Abstract
Background: Membrane-bound α-klotho functions as a co-receptor with fibroblast growth factor receptor at the renal tubule conferring specificity to fibroblast growth factor-23 (FGF-23), allowing it to inhibit tubular phosphate reabsorption at physiological concentrations. α-klotho also exists as a soluble protein. However, the complex interrelationships between soluble α-klotho (sKl), FGF-23 and phosphate reabsorption are poorly understood, with little known about the links between sKl, FGF-23 and phosphate reabsorption in chronic kidney disease (CKD). This study addresses this issue in a cohort of patients with and without CKD.
Methods: We conducted a single-centre, cross-sectional study of contemporaneously obtained samples of blood and 24-h urine biochemistry along with sKl and intact FGF-23 (iFGF-23) from non–dialysis-dependent CKD patients and healthy volunteers. Pearson’s correlation coefficients were used to determine correlations between natural log-transformed (Ln) sKl and iFGF-23 with other parameters of interest. Backward multivariate analysis was undertaken to evaluate the relationship between mineral parameters.
Results: One hundred and sixteen participants (77 with CKD and 39 healthy volunteers) were studied, of which 74 (63.8%) were male. The median age was 61 (interquartile range 49–71) years. Those with CKD had lower sKl (408 versus 542 pg/mL), higher iFGF-23 (94 versus 41 pg/mL), higher fractional excretion of phosphate (25.05 versus 10.98%) and lower daily urinary phosphate excretion (UPE) (24.8 versus 32.3 mmol/L) compared with healthy volunteers (all P ≤ 0.002). Age correlated inversely and estimated glomerular filtration rate (eGFR) correlated positively with phosphate reabsorption and Ln(sKl), while the opposite was seen with Ln(iFGF23). Upon multivariate analysis, eGFR, Ln(sKl) and parathyroid hormone were independently associated with phosphate reabsorption, whereas Ln(iFGF-23) was not, after adjustment for age.
Conclusions: Abnormalities in phosphate regulatory pathways are disturbed early in CKD. While iFGF-23 is associated with phosphate excretion on univariate analyses, sKl demonstrates a significant association with phosphate reabsorption independent of iFGF-23, and this relationship deserves further exploration.
Keywords: chronic kidney disease, fibroblast growth factor-23, phosphate reabsorption, soluble klotho, urinary phosphate excretion
Background
The discoveries of α-klotho [1] and fibroblast growth factor-23 (FGF-23) [2, 3] and their key roles in phosphate handling have broadened our understanding of mineral metabolism. Membrane-bound α-klotho (mKl) is an obligate co-receptor for physiological FGF-23 signalling [4]. FGF-23 acts on the FGF-receptor–klotho co-receptor complex to reduce apical expression of key sodium–phosphate co-transporters within the renal proximal tubules inhibiting tubular reabsorption of phosphate (TRP), thereby inducing phosphaturia [5]. However, we also now realize that α-klotho is present in a soluble circulating form (sKl) and likely interacts with this mechanism. How and why this occurs is unknown.
Chronic kidney disease (CKD) results in a number of biochemical abnormalities related to bone and mineral metabolism, including elevation of circulating FGF-23 levels and parathyroid hormone (PTH) levels accompanied by a reduction of 1,25-dihydroxycholecalciferol [6]. It is possible that these changes result in response to a reduction in phosphate excretion related to lower glomerular filtration rate (GFR) and that when renal functional decline exceeds the ability to compensate, serum phosphate (sPi) levels increase as a late maker in CKD [7].
FGF-23 has therefore been widely evaluated in CKD. Levels not only increase as GFR decreases, but also linearly correlate with increased mortality and cardiovascular disease across all stages of CKD, independent of traditional vascular risk factors [8–12]. Although the relationship between sKl and this mechanism is less well established [13], sKl has likewise been negatively correlated with mortality [14].
However, the different but potentially overlapping roles of mKl and sKl are unclear, as is the relationship between sKl and FGF-23. The only studies that have compared sKl and FGF-23 [15–21] and attempted to interpret these levels in the context of renal phosphate handling [20, 21] show contradictory results. To resolve this disparity, we measured and compared levels of sKl, intact FGF-23 (iFGF-23) and 24-h urinary phosphate (uPi) concurrently in a cohort of patients with CKD, along with healthy volunteers.
Materials and Methods
Study population
A single-centre, observational cross-sectional study in patients with CKD was conducted at the Royal Melbourne Hospital (RMH), Parkville, Australia [22]. Inclusion criteria were patients >18 years of age with a diagnosis of CKD (stages 1–5). Exclusion criteria included renal replacement therapy (any dialysis or previous transplantation) and psychological or medical illness precluding informed consent. Patients fulfilling the above criteria attending the Department of Nephrology at RMH were approached to participate in the study. The same data were obtained from a separate cohort of healthy volunteers. Single-agent-treated stable hypertension and hypercholestrolaemia were not exclusion criteria for the healthy volunteer cohort. Participants were not subject to any specific dietary restrictions for any time period during the study. Demographic information, along with medical and medication history, was recorded for all the participants. These studies were approved by the Melbourne Health Human Research Ethics Committee, Melbourne, Victoria, Australia (HREC 2012.154) and performed in accordance with the Declaration of Helsinki. All study participants provided informed consent.
Sample collections
All participants were requested to provide a 24-h urine collection commencing the day prior to blood tests. Study participants were instructed to keep the urine collected in a cool, dry area and return the specimen within 4 h of completion, at which point blood samples were obtained. Routine biochemistry was collected along with two extra tubes, one K3-EDTA tube (Vacuette, Greiner Bio-One International, Kremsmunster, Austria) and one serum separator tube (SST, BD Biosciences, Franklin Lakes, NJ, USA).
