Abstract
β-d-Salicin 1 (Mahdi et al. [8]) is an interesting medicinal phytochemical that exhibits cross functions in plants and humans immunologically. This molecule 1 (Mahdi et al. [8]) has attracted the attention of scientists in various interdisciplinary fields, including chemistry, pharmacology and medicine. The biological cross functions of β-d-salicin 1 (Mahdi et al. [8]) serve in plant survival and healing processes via salicylic acid 2 (Pierpont [23]). Thus, this raise a question whether plant biosynthesis and human metabolism crosstalk to induce therapy via molecular recognition. If so, biotechnology and bioinformatics are significant techniques for new strategies in drug development. Thus, understanding the biosynthesis, metabolism and the cross-molecular setting of recognition may encourage further discussion and research on its medicinal and biological activity virtues.
Keywords: β-d-Salicin, Biosynthesis, Metabolism, Pharmacological activities
1. Introduction
The willow tree, like any other medicinal plant species, can be considered as a bioreactor for the biosynthesis of many phytochemicals, including β-d-salicin 1. These phytochemicals are recognised as secondary metabolites, which contribute to the biology of plants, as they are essential for growth, reproduction and have important roles in the ecological survival of plants against biotic and abiotic stress [1], [2], [3], [4], [5], [6], [7]. The accumulation of knowledge on phytochemistry, pharmacology and pharmacognosy and the rapid development of analytical techniques in chemistry all have profoundly contributed to the discovery of β-d-salicin 1 and its metabolite, salicylic acid 2. The elucidation of the chemical structures of these two phytochemicals, 1 and 2, leads the discovery of the most common anti-inflammatory drug, acetylsalicylic acid 3 or aspirin [8]. In this respect, researches have recognised the essential steps for exploiting plants in drug discovery and development. These steps include the identification of natural products, characterisation of the chemical structures of the bioactive molecules, investigating their pharmacological potentials and identifying the target active sites. The ethnomedical usage of plants and the retrospective pharmacological activities have also contributed to the identification of biologically active phytochemicals [9], [10]. Per se, β-d-salicin 1 and salicylic acid 2 from willow have been identified to exert vital pharmacological roles in modulating the inflammatory process and inhibition of the activation of NF-κB, and subsequent down regulating COX-2 expression [11], [12]. In addition, our recent results using Schrödinger software showed that β-d-salicin 1 has better binding affinity with COX-2 (docking score = −9.966) and NK-κB (docking score = −9.274) compared to acetylsalicylic acid 3 (docking score for COX-2 = −5.412; for NK-κB = −5.525) [13]. Furthermore, salicylates exhibit other biological activities, including anticancer and anti-proliferation [12]. The significance of β-d-salicin 1 molecule may encourage further understanding into its cross-biological function. Therefore, the aim of this article is to explore the mechanistic biosynthetic pathways of β-d-salicin 1, its metabolism and discuss the genetic cross-talk between pants and humans.
2. Phytochemistry of β-d-Salicin 1
β-d-Salicin 1 or 2-(hydroxymethyl) phenyl-O-β-d-glucopyranoside is the first phenolic glycoside discovered in nature with a molecular mass of 286.27782 g/mol. Its IUPAC name is (2R,3S,4S,5R,6S)-2-(hydroxymethyl)-6-[2-(hydroxymethyl) phenoxy]oxane-3,4,5-triol. β-d-Glucose 4 moiety of β-d-salicin 1 contributes to all five chiral carbon centres. The chemical structure of β-d-salicin 1 encompasses β-d-glucose 4 and 2-hydroxybenzyl alcohol, or salicyl alcohol 5. β-d-Salicin 1 contains seven oxygen atoms, as H-bond acceptor and five hydroxyl groups, as H-bond donors. It 1 also possesses nine rotational bonds that control its conformational structure. In addition, the β-d-glucose 4 and salicyl alcohol 5 moieties are bonded by β-1,1′-d-glycosidic bond. These chemical features contribute to the polarity of β-d-salicin 1. Therefore, the extraction of β-d-salicin 1 requires a polar solvent system, such as boiling water and ethyl alcohol. In addition, the presence of d-glucose 4 moiety contributes to the enhancement of physcochemical properties of β-d-salicin 1.
