Abstract
This study reports the in vitro cytotoxic effect of biologically synthesized silver and gold nanoparticles against MDA-MB-231, human breast cancer cells. Formation of silver and gold nanoparticles was observed within 30 min and the various characterization techniques such as UV–vis spectrophotometer, FE-SEM, TEM and XRD studies were confirmed the synthesis of nanoparticles. Further, MTT, acridine orange and ethidium bromide (AO/EB) dual staining, caspase-3 and DNA fragmentation assays were carried out using various concentrations of silver and gold nanoparticles ranging from 1 to 100 μg/ml. At 100 μg/ml concentration, the plant extract derived nanoparticles exhibited significant cytotoxic effects and the apoptotic features were confirmed through caspase-3 activation and DNA fragmentation assays. Thus, the results of the present study indicate that biologically synthesized silver and gold nanoparticles might be used to treat breast cancer; however, it necessitates clinical studies to ascertain their potential as anticancer agents.
Keywords: Acalypha indica, Silver nanoparticles, Gold nanoparticles, MDA-MB-231 human breast cancer cells
1. Introduction
Over the past few years, synthesis and characterization of nanoparticles has gained increasing momentum due to their large surface area to volume ratio because of which nanoparticles exhibit novel and new properties than their macroscopic counterparts. Thus, nanotechnology has immense potential to revolutionize in the biomedical research by developing new and improved products for clinical diagnosis and therapy. Several noble metal nanoparticles such as silver, gold, copper and platinum were widely synthesized by employing various procedures including physical, chemical and biological methods. The physical and chemical routes of nanoparticles preparation have many disadvantages and are not eco-friendly. Hence, researchers across the globe have searched for new and environmentally benign methods for the synthesis of biocompatible nanoparticles [29].
Incidentally, biological systems have long been known to reduce metal ions into nano-sized particles [7] and many researchers have recently reported the biogenic synthesis of silver and gold nanoparticles using a wide range of biological resources like bacteria [37], fungi [30], [10] and plants [12], [2]. In the plant mediated green chemistry approach, the reduction rate of metal salts is very fast and the procedure itself requires no specific conditions unlike the physical and chemical methods [29], [32]. Besides, this biogenic method of nanoparticles synthesis appears to be reproducible and the particles, produced through this environmentally friendly approach, are found highly stable [24]. Hence, this one pot green chemistry procedure has attracted the attention of biologists and nanotechnologists in myriad ways and is recently emerged as one of the active areas of current nanobiotechnological research.
Breast cancer is the second leading cause of cancer death among women in the U.S. An estimated 39,620 breast cancer deaths and 232,340 new cases are expected among women in 2013 [5]. This data shows an increase of 100 breast cancer deaths and 1860 new cases compared to the previous report published in 2011 [4]. The existing cytotoxic agents used for the breast cancer treatment are found to be expensive and inefficient because they induce severe side effects due to their toxicity in noncancerous tissues [26], [43]. Therefore, it is of urgent need to develop novel therapeutic agents that are biocompatible and cost-effective. In recent times, nanotechnology based products such as nano-dresses, nano-cars, skin creams, tennis rackets and balls have been increasingly introduced into the global market. To date, as many as 1628 nano-based products are being extensively used for various purposes throughout the world [34]. Inorganic nanoparticles have already been utilized in wound healing and in antibacterial applications [13].
Nowadays, silver and gold nanoparticles are emerging as promising agents for cancer therapy. The anticancer activities of nano-sized silver and gold particles have been evaluated against a variety of human cancer cells. However, very few reports were available against the breast cancer cells and most of these studies have mainly used chemically made nanoparticles [21], [8], [14]. Currently, there has only been a limited data existence for the cytotoxic effects of biologically synthesized silver and gold nanoparticles against human breast cancer cells [17], [41]. The major objective of this work is to evaluate the cytotoxic effect of biosynthesized silver and gold nanoparticles against human breast cancer cell line. Our group has for the first time reported the biogenic synthesis of silver nanoparticles from Acalypha indica Linn leaves extract [28]. In continuation of this study, we screened the same plant for its ability to biosynthesize gold nanoparticles. Further, the cytotoxic effects of both silver and gold nanoparticles were tested against MDA-MB-231 cells by MTT assay and the possible mechanism for cell death was addressed through acridine orange and ethidium bromide (AO/EB) dual staining, caspase-3 and DNA fragmentation assays.