Biochemical analysis
Biochemical analysis of sPi, serum calcium (sCa), serum creatinine (sCr), uPi, urinary creatinine (uCr) and serum intact PTH, along with analytical precisions, have previously been detailed [22]. Estimated glomerular filtration rate (eGFR) for all study participants was calculated using the CKD Epidemiology Collaboration (CKD-Epi) equation [23]. The CKD stage was defined according to Kidney Dialysis: Improving Global Outcomes (KDIGO) guidelines [24].
Total urinary phosphate excretion (UPE), fractional excretion of phosphate (FEPi) and tubular maximum for phosphate reabsorption corrected for GFR (TmP/GFR) provide measures of uPi handling where the latter two variables are derived from the same parameters in different mathematical equations. All three have been used in this study. Calculations for FEPi and TRP were as follows: FEPi = {[uPi × (sCr)]/[sPi × uCr] × 100%} and TRP = 1 − [(uPi/sPi) × (sCr/uCr)]. TmP/GFR was estimated from a previously validated algorithm where TmP/GFR = 0.3 × sPi × {TRP/[1 − (0.8 × TPR)]} and TRP was > 86% or equal to TRP × sPi when TRP was ≤ 86% [25–27]. TmP/GFR is usually calculated in a fasting state as an index of phosphate replacement requirements in phosphate wasting states [28]. Thus, the estimated TmP/GFR of non-fasting study participants here only provided an estimate of renal phosphate handling independent of sPi levels and eGFR.
The K3-EDTA and SST blood tubes were allowed to stand at room temperature for 30 min prior to centrifugation (10 min, 4°C , 3000 g) and aliquoted for storage at −80 °C until batch analysis. Serum sKl concentrations were measured using the IBL soluble klotho ELISA kit (Immuno-Biological Laboratories, Gunma, Japan) according to the manufacturer’s protocol. Based on duplicate measurements, the intra- and interassay coefficients of variation (CVs) for this study were 4.6% and 9.8%, respectively. Plasma iFGF-23 levels were determined using the Kainos iFGF-23 ELISA kit (Kainos Laboratories, Tokyo, Japan), according to the manufacturer’s protocol. The intra-assay and interassay analytical CVs were 4.5% and 8.6%, respectively, for samples measured in duplicate.
Statistical analysis
Parametric continuous variables have been reported using mean and standard deviation (SD), while categorical variables have been summarized using frequency and percentage. Non-parametric variables have been reported using median and interquartile range (IQR) for descriptive purposes to ease clinical interpretation. Student’s t-test or Mann–Whitney test were first used to evaluate differences between CKD and healthy controls as appropriate.
Non-parametric variables were subsequently normalized by natural log transformation for univariate and multivariate analyses performed on all study participants (CKD and healthy group as a total cohort). Variables of interest evaluated included age, gender, body mass index (BMI), eGFR, sPi, sCa, 25-hydroxyvitamin D [25(OH)D], PTH and 24-h uPi parameters—UPE, FePi and TmP/GFR. A P-value <0.003 was used to determine significance on univariate analyses for multiple comparisons with Bonferroni adjustment. Both sKl and iFGF-23 were expected to vary with age and eGFR, therefore backward multiple linear regression analysis was undertaken using natural log-transformed sKl (Ln-sKl) or Ln-iFGF-23 as the dependent variable, along with age, eGFR and other vary of interest (listed above) that showed unadjusted univariate correlation with the dependent variable where the P-value was <0.1. Backward multiple linear regression analysis was also undertaken using either Ln-24-h UPE or TmP/GFR as the dependent variable along with age, eGFR and variables that demonstrated unadjusted univariate correlation with the dependent variable where the P-value was <0.1.
All statistical analyses were performed with SPSS Statistics Version 24.0 (IBM, Armonk, NY, USA) and all graphics were created with GraphPad Prism 6 for Macintosh (GraphPad Software, La Jolla, CA, USA). P-values <0.05 were considered significant unless otherwise stated.
Results
A total of 116 participants, 77 CKD patients and 39 healthy volunteers were included for study analysis. Seventy-four (63.8%) participants were male. The median age of participants was 61 [interquartile range (49–71)] years. Demographic information and biochemical profiles for both groups are displayed in Table 1. In this study, those with CKD were older, with a male predominance and had larger BMI compared with healthy volunteers (all P < 0.01). The distribution of CKD stage and aetiology of disease is also tabulated in Table 1.
Table 1.