3. Biosynthesis of β-d-Salicin 1
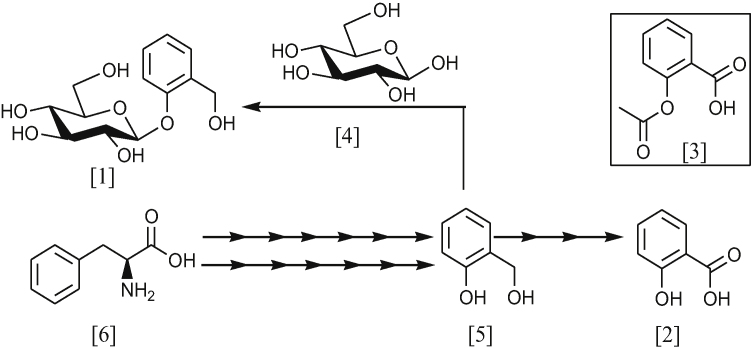
Although there have been long-standing biotic and abiotic interests in β-d-salicin 1, no defined biosynthetic pathway, genes or enzymes have been illustrated in the literature [14], [15]. Nonetheless, adapting the biotechnological approach and utilising leave tissues and radio labelled precursors have elucidated some biosynthetic aspects of β-d-salicin 1 in Salix and Populous [16], [17]. It reveals that the biosynthesis of β-d-salicin 1 is associated with phenylpropanoid pathway that starts with l-phenylalanine 6 (Scheme 1). Using radiolabled precursors indicate that the biosynthesis of β-d-salicin 1 encompasses five steps: deamination, ortho-hydroxylation, β-oxidation, C2 unite elimination and glucosylation [7], [16].
Scheme 1.
An overview of the β-d-salicin 1 biosynthesis.
4. Formation of trans cinnamic acid 7
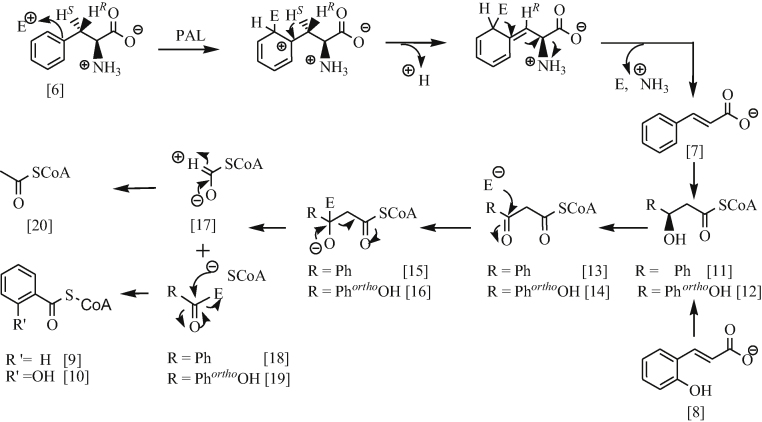
l-Phenylalanine 6 is available in plants and readily biotransforms into trans cinnamic acid 7 by phenylalanine ammonialyase (PAL) [18]. Thereby, plants produce a large number of organic compounds via this biotransformation [19]. The catalysis of l-phenylalanine 6 involves deamination of the amino group of α-amino acid. The mechanism of this biotransformation involves the formation of an enzyme-substrate complex, generating a carbonium ion intermediate which subsequently induces the elimination of the 3-pro-S proton giving trans-cinnamic acid 7 in a stereospecific manner (Scheme 2).
Scheme 2.
The stereospecific biosynthetic transformation of l-phenylalanine for generating benzoic acid-related analogues.
5. C2 unite elimination
Salix and Populous utilise trans-cinnamic acid 7 and trans-coumaric acid 8 in the synthesis of essentially benzoyl-SCoA 9 and salicyloyl-SCoA 10, respectively (Scheme 2). The incorporation of radiolabelled 7 or 8 in Salix or Populous leave tissues can readily be transformed stereospecifically to 3-hydroxy-3-phenylpropanoic acid 11 or 3-hydroxy-3-(2-hydroxyphenyl)-propanoic acid 12 via CoA-dependent β-oxidation [7], [20]. Subsequently, 3-hydroxy propanoate side chain of compounds, 13 or 14, undergo C2 unit elimination to yield 9 or 10 via retro Claisen condensation (Scheme 2). The mechanism of biotransformation, in the last two steps, is analogous to the metabolism of fatty acids in humans [16], [20], [21]. The elimination of the C2 unit involves the formation of β-oxophenyl propionyl-CoA 13 or β-oxo-orthohydroxyphenyl propionyl-CoA 14 which is followed by the nucleophilic attack by thiolase at β-carbonyl group, forming an enzyme-substrate complex 15 or 16, respectively. These two complexes, 15 and 16, subsequently, undergoes α-β-C–C cleavage, resulting in the formation of the following intermediates: 17, 18, 19. Protonation of 17 gives acetyl CoA 20 while the intermediate 18 and 19 undergo nucleophilic attack by acetyl S-CoA to release the enzyme and form benzoyl-SCoA 9 and salicyloyl-SCoA 10, respectively (Scheme 2).
