kanter Berger, 2010 [33] | 63 | Retrospective: 25(OH)D <30 at baseline | Calcidiol | 8044 ± 4087/week | 12 | VitD deficiency reduced from 61.3% to 2.1% at 6 months and 7.5% at 12 months. No change in calcium, phosphate or PTH with repletion. |
| Sgambat, 2011 [44] | 71 | Paediatric RTRs versus African American controls | Ergocalciferol or cholecalciferol | Ergocalciferol 50 000/week; cholecalciferol 28 000/week | 48 | 13% with ergocalciferol versus 82.6% with cholecalciferol achieved VitD repletion (P < 0.0001). RTR had 3.4-fold higher risk of low BMD than controls (P < 0.05). |
| Courbebaisse, 2011 [17] | 64 | Retrospective follow-up | Cholecalciferol | 100 000/2 week for 2 months, then 2 monthly for 10 months | 12 | Cholecalciferol did not prevent epithelial to mesenchymal transition, interstitial fibrosis, tubular atrophy or renal function deterioration. |
| Amer, 2013 [45] | 87 | RCT: VDRA versus no treatment | Paricalcitol | 2 µg/day | 12 | Reduced hyperparathyroidism with paricalcitol (29% versus 63%, P = 0.0005). No difference in rejection or renal function. |
| Gonzalez, 2013 [46] | 58 | Retrospective follow-up | Paricalcitol | 1 µg/alternate days | 18 | Paricalcitol was associated with significant decrease in PTH from 333 to 181 pg/mL (P = 0.02). 25(OH)D increased (43.9–45.7 nmol/L). Proteinuria significantly reduced (P < 0.01). GFR no change. |
| Trillini, 2013 [47] | 43 | Randomized cross-over trial: VDRA versus no treatment | Paricalcitol | 2 µg/day | 6 | PTH significantly declined (115.6–63.3 pg/ml, P < 0.001) with paracalcitol but not with controls. Proteinuria, ALP and osteocalcin decreased with paricalcitol. |
| Ziff, 2016 (this study) | 102 | Retrospective follow-up: repletion versus post-repletion | Cholecalciferol | 60 000/month | 18 | Repletion significantly increased 25(OH)D and reduced PTH. 12 months post-repletion significantly reduced 25(OHD) and increased PTH. |
BMD, bone mineral density; RCT, randomized controlled trial; VDRA, vitamin D receptor agonist; NS, not significant.
The VITA-D trial randomized 200 RTRs with baseline 25(OH)D <50 nmol/L to either oral cholecalciferol 6800 IU/day or placebo in the first year after transplantation. Patients were followed up for 1 year with outcomes designed to investigate the immunomodulatory and renoprotective effects of repletion [34]. Seventy-five percent of patients randomized to VitD achieved adequate 25(OH)D levels after 6 months and this was maintained at 1 year. The number of infections and acute allograft rejections were similar between groups; however, at 12 months creatinine levels was slightly higher in the VitD group than placebo (136 versus 126 µmol/L), although this effect disappeared after multivariate adjustments. In contrast to our results, 30% of patients in the VitD group required dose reduction or discontinuation due to hypercalcaemia (compared with 17% in the placebo group). This disparity may partly be explained by the much higher VitD dose used in VITA-D (6-month total dose 1 224 000 versus ∼ 240 000 IU in our study) [35]. Results from the on-going VITALE trial, which compares the effect of cholecalciferol at high versus low doses (100 000 IU/fortnight and 6000 IU/fortnight, respectively, for 2 months then monthly for 22 months) on proteinuria and allograft function, are eagerly anticipated [36].
Current guideline recommendations on VitD repletion in VitD deficient CKD patients are conflicting, with discrepancies around target 25(OH)D levels (for selection for repletion, and targets to aim for post-repletion), indications for intervention and repletion regimens to use (agents, dose and course length) [37, 38]. There are currently no specific recommendations of optimal VitD replacement strategy in RTRs. We have shown in our study that a simple gentle approach to VitD repletion seems both safe and effective, but that adaptation to the season of repletion seems sensible, and continued maintenance of vitD sufficiency will require ongoing supplementation with VitD in these patients.