Baseline demographic and biochemical characteristics of study participantsa
| Characteristics | CKD patients (n = 77) | Healthy cohort (n = 39) |
|---|---|---|
| Demographics | ||
| Age (years), median (IQR) | 68 (55–74)* | 52 (36–57) |
| Gender (male), n (%) | 57 (74)* | 17 (43.6) |
| Body mass index (kg/m2) | 29.6 ± 6.9† | 26.3 ± 4.2 |
| CKD stages, n (%) | ||
| Stage 1 CKD/no CKD (eGFR > 90 ml/min/1.73 m2) | 6 (7.8) | n/a |
| Stage 2 CKD (eGFR 60–90 ml/min/1.73 m2) | 17 (22.1) | |
| Stage 3a CKD (eGFR 45–60 ml/min/1.73 m2) | 16 (20.8) | |
| Stage 3b CKD (eGFR 30–45 ml/min/1.73 m2) | 20 (25.9) | |
| Stage 4 CKD (eGFR 15–30 ml/min/1.73 m2) | 15 (19.5) | |
| Stage 5 CKD (eGFR <15 ml/min/1.73 m2) | 3 (3.9) | |
| CKD aetiology, n (%) | ||
| Glomerulonephritis | 21 (27.3) | n/a |
| Diabetic nephropathy | 15 (19.5) | |
| Vascular disease | 17 (22) | |
| Reflux nephropathy | 3 (3.9) | |
| Other | 21 (27.3) | |
| - Nephrectomy | 9 | |
| - Obstructive uropathy | 3 | |
| - Tubulointerstitial disease | 3 | |
| Biochemical parameters | ||
| Serum potassium (mmol/L) | 4.4 ± 0.5* | 4.0 ± 0.4 |
| Serum albumin (g/L) | 35 ± 5* | 41 ± 3 |
| Serum calcium (mmol/L) | 2.35 ± 0.25 | 2.35 ± 0.24 |
| Serum alkaline phosphatase (IU/L) | 97 (73–120) | 71 (57–87) |
| Serum 25(OH)D (nmol/L) | 60 (42–80) | 51 (31–75) |
| Intact PTH (pmol/L) | 7.90 (4.6–14.1)* | 2.65 (1.97–3.85) |
| Serum phosphate (mmol/L) | 1.14 ± 0.3 | 1.08 ± 0.15 |
| Serum creatinine (μmol/L) | 168 (119–208)* | 65 (57–77) |
| eGFR (ml/min/1.73 m2) | 36.7 (26.8–50.1)* | 99.2 (95–112.4) |
| 24-h UPE (mmol/day) | 24.8 (16.55–31.8)† | 32.3 (21.75–41.35) |
| 24-h creatinine excretion (mmol/day) | 11.97 ± 4.81† | 13.89 ± 4.67 |
| 24-h FEPi | 25.05 (14.94–34.28)* | 10.98 (7.89–16.1) |
| 24-h TmP/GFR | 0.74 ± 0.35* | 0.96 ± 0.25 |
| Soluble klotho (pg/mL) | 408 (340–528)* | 542 (439–806) |
| Intact FGF-23 (pg/mL) | 94 (65–160)* | 41 (38–53) |
Independent t-test or Mann–Whitney U-test performed for continuous variables and chi-square test performed for gender.
P ≤ 0.001 and
P < 0.05 compared with control group.
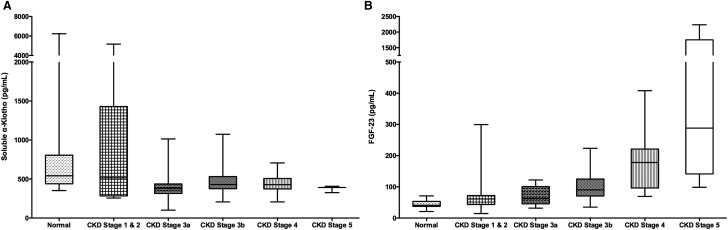
Median sKl was lower and iFGF-23 was higher in those with CKD when compared with healthy volunteers [408 (IQR 340–528) versus 542 (439–806) pg/mL and 94 (65–160) versus 41 (38–53) pg/mL; P < 0.001 for both]. sKl levels were lower in subjects with an eGFR <60 mL/min/1.73 m2 while iFGF-23 levels showed a progressive increase with CKD stage (Figure 1). While there were no significant differences in sCa and sPi levels, CKD patients were more likely to have higher FEPi, lower total daily UPE and lower phosphate reabsorption (24-h TmP/GFR) than healthy volunteers (Table 1). Notably, female CKD patients had significantly higher iFGF-23 levels than their male CKD counterparts [117 (IQR 93–170) versus 80 (61–135) pg/mL; P = 0.018].
Fig. 1.
(A) Soluble klotho (sKl) levels and (B) intact FGF-23 (iFGF-23) levels plotted across a range of renal function (median with range).
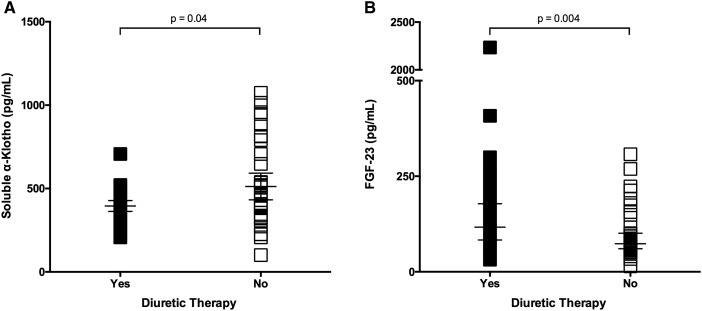
Among CKD patients, 37 (48%) were on diuretic (loop/thiazide) therapy, and this group had a lower sKl level (Δmedian = −46 pg/mL, P = 0.04) and higher iFGF-23 levels (Δmedian = 43 pg/mL, P = 0.004) than those not on diuretics (Figure 2). Diuretic use in patients with CKD did not result in differences between sCa and sPi levels, though PTH levels were higher in those on diuretic therapy [10.8 (IQR 7.5–16.5) pmol/L] compared with those not on diuretic therapy [6.6 (IQr 3.2–9.0) pmol/L; P = 0.003]. No differences in mineral parameters were noted with other therapies, namely angiotensin blockade, lipid-lowering agents or oral supplementary vitamin D—inclusive of cholecalciferol (n = 29) and calcitriol (n = 1) (Table 2).