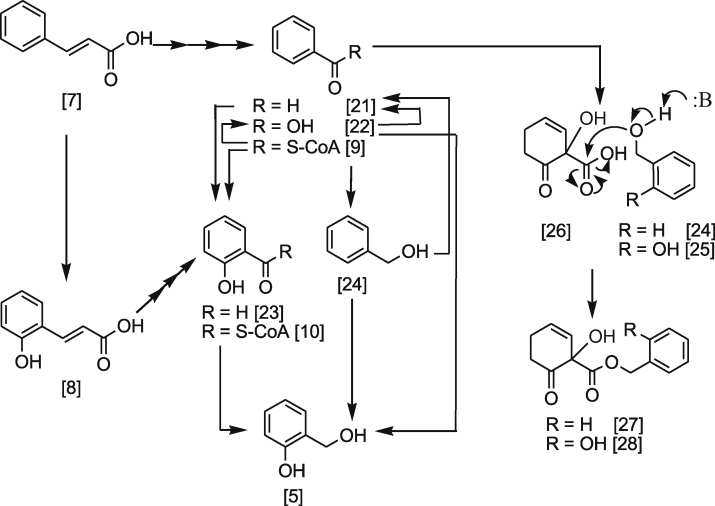
Plants modulate the phenylpropanoide pathways by interconverting benzoate secondary metabolites in response to the plant’s physiological requirement. Therefore, the exact mechanism of β-d-salicin 1 biosynthesis may seem difficult to justify. Using Salix and Populous leaf tissue indicated that the downstream of β-d-salicin 1 biosynthesis involves inter conversion of different simple phenolic molecules, including benzaldehyde 21, benzoic acid 22 and benzoyl-SCoA 9 compounds in plants [7], [16], [22], [23]. The biotic transformation of cinnamic acid 7, for example, can undergo direct ortho hydroxylation to give 2-hydroxycinnamic acid 8 or C2 elimination to give benzaldehyde 21 (Scheme 3). Benzaldehyde 21 can also be hydroxylated at ortho position to give 2-hydroxybenzaldehyde 23. Feeding the leave tissue of S. purpurea with radiolabelled benzoic acid 22 or benzyl alcohol 24 gave benzaldehyde 21 via reduction or oxidation reaction, respectively [7], [16]. Further biotic transformation of compounds 22 and 24 gave salicyl alcohol 5, the precursor of β-d-salicin 1 (Scheme 3). In addition, benzoyl-SCoA 9 undergoes a reduction reaction to give benzyl alcohol 24 or benzoic acid 22 (Scheme 3).
Scheme 3.
Incorporation of radiolabelled compounds in the biosynthesis of β-d-salicin 1.
In addition, there are other benzoate secondary metabolites that have been found in Populous, which contribute to the biosynthesis of phenolic glycosides. These benzoates are 1-hydroxy-6-oxo-2-cyclohexene-1-carboxylic acid 26, benzyl 6-hydroxy-2-cyclohexen-on-oyl 27 and salicyl 6-hydroxy-2-cyclohexen-on-oyl 28 [7], [22], [23]. The final step, in the biosynthesis of 1, involves glucosylation of salicyl alcohol 5 at the phenyl hydroxyl group.
