Abstract
Two different quantitative PCR platforms, droplet digital PCR (dd-PCR) and quantitative real-time PCR (qPCR), were compared in a mcrA-based methanogen community assay that quantifies ten methanogen sub-groups. Both technologies exhibited similar PCR efficiencies over at least four orders of magnitude and the same lower limits of detection (8 copies μL-DNA extract−1). The mcrA-based methanogen communities in three full-scale anaerobic digesters were examined using the two technologies. dd-PCR detected seven groups from the digesters, while qPCR did five groups, indicating that dd-PCR is more sensitive for DNA quantification. Linear regression showed quantitative agreements between both of the technologies (R2 = 0.59–0.98) in the five groups that were concurrently detected. Principal component analysis from the two datasets consistently indicated a substantial difference in the community composition among the digesters and revealed similar levels of differentiation among the communities. The combined results suggest that dd-PCR is more promising for examining methanogenic archaeal communities in biotechnological processes.
Keywords: Droplet-digital PCR, Quantitative real-time PCR, Methanogen community, Anaerobic digester, mcrA
Biological production of methane as a renewable energy has received extensive attention in the field of biotechnology [1]. For instance, anaerobic digestion is a typical biotechnological process for reduction of waste biomass along with production of methane-containing biogas. Methanogens (methane-producing archaea) are strictly anaerobic and slowly growing, and require different growth conditions [2]. Therefore, it is very difficult to scrutinize the methanogens present in these biotechnological processes using culture-dependent techniques. Technical advances in molecular microbial ecology have enabled rapid and complete examination of methanogen communities in anaerobic digestion systems without cultivation [10], [14], [17]. For instance, Steinberg and Regan [14] developed a methanogen community assay, based on the alpha-subunit of the methyl coenzyme M reductase (mcrA) as a phylogenetic marker. The basis of the assay is to quantify ten different groups within the methanogen community using quantitative real-time PCR (qPCR).
The nature of qPCR is to extrapolate the initial concentration of target DNA with an external DNA calibrator [5]. For the mcrA-based assay, ten different external DNA calibrators must be prepared, which is an expensive, laborious, and time-consuming process, because they are not readily available [9]. Recently, droplet digital PCR (dd-PCR) has been developed as a new platform for DNA quantification [6]. The most important advantage of dd-PCR over qPCR is to enable the absolute quantification of DNA concentrations without external calibrators [6], [13]. In addition, dd-PCR is less susceptible to PCR inhibitors present in the DNA extracts than qPCR [12]. Earlier studies have demonstrated the accuracy and precision of dd-PCR in the quantitative detection of bacteria and viruses in clinical samples [4], [7], [15]. The primary objective of this study was to compare dd-PCR and qPCR in the mcrA-based community assay. Each group was quantified from three full-scale anaerobic digesters using both technologies, and the two community datasets were compared.
Three wastewater treatment facilities are located in Seoul, South Korea. An anaerobic digester was selected from each of the facilities. They are all cylindrical and continuously stirred tank reactors, receiving municipal sewage sludge. They were designated as A (an operational temperature of 38 °C and a HRT of 19 days), B (38 °C and 43 days) and C (52.5 °C and 40 days). Sludge was collected in sterile polyethylene bottles from the recirculation loop of each digester. DNA was extracted using a NucleoSpin Soil kit (Macherey-Nagel GmbH, Düren, Germany) according to the manufacturer’s recommendations. DNA was eluted in 100 μL of the elution buffer. There were three replicates per digester.
