Abstract
Purpose
Using the Risk, Injury, Failure, Loss of kidney function, End-stage kidney disease (RIFLE), Acute Kidney Injury Network (AKIN) and Kidney Disease: Improving Global Outcomes (KDIGO) systems, the incidence of acute kidney injury (AKI) and their ability to predict in-hospital mortality in severe sepsis or septic shock was compared.
Materials and methods
We performed a retrospective analysis of 457 critically ill patients with severe sepsis or septic shock hospitalized between January 2008 and December 2014. Multivariate logistic regression was employed to evaluate the association between the RIFLE, AKIN and KDIGO systems with in-hospital mortality. Model fit was assessed by the goodness-of-fit test and discrimination by the area under the receiver operating characteristic (AUROC) curve. Statistical significance was defined as P < 0.05.
Results
RIFLE (84.2%) and KDIGO (87.5%) identified more patients with AKI than AKIN (72.8%) (P < 0.001). AKI defined by AKIN and KDIGO was associated with in-hospital mortality {AKIN: adjusted odds ratio [OR] 2.3[95% confidence interval (CI) 1.3–4], P = 0.006; KDIGO: adjusted OR 2.7[95% CI 1.2–6.2], P = 0.021} while AKI defined by RIFLE was not [adjusted OR 2.0 (95% CI 1–4), P = 0.063]. The AUROC curve for in-hospital mortality was similar between the three classifications (RIFLE 0.652, P < 0.001; AKIN 0.686, P < 0.001; KDIGO 0.658, P < 0.001).
Conclusions
RIFLE and KDIGO diagnosed more patients with AKI than AKIN, but the prediction ability for in-hospital mortality was similar between the three systems.
Keywords: acute kidney injury, definition, incidence, mortality, sepsis
Introduction
Numerous definitions have been proposed to define acute kidney injury (AKI) [1], resulting in great discrepancy in the reported incidence of AKI. This heterogeneity of data has made the comparison of various published studies focusing on AKI difficult, and in many cases impossible. The development of two new classification systems for AKI over the last decade [Risk, Injury, Failure, Loss of kidney function, End-stage kidney disease (RIFLE) and Acute Kidney Injury Network (AKIN)] [2, 3] has led to enhanced knowledge concerning the epidemiology of AKI, demonstrating greater sensitivity and specificity in the diagnosis and stratification of AKI [4]. The RIFLE classification system (Table 1) was published in 2004 [2]. It stratifies AKI according to three classes of severity—risk, injury and failure—based on serum creatinine (SCr) or glomerular filtration rate (GFR) and on changes in urine output (UO) over a predetermined period of time and two classes of outcome—loss of kidney function and end-stage kidney disease—based on time of renal replacement therapy dependency. Worsening of renal function must occur over a period of 7 days and persist for >24 h for AKI to be deemed present. In the absence of a pre-existing history of chronic kidney disease (CKD) and when baseline SCr is unknown, a baseline GFR between 75 and 100 mL/min/1.73 m2 should be assumed and the Modification of Diet in Renal Disease (MDRD) equation should be used to calculate an estimated baseline SCr. However, some modifications were warranted due to the knowledge that even small increases in SCr are associated with poor outcomes [5], the fact that formulas that estimate GFR presume a steady state that is absent in AKI and, lastly, due to differences in accessibility and indications for initiation of renal replacement therapy among different institutions and countries. Therefore, in 2007, the AKIN classification (Table 1), also known as the modified RIFLE [3], was proposed and published with the intention of improving diagnostic accuracy. Instead of depending on either SCr or GFR, the AKIN classification relies solely on the former, calling for at least two measurements over a 48-h period, thereby discarding the requirement for known baseline SCr levels. The diagnosis of AKI and classification of patients into the various groups is carried out only after the hydration status has been optimized and after excluding urinary obstruction as a cause for renal dysfunction, and the AKIN classification does not consider outcome classes, as happens with the RIFLE classification. AKIN has not proved to be advantageous over RIFLE regarding the severity and outcomes of AKI despite showing a higher diagnostic sensitivity [6–8]. Recently, the RIFLE and AKIN classifications were merged into the Kidney Disease: Improving Global Outcomes (KDIGO) classification (Table 1), with the purpose of providing integrated yet simplified criteria to be applied in clinical activity, research and public health surveillance [9]. In this classification, severity has been stratified in the same way as AKIN, except for a simplification of the criteria required to reach stage 3. Theoretically, KDIGO offers advantages over the other two systems in identifying patients with AKI and in predicting outcome.
Table 1.
| Class/stage | SCr/GFR |
UO |
||||
|---|---|---|---|---|---|---|
| RIFLE | AKIN | KDIGO | RIFLE | AKIN | KDIGO | |
| Risk/1a | ↑ SCr ×1.5 or ↓ GFR >25% | ↑ SCr ≥26.5 μmol/L (≥0.3 mg/dL) or ↑ SCr ≥150–200% (1.5–2×) |
↑ SCr ≥26.5 μmol/L (≥0.3 mg/dL) or ↑ SCr ≥150–200% (1.5–2×) |
<0.5 mL/kg/h (>6 h) | <0.5 mL/kg/h (>6 h) | <0.5 mL/kg/h (>6 h) |
| Injury/2a | ↑ SCr ×2 or ↓ GFR >50% | ↑ SCr >200–300% (>2–3×) | ↑ SCr >200–300% (>2–3×) | <0.5 mL/kg/h (>12 h) | <0.5 mL/kg/h (>12 h) | <0.5 mL/kg/h (>12 h) |
| Failure/3a | ↑ SCr ×3 or ↓ GFR >75% or if baseline SCr ≥353.6 μmol/L (≥4 mg/dL) ↑ SCr 44.2 μmol/L (>0.5 mg/dL) |
↑ SCr >300% (>3×) or if baseline SCr ≥353.6 μmol/L (≥4 mg/dL) ↑SCr ≥44.2 μmol/L (≥0.5 mg/dL) or initiation of renal replacement therapy |
↑ SCr >300% (>3×) or ↑SCr to ≥353.6 μmol/L (≥4 mg/dL) or initiation of renal replacement therapy |
<0.3 mL/kg/h (>24 h) or anuria (>12 h) |
<0.3 mL/kg/h (24 h) or anuria (12 h) |
<0.3 mL/kg/h (24 h) or anuria (12 h) |
aRisk class (RIFLE) corresponds to stage 1 (AKIN and KDIGO), injury class (RIFLE) corresponds to stage 2 (AKIN and KDIGO) and failure class (RIFLE) corresponds to stage 3 (AKIN and KDIGO).