6. Glucosylation and its stereospecificity
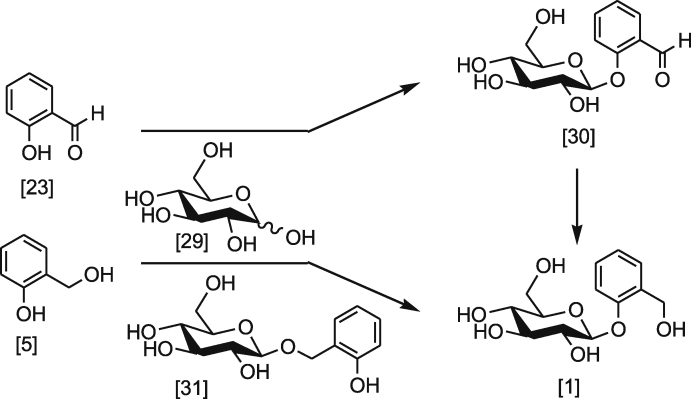
In S. populous and other plants, salicylaldehyde 23, its counterpart SCoA active form 9 or salicyl alcohol 5 can undergo glycosylation with d-glucose 29 [7], [16], [24], [25], [26]. Scheme 4 shows the direct and indirect routes that involve the formation of β-d-salicin 1. Radiolabelled salicylaldehyde 23 was readily glucosyled to yield β-d-helacin 30 when fed to S. purpurea which, subsequently underwent reduction at the carbonyl group to give β-d-salicin 1 [7], [16]. In addition, using radiolabelled β-d-helacin 30 undergoes similar reduction to give β-d-salicin 1 [27]. Research also found that using radiolabelled salicyl alcohol 5 can be directly incorporated in the synthesis of 1 (Scheme 4) [16]. However, literature indicated that salicyl alcohol 5 is not the direct precursor of β-d-salicin 1 in higher plants. Although salicyl alcohol 5 can undergo glycosylation reaction, it only underwent 46.4% incorporation into β-d-salicin 1 while 53.6% of it 22 formed ortho-hydroxybenzylglucoside 31 [16]. Chemically, there are two types of hydroxyl group that are present in salicyl alcohol 5: primary and phenolic. In physiological environments, these two hydroxyl groups are different in their chemical properties. Primary hydroxyl (pKa = ∼16–19) is amphoteric, while phenolic hydroxyl tend to be acidic (pKa = ∼8–10). These chemical properties may play an essential role in the selectivity of which type of hydroxyl group preferably undergoes glucosylation. Nonetheless, with a single enzyme, the ratio of glucosylation is controlled by the stereo-specificity or by the relative biochemical reactivity of hydroxyl groups.
Scheme 4.
Glucosylation of salicyldehyde 23 and salicyl alcohol 5.
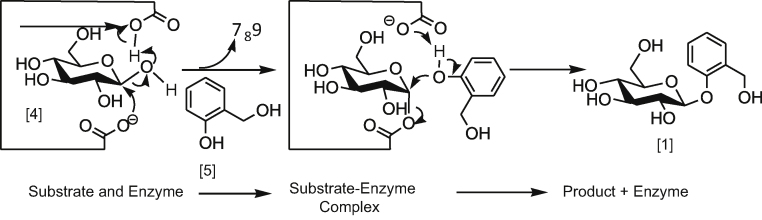
The stereochemistry of the β-glycosidic bond formation in β-d-salicin 1 is based on transglycosylation of glycan (d-glucose) with an aglycan (benzoate) compound. The mechanism that controls the configuration of the β-bond requires two carboxylate residues on the enzyme that are spatially proximal within about 6.0 Å [28]. In this mechanism, the two nucleophilic carboxgylates participate in the transglucosylation, as illustrated in Scheme 5. The nucleophilic carboxylate of glucosidase attacks the anomeric centre of d-glucose 4 to form an enzyme-substrate complex, while the acid/base residue protonates the glycosidic oxygen and subsequently activates a compound acceptor to form the transglycosylated product 1 [28].
Scheme 5.
The mechanism of enzymatic glucosylation of slicyl aalcohol 5.
7. Metabolism of β-d-Salicin 1
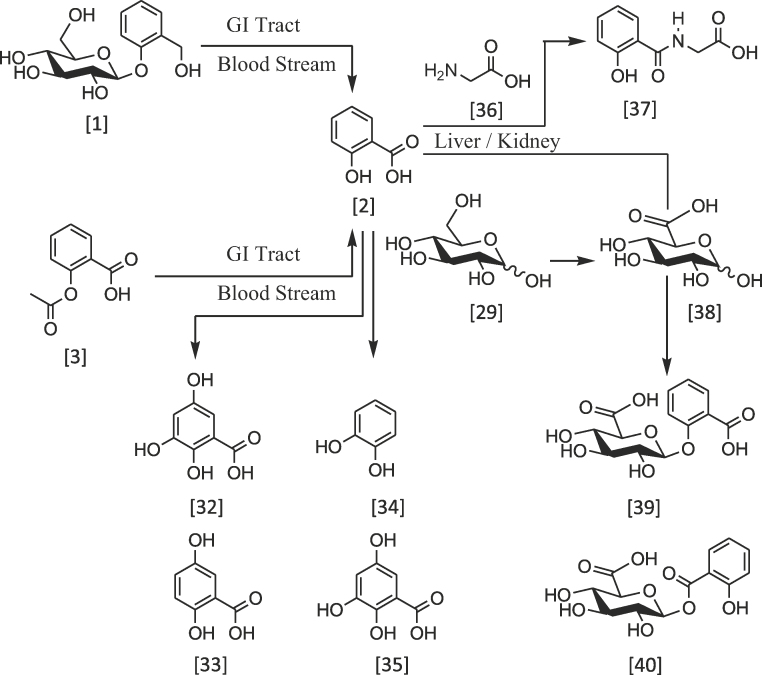
β-d-Salicin 1 is a pro-antiinflammatory drug which upon oral administration, is metabolised into the pharmacological active form, salicylic acid 2. This metabolic step takes place in the gastrointestinal tract and blood stream which involves glycon hydrolysis and oxidation of benzyl carbon. Similarly, acetylsalicylic acid 3 is also hydrolysed into salicylic acid 2 and acetic acid.
