Highlights
-
•
Toxic metals in fly ash caused morphological alteration of A. niger.
-
•
Branched and swollen fungal hyphae seen in one-step and two-step bioleaching.
-
•
Nano-sized calcium oxalate crystals observed on fungal surface.
-
•
Precipitation of crystals indirectly enhanced bioleaching efficiency.
Keywords: Aspergillusniger, Bioleaching, Incineration fly ash, Fungal morphology, Calcium oxalate
Abstract
This study examines the bioleaching of municipal solid waste incineration fly ash by Aspergillus niger, and its effect on the fungal morphology, the fate of the ash particles, and the precipitation of metallic salt crystals during bioleaching. The fungal morphology was significantly affected during one-step and two-step bioleaching; scanning electron microscopy revealed that bioleaching caused distortion of the fungal hyphae (with up to 10 μm hyphae diameter) and a swollen pellet structure. In the absence of the fly ash, the fungi showed a linear structure (with 2–4 μm hyphae diameter). Energy-dispersive X-ray spectroscopy and X-ray diffraction confirmed the precipitation of calcium oxalate hydrate crystals at the surface of hyphae in both one-step and two-step bioleaching. Calcium oxalate precipitation affects bioleaching via the weakening of the fly ash, thus facilitating the release of other tightly bound metals in the matrix.
1. Introduction
Incineration offers a management option for treating incinerable municipal solid waste (MSW). In general, the volume of waste is reduced by about 90%, and energy is recovered in the process. Although all organic matter is oxidized during incineration, the less volatile inorganic waste remains in the bottom ash while the more volatile inorganic wastes are captured as residues (termed fly ash) in air pollution control devices (for instance, electrostatic precipitator [9]). MSW incineration fly ash is a granular material that contains many hazardous constituents, amongst which are heavy metals (e.g. Cd, Cu, Ni, Pb, Zn). When in contact with water, these hazardous constituents may potentially be leached [25]. Due to its toxicity, most of the fly ash is landfilled after detoxification, or recycled as a secondary material [26]. Since some of the elements (e.g. Cu and Zn) are present in high concentration and may permit an economic recovery, fly ash may be considered as an artificial ore [5]. The leached and recovered metals may be recycled for re-use as raw materials [17].
Conventional pyro- or hydro-metallurgical techniques in fly ash detoxification and heavy metal recovery include thermal treatment, chloride evaporation process and chemical leaching. Although these techniques provide a rapid treatment and complete destruction of toxic compounds in fly ash, they are very energy intensive and result in the release of hazardous emissions during treatment. The high cost and the negative environmental impact of conventional methods have led to the investigation of bioleaching (considered a clean technology) as an alternative in the extraction of heavy metals from fly ash [24], [26].
The main focus in bioleaching was initially the recovery of metals from insoluble metal sulfide minerals in mining ores, based on the ability of microorganisms to oxidize reduced iron and sulfur compounds, via the production of organic or inorganic acids. There are patents on pilot- or commercial-scale bioleaching plants, with most focused on low-grade ore [8]. Recently, however, there have been interests in the application of bioleaching in industrial wastes as increasingly vast quantities of hazardous industrial wastes (such as spent catalyst, electronic waste, MSW incineration fly ash etc.), are generated [4], [30]. Although much has been reported on bioleaching by the chemolithoautotrophic acidophilic microorganisms of the genus Acidithiobacillus, fly ash is not a suitable substrate for bioleaching due to its high pH [26]. Acidithiobacillus sp. grow well under pH 2–3, while fungi are generally able to grow over a wide pH range, from 1.5 to 9.8 [7], [26].
Fungal bioleaching of heavy metals have been reported for solid wastes including electronic scrap material [6], spent refinery processing catalyst [2], [27] and incineration fly ash [5], [31], [33]. In general, bioleaching may be conducted using either one-step or two-step. In the former, the microorganism is incubated together with the metal-bearing waste. In two-step bioleaching, the microorganism is first cultured in the growth media and incubated for a period of time before the metal-bearing waste is added to the culture and the incubation continued.
In order to better exploit this intrinsic capability of selected microorganisms for metal leaching and recycling, more efforts are needed to understand the behavior of both the microorganisms and the metal substrate during bioleaching. The objective of this study is to examine the fungal growth behaviour and its morphology in the presence of the ash, the fate of fly ash particles, and the precipitation of nano-sized metallic salt crystals during bioleaching.
2. Materials and methods
2.1. Fly ash
Municipal solid waste (MSW) incineration fly ash used in this study was obtained from Tuas South Incineration Plant in Singapore. The fly ash was autoclaved at 121 °C for 15 min prior to use.