Sepsis is the leading cause of AKI in the intensive care unit (ICU) and patients with septic AKI are clinically distinct from those with non-septic AKI. Septic AKI is associated with high disease severity scores, non-renal organ failure, requirement for mechanical ventilation, need for vasoactive drugs, extended lengths of ICU and hospital stay, higher in-hospital mortality and increased probability of renal function recovery at hospital discharge [10, 11]. Consequently, a profound understanding of septic AKI is essential for the nephrologist and the intensivist to appropriately formulate diagnoses, treatment options and follow-up strategies, in addition to predicting patient outcome.
The KDIGO classification system has been recently assessed in several published studies [12–18]; however, its applicability to septic AKI and its comparison with the two previous classifications has not yet been performed or validated in this setting. Therefore, we sought to evaluate the incidence of AKI according to these three classifications (RIFLE, AKIN and KDIGO) and compared their ability to predict in-hospital mortality in a cohort of critically ill patients with severe sepsis or septic shock.
Materials and methods
The present study is retrospective in nature, including all patients with severe sepsis or septic shock admitted to the Division of Intensive Medicine of the Centro Hospitalar Lisboa Norte (Lisbon, Portugal) between January 2008 and December 2014. Centro Hospitalar Lisboa Norte is an academic and referral centre for 3 000 000 inhabitants.
The study was approved by the Ethical Committee at the Centro Hospitalar Lisboa Norte, EPE, in agreement with institutional guidelines. Informed consent was waived by the Ethical Committee due to the retrospective and non-interventional nature of the study.
Participants
Selection of potentially eligible patients was conducted based on the ICU patient admission register. All adult patients (≥18 years of age) with severe sepsis or septic shock admitted to the Division of Intensive Medicine were selected. Sepsis was defined by historical criteria in accordance with the American College of Chest Physicians and the Society of Critical Care Medicine consensus [19].
Exclusion criteria included (i) CKD patients already on renal replacement therapy, (ii) patients who underwent renal replacement therapy 1 week before ICU admission and (iii) patients who were discharged or died <2 days after admission in the ICU.
Variables and data sources
All variables were collected from electronic and handwritten patient clinical records. The analysed variables included patient demographic characteristics (age, gender, ethnicity and body weight), comorbidities [diabetes mellitus, hypertension, cardiovascular disease (CVD), chronic obstructive pulmonary disease (COPD), cirrhosis and malignancy], primary diagnosis (medical versus surgical), source of infection, serum haemoglobin, serum albumin, SCr, UO, disease severity according to the Simplified Acute Physiologic Score (SAPS) II [20] as determined by the worst variables recorded during the first 24 h, fluid balance, need for mechanical ventilation, vasopressor use and requirement for renal replacement therapy. Diabetes mellitus was diagnosed according to the American Diabetes Association criteria [21] and hypertension was diagnosed according to the seventh report of the Joint National Committee [22]. CVD was considered whenever a history of cerebrovascular disease, chronic heart failure, cardiac ischaemic disease and peripheral arterial disease was present, and COPD included emphysema and chronic bronchitis. For CVD and COPD, a previous diagnosis on clinical records was considered sufficient. In-hospital mortality was considered the outcome measure.
The development of AKI within the first week of ICU hospitalization was diagnosed and classified using the RIFLE, AKIN and KDIGO classifications based on both SCr and UO criteria (Table 1). The criteria that led to the worst classification were used and the maximum RIFLE, maximum AKIN and maximum KDIGO were reported. In this ICU, SCr is determined at least once a day and UO is recorded hourly for all patients. Pre-admission SCr (SCr within the previous 3 months) was considered as baseline SCr for RIFLE and KDIGO classifications and SCr on ICU admission was considered as baseline SCr for AKIN classification. When pre-admission SCr values were unavailable they were estimated from the MDRD equation [23] assuming the lower limit of a normal baseline GFR of 75 mL/min/1.73 m2. Hourly recorded urine output was available for all patients and 6-h periods were used to diagnose and classify AKI.
Statistical methods
Continuous variables were presented as the mean ± standard deviation and categorical variables as the total number and percentage of cases for each category. RIFLE classes, AKIN stages and KDIGO stages were compared using Student's t-test for normally distributed continuous variables, Mann–Whitney U test for non-normally distributed continuous variables and chi-square test for categorical variables.
Multivariate logistic regression analysis was employed to evaluate the association between RIFLE criteria, AKIN criteria and KDIGO criteria with in-hospital mortality. Model fit was assessed by the goodness-of-fit test and discrimination was assessed by the area under the receiver operating characteristic (AUROC) curve. Data were expressed as odds ratios (ORs) with 95% confidence intervals (CIs). Statistical significance was defined as a P-value <0.05. Analyses were performed with the statistical software package SPSS for Windows (version 21.0; SPSS, Chicago, IL, USA). The comparison between AUROC curves was made using the method of DeLong with the statistical software MedCalc for Windows (version 16.2; MedCalc Software, Ostend, Belgium).
Results
Participants
After analysis of the ICU patient admission register, 723 patients were selected as potentially eligible. Of these, 266 were excluded: 122 had CKD on renal replacement therapy and 144 had been hospitalized for less than 48 h. None required renal replacement therapy in the week preceding ICU admission. Consequently, we focused on a final cohort of 457 patients. Patient baseline characteristics are described in Table 2.
Table 2.