The mcrA-based community assay consists of a single forward/reverse primer set and 10 different hydrolysis probes targeting Methanobacteriaceae mcrA (mbac), Methanobacteriaceae mrtA (mrtA), Methanocorpusculaceae (mcp), Methanospirillaceae (msp), Methanosarcina (msar), Methanosaetaceae (msa), uncultured mcr-7 group (mcr-7), uncultured mcr-2a group (mcr-2a), uncultured mcr-2b group (mcr-2b), and uncultured Fen cluster (Fen) [14]. dd-PCR was performed using a QX100™ droplet digital PCR system (Bio-Rad, Pleasanton, USA) according to the manufacturer’s recommendations. The reaction mixture (20 μL) contained 1× dd-PCR master mix (Bio-Rad), 0.9 μM each primer, 1 μM probe and 1 μL template DNA. PCR amplification was carried out on a 2700 GeneAmp® PCR system (Applied Biosystems, Foster, USA). PCR was initiated at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 90 s, and 1 cycle at 98 °C for 10 min. Data were obtained and analyzed using the QX100™ droplet reader (Bio-Rad) and QuantaSoft software (Bio-Rad). The QuantaSoft program generates absolute quantities per microliter-reaction mixture (a total of 20 μL-reaction volume) from given numbers of positive droplets and negative droplets. The obtained values were multiplied by 20 to calculate quantities in microliter-DNA extracts. qPCR was performed using an Applied Biosystems 7300 system as previously described [9]. dd-PCR was used in order to determine the concentrations of the external DNA calibrators with multiple probe sites [9] for qPCR because it accurately provides absolute quantification of target DNA [3], [4], [6]. The 25-μL reaction mixture contained 1× PCR buffer, 0.2 μL Ace-Taq (Genenmed, Seoul, Korea), 0.3 mM dNTPs mix, 0.25 μM each primer, 0.15 μM probe, 1× ROX (Invitrogen, Carlsbad, USA), 1× SYBR green I (Invitrogen) and 1 μL template DNA. PCR was initiated at 95 °C for 3 min, followed by 40 cycles at 95 °C for 15 s and 55 °C for 90 s.
Two artificial DNA templates with multiple probe sites were developed as reference DNA templates for qPCR of the 10 groups [9]. The two artificial sequences (509 bp long) contain the target DNA region (amplified by the primer pair), with additional flanking 20-bp DNA regions at the both ends. Plasmids with the artificial DNA templates were used to construct standard curves. They were serially diluted 10-fold. The two technologies did not detect DNA at <10−8 dilution (equivalent to 8 copies μL−1 as measured by dd-PCR). The 10 standard curves constructed by qPCR over the 10-fold serial dilution series (10−5–10−8) showed a slope value of 3.39 ± 0.14 (R2 = 0.99 ± 0.01), corresponding to a PCR efficiency of 97%. In order to compare the quantitative limits of detection, linearity and PCR efficiencies, the standard curves of several probes including msar, mcp, and msa were constructed using dd-PCR. The dd-PCR showed a slope value of 1.00 ± 0.03 (R2 = 0.99 ± 0.01), equivalent to 100% efficiency, over at least 4 orders of magnitude. Both technologies exhibited very similar levels of efficiency and linearity, with the same lower limits of detection. Quantification results were expressed as copy number microliter-DNA extract−1 for direct comparison.
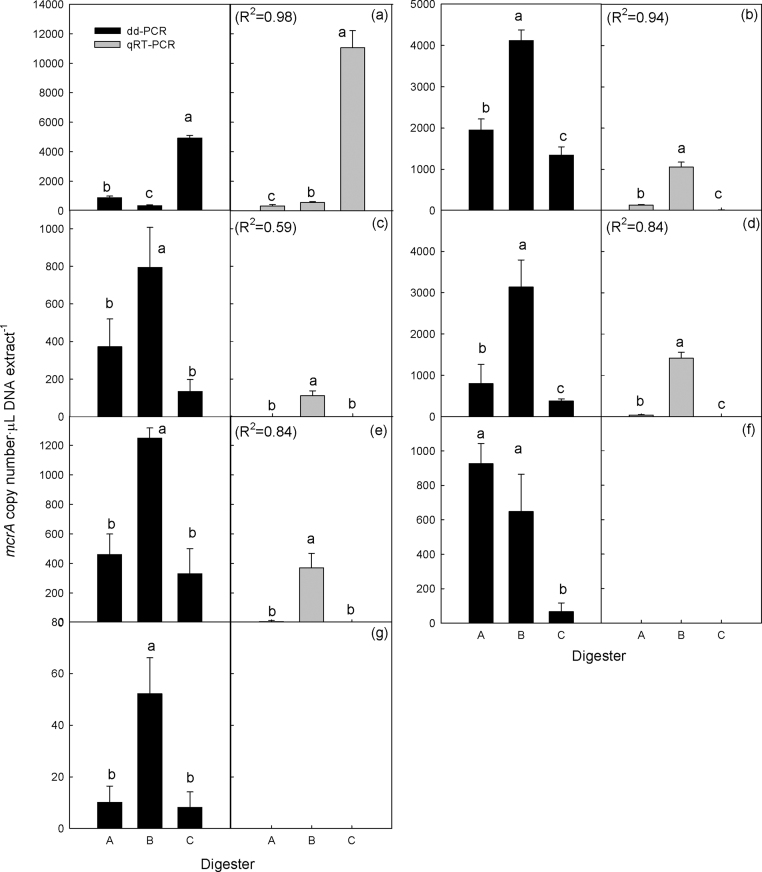