2.2. Fungi inoculum preparation
A. niger was obtained from Dr H. Brandl (University of Zürich, Switzerland) and was cultured as previously described [32]. 7-day old conidia were harvested from the surface of potato dextrose agar (Becton Dickinson Co.) using sterile deionized (DI) water. The number of spores was counted under a microscope (Olympus CX40) at 400× magnification using a Superior Marienfeld 0.1 mm depth haemocytometer. The spore suspension was diluted with DI water to the desired spore suspension concentration (107 spores/ml).
2.3. Shake flask pure culture
1 ml of spore suspension was added to 100 ml of standard sucrose medium with composition (g/l): sucrose (100), NaNO3 (1.5), KH2PO4 (0.5), MgSO4∙7H2O (0.025), KCl (0.025), yeast extract (1.6), and incubated at 30 °C with rotary shaking at 120 rpm [32]. All reagents were of analytical grade. The liquid medium was autoclaved at 121 °C for 15 min prior to inoculation.
2.4. Shake flask one-step bioleaching
One-step bioleaching was conducted following reported protocol [32]. In one-step bioleaching, the fungus was incubated with ash at 1% pulp density. Sterile medium was added to autoclaved flasks containing the fly ash, followed by inoculation of fungal spore suspension. Samples of fungi pellet were withdrawn after Day 7, 8, 17, and 27 for SEM, EDX and XRD analyses.
2.5. Shake flask two-step bioleaching
In two-step bioleaching, the fungus was first cultured in an autoclaved sucrose medium (as in pure culture) and incubated at 30 °C with rotary shaking at 120 rpm without fly ash. After 2 days, when a large pH drop occurred, sterile fly ash at 1% pulp density was added to the culture and the incubation was continued. Samples of fungi pellet were withdrawn after Day 2, 3, 7, 8, 17 and 27 for SEM, EDX and XRD analyses.
2.6. Scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX)
Fungi pellet taken from pure culture, one-step bioleaching, and two-step bioleaching were washed with deionized water for three changes. The pellets were fixed with 3% (v/v) glutaraldehyde in deionized water at 4 °C overnight before being washed with deionized water and dehydrated over an ethanol gradient. Samples were dried using a critical point dryer, mounted on copper stub and sputter-coated for 120 s using a JEOL JFC-1300 Auto Fine Coater fitted with a Pt target. A JEOL JSM-5600LV scanning electron microscope (SEM) was used to examine the morphology of the fungi and fly ash. For high magnifications, field emission scanning electron microscope (FESEM), JEOL JSM-6700F was used. The images obtained were analyzed using Image-Pro Premier software to obtain the size of particles and fungal hyphae. Energy-dispersive X-ray spectroscopy (EDX) (OXFORD Instruments 6647) was coupled to the SEM for surface elemental analysis of the fungal samples. The EDX data were analyzed using INCA Suite Version 4.01.
2.7. X-ray diffraction (XRD)
Fungal pellet samples taken from pure culture, one-step bioleaching, and two-step bioleaching were washed with DI water (three changes). After lyophilization, the pellet was mixed with liquid nitrogen, ground in a mortar and pestle, and placed in the sample holder for X-ray diffraction (XRD) analysis using a SHIMADZU X-ray diffractometer (XRD-6000). The diffraction data from the fungal samples were compared with that obtained from JCPDS-International Center for Diffraction Data.
2.8. Analysis of organic acid
Citrate, oxalate and gluconate were analyzed using HP 1100 series high performance liquid chromatography with variable wavelengths detector at 210 nm, and carried out at 30 °C. The mobile phase used was 5 mM sulphuric acid (Merck, analytical grade), at a flow rate of 0.5 ml/min. Standards of the compounds mixture were prepared using analytical grade reagents of citric acid (Aldrich Chemical Co.), disodium oxalate (Merck) and d-gluconic potassium salt (Sigma Chemical Co.) at concentrations of 0, 5, 50, 100, 200 mM for citrate and gluconate; and 0, 5, 10, 20, 50 mM for oxalate.
3. Results and discussion
3.1. Characteristics of fly ash
Fly ash obtained from the Tuas incineration plant in Singapore was of very small particle size (averaging 26 μm) and was rich in metals. Ca was the most dominant followed by K, Mg and Zn. Pb, Al and Fe were also found in significantly amounts. A more detailed description of the physical and chemical characteristics of fly ash has been given in the supplementary material (Tables S1 and S2).