The route to the metabolism of these drugs has been associated with esterases that are found in the intestinal mucosa and serum cytosol [29]. Salicylic acid 2 undergoes further metabolism in the liver and kidney, as part of drug clearance (Scheme 6). Cytochrome p-450 enzyme system in mitochondria is capable of catalysing salicylate into different metabolites, including 2,3,5-trihydroxy benzoic acid 32, 2,5-dihydroxy benzoic 33 acid and catechol 34; the decarboxylated analogue of compound 35 [30], [31], [32]. The biotransformation is likely to involve enzymatic hydroxylation or radical oxidation. In addition, salicylic acid 2 is readily available for conjugation reaction with glycine 36 to form salicyluric acid 37 or d-glucuronic acid 38 to form salicylacyl glucuronide 39 or 1-salicylate glucuronide 40 via the formation of ether or ester bonds (Scheme 6).
Scheme 6.
An overview of the metabolism of β-d-salicin 1 and related compounds.
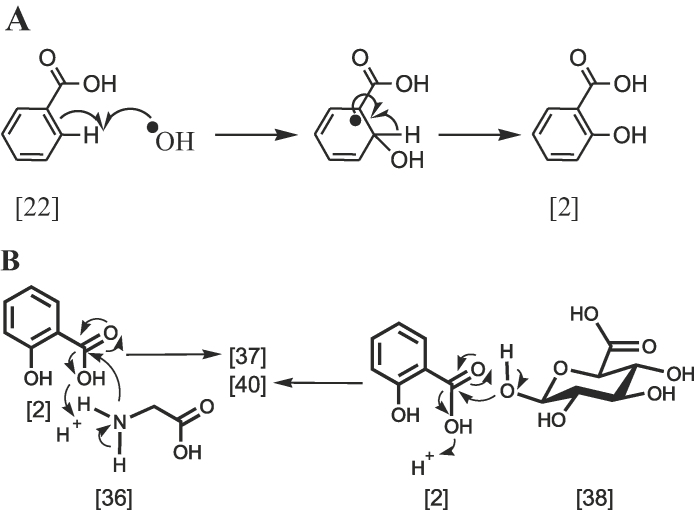
The mechanism of aryl hydroxylation involves a cyclohexadienyl radical intermediate followed by hydroxy radical attack (Scheme 7A). While the biotransformation of 2 compound, in the presence of glycine 36 or d-glucuronic acid 38, in the liver and kidney gives, respectively, 37 and 39 and 40 compounds. The mechanism of these reactions involves nucleophilic addition of amino group or hydroxyl group to the carbonyl group of salicylic acid 2, as illustrated in Scheme 7B. In addition, the carboxylic group (pKa = 4.5) is characterised as a week acid, and it is readily available to interact with any congruent amino group (pKa = 10.5) or hydroxyl group (pKa = 10) forming amide or ester bonds, respectively (Scheme 7B).
Scheme 7.
The mechanism of free radical hydroxylation and glycosidation.
The interactions of carboxylic group in salicylic acid 2 with macromolecules must be tightly associated with the cellular recognition which mainly depends on the compatibility of the inter-relationship between compounds to interact with each other. Salicylic acid 2 possesses three functional groups, as indicated above, which allow different biochemical interactions to take place within the cellular molecular biology. The functional groups also allow appropriate modifications, with the aim to improve its anti-inflammatory and pro-apoptotic efficacy.