Fig. 2.
In those with CKD, a significant difference is seen in (A) sKl and (B) iFGF-23 levels between those on diuretic therapy compared with those not on diuretic therapy (median with IQR).
Table 2.
sKl, iFGF-23 and UPE measurements in those with CKD grouped according to medication use (n = 77)
| Medication group | Absolute number (% of total) | sKl (pg/mL), median (IQR) | iFGF-23 (pg/mL), median (IQR) | 24-h UPE (mmol/day), median (IQR) |
|---|---|---|---|---|
| Diuretic therapy | ||||
| Yes | 37 (48) | 397 (345–435)* | 116 (82–172)† | 21.7 (16.7–31.5) |
| No | 40 (52) | 443 (335–663) | 73 (60–101) | 25.8 (14.3–34.4) |
| Angiotensin blockade | ||||
| Yes | 53 (69) | 408 (341–507) | 98 (70–163) | 24.5 (17.6–34.0) |
| No | 24 (31) | 408 (385–516) | 73 (57–116) | 25.4 (11.4–31.1) |
| Statin therapy | ||||
| Yes | 44 (57) | 427 (373–478) | 98 (70–160) | 23.6 (16.4–35.4) |
| No | 33 (43) | 408 (320–521) | 76 (60–122) | 25.3 (15.9–30.3) |
| Oral vitamin D supplementationa | ||||
| Yes | 30 (40) | 430 (385–509) | 107 (70–165) | 21.9 (13.8–31.9) |
| No | 47 (60) | 403 (315–515) | 80 (61–133) | 25.4 (17.7–31.8) |
Inclusive of oral cholecalciferol (n = 29, average dose = 1231 ± 583 IU/day) and calcitriol (n = 1, dose 0.75 μg/week).
P < 0.05 compared with those not on treatment using Mann–Whitney U-test;
P < 0.01.
Soluble klotho correlates with phosphate reabsorption
Univariate correlations are tabulated in Table 3. There was no significant correlation observed between Ln-sKl and Ln-iFGF-23 or Ln-PTH. An inverse correlation was noted between Ln-sKl with age, while a positive correlation between Ln-sKl and eGFR was detected (R = −0.354 and R = 0.324, respectively; both P < 0.001). Relationships between Ln-sKl and both BMI and FEPi were also demonstrated. A significant correlation was seen between Ln-sKl and 24-h TmP/GFR (R = 0.399, P < 0.001). Independent parameters in the backward multivariate analysis model using Ln-sKl as the dependent variable included age, eGFR, BMI, Ln-PTH, Ln-iFGF-23, sPi, Ln-25(OH)D and 24-h TmP/GFR. Only age and 24-h TmP/GFR were selected as significant in the final Ln-sKl predictive model with F (2,94) = 12.849, P < 0.001, R2 = 0.215, where P ≤ 0.01 for both variables (Table 4).
Table 3.
Univariate correlation analyses between phosphate/mineral parameters of interesta
| Ln-sKl | Ln-iFGF-23 | Ln-24-h UPE | Ln-24-h FEPi | 24-h TmP/GFR | |
|---|---|---|---|---|---|
| Parameter | Correlation coefficient | ||||
| Ln-sKl | −0.243* | −0.428† | 0.399† | ||
| Ln-iFGF-23 | −0.138 | −0.268* | 0.514† | −0.198* | |
| Age | −0.354† | 0.407† | −0.118 | 0.546† | −0.259† |
| eGFR | 0.324† | −0.684† | 0.299† | −0.761† | 0.342† |
| BMI | −0.258* | 0.164 | 0.085 | 0.252* | −0.222* |
| Ln-PTH | −0.201* | 0.645† | −0.332† | 0.628† | −0.362† |
| Serum phosphate (sPi) | 0.171 | 0.388† | −0.486† | −0.136 | 0.344† |
| Serum calcium (sCa) | −0.167 | −0.076 | 0.236 | 0.037 | −0.054 |
| Ln-25(OH)D | −0.211* | 0.163 | 0.017 | 0.176 | −0.162 |
| Ln-24-h UPE | −0.243* | −0.268* | |||
| Ln-24-h FEPi | −0.428† | 0.514† | 0.103 | ||
| 24-h TmP/GFR | 0.399† | −0.198* | −0.276† | −0.794† | |
Significant values in bold text where
P < 0.05,
P ≤ 0.003 (P < 0.003 considered significant, see text).
Table 4.