3.2. Acid formation in the presence and absence of ash
The quantity of acids produced by the fungi in the presence and absence of ash is given in Table 1. The growth of fungi in sugar-containing media results in the production of organic acids such as oxalic acid, citric acid and gluconic acid. A. niger produces citric acid at a higher concentration in the absence of fly ash, while gluconic acid is produced at a higher concentration in its presence. When the fungus is grown in the absence of fly ash and in a manganese-deficient medium, the enzyme isocitrate dehydrogenase is unable to catalyse the oxidative decarboxylation of isocitrate to alpha-ketoglutarate (in the Krebs cycle) and citric acid is accumulated in the medium. In the presence of fly ash however, manganese (from the fly ash) which functions as a cofactor for isocitrate dehydrogenase is released into the medium, and citrate is converted to organic acids (succinate, fumarate, malate etc.). As a result, the accumulation of citric acid is significantly reduced. Moreover, when fly ash is inoculated with fungal spores, the alkaline calcium oxide present in the ash is hydrated to form calcium hydroxide which increases the pH. Fig. 1 shows that while the pure culture has a pH ≤ 3, the addition of fly ash increases the pH in the bioleaching medium to about 11. The alkaline medium activates glucose oxidase which converts glucose to gluconolactone which is finally hydrolyzed to gluconic acid [11]. Gluconic acid and citric acid have been reported to be the major lixiviants in leaching metals from fly ash in one-step and two-step bioleaching, respectively [5]. The concentration of oxalic acid was quite low in the medium, although studies have shown that the alkaline medium favours its production [18] (one possible reason for this is discussed in Section 3.3.2).
Table 1.
Maximum acid produced by the organism under different conditions.
| Acid produced | Pure culture (mM) | 1% pulp density |
|
|---|---|---|---|
| 1-step bioleaching (mM) | 2-step bioleaching (mM) | ||
| Citrate | 51.6 | 38.6 | 33 |
| Gluconate | 16 | 171.16 | 163.7 |
| Oxalate | 4.62 | 1.93 | 1.27 |
Aspergillus niger produces citrate in the absence of fly ash, and gluconate in its presence.
Fig. 1.
pH profile during growth of pure culture, one-step and two-step bioleaching. Day 0 in two-step bioleaching refers to time of addition of fly ash.
3.3. Formation of oxalate crystals
3.3.1. Pure culture
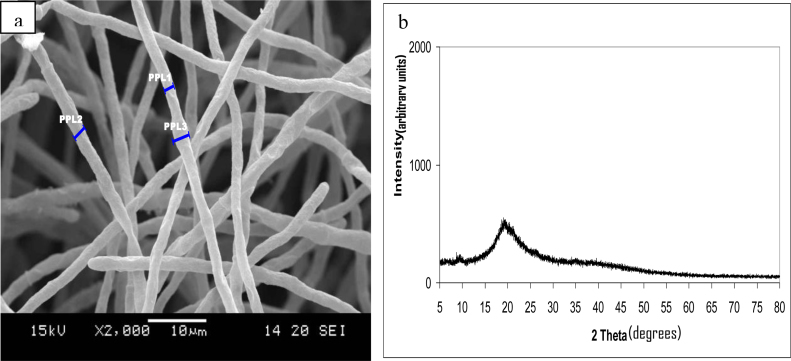
Fig. 2a and b shows the SEM and XRD of the pure fungal culture taken after two days incubation. The fungal filaments (or hyphae) are long, thread-like and connect end to end and showed a complete absence of any crystal structures within the fungal pellet. The fungal mycelium aggregated and grew as pellets (or beads). XRD pattern of the pure fungal culture shows the absence of a crystal structure.
Fig. 2.
(a) Fungal morphology of pure culture of Aspergillusniger on Day 2 showing linear and intact hyphae (PPL1 = 1.63 μm, PPL2 = 2.15 μm and PPL3 = 2.87 μm), (b) XRD analyses of the pure A.niger culture showing a complete absence of any metal and other crystal structures within the fungal pellet.
3.3.2. One-step bioleaching
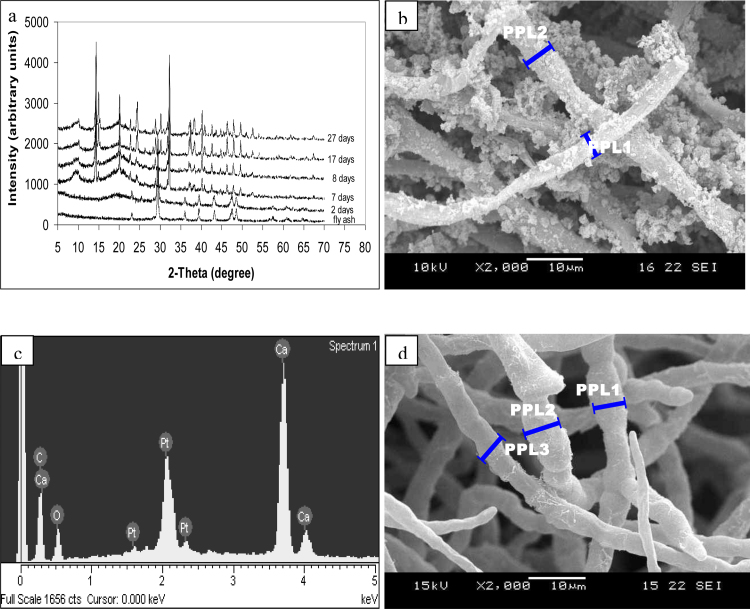
Fig. 3a shows a section of a fungal pellet, with small particles on the hyphae and a larger particle (of diameter about 50 μm) on Day 7 of bioleaching. The latter is likely to be a fly ash particle as its diameter was close to the mean particle size of the fly ash (i.e. 26 μm). The surface composition of the large particle was comparable to that of fly ash as revealed in the EDX analysis (Fig. 3b) which confirmed the presence of C, O and Ca, along with S, Al, Fe and Zn. Higher magnification of the small particles (Fig. 3c) and the hyphae (Fig. 3d) shows that the small particles were likely to be oxalate crystals that had precipitated on the hyphal surface. The diameter of the small (nano) particles was about 50 nm. EDX analysis (Fig. 3e) confirmed the presence of only C, O and Ca, indicating that the particles were calcium oxalate. These results suggest the adsorption of calcium oxalate precipitates and fly ash particles on the surface of the fungi.