Each group was quantified from the three digesters using both technologies (Fig 1). mrtA, mcr-2b and Fen were not detected by either technology. dd-PCR detected seven groups from the digesters, while qPCR detected five groups. dd-PCR could detect mcr-2a (<940 copies μL-DNA extract−1) and mrtA (<54 copies μL-DNA extract−1) that were not detected by qPCR. These results indicate that dd-PCR is more sensitive for the detection and quantification of DNA from digester samples, which is consistent with a observation by Kim et al. [8] that dd-PCR was more sensitive for quantifying DNA from soil than qPCR. PCR inhibitors co-extracted with nucleic acids from environmental samples can adversely affect qPCR quantification [16]. dd-PCR may be less sensitive to PCR inhibitors than the qPCR because the post-PCR quantification regime after 40 cycles can tolerate wide variations in PCR amplification efficiencies [6], [12]. The technologies detected five groups: msar, msa, mcp, msp, and mcr7 (Figs. 1a–e), and therefore, they were compared based on these groups. T-test revealed that the technologies identically indicated the digesters in which the target groups were most abundant at p < 0.05. Both technologies also showed the same order among the digesters in order of abundance of msa, mcp, msp, and mcr7 (p < 0.05). In the case of msar, both technologies showed that it was much greater in digester C than in digesters A and B, and dd-PCR showed it was greater in digester A than in digester B, while qPCR showed the opposite (p < 0.05). The linear regression (y = ax + b) was conducted in order to determine whether or not there were quantitative agreements between the dd-PCR and qPCR measurements. Similar to previous observations showing quantitative agreements between both technologies [4], [15], there were R2 values ranging from 0.59–0.98 in all of the groups (Fig. 1). However, slope values substantially varied between the groups.
Fig. 1.
dd-PCR and qPCR quantification results. (a) msar; (b) msa; (c) mcp; (d) msp; (e) mcr7; (f) mcr-2a; and (g) mbac. The three groups (mrtA, mcr-2b, and Fen) were not detected by either of the technologies. Error bars represent ±1 standard deviation of the mean. Different letters (a, b and c) indicate significant difference at p < 0.05. Correlation coefficients (R2) between dd-PCR and qPCR are shown in parentheses.
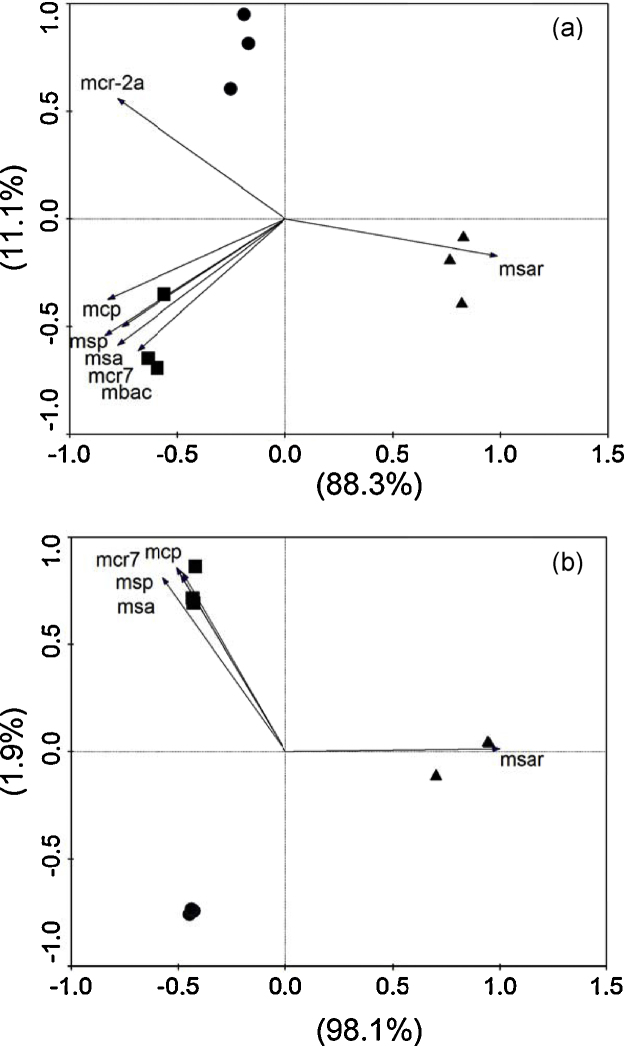
Both technologies quantitatively agreed, although their quantitative differences were quite varied. In order to determine whether or not both datasets represent similar relationships among the digester communities, principal component analysis (PCA), a multivariate approach to compare microbial communities, was performed using CANOCO version 4.5 [18]. The PCA plot of dd-PCR shows that the first and second principal component axes account for 88.3 and 11.1% of the compositional variance in the data, respectively (Fig. 2a), whereas that of qPCR shows that the first and second axes account for 98.1 and 1.9% (Fig. 2b). Both plots indicate a substantial difference in the community composition among the digesters, and exhibit similar levels of differentiation among the communities. Both plots also indicate that operational temperature (from 38 to 52.5 °C) coincides with the score of the first axis (from approximately −0.5 to 1.0). Both plots indicate that the community of the thermophilic digester C was distinct from those of the mesophilic digesters A and B, primarily because msar dominated the C community. The msar (Methanosarcina) abundance increased along with the temperature, since Methanosarcina is better established in thermophilic regimes than in mesophilic regimes [1]. Both plots