2. Materials and methods
2.1. Materials
Silver nitrate (AgNO3) and chloroaurate (HAuCl4) were purchased from Hi Media Laboratories Pvt. Ltd. Mumbai, India. MTT was obtained from Invitrogen, USA and acridine orange, ethidium bromide and all other fine chemicals were obtained from Sigma–Aldrich, St. Louis, USA. The fresh and healthy leaves of A. indica were collected from the Guindy campus of University of Madras, Chennai, India.
2.2. Preparation of leaves extract for nanoparticles biosynthesis
Ten grams of freshly collected A. indica leaves were surface cleaned with running tap water followed by distilled water and boiled in 100 ml of distilled water at 60 °C for 5 min. Then, the extract was filtered and used for the biogenic synthesis of both silver and gold nanoparticles.
2.3. Biogenic synthesis and characterization of silver nanoparticles
The biogenic synthesis of silver and gold nanoparticles was performed according to the standard published procedure with slight modifications [9]. The methods for the biosynthesis and characterization of silver nanoparticles from the leaves extract of A. indica were given in our previously published paper [28].
2.4. Biogenic synthesis and characterization of gold nanoparticles
For gold nanoparticles biosynthesis, 1 mM HAuCl4 was added to the broth containing 36 ml of leaf extract and 64 ml of distilled water at neutral pH. After this, the solution was kept at 37 °C under static condition. Simultaneously, a control setup was maintained without adding HAuCl4. The pinkish violet colour formed after the addition of HAuCl4 was characterized using UV–vis spectrophotometer (Beckman DU-20 Spectrophotometer) in the range of 200–700 nm. Further, the reaction mixture was subjected to centrifugation at 75,000 × g for 30 min and the resulting pellet was dissolved in deionized water and filtered through Millipore filter (0.45 μm). An aliquot of this filtrate containing gold nanoparticles was used for FE–SEM (Field Emission–Scanning Electron Microscopy), TEM (Transmission Electron Microscopy) and XRD (X-Ray Diffraction) analyses. For electron microscopic studies, 25 μl of sample was sputter coated on copper stub and the size as well as shape of the gold nanoparticles was studied using FE-SEM and TEM. For XRD studies, dried gold nanoparticles were coated on XRD grid and the spectra were recorded by using Philips PW 1830 X-Ray generators operated at a voltage of 40 kV and a current of 30 mA with Cu Kα1 radiation.
2.5. Cell culture
Human breast cancer cells (MDA-MB-231) were procured from National Centre for Cell Science, Pune, India. The cell lines were grown as a monolayer in Roswell Park Memorial Institute medium (RPMI) supplemented with 10% fetal bovine serum (FBS), penicillin/streptomycin (250 U/ml), gentamycin (100 μg/ml) and amphotericin B (1 mg/ml) and incubated at 37 °C in a humidified atmosphere of 5% CO2. Cells were grown confluence for 24 h before use.
2.6. MTT assay
To determine the cytotoxic effect of both silver and gold nanoparticles, cell viability study was done with the conventional MTT-reduction assay with slight modifications [27]. Briefly, MDA-MB-231 cells were seeded in a 96-well plate at the density of 5 × 103 cells/well. The cells were allowed to attach and were grown in a 96-well plate for 24 h, in 200 μl of RPMI with 10% FBS. After that the media was removed and replaced with suspension of various concentrations of AgNO3, HAuCl4, silver nanoparticles and gold nanoparticles viz., 1, 10, 50 and 100 μg/ml (minimum 3 wells were seeded with each concentration). Equal concentrations of A. indica leaves extract were used as positive control and the cells were incubated for 48 h. After the addition of MTT (10 μl, 5 mg/ml), the cells were incubated at 37 °C for another 4 h. Optical density of the formazan product was read at 495 nm using scanning multi well spectrophotometer. The results were given as mean of three independent experiments.
2.7. Acridine orange/ethidium bromide dual staining
Acridine orange/ethidium bromide (AO/EB) dual staining was carried out to detect the morphological evidence of apoptosis in silver and gold nanoparticles treated cells. Twenty five microliters of treated and untreated cell suspension (5 × 106 cells/mL) was stained with 1 μl of acridine orange and ethidium bromide dye mix (100 μg/ml of acridine orange and ethidium bromide prepared in PBS separately) [42]. Then the samples were examined under fluorescent microscopy (Nikon Eclipse TS 100).