Fig. 3.
(a) Section of the fungal pellet in one-step bioleaching at Day 7 showing small calcium oxalate particles on the swollen hyphae (PPL2 = 6.43 μm) and a larger fly ash particle (PPL1 = 50.92 μm), (b) EDX of the large particles in fungal pellet in one-step bioleaching on Day 7 confirming the presence of C, O and Ca, along with S, Al, Fe and Zn (metals found in fly ash), (c) a magnified view of the small particles (PPL = 82.32 nm) showing that diameter of the small (nano) particles was about 50–100 nm, (d) surface of the fungal pellet in one-step bioleaching on Day 7 showing increased diameter of fungal hyphae (PPL1 = 9.396 μm and PPL2 = 9.94 μm) and calcium oxalate particles on the hyphae, (e) EDX analyses of the small particles in fungal pellet in one-step bioleaching on Day 7 showing peaks corresponding to Ca, C and O confirming the presence of calcium oxalate crystals in the particles, (f) XRD analyses of the fungal pellet in one-step bioleaching showing formation of calcium oxalate crystals and dissolution of fly ash particles after Day 8, (g) surface of the fungal pellet in one-step bioleaching on Day 17 (PPL1 = 4.25 μm, PPL2 = 9.47 μm and PPL3 = 9.99 μm) showing abnormally short, swollen and highly-branched distortion, (h) a magnified view of the small particles section of the fungal pellet in one-step bioleaching on Day 27 showing increased diameter of calcium oxalate crystals (PPL1 = 229.06 nm and PPL2 = 204.27 nm).
XRD (Fig. 3f) corroborates these findings; the peak pattern (Day 7) was similar to that of fly ash. XRD on Day 8 (Fig. 3f) confirmed that the small particles were calcium oxalate. Interestingly, it was noted that the fly ash peak was absent from Day 8, thus suggesting that the ash particles, entrapped within the pellet, were completely absent (i.e. dissolved) by that time. Samples taken on Day 17 and 27 for SEM (Fig. 3g), EDX (data not shown) and XRD (Fig. 3f) show results similar to that at Day 8. It was also evident that the calcium oxalate precipitates were present throughout the one-step bioleaching process. Fig. 3h shows that the diameter of particle (about 130 nm) at Day 27 was larger than that at Day 7 (about 50 nm); the calcium oxalate crystal grew during bioleaching, and peak intensity at Day 27 was higher than that at Day 8 (Fig. 3f).
Despite oxalic acid formation being favoured in the alkaline medium, the amount of acid detected in the liquid medium during the lag phase was very low, possibly due to the immediate precipitation of insoluble metal oxalates, including calcium oxalate [31]. The dominance of calcium oxalate over calcium gluconate and calcium citrate can be attributed to the significantly lower solubility product (Ksp) of calcium oxalate (about ×10−9) compared to calcium gluconate (about ×10−3). Precipitation of calcium oxalate crystals is also favoured by the pH of the bioleaching medium (Fig. 1 and Table 2). For instance, the pH of the medium was initially around 11 and gradually decreased to around 8.5 on Day 7 during one-step bioleaching, and calcium oxalate precipitation occurs (Table 2). Indeed, the precipitation of calcium oxalate by several mycorrhizal species have also been documented, with one ascribed function being detoxification of calcium, since it is known that high concentration of free Ca2+ within cells is toxic [14], [15], [20], [21]. Such a detoxification mechanism may also be the reason for the observed calcium oxalate precipitation in this present study. Interestingly, EDX data (Fig. 3b and e) and XRD data (Fig. 3f and Fig. 4a) show no evidence of precipitation of oxalates of aluminium, copper, iron, manganese, lead, and zinc during bioleaching, although others have reported fungal precipitation of oxalates and citrates of cobalt, copper, chromium, and nickel [12], [13], [22], [28], [29]. This is expected since the concentration of these metal ions (at 101 ppm) in the fly ash is lower than that of calcium (103 ppm) in the liquid medium.