Patients' baseline characteristics
| Characteristic | Value |
|---|---|
| Age (years), mean ± SD | 64.1 ± 16.4 |
| Gender (Male), n (%) | 264 (57.9) |
| Race (Caucasian), n (%) | 433 (94.7) |
| Weight (kg), mean ± SD | 76.2 ± 18.2 |
| Hypertension, n (%) | 212 (46.5) |
| Co-morbidities, n (%) | |
| Diabetes | 103 (22.6) |
| CVD | 125 (27.4) |
| COPD | 38 (8.3) |
| Cirrhosis | 18 (3.9) |
| Malignancy | 109 (23.9) |
| Medical admission, n (%) | 253 (55.5) |
| Infection source, n (%) | |
| Abdominal | 187 (41.0) |
| Respiratory | 138 (30.3) |
| Kidney | 57 (12.5) |
| Skin | 34 (7.5) |
| Other | 26 (5.7) |
| Unknown | 14 (3.1) |
| SAPS II, mean ± SD | 49.4 ± 17.3 |
| Baseline SCr (mg/dL)a, mean ± SD | 1.3 ± 0.6 |
| Admission SCr (mg/dL)b, mean ± SD | 2.3 ± 1.5 |
| Haemoglobin (g/dL)c, mean ± SD | 10.4 ± 2.0 |
| Serum albumin (g/dL)c, mean ± SD | 1.9 ± 0.6 |
| Mechanical ventilation, n (%)d | 350 (76.8) |
| Vasopressors, n (%)d | 316 (69.3) |
| Fluid balance (L)d, mean ± SD | 4.5 ± 5.7 |
| RRT, n (%)d | 108 (23.7) |
| LOS in hospital (days), mean ± SD | 37.1 ± 39.4 |
| LOS in ICU (days), mean ± SD | 10.0 ± 10.0 |
| ICU mortality, n (%) | 108 (23.7) |
| In-hospital mortality, n (%) | 153 (33.6) |
LOS, length of stay; RRT, renal replacement therapy; SD, standard deviation.
aUsed in RIFLE and KDIGO classifications.
bUsed in AKIN classification.
cOn ICU admission.
dDuring ICU stay.
Demographic and clinical characteristics of the studied population according to AKI development are described in Table 3. Pre-admission SCr was available in 185 patients (40.6%) and in the remaining cases [n = 272(59.4%)] it was estimated using the MDRD formula, assuming a baseline eGFR of 75 mL/min/1.73 m2. As expected, when compared with patients not developing AKI, AKI patients were more likely to have significantly higher SAPS II values (P < 0.001 for RIFLE and AKIN; P = 0.002 for KDIGO) and to require vasopressors (P < 0.001 for RIFLE, AKIN and KDIGO). Lower serum albumin values were also more commonly found among AKI patients (P = 0.018 for RIFLE; P = 0.003 for AKIN and KDIGO). Furthermore, ICU mortality (P = 0.033 for RIFLE; P = 0.005 for AKIN; P = 0.03 for KDIGO) and in-hospital mortality (P = 0.026 for RIFLE; P = 0.001 for AKIN; P = 0.006 for KDIGO) were also higher in AKI patients than in non-AKI patients.
Table 3.
Demographic and clinical characteristics of patients according to the development of AKI defined by the RIFLE, AKIN and KDIGO classifications
| Characteristic | RIFLE |
AKIN |
KDIGO |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No AKI (n = 72) | AKI (n = 384) | P-value | No AKI (n = 124) | AKI (n = 332) | P-value | No AKI (n = 57) | AKI (n = 399) | P-value | |
| Age (years), mean ± SD | 62.4 ± 18.2 | 64.4 ± 15.6 | 0.321 | 62.6 ± 17.1 | 64.6 ± 15.6 | 0.215 | 64.1 ± 17.4 | 64.1 ± 16.0 | 0.994 |
| Gender (male), n (%) | 16 (63.9) | 218 (56.8) | 0.262 | 73 (58.9) | 191 (57.5) | 0.796 | 35 (61.4) | 229 (57.4) | 0.566 |
| Race (Caucasian), n (%) | 67 (93.1) | 366 (95.3) | 0.422 | 116 (93.5) | 317 (95.5) | 0.401 | 54 (94.7) | 379 (95.0) | 0.936 |
| Weight (kg), mean ± SD | 71.01 ± 14.5 | 77.2 ± 18.7 | 0.009 | 73.1 ± 15.7 | 77.4 ± 19.0 | 0.024 | 70.9 ± 14.3 | 77.0 ± 18.6 | 0.019 |
| Co-morbidities, n (%) | |||||||||
| Hypertension | 34 (47.2) | 178 (46.4) | 0.892 | 59 (47.6) | 153 (46.1) | 0.776 | 30 (50.2) | 182 (45.6) | 0.320 |
| Diabetes | 17 (23.6) | 86 (22.4) | 0.821 | 29 (23.4) | 74 (23.3) | 0.803 | 15 (26.3) | 88 (22.1) | 0.472 |
| CVD | 17 (23.6) | 108 (28.1) | 0.431 | 29 (23.4) | 96 (28.9) | 0.239 | 14 (24.6) | 111 (27.8) | 0.606 |
| COPD | 6 (8.3) | 32 (8.3) | 1.000 | 12 (9.7) | 26 (7.8) | 0.526 | 5 (8.8) | 33 (8.3) | 0.898 |
| Cirrhosis | 2 (2.8) | 16 (4.2) | 0.579 | 3 (2.4) | 15 (4.5) | 0.306 | 1 (1.8) | 17 (4.3) | 0.363 |
| Malignancy | 18 (25) | 91 (23.7) | 0.812 | 33 (26.6) | 76 (22.9) | 0.407 | 13 (22.8) | 96 (24.1) | 0.836 |
| Medical admission, n (%) | 41 (56.9) | 212 (55.2) | 0.768 | 67 (54.0) | 186 (56.0) | 0.703 | 31 (54.4) | 222 (55.6) | 0.859 |
| Infection source, n (%) | |||||||||
| Abdominal | 23 (31.9) | 164 (42.7) | 0.088 | 47 (37.9) | 140 (42.2) | 0.410 | 19 (33.3) | 168 (42.1) | 0.208 |
| Respiratory | 22 (30.6) | 116 (30.2) | 0.953 | 40 (32.3) | 98 (29.5) | 0.571 | 16 (28.3) | 122 (30.6) | 0.700 |
| Kidney | 16 (22.2) | 41 (10.7) | 0.007 | 24 (19.4) | 33 (9.9) | 0.007 | 14 (24.6) | 43 (10.8) | 0.003 |