Multivariate linear regression analyses
| Predictors | Univariate model |
Backward multivariate model |
||||
|---|---|---|---|---|---|---|
| B coefficient | 95% confidence interval | P-value | B coefficient | 95% confidence interval | P-value | |
| Dependent: Ln-sKl | ||||||
| Age | −0.005 | −0.016–0.005 | 0.321 | −0.009 | −0.016 to − 0.002 | 0.013 |
| eGFR | 0.003 | −0.003–0.009 | 0.329 | |||
| BMI | −0.005 | −0.023–0.014 | 0.638 | |||
| Ln-PTH | 0.028 | −0.252–0.196 | 0.806 | |||
| Ln-iFGF-23 | 0.199 | −0.062–0.460 | 0.134 | |||
| sPi | −0.049 | −0.629–0.531 | 0.867 | |||
| Ln-25(OH)D | −0.144 | −0.388–0.100 | 0.243 | |||
| 24-h TmP/GFR | 0.567 | 0.154–0.979 | 0.008 | 0.618 | 0.293–0.943 | <0.001 |
| Dependent: Ln-iFGF-23 | ||||||
| Age | −0.001 | −0.009–0.008 | 0.846 | |||
| eGFR | −0.008 | −0.014 to − 0.002 | 0.012 | −0.009 | −0.013 to − 0.005 | <0.001 |
| Ln-sKl | 0.044 | −0.12–0.208 | 0.594 | |||
| Ln-PTH | 0.15 | −0.019–0.319 | 0.081 | 0.166 | 0.004–0.329 | 0.045 |
| Serum phosphate (sPi) | 0.637 | 0.106 to 1.167 | 0.019 | 0.524 | 0.111–0.937 | 0.014 |
| Ln-24-h FEPi | 0.164 | −0.132–0.46 | 0.274 | |||
| Dependent: 24-h TmP/GFR | ||||||
| Age | 0.006 | 0.001–0.011 | 0.020 | 0.005 | 0.000–0.009 | 0.03 |
| eGFR | 0.001 | −0.002–0.004 | 0.375 | |||
| BMI | −0.008 | −0.017–0.000 | 0.063 | −0.009 | −0.018–0.000 | 0.054 |
| Ln-sKl | 0.124 | 0.031–0.217 | 0.009 | 0.131 | 0.04–0.222 | 0.005 |
| Ln-iFGF-23 | −0.088 | −0.210–0.033 | 0.153 | −0.11 | −0.221–0.001 | 0.052 |
| Ln-PTH | −0.124 | −0.224 to − 0.024 | 0.016 | −0.143 | −0.234 to − 0.052 | 0.002 |
| Serum phosphate (sPi) | 0.643 | 0.397–0.889 | <0.001 | 0.641 | 0.395–0.886 | <0.001 |
| Dependent: Ln-24-h UPE | ||||||
| Age | 0.002 | −0.008–0.011 | 0.742 | |||
| eGFR | 0.005 | −0.001–0.011 | 0.093 | 0.005 | 0.002–0.009 | 0.001 |
| Ln-sKl | −0.246 | −0.431–0.061 | 0.010 | −0.255 | −0.437 to − 0.072 | 0.007 |
| Ln-iFGF-23 | 0.144 | −0.087–0.376 | 0.219 | |||
| Ln-PTH | −0.144 | −0.338–0.05 | 0.145 | |||
| Serum phosphate (sPi) | −1.193 | −1.724 to − 0.662 | <0.001 | −1.158 | −1.666 to − 0.65 | <0.001 |
Intact FGF-23 and its relationship with phosphate parameters
Unadjusted univariate analysis demonstrated strong correlations between Ln-iFGF-23 with various parameters, including age, eGFR, Ln-PTH, sPi, Ln-UPE and Ln-FEPi (Table 3). Backward multivariate analysis for Ln-iFGF-23 used the former four independent variables, Ln-sKl and a uPi marker. The analysis was performed with Ln-24-h FEPi and not Ln-24-h UPE or 24-h TmP/GFR, as this uPi variable demonstrated the strongest univariate association with Ln-iFGF-23. Only eGFR, Ln-PTH and sPi remained in the final Ln-iFGF-23 model, with F (3,97) = 38.442, P < 0.001, R2 = 0.543, where all three variables added to the model significantly and all P-values were < 0.05 (Table 4).
Phosphate handling
Unadjusted univariate analysis demonstrated strong correlations between 24-h TmP/GFR with various parameters, including age, eGFR, Ln-sKl and Ln-iFGF-23, Ln-PTH and sPi (Table 3). Independent parameters in the backward multivariate analysis model using Ln-24-h TmP/GFR as the dependent variable included age, eGFR, Ln-sKl, Ln-iFGF-23, BMI, Ln-PTH and sPi. Age, Ln-sKl, Ln-PTH and sPi were selected as significant in the final predictive model, though Ln-iFGF-23 and BMI remained in the model, with F (6,92) = 11.354, P < 0.001, R2 = 0.36 (Table 4).
Discussion
In this observational cross-sectional study, a substantial decrease in sKl is demonstrated when eGFR falls below 60 mL/min/1.73 m2. The concurrent evaluation of sKl, iFGF-23 and 24-h uPi measurements have facilitated further appraisal of mineral metabolism in subjects with non–dialysis-dependent CKD and healthy volunteers. The independent association between sKl and phosphate reabsorption (24-h TmP/GFR), following multivariate analysis adjusting for eGFR and age, suggests sKl may be a promising marker of phosphate reabsorption.
Progressive elevation of FGF-23 levels in the CKD population and its association with cardiovascular and all-cause mortality is well-described [8–12]. Similarly, the observed differences in FEPi and total UPE in those with CKD, compared with healthy volunteers, have independently been described [29, 30]. Lower sKl levels in CKD, as reported here, are consistent with several medium to large observational studies evaluating sKl in patients with CKD stages 1–5, including one paediatric study [31–36]. Nevertheless, several studies have yielded different results [13]. These inconsistencies could relate to different assays used [37, 38], smaller sample sizes [39] or the absence of a wider CKD range [40]. Given the marked reduction observed at eGFR ∼60 mL/min/1.73 m2 or less in this study, studies that targeted a narrower range of renal function despite larger sample sizes [40] may have been biased towards the null and therefore reported no association between sKl and CKD.