consistently indicate that abundances of msa, mcp, msp, and mcr7 were greater in digester B. The dd-PCR plot shows that mcr-2a and mbac, which were not detected by RT-PCR, were more abundant in digesters A and B, respectively. Both datasets indicated that operational temperature was an important factor for explaining the community variation, which is consistent with previous observations by Levén et al. [11] and Zielinska et al. [19], who reported that temperature is the key determinant of growth of specific methanogens when the microbial communities of mesophilic and thermophilic digesters were compared.
Fig. 2.
Principle component analysis of dd-PCR (a) and qPCR (b) datasets. ●, digester A; ■, digester B; and ▲, digester C. Percentage variance of methanogen composition explained by each axis is shown in parentheses. Arrows indicate the direction of increase for the methanogen groups.
In summary, both technologies exhibited nearly identical PCR efficiencies and the same detection limits of detection. However, dd-PCR was more sensitive for DNA quantification than qPCR. The two technologies showed quantitative agreement on the methanogen groups that were detected by both of them. In addition, both datasets revealed similar community comparison results. Therefore, dd-PCR is very promising for examining mcrA-based methanogen communities as an alternative to qPCR.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIP) (No. 2012R1A2A03046724) and the RP-Grant 2014 of the Ewha Womans University.
References
- 1.Demirel B., Scherer P. The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: a review. Rev. Environ. Sci. Biotechnol. 2008;7:173–190. [Google Scholar]
- 2.Garcia J.-L., Patel B.K.C., Ollivier B. Taxonomic, phylogenetic, and ecological diversity of methanogenic archaea. Anaerobe. 2000;6:205–226. [Google Scholar]
- 3.Hayden R.T., Gu Z., Ingersoll J., Abdul-Ali D., Shi L., Pounds S., Caliendo A.M. Comparison of droplet digital PCR to real-time PCR for quantitative detection of Cytomegalovirus. J. Clin. Microbiol. 2013;51:540–546. doi: 10.1128/JCM.02620-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henrich T.J., Gallien S., Li J.Z., Pereyra F., Kuritzkes D.R. Low-level detection and quantitation of cellular HIV-1 DNA and 2-LTR circles using droplet digital PCR. J. Virol. Methods. 2012;186:68–72. doi: 10.1016/j.jviromet.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higuchi R., Fockler C., Dollinger G., Watson R. Kinetic PCR analysis: real-time monitoring of DNA amplification reactions. Nat. Biotechnol. 1993;11:1026–1030. doi: 10.1038/nbt0993-1026. [DOI] [PubMed] [Google Scholar]
- 6.Hindson B.J., Ness K.D., Masquelier D.A., Belgrader P., Heredia N.J., Makarewicz A.J., Bright I.J., Lucero M.Y., Hiddessen A.L., Legler T.C., Kitano T.K., Hodel M.R., Petersen J.F., Wyatt P.W., Steenblock E.R., Shah P.H., Bousse L.J., Troup C.B., Mellen J.C., Wittmann D.K., Erndt N.G., Cauley T.H., Koehler R.T., So A.P., Dube S., Rose K.A., Montesclaros L., Wang S., Stumbo D.P., Hodges S.P., Romine S., Milanovich F.P., White H.E., Regan J.F., Karlin-Neumann G.A., Hindson C.M., Saxonov S., Colston B.W. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal. Chem. 2011;83:8604–8610. doi: 10.1021/ac202028g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelley K., Cosman A., Belgrader P., Chapman B., Sullivan D.C. Detection of methicillin-resistant Staphylococcus aureus by a duplex droplet digital PCR assay. J. Clin. Microbiol. 2013;51:2033–2039. doi: 10.1128/JCM.00196-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim T.G., Jeong S.-Y., Cho K.-S. Comparison of droplet digital PCR and quantitative real-time PCR for examining population dynamics of bacteria in soil. Appl. Microbiol. Biotechnol. 2014;98:6105–6113. doi: 10.1007/s00253-014-5794-4. [DOI] [PubMed] [Google Scholar]