2.8. Capase-3 assay
Caspase-3 assay was carried out according to the procedure of Sutter et al. (2003) with slight modification [39]. The activity of caspase-3 was calculated from the cleavage of fluorogenic substrate Ac-DEVD-AMC (acetyl Asp-Glu-Val-Asp 7-amido-4-methylcoumarin). After 24, 36 and 48 h of incubation, silver and gold nanoparticles treated cell lysates were incubated with substrate solution (caspase-3 substrate Ac-DEVD-AMC 20 mg/ml, HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) 20 mM, glycerol 10%, dithiotheritol 2 mM, pH 7.5) for 1 h at 37 °C and the cleavage of caspase-3 substrate was measured at an excitation wavelength of 390 nm and an emission wavelength of 460 nm. The activity was expressed as relative fluorescence unit (RFU).
2.9. DNA fragmentation assay
To investigate the internucleosomal DNA fragmentation caused by both silver and gold nanoparticles, DNA laddering assay was performed according to the standard procedure described by Su et al. (2005) with little modification [38]. A total of 1 × 106 cells was treated with silver and gold nanoparticles (100 μg/ml) for 48 h and then collected by centrifugation. Further, the DNA was isolated using commercially available kit (Genei, Bangalore, India) following the manufacturer’s instructions. DNA was resolved on 1.5% agarose gel (containing 3 μg/ml of ethidium bromide in 1 × TAE buffer of pH 8.5) at 90 V for 1.5 h and the bands were visualized using UV transilluminator.
3. Results
In this present study, gold nanoparticles were rapidly synthesized using A. indica leaves extract as bio-reductants. Similar to silver nanoparticles formation, the bio-reduction of HAuCl4 into gold nanoparticles was completed within 30 min of incubation.
3.1. Biogenic synthesis and characterization of gold nanoparticles
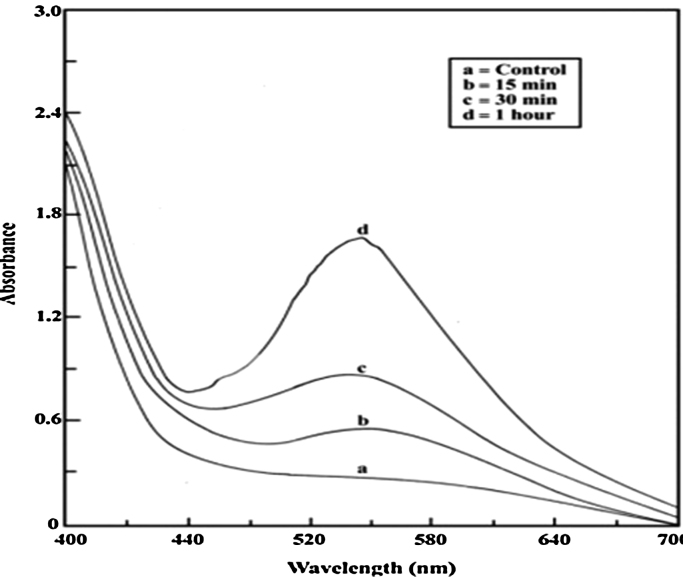
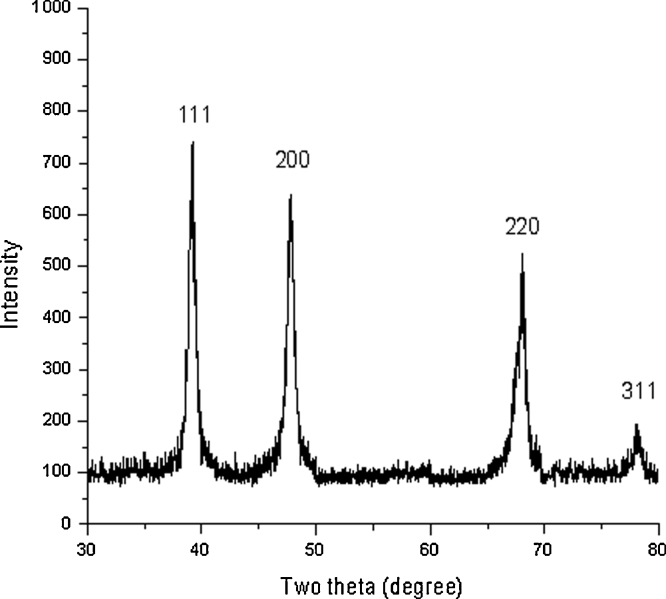
The very first indication for nanoparticles formation is colour change. A clear pinkish violet colour was formed within 30 min when 1 mM HAuCl4 was added into the aqueous leaves extract of A. indica, which indicates the biogenic synthesis of gold nanoparticles (Fig. 1). The intensity of pinkish violet colour was increased with the incubation period and it was due to the excitation of surface plasmon vibrations. On the other hand, control (leaf extract alone) showed no change of colour (Fig. 1). Very recently, Karuppaiya et al. (2013) have reported that the aqueous extract of Dysosma pleiantha rhizome rapidly biosynthesized gold nanoparticles within 20 min [25]. A characteristic absorption peak at 540 nm further confirmed the formation of nano-sized gold particles (Fig. 2). The formation of gold nanoparticles was started at 15 min and was completed at 30 min. Interestingly, the peak was found to be stable at the same wave length for up to 1 h, indicating that phytochemicals may have stabilized the synthesized gold nanoparticles (Fig. 2). Fig. 3a and b depict digitalized FE–SEM and TEM images of biosynthesized gold nanoparticles, respectively. These two images showed spherical shaped gold nanoparticles with a size of less than 30 nm. XRD analysis showed three distinct diffraction peaks at 38.1°, 44.1° and 64.1° which indexed the planes 1 1 1, 2 0 0 and 2 2 0 of the cubic face-centred gold. The obtained data was matched well with the Joint Committee on Powder Diffraction Standards (JCPDS) file no. 04–0784, which suggest the crystalline nature of gold nanoparticles (Fig. 4).