Table 2.
Optimal pH for precipitation of oxalates [23].
| Metal (H2C2O4) | pH |
|---|---|
| Cu2+ | 1.2–1.6 |
| Ca2+ | >4.9 |
| Sr2+ | >5.1 |
| Pb2+ | 1.5–3.0 |
Fig. 4.
(a) Undefined XRD analyses of the fungal pellet in two-step bioleaching confirming the formation of calcium oxalate crystals and dissolution of fly ash particles from Day 7, (b) section of the fungal pellet in two-step bioleaching on Day 7 showing small particles on the hyphae and increased diameter of hyphae (PPL1 = 4.45 μm and PPL2 = 5.97 μm), (c) EDX analyses of the fungal pellet in two-step bioleaching at Day 7 showing peaks corresponding to Ca, C and O confirming the presence of calcium oxalate crystals on the fungal surface, (d) surface of the fungal pellet in two-step bioleaching at Day 8 showing abnormally short, swollen and high-branched hyphae (PPL1 = 5.59 μm, PPL2 = 6.32 μm and PPL3 = 5.32 μm).
3.3.3. Two-step bioleaching
Similar results were observed in two-step bioleaching. Samples were taken immediately after the addition of fly ash in two-step bioleaching. SEM photomicrographs confirmed the absence of solid particles on the fungal surface and within the section of the fungi pellet (data not shown). EDX results confirmed the presence of only carbon and oxygen; no metal element was detected within or outside the fungi pellet (data not shown). This confirmed the absence of metal salt precipitation at the start of the two-step bioleaching. Fig. 4b shows the section of fungal pellet at Day 7. The SEM photomicrograph at Day 7 (Fig. 4b) in two-step bioleaching was similar to Fig. 3d (i.e. one-step bioleaching), with small particles on the surface of the hyphae within the fungal pellet. Precipitation of particles on the surface of the hyphae both within and outside the pellet is evident. EDX analysis (Fig. 4c) shows results similar to Fig. 3e and confirmed that the particles were composed of calcium, carbon and oxygen. This is supported by the XRD spectrum (Fig. 4a) which shows evidence of calcium oxalate precipitation on Day 7. SEM photomicrographs on Day 8, Day 17, and Day 27 (data not shown) in two-step bioleaching were similar to Fig. 4b. EDX results showed that the fungal pellet on Day 8, Day 17, and Day 27 contained carbon, oxygen and calcium (data not shown), similar to Fig. 3e. XRD spectra on Day 8, Day 17 and Day 27 (Fig. 4a) shows that the peak pattern in the crystal structure matched that of calcium oxalate hydrates.
Although the mechanism of calcium oxalate precipitation was similar in both one-step and two-step bioleaching, the dissolution rate of fly ash was different. XRD results at Day 7 in one-step bioleaching (Fig. 3f) confirmed the presence of fly ash particles. However, XRD results at Day 7 in two-step bioleaching (Fig. 4a) shows the absence of fly ash. Instead, the fly ash peak is replaced by calcium oxalate hydrate peak. The fly ash in two-step bioleaching dissolved earlier than that in one-step bioleaching while the calcium oxalate hydrate in two-step bioleaching formed earlier than that in one-step bioleaching.
3.4. Adherence of oxalate crystals to fungus
3.4.1. Pure culture
As there were minimal amount of metal ions in the medium, the formation of oxalate salts was insignificant in the pure culture and hence could not be detected in SEM, EDX and XRD analyses.
3.4.2. One-step bioleaching
The speculated growth mechanism in one-step bioleaching is the aggregation of swollen spores with fly ash particles after inoculation, resulting in relatively large pellet nuclei. Adhesion of un-germinated spores and fly ash particle to the large pellet nuclei which contained newly-germinated spores and hyphae also occurred and resulted in a tendency to reduce the overall number of pellets in the medium [16], [10]. This observation is consistent with the early findings of free spore aggregation of A. niger in batch flask culture and bubble-column fermenters [10].
Calcium oxalate precipitation affects bioleaching in several ways. Due to the heavy leaching of calcium from fly ash, the fly ash matrix may be weakened, thus facilitating the release of other tightly bound metals in the matrix. In addition, the bioleaching rate may also be enhanced as the organic acids released into the media by the fungus are available for complexation with other metals as the competition from calcium in the bioleaching of other metals in reduced.