Lack of correlation was noted between Ln-sKl and Ln-iFGF-23 in this study, which is consistent with most studies investigating both markers concurrently, irrespective of patient population. In patients with CKD, whether adults or children, dialysis dependent or not, no clear relationship has been established between sKl and FGF-23 [15–20]. Only one study, by Rotondi et al. [21], has described a strong negative correlation between FGF-23 and sKl levels in 68 CKD patients. The cohort presented in our study included older and more male participants compared with participants in this study, where female CKD participants were observed to have higher iFGF-23 levels compared with CKD males, providing a potential explanation for the disparity. This gender difference in FGF-23 levels is consistent with reports in both the very young [41] and adults [42, 43]. One postulation is that estradiol is a phosphaturic agent [44] and the loss of this hormone with onset of menopause may result in higher sPi levels, contributing to a compensatory increase in FGF-23. Although information on menopausal status of CKD females was lacking, females with CKD commonly experience the onset of menopause before the age of 45 years [45], and the median age of CKD females in this study was 65 (range 38–75) years.
The multivariate analyses reveal a more interesting picture in the context of 24-h uPi measurements. We have previously reported bias in uPi measurements between different collection methods, where spot urine collections underestimated phosphate excretion and overestimated phosphate reabsorption compared with 24-h urine collections [22]. Hence, 24-h urine phosphate parameters evaluated in this study may provide more comprehensive assessments of relationships with sKl and iFGF-23 compared with two prior studies that evaluated spot urine phosphate [20, 21]. No other study to date has assessed FGF-23, sKl and 24-h uPi.
The positive correlation between Ln-sKl and 24-h TmP/GFR was observed on unadjusted analysis and remained after multivariate regression analysis adjusting for both age and eGFR. This suggests a robust relationship, with sKl as either a dominant influence on or a prominent marker of phosphate reabsorption, or both. No correlation was detected between Ln-sKl and sPi or UPE on multivariate analysis. Given the reported links between phosphate, PTH and FGF-23, where both phosphate and PTH are positive regulators of the latter [8], the association seen between Ln-iFGF-23 and both variables was not surprising. The loss of correlation between Ln-iFGF-23 and urinary markers of phosphate regulation within the multivariate analysis is striking, as it may suggest the kidney’s phosphate reabsorption capacity is more closely linked to sKl than iFGF-23.
Interestingly, multivariate analyses using either Ln-24-h UPE or 24-h TmP/GFR as dependent variables indicated an independent association with sKl but not iFGF-23, corroborating the signal between Ln-sKl and phosphate handling, specifically TmP/GFR. Hence, it is postulated that sKl levels reflect the ‘true’ FGF-23 and FGF receptor action, which is downregulation of sodium phosphate co-transporters at the proximal tubules, resulting in increased UPE or reduced phosphate reabsorption, as sKl would mirror the physiologically available klotho serving as co-receptor.
Klotho also acts as an obligatory co-receptor to FGF-23 actions at the FGF receptor present in the parathyroid gland [46]. Following this speculation of sKl levels reflecting the true FGF-23 action, one might assume the same is true of PTH levels and PTH secretion. Multivariate analyses conducted using Ln-PTH as a dependent variable (Supplementary data, Table S1) suggest that Ln-sKl is not associated with Ln-PTH. Both sPi and sCa were also entered into the statistical model. However, a major limitation to this multivariate analysis is the lack of active vitamin D levels. 1,25-dihydroxyvitamin D is a major regulator of the calcium–PTH axis and, as such, the evaluation of this axis is incomplete without these values.
Lower sKl and higher iFGF-23 levels in patients with CKD treated with diuretics is noteworthy. The use of loop diuretic and thiazide diuretic have been analysed together, as there were no appreciable clinical or biochemical differences between these patient groups. High sodium exposure to the distal convoluted tubules (DCTs) can result from chronic loop diuretic therapy leading to DCT cellular hypertrophy [47], while chronic thiazide diuretic use can negatively affect the morphology and behaviour of DCT cells [48, 49]. As renal α-klotho is predominantly found within the DCTs, this finding suggests a possible link between diuretic use and changes to α-klotho expression within the DCTs. Theoretically, cellular hypertrophy could lead to higher mKl and sKl levels, although it is conceivable that this pathological diuretic DCT response or the underlying pathophysiological CKD changes may alter cleavage and/or metabolism of renal mKl, resulting in lower than expected sKl levels. The lack of temporal information, specifically chronicity, of diuretic therapy is a limitation of this hypothesis.
Taken together, this clinical dataset provides a basis to postulate relationships between these mineral parameters. Klotho and FGF-23 likely work together to provide a well-balanced regulated phosphate handling system. In health, iFGF-23 signals lower phosphate reabsorption, and the tubular cell is able to respond with healthy levels of mKl [4]. Assuming sKl is a reflection of mKl, the robust relationship seen between sKl and TmP/GFR supports the notion of sKl reflecting true FGF-23 phosphaturic action. In CKD, as the kidney function progressively declines, fading sKl levels could indicate diminished total kidney capacity to reabsorb/excrete phosphate despite elevated iFGF-23 levels.
There are several limitations to this study. The cohort is small in size and therefore the findings from this study require validation within a larger cohort. Potential bias as a consequence of disease aetiology cannot be excluded in this study. As mentioned already, the lack of active vitamin D levels could limit the complex evaluation of mineral relationships and the lack of extended clinical information limits the discussion surrounding associations between klotho levels and oestrogen or diuretic use.
Conclusion
In summary, we have shown increasing sKl and decreasing iFGF-23 levels in patients with CKD, which is particularly marked when renal function falls below an eGFR of 60 mL/min/1.73 m2. A significant association was noted between sKl levels and uPi reabsorption, indicating a relationship between circulating sKl levels and true phosphaturic actions of FGF-23 on the FGF receptor. However, the cross-sectional nature of these findings cannot provide temporal interpretation. Further studies are critical for improved comprehension and translation of these concepts into clinical practice and a better understanding of the cellular effects of sKl.