| Skin | 3 (4.2) | 31 (8.1) | 0.247 | 5 (4.0) | 29 (8.7) | 0.089 | 2 (3.5) | 32 (8.0) | 0.225 |
| Other | 7 (9.7) | 19 (4.9) | 0.109 | 7 (5.6) | 19 (5.7) | 0.975 | 6 (10.5) | 20 (5.0) | 0.093 |
| Unknown | 1 (1.4) | 13 (3.4) | 0.367 | 1 (0.8) | 13 (3.9) | 0.087 | 0 (0.0) | 14 (3.5) | 0.151 |
| SAPS II, mean ± SD | 42.8 ± 15.3 | 50.7 ± 17.4 | <0.001 | 44.3 ± 15.3 | 51.3 ± 17.6 | <0.001 | 42.8 ± 15.6 | 50.4 ± 17.3 | 0.002 |
| Baseline SCr (mg/dL)a, mean ± SD | 1.4 ± 0.6 | 1.3 ± 0.6 | 0.169 | 1.4 ± 0.7 | 1.3 ± 0.6 | 0.185 | |||
| Admission SCr (mg/dL)b, mean ± SD | 2.1 ± 1.5 | 2.3 ± 1.5 | 0.176 | ||||||
| Haemoglobin (g/dL)c, mean ± SD | 10.1 ± 2.1 | 10.4 ± 2.0 | 0.248 | 10.1 ± 1.8 | 10.5 ± 2.1 | 0.047 | 10.0 ± 1.7 | 10.5 ± 2.0 | 0.078 |
| Serum albumin (g/dL)c, mean ± SD | 2.1 ± 0.6 | 1.9 ± 0.6 | 0.018 | 2.1 ± 0.6 | 1.9 ± 0.6 | 0.003 | 2.1 ± 0.6 | 1.9 ± 0.6 | 0.003 |
| Mechanical ventilation, n (%)d | 51 (70.8) | 85 (77.9) | 0.195 | 88 (71) | 262 (78.9) | 0.074 | 41 (71.9) | 309 (77.4) | 0.357 |
| Vasopressors, n (%)d | 35 (48.6) | 281 (73.2) | <0.001 | 66 (53.2) | 250 (75.3) | <0.001 | 26 (45.6) | 290 (72.7) | <0.001 |
| Fluid balance (L)d, mean ± SD | 3.9 ± 6.9 | 4.6 ± 5.4 | 0.335 | 3.7 ± 5.9 | 4.8 ± 5.6 | 0.065 | 3.6 ± 6.9 | 4.6 ± 5.4 | 0.180 |
| RRT, n (%)d | 108 (28.1) | 108 (32.5) | 108 (27.1) | ||||||
| LOS in hospital (days), mean ± SD | 41.4 ± 54.3 | 36.3 ± 35.9 | 0.314 | 36.6 ± 37.2 | 37.2 ± 40.2 | 0.879 | 39.5 ± 44.8 | 36.7 ± 38.6 | 0.625 |
| LOS in ICU (days), mean ± SD | 9.9 ± 9.9 | 10.0 ± 10.1 | 0.913 | 9.4 ± 9.4 | 10.2 ± 10.3 | 0.431 | 9.8 ± 10.3 | 10.0 ± 10.0 | 0.881 |
| ICU mortality, n (%) | 10 (13.9) | 98 (25.5) | 0.033 | 18 (14.5) | 90 (27.1) | 0.005 | 7 (12.3) | 101 (25.3) | 0.030 |
| Hospital mortality, n (%) | 16 (22.2) | 137 (35.7) | 0.026 | 26 (21.0) | 127 (38.3) | 0.001 | 10 (17.5) | 143 (87.5) | 0.006 |
aUsed in RIFLE and KDIGO classifications.
bUsed in AKIN classification.
cOn ICU admission.
dDuring ICU stay.
AKI stratified by the RIFLE, AKIN and KDIGO criteria
AKI occurred in 84.2% of patients with a maximum RIFLE category: risk in 16.9%, injury in 21.9% and failure in 45.4%. According to AKIN and KDIGO criteria, AKI occurred in 72.8% of patients (24.1% with stage 1, 15.4% with stage 2 and 33.3% with stage 3) and 87.5% of patients (19.5% with stage 1, 22.6% with stage 2 and 45.4% with stage 3), respectively (Table 4). RIFLE and KDIGO criteria allowed for the identification of more patients as having AKI than AKIN criteria (P < 0.001, respectively) and classified more patients with injury (P = 0.011)/stage 2 of KDIGO (P = 0.005) and failure (P = 0.002)/stage 3 of KDIGO (P < 0.001), although AKIN identified more patients with stage 1 than RIFLE (P = 0.007). There were no significant differences in AKI incidence (overall AKI and severity classes/stages) between RIFLE and KDIGO classifications (Table 5).
Table 4.
Patients with AKI classified by creatinine criteria, UO criteria or both
| SCr | UO | SCr + UO | |
|---|---|---|---|
| RIFLE classification, % | |||
| Risk | 53.2 | 31.2 | 15.6 |
| Injury | 65.4 | 26.7 | 7.9 |
| Failure | 46.2 | 38.3 | 15.5 |
| Any category | 52.6 | 33.9 | 13.5 |
| AKIN classification, % | |||
| Stage 1 | 50.5 | 32.1 | 17.4 |
| Stage 2 | 44.1 | 55.9 | 0 |
| Stage 3 | 28.1 | 64.7 | 7.2 |
| Any category | 38.8 | 52.1 | 9.1 |
| KDIGO classification, % | |||
| Stage 1 | 49.1 | 34.2 | 16.7 |
| Stage 2 | 31.0 | 67.3 | 1.7 |
| Stage 3 | 34.7 | 55.9 | 9.4 |
| Any category | 38.7 | 50.9 | 10.4 |
Table 5.
Incidence of AKI according to RIFLE, AKIN and KDIGO criteria
| RIFLE | AKIN | P-value | |
| Risk/stage 1 | 77 (16.9) | 110 (24.1) | 0.007 |
| Injury/stage 2 | 100 (21.9) | 70 (15.5) | 0.011 |
| Failure/stage 3 | 207 (45.4) | 152 (33.3) | 0.002 |
| Any category | 384 (84.2) | 332 (72.8) | <0.001 |
| RIFLE | KDIGO | P-value | |
| Risk/stage 1 | 77 (16.9) | 89 (19.5) | 0.303 |
| Injury/stage 2 | 100 (21.9) | 103 (22.6) | 0.811 |
| Failure/stage 3 | 207 (45.4) | 207 (45.4) | 1 |
| Any category | 384 (84.2) | 399 (87.5) | 0.154 |
| AKIN | KDIGO | P-value | |
| Risk/stage 1 | 110 (24.1) | 89 (19.5) | 0.092 |
| Injury/stage 2 | 70 (15.5) | 103 (22.6) | 0.005 |
| Failure/stage 3 | 152 (33.3) | 207 (45.4) | <0.001 |
| Any category | 332 (72.8) | 399 (87.5) | <0.001 |
Values given as n (%).