3.4.3. Two-step bioleaching
Although the mechanism of calcium oxalate hydrate precipitation in two-step bioleaching was similar to that of one-step bioleaching discussed earlier, the leaching rate of metals from fly ash was different. Metals from fly ash were bioleached more rapidly in two-step bioleaching compared to one-step bioleaching, resulting in earlier formation of calcium oxalate hydrate. A more rapid decrease in pH occurred in two-step bioleaching since organic acids were already present in the medium prior to the addition of fly ash (Fig. 1). Besides, the addition of fly ash after fungal germination in two-step bioleaching effectively reduces the toxic effects on the spore germination and fungal growth, and accelerates bioleaching process [5], [31]. This was also observed in the two-step bioleaching of electronic scrap materials [6]. Moreover, in contrast to one-step leaching, aggregation of calcium oxalate salt, fly ash and fungi hyphae did not occur in two-step bioleaching.
3.5. Changes in fungal morphology
3.5.1. Pure culture
Fig. 2a shows the mycelial structure of the pure fungal culture in the medium after 2 days. The hyphae were linear, with a diameter of about 2 μm, which is the normal structure for A. niger [22]. SEM photomicrographs of the pure culture at 3 days, 7 days and 17 days (data not shown) show similar morphology. Due to the absence of any stress factors in the pure culture, the fungi achieved exuberant growth and were morphologically intact.
3.5.2. One-step bioleaching
In one-step bioleaching, the fungus showed a 6 day lag phase, and samples were taken at 7, 8, 17, and 27 days. Fig. 3d shows the surface of the fungal pellet at Day 7. Compared to the diameter of the fungal hyphae observed in the absence of fly ash (i.e. the control, with a diameter of 2 μm, as discussed in Section 3.5.1), the fungal hyphae in one-step bioleaching were much larger in diameter (∼10 μm). SEM photomicrographs show that the morphology of the fungus on Day 7 and Day 8 was similar. Although the diameter of fungal hyphae observed on Day 17 (Fig. 3g) and Day 27 was similar to that on Day 7, some hyphae had lost the linear structure and were abnormally short, swollen and showed highly-branched distortion. Swelling of fungal cells in the presence of fly ash has been reported (for e.g., Yarrowia lipolytica) and attributed to the presence of heavy metals from the fly ash in the medium [3]. In the present study, the heavy metals included zinc, iron, lead and copper whose concentrations were 15 ppm, 1 ppm, 4 ppm and 1 ppm, respectively, in Day 7 of one-step bioleaching. Although the concentration of lead and copper were not high compared with that of calcium (4000 ppm), these metals are very toxic to the fungus and their effect may indeed be synergistic. For instance, a study in 2004 reported luxurious growth and good metabolite production by A. niger in the presence of Pb at a concentration as high as 40 ppm [1].
Swollen morphological structure of A. niger in a nickel-containing medium (similar to that observed in this study) has also been reported [22]. Apical growth usually occurs in fungi where a complex network of internal and external signals is involved. Changes in the network components affect the shape as well as direction of growth. Excess metal ions in the growth environment may cause swelling at the tips, and an increase in branching and thickness of transverse walls at subapical parts. This was a strategy adopted to survive adverse conditions by increasing fungal branching. Hyphal growth requires enzymes such as chitin synthases and chitinases involved in chitin synthesis and degradation. Excessive degradation or reduced synthesis of cell wall components may result in loosening of cell wall, which in turn leads to swelling [19]. All these factors may be the reason for the observed morphology although this may not have a significant impact on the organic acid production or leaching unless the enzymes involved in the mechanism are affected.
Fig. 3g shows no precipitated particles on the hyphal surface. XRD results (Fig. 3f), however, show calcium oxalate crystal peak at Day 17; the growth of new fungal hyphae encapsulated the precipitated calcium oxalate salt, fly ash and old hyphae and no new calcium oxalate precipitated on the newly germinated hyphae.
3.5.3. Two-step bioleaching
In two-step bioleaching, the fungus was first cultured for two days before the addition of fly ash to the medium. Samples of fungi pellet were withdrawn on Day 2, 3, 7, 17 and 27. SEM photomicrographs of the fungi on Day 2 and Day 3 (data not shown) show that the fungi had long, thread-like hyphae (diameter around 2 μm; as discussed in Section 3.5.1), which were very similar to what was observed in pure culture (Fig. 2a). Fig 4b shows swollen hyphae and precipitated calcium oxalate crystals on the fungal surface on Day 7 in two-step bioleaching, similar to what was observed on Day 7 in one-step bioleaching. However, the diameter of hyphae in two-step bioleaching was around 5 μm smaller than what was observed in one-step bioleaching (10 μm; as discussed in Section 3.5.2). As the fungi had already grown and germinated before the addition of fly ash, the effect of fly ash on the fungus was not pronounced. On Day 8 however, the fungal morphology (Fig. 4d) was similar to the fungal morphology observed on Day 17 in one-step bioleaching (Fig. 3g). The diameter of the hyphae (about 7 μm) was larger than the diameter of the hyphae observed in the pure culture (2 μm) but no oxalate crystals were seen on the hyphal surface. Again, some hyphae had lost the linear structure and were more highly branched and swollen, probably due to the presence of toxic metals in the bioleaching broth as was the case in one-step bioleaching. The fungal morphology on Day 17 and Day 27 in the two-step bioleaching was similar to that on Day 8. The on-set of the distortion and swollen structure of the hyphae occurred earlier in two-step bioleaching compared with one-step leaching. It is likely due to the earlier on-set of growth in the former. Despite this, the effect on bioleaching appears insignificant, possibly due to the high production of organic acids before the addition of fly ash and exposure to toxic conditions.