8. Molecular recognition and cross-function of β-d-Salicin 1
The molecular recognition is a fundamental concept of how molecules communicate with their partners in micro-environments. The tool for recognition mainly involves molecular interactions, whereas, functional groups in molecules are the main sources for molecules to interact with each other. The simplest example is water (H—O—H) whereas both hydrogen and oxygen atoms contribute to the formation of hydrogen bonding with other water molecules. In addition, molecules containing functional groups (e.g. —OH, —SH, —NH) also may interact with each other. β-d-Salicin 1 and salicylic acid 2 contain mainly hydroxyl groups (—OH) that can interact with cyclooxygenase-2, causing the inhibition of this enzyme and subsequently downregulating inflammation [11], [12]. Thus, molecular recognition is often the main dynamic process of any signalling cascade. As such, the molecular recognition is vital for understanding drug-receptor interaction, and drug development. In order for the molecule to achieve suitable interaction, the molecular geometry and shape of the interacted molecules must mach. Thus, for salicylic acid 2 to be recognised by the active site of cyclooxygenase-2, both must have matched geometry that allows the interaction. However, it is not yet clear how salicylic acid 2 is directly recognised by some inflammatory mediators while β-d-salicin 1 must be metabolised to exert its anti-inflammatory potential. Owing to the random nature of macromolecules to recognise xenobiotic molecules, they may generate an expression on how molecules communicate with each other to produce specific function. However, random interaction may not be suitable in a complex dynamic biological system. It seems most likely that a genetic match occurs between specific phyto-biosynthesis and therapeutic activities to restore inflammatory problems clinically. Per se, humans have identified the diversity of herbal medication according to the type of plant. The earliest explanation to the therapeutic potential of plants goes back to the Doctorine of Signatures, a philosophy that rationalizes the similarity in colour or shape between matched parts of plant and human bodies to coordinate treating an ailment. The other explanation is related to the co-evolution that is associated with close proximity between plant and human. In both point of views, the cross-talk may exist in engineering DNAs in plant and human in a way to complement each other.
Although the structure of DNA, in all living things is a complicated structure, it simply encompasses of only four repeating nucleotide units; adenine, cytosine, guanine and thymine, or respectively ACGT. Therefore, plant and human DNAs are structurally identical in their monomeric composition, but different in the sequence patterns of these monomers, the nucleotides. In order to understand the relationship between biosynthesis and pharmacological properties of specific phytomolecule, it is important to consider the pattern of the encoded enzymes in biosynthetic and pharmacological pathways. The interaction of phyto-molecule with an enzyme requires recognition of amino acid consensus motifs of this enzyme. In addition, the pattern of recognition must have its root in the encoded gene(s) that control both biosynthesis and pharmacology pathways. In this respect, the availability of high-throughput technologies in genome and various databases is considered vital for bioinformatics approach for the analysis of DNA sequence bioinformatically. The genetic approach that encompasses encoded specific gene and or the corresponding expressed proteins may help us to understand the complementary functional relationship of phyto-secondary metabolites. This may encourage the development of new biotechnological strategies for therapeutic intervention of certain clinical cases. Mapping of encoded-related genes and analysis of the nucleotides/amino acids sequences of cascade networks bioinformatically may also facilitate a quick understanding into the pattern of the cross-talk between biosynthesis of a phytomolecule and its pharmacological potential. High-throughput [33], serial analysis of gene expression [34] and massively parallel signature sequencing [35] provide good opportunities for studying sequences and expressions.
9. Conclusion
β-d-Salicin 1 and salicylic acid 2 are interesting phytochemicals that exert cross-biological functions in plants and humans. This cross-function may be linked to the homological nature of DNAs in both plants and humans and can be extended to animals and insects. In this respect, both cell regulatory proteins and nucleic acids, for example, possess the same amino acids or nucleotides repeating units, respectively. The match-up between a phytochemical and the corresponding receptor depend on the molecular recognitions and the stereo-compatibility of the interacted molecules. Therefore, mapping and analysing gene and expressed protein sequences of certain biosynthetic/pharmacologyical related pathways of certain phytochemical bioinformatically may contribute to devising new strategies in drug production. As such, β-d-salicin 1 and salicylic acid 2 may represent good examples in this respect, as both molecules exert biological activities in plants and humans to antagonise cell molecular dysfunction.
Conflict of interests
Author declare that there is no any conflict of interests.