Creatinine criteria led to a maximum RIFLE in 52.6% of patients, a maximum AKIN in 38.8% of patients and a maximum KDIGO in 38.7% of patients, while UO criteria led to a maximum RIFLE in 33.9% of patients, a maximum AKIN in 52.1% of patients and a maximum KDIGO in 50.9% of patients, whereas in almost 10% of patients it was both the UO and creatinine criteria that led to a maximum RIFLE, a maximum AKIN and a maximum KDIGO (Table 4).
In-hospital mortality
AKI defined by AKIN and KDIGO based on SCr and UO criteria was independently associated with increased in-hospital mortality [AKIN: any category 38.3%, adjusted OR 2.3 (95% CI 1.3–4), P = 0.006; KDIGO: any category 35.8%, adjusted OR 2.7 (95% CI 1.2–6.2), P = 0.021], while AKI defined by RIFLE was not [any category 35.7%, adjusted OR 2.0 (95% CI 1–4), P = 0.063]. In addition, failure [48.3%, adjusted OR 1.4 (95% CI 1.1–1.8), P = 0.009], as well as stage 3 of AKIN and KDIGO [AKIN 56.6%, adjusted OR 1.6 (95% CI 1.3–2.1), P < 0.001; KDIGO 48.3%, adjusted OR 2.7 (95% CI 1.2–6.2), P = 0.021], was also independently associated with an increased risk for death. In contrast, risk and stage 1 of AKIN and KDIGO, as well as injury and stage 2 of AKIN and KDIGO, were not associated with increased in-hospital mortality (Tables 6 and 7).
Table 6.
In-hospital mortality for AKI defined and classified according to RIFLE, AKIN and KDIGO criteria
| RIFLE | AKIN | P-value | |
| Risk/stage 1 | 25 (18.2) | 23 (18.2) | 0.767 |
| Injury/stage 2 | 32 (23.0) | 38 (30.0) | 0.455 |
| Failure/stage 3 | 66 (48.3) | 72 (56.6) | 0.579 |
| Any category | 49 (35.7) | 49 (38.3) | 1 |
| RIFLE | KDIGO | P-value | |
| Risk/stage 1 | 25 (18.2) | 30 (21.3) | 0.487 |
| Injury/stage 2 | 32 (23.0) | 33 (23.3) | 0.898 |
| Failure/stage 3 | 66 (48.3) | 69 (48.3) | 0.78 |
| Any category | 49 (35.7) | 51 (35.8) | 0.832 |
| AKIN | KDIGO | P-value | |
| Risk/stage 1 | 23 (18.2) | 30 (21.3) | 0.425 |
| Injury/stage 2 | 38 (30.0) | 33 (23.3) | 0.537 |
| Failure/stage 3 | 72 (56.6) | 69 (48.3) | 0.783 |
| Any category | 49 (38.3) | 51 (35.8) | 0.832 |
Values given as n (%).
Table 7.
Multivariate regression analysis of in-hospital mortality for the RIFLE, AKIN and KDIGO classifications
| Criteria | OR (95% CI) | P-value | AUROC curve (95% CI) |
|---|---|---|---|
| RIFLE criteria (SCr + UO) | |||
| Risk | 0.9 (0.3–2.4) | 0.820 | 0.652 (0.607–0.696) |
| Injury | 1.3 (0.8–2.1) | 0.284 | |
| Failure | 1.4 (1.1–1.8) | 0.009 | |
| Any category | 2.0 (1.0–4.0) | 0.063 | |
| AKIN criteria (SCr + UO) | |||
| Stage 1 | 0.9 (0.4–1.9) | 0.768 | 0.686 (0.642–0.729) |
| Stage 2 | 1.3 (0.8–2.0) | 0.234 | |
| Stage 3 | 1.6 (1.3–2.1) | <0.000 | |
| Any stage | 2.3 (1.3–4.0) | 0.006 | |
| KDIGO criteria (SCr + UO) | |||
| Stage 1 | 1.5 (0.5–4.3) | 0.486 | 0.658 (0.612–0.701) |
| Stage 2 | 1.7 (0.9–3.1) | 0.085 | |
| Stage 2 | 1.6 (1.1–2.1) | 0.005 | |
| Any stage | 2.7 (1.2–6.2) | 0.021 | |
| RIFLE criteria (SCr) | |||
| Risk | 1.1 (0.6–2.2) | 0.737 | 0.506 (0.449–0.562) |
| Injury | 1.1 (0.8–1.5) | 0.456 | |
| Failure | 1.0 (0.8–1.3) | 0.779 | |
| Any category | 1.1 (0.7–1.9) | 0.626 | |
| AKIN criteria (SCr) | |||
| Stage 1 | 1.0 (0.6–1.7) | 0.955 | 0.528 (0.472–0.585) |
| Stage 2 | 0.9 (0.5–1.4) | 0.508 | |
| Stage 3 | 1.4 (1.1–1.9) | 0.014 | |
| Any stage | 1.6 (1.0–2.6) | 0.057 | |
| KDIGO criteria (SCr) | |||
| Stage 1 | 1.1 (0.5–2.3) | 0.872 | 0.605 (0.549–0.661) |
| Stage 2 | 0.8 (0.5–1.3) | 0.466 | |
| Stage 2 | 1.2 (1.0–1.5) | 0.076 | |
| Any stage | 1.3 (0.7–2.4) | 0.322 | |
| RIFLE, AKIN and KDIGO criteria (UO) | |||
| Risk | 0.8 (0.4–1.6) | 0.466 | 0.701 (0.647–0.754) |
| Injury | 1.8 (1.2–2.7) | 0.004 | |
| Failure | 1.8 (1.4–2.3) | <0.000 | |
| Any category | 2.7 (1.7–4.5) | <0.000 | |
Multivariate analysis included age, gender, race, diabetes mellitus, hypertension, CVD, COPD, cirrhosis, malignancy, medical admission, illness severity evaluated by SAPS II, haemoglobin, serum albumin, need for vasopressors or mechanical ventilation and fluid balance.