4. Conclusion
This study investigated the morphology of A. niger and the precipitation of metals in one-step and two-step bioleaching. Unlike in control cultures, branched and swollen fungal hyphae were formed during one-step and two-step bioleaching, due to the high toxic metal concentration (concentration of heavy metals at the end of bioleaching: zinc (40 ppm), iron (7 ppm), lead (5 ppm) and copper (2 ppm)). Calcium oxalate was precipitated in both one-step and two-step bioleaching, possibly as a strategy to decrease calcium toxicity to the fungi. Other metal oxalates were not detected in both one-step and two-step bioleaching. Fly ash particles were found within the fungi pellet in one-step bioleaching due to the aggregation of newly-germinated spores with fly ash particles.
Footnotes
Available online 29 May 2014
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.btre.2014.05.009.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Angumeenal A., Venkappayya D. Growth kinetics of heavy metal adapted Aspergillusniger during citric acid biosynthesis. J. Sci. Ind. Res. 2004;63:610–613. [Google Scholar]
- 2.Aung K.M.M., Ting Y.-P. Bioleaching of spent fluid catalytic cracking catalyst using Aspergillusniger. J. Biotechnol. 2005;116:159–170. doi: 10.1016/j.jbiotec.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Bankar A., Winey M., Prakash D., Kumar A.R., Gosavi S., Kapadnis B., Zinjarde S. Bioleaching of fly ash by the tropical marine yeast, Yarrowia lipolytica NCIM 3589. Appl. Biochem. Biotechnol. 2012;168:2205–2217. doi: 10.1007/s12010-012-9930-2. [DOI] [PubMed] [Google Scholar]
- 4.Bharadwaj A., Ting Y.-P. Bioleaching of spent hydrotreating catalyst by acidophilic thermophile Acidianus brierleyi: leaching mechanism and effect of decoking. Bioresour. Technol. 2012;130:673–680. doi: 10.1016/j.biortech.2012.12.047. [DOI] [PubMed] [Google Scholar]
- 5.Bosshard P.P., Bachofen R., Brandl H. Metal leaching of fly ash from municipal waste incineration by Aspergillusniger. Environ. Sci. Technol. 1996;30:3066–3070. [Google Scholar]
- 6.Brandl H., Bosshard R., Wegmann M. Computer-munching microbes: metal leaching from electronic scrap by bacteria and fungi. Hydrometallurgy. 2001;59:319–326. [Google Scholar]
- 7.Brandl H., Faramarzi M.A. Microbe-metal-interactions for the biotechnological treatment of metal-containing solid waste. China Particuology. 2006;4:93–97. [Google Scholar]
- 8.Brombacher C., Bachofen R., Brandl H. Biohydrometallurgical processing of solids: a patent review. Appl. Microbiol. Biotechnol. 1997;48:577–587. [Google Scholar]
- 9.Buekens A. Springer; New York: 2013. Incineration Technologies. [Google Scholar]
- 10.Cocker R., Greenshields R.N. Fermenter cultivation of Aspergillus. In: Smith J.E., Pateman J.A., editors. Genetics and Physiology of Aspergillus. Academic Press; London: 1977. pp. 361–390. [Google Scholar]
- 11.Dellweg H. Biotechnology vol. 3. Verlag Chemie Weinheim; Germany: 1983. [Google Scholar]
- 12.Gadd G.M. Fungal production of citric and oxalic acid: importance in metal speciation, physiology and biogeochemical processes. Adv. Microb. Physiol. 1999;41:47–92. doi: 10.1016/s0065-2911(08)60165-4. [DOI] [PubMed] [Google Scholar]
- 13.Gharieb M.M., Sayer J.A., Gadd G.M. Solubilization of natural gypsum (CaSO4∙ 2H2O) and the formation of calcium oxalate by Aspergillusniger and Serpulahimantioides. Mycol. Res. 1998;102:825–830. [Google Scholar]
- 14.Gourlay I., Grime G. Calcium oxalate crystals in African acacia species and their analysis by scanning proton microprobe (SPM) IAWA J. 1994;25:137–148. [Google Scholar]
- 15.Gadd G.M. Signal transduction in fungi. In: Gow N.A., Gadd G.M., editors. The Growing Fungus Part 3. Springer; Netherlands: 1995. pp. 183–210. [Google Scholar]