Footnotes
Available online 28 August 2014
References
- 1.Osier T.L., Lindroth R.L. Effects of genotype, nutrient availability, and defoliation on aspen phytochemistry and insect performance. J. Chem. Ecol. 2001;27:1289–1313. doi: 10.1023/a:1010352307301. [DOI] [PubMed] [Google Scholar]
- 2.Warren J.M., Bassman J.H., Fellman J.K., Mattinson D.S., Eigenbrode S. Ultraviolet-B radiation alters phenolic salicylate and flavonoid composition of Populus trichocarpa leaves. Tree Physiol. 2003;23:527–535. doi: 10.1093/treephys/23.8.527. [DOI] [PubMed] [Google Scholar]
- 3.Peltonen P.A., Vapaavuori E., Julkunen-Tiitto R. Accumulation of phenolic compounds in birch leaves is changed by elevated carbon dioxide and ozone. Global Change Biol. 2005;11:1305–1324. [Google Scholar]
- 4.Lattanzio V., Lattanzio V.M.T., Cardinali A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In: Imperato F., editor. Phytochemistry: Advances in Research. Research Signpost; Kerala, India: 2006. pp. 23–67. [Google Scholar]
- 5.Tsai C.-J., Harding S.A., Tschaplinski T.J., Lindroth R.L., Yuan Y. Genome-wide analysis of the structural genes regulating defense phenylpropanoid metabolism in Populus. New Phytol. 2006;172:47–62. doi: 10.1111/j.1469-8137.2006.01798.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen F., Liu C.-J., Tschaplinski T.J., Zhao N. Genomics of secondary metabolism in populus: interactions with biotic and abiotyic environments. Crit. Rev. Plant Sci. 2009;28:375–392. [Google Scholar]
- 7.Babst B.A., Harding S.A., Tsai C.J. Biosynthesis of phenolic glycosides from phenylpropanoid and benzenoid precursors in Populous. J. Chem. Ecol. 2010;36:286–297. doi: 10.1007/s10886-010-9757-7. [DOI] [PubMed] [Google Scholar]
- 8.Mahdi J.G., Mahdi A.J., Bowen I.D. Historical analysis of aspirin discovery, its relation to the willow tree and antiproliferative potential. Cell Prolif. 2006;39:147–155. doi: 10.1111/j.1365-2184.2006.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farnsworth N.R. The role of ethnopharmacology in drug development. Ciba Found. Symp. 1990;154:2–11. doi: 10.1002/9780470514009.ch2. [DOI] [PubMed] [Google Scholar]
- 10.Fabricant D.S., Farnsworth N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001;109:69–75. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pepper C.J., Mahdi J.G., Buggins A.G.S., Hewamana S., Walsby E., Mahdi E.J., Al-Haza'a A., Mahdi A.J., Lin T.T., Pearce L., Morgan L., Bowen I.D., Brennan P., Fegan C. Two novel aspirin analogues show selective cytotoxicity in primary chronic lymphocytic leukaemia cells that is associated with dual inhibition of Rel A and COX-2. Cell Prolif. 2011;44:380–390. doi: 10.1111/j.1365-2184.2011.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahdi J.G., Al-Musayeib N.M., Mahdi E.J., Pepper C.J. Pharmacological importance of hydroxybenzoates in modulating cell inflammation, proliferation and apoptosis with a special reference to β-d-salicin and salicylic acid. Eur. J. Inflame. 2013;11:327–336. [Google Scholar]
- 13.J.G. Mahdi, E.J. Mahdi, I. Hamad, (2014) Unpublished results.
- 14.Johnson L.A., Douglas C.J. Populus trichocarpa monopteros/auxin response factor 5 (ARF5) genes: comparative structure, sub-functionalization, and Populus–Arabidopsis microsynteny. Can. J. Bot. 2007;85:1058–1070. [Google Scholar]
- 15.Boeckler A., Gershenzon J., Unsicker S. Phenolic glycosides of the Salicaceae and their role as anti-herbivore defences. Photochemistry. 2011;72:1497–1509. doi: 10.1016/j.phytochem.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 16.Zenk M.H. Pathways of salicyl alcohol and salicin formation in Salix purpurea L. Phytochemistry. 1967;6:245–252. [Google Scholar]
- 17.Boeckler A., Gershenzon J., Unsicker S. Phenolic glycosides of the Salicaceae and their role as anti-herbivore defenses. Photochemistry. 2011;2011(72):1497. doi: 10.1016/j.phytochem.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 18.Mahdi J.G., Kelly D.R. Lyases. In: Rehm H.J., Reed G., editors. Biotechnology. second ed. Wiley-VCH; Germany: 2000. pp. 41–171. [Google Scholar]