Multivariate analysis was repeated using the RIFLE, AKIN and KDIGO classifications based either on creatinine criteria or on UO criteria (Table 7). On the one hand, AKI defined by SCr criteria, as well as any of its classes/stages, was not associated with increased risk for in-hospital death. On the other hand, when AKI was defined and categorized by UO criteria, any category of AKI [adjusted OR 2.7 (95% CI 1.7–4.5), P < 0.001], as well as injury/stage 2 [adjusted OR 1.8 (95% CI 1.2–2.7), P = 0.004] and failure/stage 3 [adjusted OR 1.8 (95% CI 1.4–2.3), P < 0.001], was significantly associated with increased in-hospital mortality.
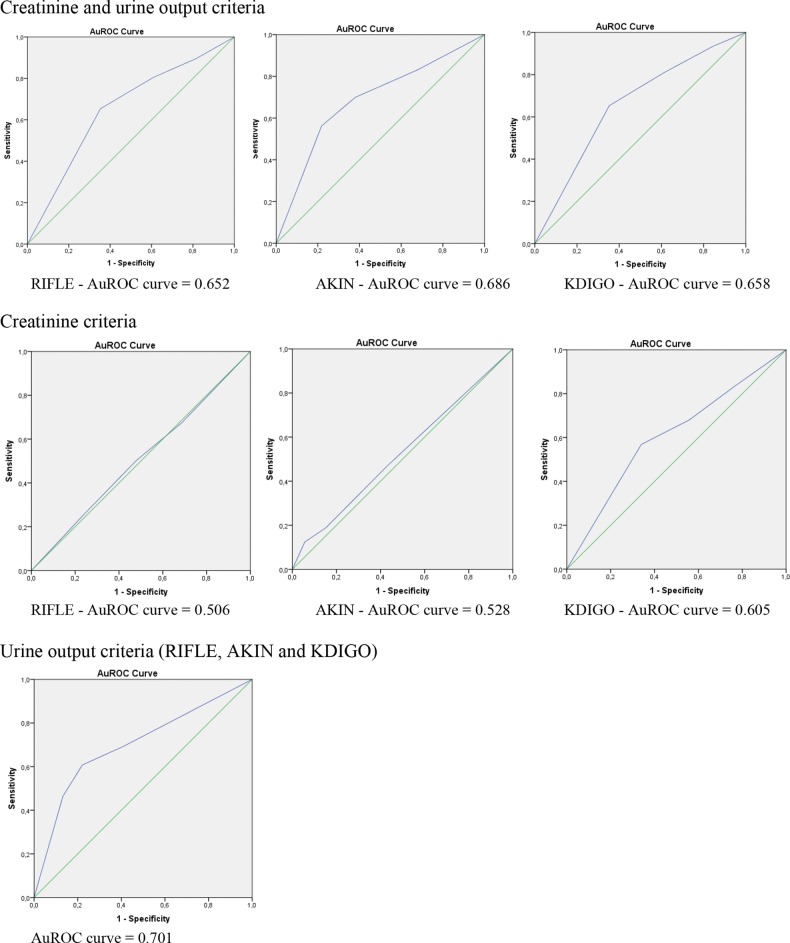
When considering both creatinine and UO criteria, the AUROC curve for in-hospital mortality was 0.652 for RIFLE criteria (P < 0.001), 0.686 for AKIN criteria (P < 0.001) and 0.658 for KDIGO criteria (P < 0.001) (Figure 1). There were no statistically significant differences in AUROC curves between the three classifications (RIFLE versus AKIN, P = 0.227; RIFLE versus KDIGO, P = 0.156; AKIN versus KDIGO, P = 0.147). The AUROC curves of these classifications for in-hospital mortality were significantly higher when defining AKI only by UO criteria as compared with SCr criteria (RIFLE, P < 0.001; AKIN, P < 0.001; KDIGO, P = 0.004).
FIGURE 1:
Area under the receiver operating characteristic (AUROC) curves for in-hospital mortality according to the Risk, Injury, Failure, Loss of kidney function, End-stage kidney disease (RIFLE), the Acute Kidney Injury Network (AKIN) and Kidney Disease: Improving Global Outcomes (KDIGO) classifications based on serum creatinine and urine output criteria.
Discussion
We conducted a single-centre study comprising 457 critically ill patients admitted to the ICU with severe sepsis or septic shock, aiming to evaluate the incidence of AKI according to the new definitions/classifications for AKI—the RIFLE, AKIN and KDIGO systems—and to compare their ability in predicting in-hospital mortality of those patients.
In the present study, the incidence of AKI was high, varying between 72.8 and 87.5% depending on the definition used. These results are in accordance with other retrospective studies carried out primarily in sepsis cohorts and that have also reported a high occurrence of septic-associated AKI. In these studies, >60–70% of patients with septic shock suffered AKI [24, 25].
A small number of recently published studies have compared the incidence of AKI as defined and categorized by the RIFLE, AKIN and KDIGO systems, as well as their prediction performance in different medical settings. In the FINNAKI study, the incidence of AKI defined by the AKIN and KDIGO criteria among the 2901 ICU patients studied was identical (39%) [12]. In a prospective study enrolling 637 patients hospitalized for acute decompensated heart failure, the incidence of AKI as defined by RIFLE, AKIN and KDIGO criteria was also similar and there were only slight differences in the predictive ability between RIFLE and KDIGO in relation to clinical outcomes at 30 days (AUROC 0.76 and 0.74, respectively) [13]. In a cohort of 1050 patients diagnosed with acute myocardial infarction, KDIGO criteria detected significantly more AKI patients than RIFLE criteria (36.6 versus 14.8%) and those patients diagnosed as having AKI by KDIGO criteria, but not RIFLE criteria, had a substantially higher early and late mortality (adjusted hazard ratio for 30-day death of 3.51 by RIFLE and 3.99 by KDIGO; adjusted hazard ratio for 1-year mortality of 1.84 by RIFLE and 2.43 by KDIGO) [14]. The incidence of AKI was identical between the RIFLE, AKIN and KDIGO classification systems in a retrospective analysis of 1881 adult patients who underwent cardiac surgery, however, the AUROC curve was significantly greater when using the AKIN as compared with the RIFLE criteria (0.86 versus 0.78, P < 0.001) [15]. In a recent retrospective cohort study considering 31 970 hospitalizations, the incidence of AKI was similar between the RIFLE, AKIN and KDIGO systems, as were their adjusted ORs for in-hospital death [16]. In a Chinese prospective study of 3107 ICU patients, KDIGO criteria diagnosed more AKI patients than both RIFLE (51 versus 46.9%, P = 0.001) and AKIN criteria (51 versus 38.4%, P < 0.001) and the AUROC curves for in-hospital mortality were 0.738 (P < 0.001) for RIFLE, 0.746 (P < 0.001) for AKIN and 0.757 (P < 0.001) for KDIGO. KDIGO was shown to be more predictive than RIFLE for in-hospital mortality (P < 0.001), but there were no differences between KDIGO and AKIN (P = 0.12) regarding this outcome [17]. Fujii et al. [18] recently completed a retrospective analysis of 49 518 hospitalizations and reported that the RIFLE and KDIGO criteria identified more patients with AKI than AKIN (11 versus 4.8%) and proved to have a higher discrimination ability for in-hospital mortality (AUROC curve for RIFLE 0.77; AUROC curve for KDIGO 0.78; AUROC curve for AKIN 0.69).