- 16.Grimm L., Kelly S., Hengstler J., Gobel A., Krull R., Hempel D. Kinetic studies on the aggregation of Aspergillusniger conidia. Biotechnol. Bioeng. 2004;87:213–218. doi: 10.1002/bit.20130. [DOI] [PubMed] [Google Scholar]
- 17.Krebs W., Brombacher C., Bosshard P.P., Bachofen R., Brandl H. Microbial recovery of metals from solids. FEMS Microbiol. Rev. 1997;20:605–617. [Google Scholar]
- 18.Kubicek C., Schreferl-Kunar G., Wohrer W., Rohr M. Evidence for a cytoplasmic pathway of oxalate biosynthesis in Aspergillusniger. Appl. Environ. Microbiol. 1988;54:633–637. doi: 10.1128/aem.54.3.633-637.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanfranco L., Balsamo R., Martino E., Perotto S., Bonfante P. Zinc ions alter morphology and chitin deposition in an ericoid fungus. Eur. J. Histochem. 2010;46:341–350. doi: 10.4081/1746. [DOI] [PubMed] [Google Scholar]
- 20.Lapeyrie F., Ranger J., Vairelles D. Phosphate-solubilizing activity of ectomycorrhizal fungi in vitro. Can. J. Bot. 1991;69:342–346. [Google Scholar]
- 21.Lovley D.R. ASM press; Washington, DC: 2000. Environmental Microbe-Metal Interactions. [Google Scholar]
- 22.Magyarosy A., Laidlaw R., Kilaas R., Echer C., Clark D., Keasling J. Nickel accumulation and nickel oxalate precipitation by Aspergillusniger. Appl. Microbiol. Biotechnol. 2002;59:382–388. doi: 10.1007/s00253-002-1020-x. [DOI] [PubMed] [Google Scholar]
- 23.Marta L., Horovitz O., Zaharescu M. Analytical study of oxalates coprecipitation. Leonardo J. Sci. 2003;2:72–82. [Google Scholar]
- 24.Mishra D., Rhee Y.-H. Current Research, Technology and Education topics in Applied Microbiology and Microbial Biotechnology. Formatex Research Center; Badajoz, Spain: 2010. Current research trends of microbiological leaching for metal recovery from industrial wastes; pp. 1289–1296. [Google Scholar]
- 25.Mizutani S., Yoshida T., Sakai S.I., Takatsuki H. Release of metals from MSWI fly ash and availability in alkali conditions. Waste Manage. 1996;16:537–544. [Google Scholar]
- 26.Ramanathan T., Ting Y.P. Fly Ash: Chemical Composition, Sources and Potential Environmental Impacts. Nova publishers; New York: 2014. Fly ash and the use of bioleaching for fly ash detoxification. [Google Scholar]
- 27.Santhiya D., Ting Y.-P. Bioleaching of spent refinery processing catalyst using Aspergillus niger with high-yield oxalic acid. J. Biotechnol. 2005;116:171–184. doi: 10.1016/j.jbiotec.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Sayer J.A., Gadd G.M. Solubilization and transformation of insoluble inorganic metal compounds to insoluble metal oxalates by Aspergillusniger. Mycol. Res. 1997;101:653–661. [Google Scholar]
- 29.Sayer J.A., Kierans M., Gadd G.M. Solubilisation of some naturally occurring metal-bearing minerals, limescale and lead phosphate by Aspergillusniger. FEMS Microbiol. Lett. 1997;154:29–35. doi: 10.1016/s0378-1097(97)00296-6. [DOI] [PubMed] [Google Scholar]
- 30.Tay S.B., Natarajan G., bin Abdul Rahim M.N., Tan H.T., Chung M.C.M., Ting Y.P., Yew W.S. Enhancing gold recovery from electronic waste via lixiviant metabolic engineering in Chromobacterium violaceum. Sci. Rep. 2013:3. doi: 10.1038/srep02236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu H.-Y., Ting Y.-P. Metal extraction from municipal solid waste (MSW) incinerator fly ash—Chemical leaching and fungal bioleaching. Enzyme Microb. Technol. 2006;38:839–847. [Google Scholar]
- 32.Xu T.-J., Ting Y.-P. Fungal bioleaching of incineration fly ash: metal extraction and modeling growth kinetics. Enzyme Microb. Technol. 2009;44:323–328. [Google Scholar]
- 33.Yang J., Wang Q., Wang Q., Wu T. Heavy metals extraction from municipal solid waste incineration fly ash using adapted metal tolerant Aspergillusniger. Bioresour. Technol. 2009;100:254–260. doi: 10.1016/j.biortech.2008.05.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.