Limitations
This was a retrospective analysis of VitD status of 98 patients (102 repletion courses), of which 72 were available for post-repletion analysis (75 repletion courses); patients with no post-repletion data-point close to the 12 months post-repletion time-point were excluded from the cohort. Although there was no control group each individual acted as their own control in a repeated-measures analysis. We did not collect dietary VitD intake information; however, it is documented that diet, except in extreme conditions, is only a modest contributor to total VitD status, especially in the UK [39]. We did not correlate biochemical changes to objective relevant clinical end-points such as hospitalization and mortality rates. Additionally, specific clinically relevant outcomes relating to bone mineral disease including DEXA scores, bone biopsies and fractures are not reported (and would require a study of 1000s of patients, and of many years duration).
Conclusions
Hypovitaminosis D is highly prevalent in long-term stable RTRs and is independently associated with abnormalities in bone turnover biomarkers. Six months of VitD repletion effectively and safely corrected these abnormalities; however, repletion without on-going supplementation is followed by a slow return to VitD insufficiency and deficiency. We report for the first time that the season of repletion significantly influences repletion-end and post-repletion 25(OH)D values, with winter repletion associated with an inadequate response. These findings support the use of an individualized VitD repletion strategy followed by long-term maintenance in RTRs to prevent continued pathological chronic rises in PTH. Future randomized trials of VitD are urgently required to identify the optimal repletion strategy and any long-term skeletal and general health benefits therefrom.
Authors' contributions
O.J.Z. performed data extraction, statistical analysis and drafting of the manuscript; H.P., S.F. and A.C. collected and analyzed data and helped to draft the manuscript and D.G. designed the study concept, led the study group and revised the manuscript.
Acknowledgements
We acknowledge the highly professional nature of the extended multi-disciplinary team that cares for renal transplant patients at our institution.
O.J.Z. (lead author) affirms that the manuscript is an honest, accurate and transparent account of the study being reported, that no important aspects of the study have been omitted and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Conflict of interest statement.
D.G. has received speaking and consulting fees from Abbvie, Amgen, Genzyme, Sanofi and Shire.
References
- 1. Pludowski P, Holick MF, Pilz S. et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality—a review of recent evidence. Autoimmun Rev 2013; 12: 976–989 [DOI] [PubMed] [Google Scholar]
- 2. Pilz S, Iodice S, Zittermann A. et al. Vitamin D status and mortality risk in CKD: a meta-analysis of prospective studies. Am J Kidney Dis 2011; 58: 374–382 [DOI] [PubMed] [Google Scholar]
- 3. Palmer SC, McGregor DO, Strippoli GF.. Interventions for preventing bone disease in kidney transplant recipients. Cochrane Database Syst Rev 2007; Cd005015. [DOI] [PubMed] [Google Scholar]
- 4. Courbebaisse M, Souberbielle JC, Thervet E.. Potential nonclassical effects of vitamin D in transplant recipients. Transplantation 2010; 89: 131–137 [DOI] [PubMed] [Google Scholar]
- 5. Sadlier DM, Magee CC.. Prevalence of 25(OH) vitamin D (calcidiol) deficiency at time of renal transplantation: a prospective study. Clin Transplant 2007; 21: 683–688 [DOI] [PubMed] [Google Scholar]
- 6. Baia LC, Humalda JK, Vervloet MG. et al. Fibroblast growth factor 23 and cardiovascular mortality after kidney transplantation. Clin J Am Soc Nephrol 2013; 8: 1968–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eyal O, Aharon M, Safadi R. et al. Serum vitamin D levels in kidney transplant recipients: the importance of an immunosuppression regimen and sun exposure. Isr Med Assoc J 2013; 15: 628–633 [PubMed] [Google Scholar]
- 8. Cianciolo G, Galassi A, Capelli I. et al. Vitamin D in kidney transplant recipients: mechanisms and therapy. Am J Nephrol 2016; 43: 397–407 [DOI] [PubMed] [Google Scholar]