In our analysis, we found that RIFLE and KDIGO criteria permitted the identification of more patients as having AKI than AKIN criteria and classified more patients with injury/stage 2 of KDIGO and failure/stage 3 of KDIGO. However, AKIN recognized more patients with stage 1 AKI than RIFLE criteria. No significant differences in AKI incidence (overall AKI and severity classes/stages) were found when comparing RIFLE and KDIGO classifications. Furthermore, the prediction performance for in-hospital mortality was similar between the three classifications.
The pre-admission SCr level (1.3 ± 0.6 mg/dL) was much lower than the mean SCr level on the day of admission to the ICU (2.3 ± 1.5 mg/dL), implying that AKI may have already been present on the day of ICU admission or even earlier. In accordance with the definition of AKI by AKIN criteria, AKI was diagnosed by two creatinine measurements within 48 h. Unfortunately, most patients did not have a daily creatinine measurement prior to the ICU admission. Therefore, when using creatinine at ICU admission, some AKI cases may have been undeniably overlooked [26–28]. In addition, patients with a slow but progressive decrease in renal function may have also gone unnoticed when using the AKIN criteria for diagnosis [29].
In the current study, approximately one-third of patients with AKI as defined by the RIFLE classification system and one-half of patients with AKI defined by the KDIGO and AKIN systems were diagnosed as so based merely on UO criteria. We speculate that the higher percentage of AKI patients identified by UO criteria alone compared with those in whom AKI was diagnosed only by SCr criteria could be related to more pronounced oliguria often seen in septic patients, hemodilution due to aggressive fluid resuscitation and greater degrees of positive fluid balance usually achieved in septic patients, resulting in lower SCr values, and reduced production of endogenous creatinine in sepsis [30–32].
There is a lack of conclusive data on the impact of AKI defined by SCr and/or UO on mortality. For example, in a systematic review, the relative risk for death among studies that used RIFLE based on SCr and UO was lower than in those using RIFLE based exclusively on SCr [33]. Nevertheless, in a previous study we did not find any difference in terms of mortality for RIFLE based on SCr and UO and RIFLE based on SCr alone [6] and, similar to a North East Italian multicentre prospective study in 2164 ICU patients concerning AKI classified by the RIFLE criteria [34], the SCr criteria appeared to be a better predictor of mortality than UO. In contrast, in a recent prospective cohort study of critically ill patients, in those with AKI diagnosed exclusively by UO criteria, the need for renal replacement therapy was more frequent, the length of ICU stay was longer and the mortality rate was higher than in those patients without AKI [35]. We determined that AKI defined only by UO criteria was superior in the prediction of in-hospital mortality than AKI defined either by SCr itself or by both SCr and UO. Hence, UO seems to be a valid criterion with prognostic value in patients with septic AKI.
The present study has some limitations, including, first, its single-centre retrospective nature and relatively small cohort of patients. Second, the pre-admission SCr level was unknown to us in almost 60% of patients, compelling us to calculate an estimated baseline function using the MDRD equation, as recommended (assuming the lower limit of the normal baseline GFR of 75 mL/min/1.73 m2) and previously applied [2, 6, 36, 37]. The estimation of a baseline SCr from the MDRD equation when pre-admission SCr is unavailable appears to perform reasonably well only if and when pre-admission GFR is near normal; however, in patients with supposed CKD, use of the MDRD equation to estimate baseline SCr overestimates the incidence of AKI and should not be employed [38]. Third, notwithstanding having measurements of UO on an hourly basis, data regarding additional factors that could influence UO, such as the use of diuretic therapy, was not available to us. Overall, we recognize that the presence of any biases would ultimately influence all three classifications, and thus would not significantly impact our conclusions.
Despite these limitations, our study has numerous strengths. To the best of our knowledge, this is the first study comparing the incidence of AKI as defined by the RIFLE, AKIN and KDIGO criteria and comparing their prognostic capability in critically ill patients with severe sepsis or septic shock. Moreover, we used both creatinine criteria and UO criteria to define and categorize AKI. Finally, notwithstanding the retrospective nature of the study, most of the studied variables were routinely registered during daily clinical practice.
Conclusions
Granting that the RIFLE and KDIGO criteria potentially diagnose more ICU patients with severe sepsis or septic shock as having AKI than the AKIN criteria, the prediction ability for discerning in-hospital mortality was similar between the three systems. Nonetheless, future prospective studies enrolling a larger number of patients are still warranted to better determine the sensitivity and prognostic performance of these classifications in critically ill patients with severe sepsis or septic shock.
Conflict of interest statement
None declared.