Supplementary data
Supplementary data are available online at http://ckj.oxfordjournals.org.
Supplementary Material
Acknowledgements
S.J.T. is a recipient of a National Health and Medical Research Council (NHMRC) Postgraduate Research Scholarship. N.D.T. is supported by a Jacquot Foundation Research Establishment Award. The contents of this article are solely the views of the individual authors and do not reflect the views of NHMRC or the Jacquot Foundation.
Conflict of interest statement
This manuscript has not been published previously, in whole or part, except in abstract format. Data pertaining to differences in uPi collection methods from this dataset/cohort has recently been published (International Urology and Nephrology). This has been appropriately cited within this current manuscript. S.J.T. has received speaking honoraria from Shire. E.R.S. has received research funding from Amgen and Baxter, honoraria from Shire and served as a consultant for Vifor Pharma. S.G.H. has received research funding or honoraria from Amgen, Baxter, Gilead, Novartis and Shire. N.D.T. has received consultancy fees, honoraria and research funding from Amgen and Shire Pharmaceuticals.
References
- 1. Kuro-o MY, Matsumura H, Aizawa H. et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997; 390: 45–51 [DOI] [PubMed] [Google Scholar]
- 2. Yamashita T, Yoshioka M, Itoh N.. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem Biophys Res Commun 2000; 277: 494–498 [DOI] [PubMed] [Google Scholar]
- 3. ADHR Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 2000; 26: 345–348 [DOI] [PubMed] [Google Scholar]
- 4. Nakatani TB, Sarraj M, Ohnishi MJ. et al. In vivo genetic evidence for klotho-dependent, fibroblast growth factor 23 (Fgf23)-mediated regulation of systemic phosphate homeostasis. FASEB J 2009; 23: 433–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shimada TM, Kakitani Y, Yamazaki H. et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 2004; 113: 561–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Isakova TP, Wahl GS, Vargas OM. et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 2011; 79: 1370–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Slatopolsky E. The intact nephron hypothesis: the concept and its implications for phosphate management in CKD-related mineral and bone disorder. Kidney Int Suppl 2011; 79(Suppl 121): S3–S8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smith ER, McMahon LP, Holt SG.. Fibroblast growth factor 23. Ann Clin Biochem 2014; 51: 203–227 [DOI] [PubMed] [Google Scholar]
- 9. Gutierrez OM, Mannstadt M, Isakova T. et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008; 359: 584–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Isakova T, Xie H, Yang W. et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 2011; 305: 2432–2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kendrick J, Cheung AK, Kaufman JS. et al. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 2011; 22: 1913–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakano C, Hamano T, Fujii N. et al. Intact fibroblast growth factor 23 levels predict incident cardiovascular event before but not after the start of dialysis. Bone 2012; 50: 1266–1274 [DOI] [PubMed] [Google Scholar]
- 13. Tan SJ, Smith ER, Hewitson TD. et al. The importance of klotho in phosphate metabolism and kidney disease. Nephrology (Carlton) 2014; 19: 439–449 [DOI] [PubMed] [Google Scholar]
- 14. Semba RD, Cappola AR, Sun K. et al. Plasma klotho and mortality risk in older community-dwelling adults. J Gerontol A Biol Sci Med Sci 2011; 66: 794–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cano FJ, Freundlich M,, Ceballos ML. et al. Longitudinal FGF23 and klotho axis characterization in children treated with chronic peritoneal dialysis. Clin Kidney J 2014; 7: 457–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buiten MS, de Bie MK, Bouma-de Krijger ML. et al. Soluble klotho is not independently associated with cardiovascular disease in a population of dialysis patients. BMC Nephrol 2014; 15: 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hage V, Pelletier S, Dubourg L. et al. In chronic kidney disease, serum alpha-klotho is related to serum bicarbonate and proteinuria. J Ren Nutr 2014; 24: 390–394 [DOI] [PubMed] [Google Scholar]
- 18. Sawires HK, Essam RM, Morgan MF. et al. Serum klotho: relation to fibroblast growth factor-23 and other regulators of phosphate metabolism in children with chronic kidney disease. Nephron 2015; 129: 293–299 [DOI] [PubMed] [Google Scholar]
- 19. Okamoto Y, Fujita SI, Morita H. et al. Association between circulating FGF23, alpha-klotho, and left ventricular diastolic dysfunction among patients with preserved ejection fraction. Heart Vessels 2016; 31: 66–73 [DOI] [PubMed] [Google Scholar]
- 20. Ozeki MS, Fujita S, Kizawa H. et al. Association of serum levels of FGF23 and alpha-klotho with glomerular filtration rate and proteinuria among cardiac patients. BMC Nephrol 2014; 15: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rotondi S, Pasquali M, Tartaglione L. et al. Soluble alpha-klotho serum levels in chronic kidney disease. Int J Endocrinol 2015; 2015: 872193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tan SJ, Smith ER, Cai MM. et al. Relationship between timed and spot urine collections for measuring phosphate excretion. Int Urol Nephrol 2016; 48: 115–124 [DOI] [PubMed] [Google Scholar]