- 9.Kim T.G., Yi T., Cho K.-S. Use of artificial DNA with multiple probe sites as reference DNA templates for quantitative real-time PCR to examine methanogen communities. J. Environ. Sci. Health Part A. 2013;48:417–421. doi: 10.1080/10934529.2013.728915. [DOI] [PubMed] [Google Scholar]
- 10.Leclerc M., Delgènes J.-P., Godon J.-J. Diversity of the archaeal community in 44 anaerobic digesters as determined by single strand conformation polymorphism analysis and 16S rDNA sequencing. Environ. Microbiol. 2004;6:809–819. doi: 10.1111/j.1462-2920.2004.00616.x. [DOI] [PubMed] [Google Scholar]
- 11.Levén L., Eriksson A.R.B., Schnürer A. Effect of process temperature on bacterial and archaeal communities in two methanogenic bioreactors treating organic household waste. FEMS Microbiol. Ecol. 2007;59:683–693. doi: 10.1111/j.1574-6941.2006.00263.x. [DOI] [PubMed] [Google Scholar]
- 12.Morisset D., Štebih D., Milavec M., Gruden K., Žel J. Quantitative analysis of food and feed samples with droplet digital PCR. PLoS One. 2013;8:e62583. doi: 10.1371/journal.pone.0062583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinheiro L.B., Coleman V.A., Hindson C.M., Herrmann J., Hindson B.J., Bhat S., Emslie K.R. Evaluation of a droplet digital polymerase chain reaction format for DNA copy number quantification. Anal. Chem. 2012;84:1003–1011. doi: 10.1021/ac202578x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinberg L.M., Regan J.M. mcrA-targeted real-time quantitative PCR method to examine methanogen communities. Appl. Environ. Microbiol. 2009;75:4435–4442. doi: 10.1128/AEM.02858-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strain M.C., Lada S.M., Luong T., Rought S.E., Gianella S., Terry V.H., Spina C.A., Woelk C.H., Richman D.D. Highly precise measurement of HIV DNA by droplet digital PCR. PLoS One. 2013;8:e55943. doi: 10.1371/journal.pone.0055943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stults J.R., Snoeyenbos-West O., Methe B., Lovley D.R., Chandler D.P. Application of the 5′ fluorogenic exonuclease assay (taqMan) for quantitative ribosomal DNA and rRNA analysis in sediments. Appl. Environ. Microbiol. 2001;67:2781–2789. doi: 10.1128/AEM.67.6.2781-2789.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sundberg C., Al-Soud W.A., Larsson M., Alm E., Yekta S.S., Svensson B.H., Sørensen S.J., Karlsson A. 454 pyrosequencing analyses of bacterial and archaeal richness in 21 full-scale biogas digesters. FEMS Microbiol. Ecol. 2013;85:612–626. doi: 10.1111/1574-6941.12148. [DOI] [PubMed] [Google Scholar]
- 18.ter Braak C.J.F., Šmilauer P. Microcomputer Power; Ithaca, NY: 2002. CANOCO reference manual and CanoDraw for Windows user’s guide: Software for canonical community ordination (version 4.5) [Google Scholar]
- 19.Zielińska M., Cydzik-Kwiatkowska A., Zieliński M., Dębowski M. Impact of temperature, microwave radiation and organic loading rate on methanogenic community and biogas production during fermentation of dairy wastewater. Bioresour. Technol. 2013;129:308–314. doi: 10.1016/j.biortech.2012.11.093. [DOI] [PubMed] [Google Scholar]