References
- 1. Kellum JA, Levin N, Bouman C et al. Developing a consensus classification system for acute renal failure. Curr Opin Crit Care 2002; 8: 509–514 [DOI] [PubMed] [Google Scholar]
- 2. Bellomo R, Ronco C, Kellum JA et al. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8: R204–R212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kellum JA, Mehta RL, Levin A et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11: R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lopes JA, Jorge S. The RIFLE and AKIN classifications for acute kidney injury: a critical and comprehensive review. Clin Kidney J 2013; 6: 8–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coca SG, Peixoto AJ, Garg AX et al. The prognostic importance of a small acute decrement in kidney function in hospitalized patients: a systematic review and meta-analysis. Am J Kidney Dis 2007; 50: 712–720 [DOI] [PubMed] [Google Scholar]
- 6. Lopes JA, Fernandes P, Jorge S et al. Acute kidney injury in intensive care unit patients: a comparison between the RIFLE and the Acute Kidney Injury Network classifications. Crit Care 2008; 12: R110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Joannidis M, Metnitz B, Bauer P et al. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med 2009; 35: 1692–1697 [DOI] [PubMed] [Google Scholar]
- 8. Haase M, Bellomo R, Matalanis G et al. A comparison of the RIFLE and Acute Kidney Injury Network classifications for cardiac surgery-associated acute kidney injury: a prospective cohort study. J Thorac Cardiovasc Surg 2009; 138: 1370–1376 [DOI] [PubMed] [Google Scholar]
- 9. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012; 2: S1–S138 [Google Scholar]
- 10. Uchino S, Kellum JA, Bellomo R et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 2005; 294: 813–818 [DOI] [PubMed] [Google Scholar]
- 11. Bagshaw SM, Uchino S, Bellomo R et al. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol 2007; 2: 431–439 [DOI] [PubMed] [Google Scholar]
- 12. Nisula S, Kaukonen KM, Vaara ST et al. Incidence, risk factors and 90-day mortality of patients with acute kidney injury in Finnish intensive care units: the FINNAKI study. Intensive Care Med 2013; 39: 420–428 [DOI] [PubMed] [Google Scholar]
- 13. Roy AK, Mc Gorrian C, Treacy C et al. A comparison of traditional and novel definitions (RIFLE, AKIN, and KDIGO) of acute kidney injury for the prediction of outcomes in acute decompensated heart failure. Cardiorenal Med 2013; 3: 26–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rodrigues FB, Bruetto RG, Torres US et al. Incidence and mortality of acute kidney injury after myocardial infarction: a comparison between KDIGO and RIFLE criteria. PLoS One 2013; 8: e69998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bastin AJ, Ostermann M, Slack AJ et al. Acute kidney injury after cardiac surgery according to Risk/Injury/Failure/Loss/End-stage, Acute Kidney Injury Network, and Kidney Disease: Improving Global Outcomes classifications. J Crit Care 2013; 28: 389–396 [DOI] [PubMed] [Google Scholar]
- 16. Zeng X, McMahon GM, Brunelli SM et al. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol 2014; 9: 12–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Luo X, Jiang L, Du B et al. A comparison of different diagnostic criteria of acute kidney injury in critically ill patients. Crit Care 2014; 18: R144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fujii T, Uchino S, Takinami M et al. Validation of the Kidney Disease Improving Global Outcomes criteria for AKI and comparison of three criteria in hospitalized patients. Clin J Am Soc Nephrol 2014; 9: 848–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levy MM, Fink MP, Marshall JC et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003; 4: 1250–1256 [DOI] [PubMed] [Google Scholar]
- 20. Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993; 270: 2957–2963 [DOI] [PubMed] [Google Scholar]
- 21. ADA. Standards of medical care in diabetes—2009. Diabetes Care 2009; 32(Suppl 1): S13–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chobanian AV, Bakris GL, Black HR et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289: 2560–2572 [DOI] [PubMed] [Google Scholar]
- 23. Manjunath G, Sarnak MJ, Levey AS. Prediction equations to estimate glomerular filtration rate: an update. Curr Opin Nephrol Hypertens 2001; 10: 785–792 [DOI] [PubMed] [Google Scholar]
- 24. Bagshaw SM, Lapinsky S, Dial S et al. Acute kidney injury in septic shock: clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med 2009; 35: 871–881 [DOI] [PubMed] [Google Scholar]
- 25. Sood MM, Shafer LA, Ho J et al. Early reversible acute kidney injury is associated with improved survival in septic shock. J Crit Care 2014; 29: 711–717 [DOI] [PubMed] [Google Scholar]
- 26. Srisawat N, Kellum JA. Acute kidney injury: definition, epidemiology, and outcome. Curr Opin Crit Care 2011; 17: 548–555 [DOI] [PubMed] [Google Scholar]
- 27. Bagshaw SM. Acute kidney injury: diagnosis and classification of AKI: AKIN or RIFLE? Nat Rev Nephrol 2010; 6: 71–73 [DOI] [PubMed] [Google Scholar]
- 28. Siew ED, Matheny ME, Ikizler TA et al. Commonly used surrogates for baseline renal function affect the classification and prognosis of acute kidney injury. Kidney Int 2010; 77: 536–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ostermann M, Chang R; Riyadh ICU Program Users Group. Correlation between the AKI classification and outcome. Crit Care 2008; 12: R144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Angus DC, Linde-Zwirble WT, Lidicker J et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29: 1303–1310 [DOI] [PubMed] [Google Scholar]
- 31. Bagshaw SM, George C, Bellomo R; ANZICS Database Management Committee. Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care 2008; 12: R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kolhe NV, Stevens PE, Crowe AV et al. Case mix, outcome and activity for patients with severe acute kidney injury during the first 24 hours after admission to an adult, general critical care unit: application of predictive models from a secondary analysis of the ICNARC Case Mix Programme database. Crit Care 2008; 12(Suppl 1): S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int 2008; 73: 538–546 [DOI] [PubMed] [Google Scholar]
- 34. Cruz DN, Bolgan I, Perazella MA et al. North East Italian Prospective Hospital Renal Outcome Survey on Acute Kidney Injury (NEiPHROS-AKI): targeting the problem with the RIFLE criteria. Clin J Am Soc Nephrol 2007; 2: 418–425 [DOI] [PubMed] [Google Scholar]
- 35. Macedo E, Malhotra R, Claure-Del Granado R et al. Defining UO criterion for acute kidney injury in critically ill patients. Nephrol Dial Transplant 2011; 26: 509–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Uchino S, Bellomo R, Goldsmith D et al. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med 2006; 34: 1913–1917 [DOI] [PubMed] [Google Scholar]
- 37. Hoste EA, Clermont G, Kersten A et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care 2006; 10: R73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bagshaw SM, Uchino S, Cruz D et al. A comparison of observed versus estimated baseline creatinine for determination of RIFLE class in patients with acute kidney injury. Nephrol Dial Transplant 2009; 24: 2739–2744 [DOI] [PubMed] [Google Scholar]