- 19.Jones D.H. Phenylalanine ammonia-lyase: regulation of its production, and its role in plant development. Phytochemistry. 1984;23:1349–1359. [Google Scholar]
- 20.Wildermuth M.C. Variations on theme: synthesis and modification of plant benzoic acids. Curr. Opin. Plant Biol. 2006;9:288–296. doi: 10.1016/j.pbi.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Moore B.S., Hertweck C., Hopke J.N., Izumikawa M., Kalaitzis J.A., Nilsen G., O'Hare T., Piel J., Shipley P.R., Xiang L., Austin M.B., Noel J.P. Plant-like biosynthetic pathways in bacteria: from benzoic acid to chalcone. J. Nat. Prod. 2002;65:1956–1962. doi: 10.1021/np020230m. [DOI] [PubMed] [Google Scholar]
- 22.Palo R.T. Distribution of birch (Betula spp.), willow (Salix spp.), and poplar (Populus spp.) secondary metabolites and their potential role as chemical defense against herbivores. J. Chem. Ecol. 1984;10:499–520. doi: 10.1007/BF00988096. [DOI] [PubMed] [Google Scholar]
- 23.Pierpont W.S. Salicylic acid and its derivatives in plants: medicines, metabolites and messenger molecules. Adv. Bot. Res. 1994;20:163–235. [Google Scholar]
- 24.Ruuhola T., Julkunen-Tiitto R. Trade-off between synthesis of salicylates and growth of micropropagated Salix pentandra. J. Chem. Ecol. 2003;29:1565–1588. doi: 10.1023/a:1024266612585. [DOI] [PubMed] [Google Scholar]
- 25.Orlova I., Marshall-Colon A., Schnepp J., Wood B., Varbanova M., Fridman E., Blakeslee J.J., Peer W.A., Murphy A.S., Rhodes D., Pichersky E., Dudareva N. Reduction in the synthesis of benzenoids in petunia flowers reveals multiple pathways to benzoic acid and an unexpected enhancement in auxin transport. Plant Cell. 2006;18:3458–3475. doi: 10.1105/tpc.106.046227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long M.C., Nagegowda D.A., Kaminaga Y., Ho K.K., Kish C.M., Schnepp J., Sherman D., Weiner H., Rhodes D., Dudareva N. Involvement of snapdragon benzaldehyde dehydrogenase in benzoic acid biosynthesis. Plant J. 2009;59:256–265. doi: 10.1111/j.1365-313X.2009.03864.x. [DOI] [PubMed] [Google Scholar]
- 27.Payyavula R.S., Babst B.A., Nelsen M.P., Harding S.A., Tsai C.J. Glycosylation-mediated phenylpropanoid partitioning in Populus tremuloides cell cultures. BMC Plant Biol. 2009;9:151. doi: 10.1186/1471-2229-9-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L.X., Huang W. Enzymatic transglycosylation for glycoconjugate synthesis. Curr. Opin. Chem. Biol. 2009;13:592–600. doi: 10.1016/j.cbpa.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim D., Yang Y., Jakoby W.B. Aspirin hydrolizing esterases from rat liver cytosol. Biochem. Pharmacol. 1990;40:481–487. doi: 10.1016/0006-2952(90)90546-w. [DOI] [PubMed] [Google Scholar]
- 30.Kyle M.E.M., Kocsis J.J. Salicylate metabolism: effect of age and sex in adults. Clin. Pharmacol. Ther. 1986;39:571–576. doi: 10.1038/clpt.1986.98. [DOI] [PubMed] [Google Scholar]
- 31.Patel D.K., Notarianni L.J., Bennett P.N. Compara tive metabolism of high doses of aspirin in man and rat. Xenobiotica. 1990;20:847–854. doi: 10.3109/00498259009046898. [DOI] [PubMed] [Google Scholar]
- 32.Patel D.K., Hesse A., Ogunbona A., Notarianni L.J., Bennett P.N. Metabolism of aspirin after therapeutic and toxic doses. Hum. Exp. Toxicol. 1990;9:131–136. doi: 10.1177/096032719000900302. [DOI] [PubMed] [Google Scholar]
- 33.Sittampalam G.S., Kahl S.D., Janzen W.P. High-throughput screening: advances in assay technologies. Curr. Opin. Chem. Biol. 1997;1:384–391. doi: 10.1016/s1367-5931(97)80078-6. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto M., Wakatsuki T., Hada A., Ryo A. Use of serial analysis of gene expression (SAGE) technology. J. Immunol. Methods. 2001;250:45–66. doi: 10.1016/s0022-1759(01)00305-2. [DOI] [PubMed] [Google Scholar]
- 35.Brenner S., Johnson M., Bridgham J., Golda G., Lloyd D.H., Johnson D., Luo S., McCurdy S., Foy M., Ewan M., Roth R., George D., Eletr S., Albrecht G., Vermaas E., Williams S.R., Moon K., Burcham T., Pallas M., DuBridge R.B., Kirchner J., Fearon K., Mao J., Corcoran K. Gene expression analysis by massively parallel signature sequencing (MPSS) on microbead arrays. Nat. Biotechnol. 2000;18:630–634. doi: 10.1038/76469. [DOI] [PubMed] [Google Scholar]