- 23. Levey AS, Stevens LA, Schmid CH. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. KDIGO Working Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [DOI] [PubMed] [Google Scholar]
- 25. Walton RJ, Bijvoet OL.. Nomogram for derivation of renal threshold phosphate concentration. Lancet 1975; 2: 309–310 [DOI] [PubMed] [Google Scholar]
- 26. Kenny AP, Glen AC.. Tests of phosphate reabsorption. Lancet 1973; 2: 158. [DOI] [PubMed] [Google Scholar]
- 27. Barth JH, Jones RG, Payne RB.. Calculation of renal tubular reabsorption of phosphate: the algorithm performs better than the nomogram. Ann Clin Biochem 2000; 37: 79–81 [DOI] [PubMed] [Google Scholar]
- 28. Chong WH, Molinolo AA, Chen CC. et al. Tumor-induced osteomalacia. Endocr Relat Cancer 2011; 18: R53–R77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gutierrez OM, Isakova T, Rhee E. et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 2005; 16: 2205–2215 [DOI] [PubMed] [Google Scholar]
- 30. Craver L, Marco MP, Martinez M. et al. Mineral metabolism parameters throughout chronic kidney disease stages 1-5–achievement of K/DOQI target ranges. Nephrol Dial Transplant 2007; 22: 1171–1176 [DOI] [PubMed] [Google Scholar]
- 31. Kim HR, Nam BY, Kim DW. et al. Circulating alpha-klotho levels in CKD and relationship to progression. Am J Kidney Dis 2013; 61: 899–909 [DOI] [PubMed] [Google Scholar]
- 32. Pavik I, Jaeger P, Ebner L. et al. Secreted Klotho and FGF23 in chronic kidney disease stage 1 to 5: a sequence suggested from a cross-sectional study. Nephrol Dial Transplant 2013; 28: 352–359 [DOI] [PubMed] [Google Scholar]
- 33. Sakan H, Nakatani K, Asai O. et al. Reduced renal alpha-klotho expression in CKD patients and its effect on renal phosphate handling and vitamin D metabolism. PLoS One 2014; 9: e86301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shimamura Y, Hamada K, Inoue K. et al. Serum levels of soluble secreted alpha-klotho are decreased in the early stages of chronic kidney disease, making it a probable novel biomarker for early diagnosis. Clin Exp Nephrol 2012; 16: 722–729 [DOI] [PubMed] [Google Scholar]
- 35. Akimoto T, Yoshizawa H, Watanabe Y. et al. Characteristics of urinary and serum soluble klotho protein in patients with different degrees of chronic kidney disease. BMC Nephrol 2012; 13: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wan M, Smith C, Shah V. et al. Fibroblast growth factor 23 and soluble klotho in children with chronic kidney disease. Nephrol Dial Transplant 2013; 28: 153–161 [DOI] [PubMed] [Google Scholar]
- 37. Devaraj S, Syed B, Chien A. et al. Validation of an immunoassay for soluble klotho protein: decreased levels in diabetes and increased levels in chronic kidney disease. Am J Clin Pathol 2012; 137: 479–485 [DOI] [PubMed] [Google Scholar]
- 38. Kacso IM, Bondor CI, Kacso G.. Soluble serum klotho in diabetic nephropathy: relationship to VEGF-A. Clin Biochem 2012; 45: 1415–1420 [DOI] [PubMed] [Google Scholar]
- 39. Sugiura H, Tsuchiya K, Nitta K.. Circulating levels of soluble alpha-klotho in patients with chronic kidney disease. Clin Exp Nephrol 2011; 15: 795–796 [DOI] [PubMed] [Google Scholar]
- 40. Seiler S, Wen M, Roth HJ. et al. Plasma klotho is not related to kidney function and does not predict adverse outcome in patients with chronic kidney disease. Kidney Int 2013; 83: 121–128 [DOI] [PubMed] [Google Scholar]
- 41. Holmlund-Suila E, Viljakainen H, Ljunggren O. et al. Fibroblast growth factor 23 concentrations reflect sex differences in mineral metabolism and growth in early infancy. Horm Res Paediatr 2016; 85: 232–241 [DOI] [PubMed] [Google Scholar]
- 42. Atta MG, Estrella MM, Fine DM. et al. Correlates and longitudinal renal and cardiovascular implications of FGF23 levels in HIV-positive individuals. PLoS One 2016; 11: e0155312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ix JH, Chonchol M,, Laughlin GA. et al. Relation of sex and estrogen therapy to serum fibroblast growth factor 23, serum phosphorus, and urine phosphorus: the Heart and Soul Study. Am J Kidney Dis 2011; 58: 737–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Faroqui S, Levi M, Soleimani M. et al. Estrogen downregulates the proximal tubule type IIa sodium phosphate cotransporter causing phosphate wasting and hypophosphatemia. Kidney Int 2008; 73: 1141–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cheung KL, Stefanick ML, Allison MA. et al. Menopausal symptoms in women with chronic kidney disease. Menopause 2015; 22: 1006–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V. et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest 2007; 117: 4003–4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ellison DH, Velazquez H, Wright FS.. Adaptation of the distal convoluted tubule of the rat. Structural and functional effects of dietary salt intake and chronic diuretic infusion. J Clin Invest 1989; 83: 113–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Loffing JD, Loffing-Cueni I, Hegyi MR. et al. Thiazide treatment of rats provokes apoptosis in distal tubule cells. Kidney Int 1996; 50: 1180–1190 [DOI] [PubMed] [Google Scholar]
- 49. Morsing P, Velazquez H, Wright FS. et al. Adaptation of distal convoluted tubule of rats. II. Effects of chronic thiazide infusion. Am J Physiol 1991; 261: F137–F143 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.