- 9. McGregor R, Li G, Penny H. et al. Vitamin D in renal transplantation—from biological mechanisms to clinical benefits. Am J Transplant 2014; 14: 1259–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Falkiewicz K, Boratynska M, Speichert-Bidzinska B. et al. 1,25-dihydroxyvitamin D deficiency predicts poorer outcome after renal transplantation. Transplant Proc 2009; 41: 3002–3005 [DOI] [PubMed] [Google Scholar]
- 11. Kim H, Kang SW, Yoo TH. et al. The impact of pretransplant 25-hydroxy vitamin D deficiency on subsequent graft function: an observational study. BMC Nephrol 2012; 13: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bienaime F, Girard D, Anglicheau D. et al. Vitamin D status and outcomes after renal transplantation. J Am Soc Nephrol 2013; 24: 831–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Palmer SC, McGregor DO, Craig JC. et al. Vitamin D compounds for people with chronic kidney disease not requiring dialysis. Cochrane Database Syst Rev 2009; Cd008175. [DOI] [PubMed] [Google Scholar]
- 14. Tanaci N, Karakose H, Guvener N. et al. Influence of 1,25-dihydroxyvitamin D3 as an immunomodulator in renal transplant recipients: a retrospective cohort study. Transplant Proc 2003; 35: 2885–2887 [DOI] [PubMed] [Google Scholar]
- 15. Uyar M, Sezer S, Arat Z. et al. 1,25-dihydroxyvitamin D(3) therapy is protective for renal function and prevents hyperparathyroidism in renal allograft recipients. Transplant Proc 2006; 38: 2069–2073 [DOI] [PubMed] [Google Scholar]
- 16. Ozdemir BH, Ozdemir AA, Sezer S. et al. Influence of 1,25-dihydroxyvitamin D3 on human leukocyte antigen-DR expression, macrophage infiltration, and graft survival in renal allografts. Transplant Proc 2011; 43: 500–503 [DOI] [PubMed] [Google Scholar]
- 17. Courbebaisse M, Xu-Dubois YC, Thervet E. et al. Cholecalciferol supplementation does not protect against renal allograft structural and functional deterioration: a retrospective study. Transplantation 2011; 91: 207–212 [DOI] [PubMed] [Google Scholar]
- 18. Taylor AK, Lueken SA, Libanati C. et al. Biochemical markers of bone turnover for the clinical assessment of bone metabolism. Rheum Dis Clin North Am 1994; 20: 589–607 [PubMed] [Google Scholar]
- 19. Godar DE, Pope SJ, Grant WB. et al. Solar UV doses of adult Americans and vitamin D(3) production. Dermatoendocrinol 2011; 3: 243–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Penny H, Frame S, Dickinson F. et al. Determinants of vitamin D status in long-term renal transplant patients. Clin Transplant 2012; 26: E617–E623 [DOI] [PubMed] [Google Scholar]
- 21. Boudville NC, Hodsman AB.. Renal function and 25-hydroxyvitamin D concentrations predict parathyroid hormone levels in renal transplant patients. Nephrol Dial Transplant 2006; 21: 2621–2624 [DOI] [PubMed] [Google Scholar]
- 22. Querings K, Girndt M, Geisel J. et al. 25-hydroxyvitamin D deficiency in renal transplant recipients. J Clin Endocrinol Metab 2006; 91: 526–529 [DOI] [PubMed] [Google Scholar]
- 23. Keyzer CA, Riphagen IJ, Joosten MM. et al. Associations of 25(OH) and 1,25(OH)2 vitamin D with long-term outcomes in stable renal transplant recipients. J Clin Endocrinol Metab 2015; 100: 81–89 [DOI] [PubMed] [Google Scholar]
- 24. Obi Y, Hamano T, Ichimaru N. et al. Vitamin D deficiency predicts decline in kidney allograft function: a prospective cohort study. J Clin Endocrinol Metab 2014; 99: 527–535 [DOI] [PubMed] [Google Scholar]
- 25. Lee JR, Dadhania D, August P. et al. Circulating levels of 25-hydroxyvitamin D and acute cellular rejection in kidney allograft recipients. Transplantation 2014; 98: 292–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mazzaferro S, Pasquali M, Pugliese F. et al. Distinct impact of vitamin D insufficiency on calcitriol levels in chronic renal failure and renal transplant patients: a role for FGF23. J Nephrol 2012; 25: 1108–1118 [DOI] [PubMed] [Google Scholar]
- 27. Douthat WG, Chiurchiu CR, Massari PU.. New options for the management of hyperparathyroidism after renal transplantation. World J Transplant 2012; 2: 41–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sardiwal S, Magnusson P, Goldsmith DJ. et al. Bone alkaline phosphatase in CKD-mineral bone disorder. Am J Kidney Dis 2013; 62: 810–822 [DOI] [PubMed] [Google Scholar]
- 29. Aterrado S, Ono G, Kanehira-Mar S. et al. Evaluating Vitamin D repletion regimens and effects in veteran patients. Ann Pharmacother 2015; 49: 969–977 [DOI] [PubMed] [Google Scholar]
- 30. Gallagher JC, Sai A, Templin T 2nd, Smith L.. Dose response to vitamin D supplementation in postmenopausal women: a randomized trial. Ann Intern Med 2012; 156: 425–437 [DOI] [PubMed] [Google Scholar]
- 31. Heaney RP, Davies KM, Chen TC. et al. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 2003; 77: 204–210 [DOI] [PubMed] [Google Scholar]
- 32. Tripkovic L, Lambert H, Hart K. et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr 2012; 95: 1357–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kanter Berga J, Crespo Albiach J, Beltran Catalan S. et al. Vitamin D deficiency in a renal transplant population: safe repletion with moderate doses of calcidiol. Transplant Proc 2010; 42: 2917–2920 [DOI] [PubMed] [Google Scholar]
- 34. Thiem U, Heinze G, Segel R. et al. VITA-D: cholecalciferol substitution in vitamin D deficient kidney transplant recipients: a randomized, placebo-controlled study to evaluate the post-transplant outcome. Trials 2009; 10: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thiem U, Heinze G, Segel R. et al. Vitamin D3 Does Not Improve Outcomes After Kidney Transplant. European Renal Association–European Dialysis and Transplant Association (ERA-EDTA), 52nd Congress, 28–31 May 2015, London
- 36. Courbebaisse M, Alberti C, Colas S. et al. Vitamin D supplementation in renal transplant recipients (VITALE): a prospective, multicentre, double-blind, randomized trial of vitamin D estimating the benefit and safety of vitamin D3 treatment at a dose of 100,000 UI compared with a dose of 12,000 UI in renal transplant recipients: study protocol for a double-blind, randomized, controlled trial. Trials 2014; 15: 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stevens PE, Levin A.. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158: 825–830 [DOI] [PubMed] [Google Scholar]
- 38. NICE; National Institute of Health and Care Excellence guideline NG182. Chronic Kidney Disease: Early Identification and Management of Chronic Kidney Disease in Adults in Primary and Secondary Care, 2014. [PubMed]
- 39. Holick MF. Vitamin D deficiency. N Engl J Med 2007; 357: 266–281 [DOI] [PubMed] [Google Scholar]
- 40. Torres A, Garcia S, Gomez A. et al. Treatment with intermittent calcitriol and calcium reduces bone loss after renal transplantation. Kidney Int 2004; 65: 705–712 [DOI] [PubMed] [Google Scholar]
- 41. Wissing KM, Broeders N, Moreno-Reyes R. et al. A controlled study of vitamin D3 to prevent bone loss in renal-transplant patients receiving low doses of steroids. Transplantation 2005; 79: 108–115 [DOI] [PubMed] [Google Scholar]
- 42. Sahin G, Yasar NS, Sirmagul B. et al. The effect of low-dose cholecalciferol and calcium treatment on posttransplant bone loss in renal transplant patients: a prospective study. Ren Fail 2008; 30: 992–999 [DOI] [PubMed] [Google Scholar]
- 43. Courbebaisse M, Thervet E, Souberbielle JC. et al. Effects of vitamin D supplementation on the calcium–phosphate balance in renal transplant patients. Kidney Int 2009; 75: 646–651 [DOI] [PubMed] [Google Scholar]
- 44. Sgambat K, Tuchman S, Ryan L. et al. Low bone mineral density and nutritional vitamin D deficiency in pediatric renal transplant recipients: assessment of risk factors and response to oral vitamin D therapy. Pediatr Transplant 2011; 15: 790–797 [DOI] [PubMed] [Google Scholar]
- 45. Amer H, Griffin MD, Stegall MD. et al. Oral paricalcitol reduces the prevalence of posttransplant hyperparathyroidism: results of an open label randomized trial. Am J Transplant 2013; 13: 1576–1585 [DOI] [PubMed] [Google Scholar]
- 46. Gonzalez E, Rojas-Rivera J, Polanco N. et al. Effects of oral paricalcitol on secondary hyperparathyroidism and proteinuria of kidney transplant patients. Transplantation 2013; 95: e49–e52 [DOI] [PubMed] [Google Scholar]
- 47. Trillini M, Cortinovis M, Ruggenenti P. et al. Paricalcitol for secondary hyperparathyroidism in renal transplantation. J Am Soc Nephrol 2015; 26: 1205–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]