Fig. 1.
Reaction of aqueous A. indica leaves extract with 1 mM HAuCl4 solution. (a) Leaves extract alone; (b) pinkish violet colour formation after the addition of 1 mM HAuCl4 solution at 30 min of incubation.
Fig. 2.
UV–vis spectra recorded for gold nanoparticles at different time intervals. The formation of gold nanoparticles was started within 15 min (b) and the reaction was completed at 30 min (c), no change in absorbance was observed even after 1 h (d).
Fig. 3.
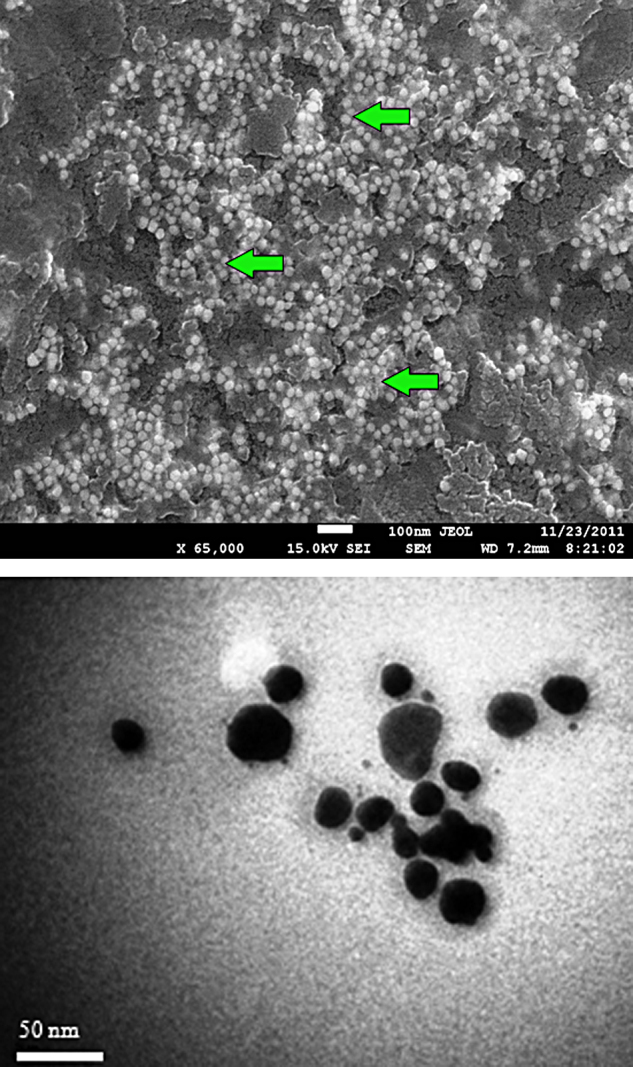
(a) FE-SEM image of biogenic gold nanoparticles showing spherical shapes; (b) TEM image of spherical shaped gold nanoparticles with an average size of 20–30 nm (Scale bar = 50 nm).
Fig. 4.
XRD pattern of biosynthesized gold nanoparticles index at (1 1 1), (2 0 0) and (2 2 0) exhibiting the facets of crystalline gold.
3.2. Cytotoxic activity of biologically synthesized silver and gold nanoparticles against MDA-MB-231 cells
The biogenic silver and gold nanoparticles were tested for their potent cytotoxic activity against MDA-MB-231, breast cancer cells. The results of the mechanistic studies indicated that silver and gold nanoparticles induced apoptosis through caspase-3 activation and DNA fragmentation.
3.2.1. Determination of cell viability by MTT assay
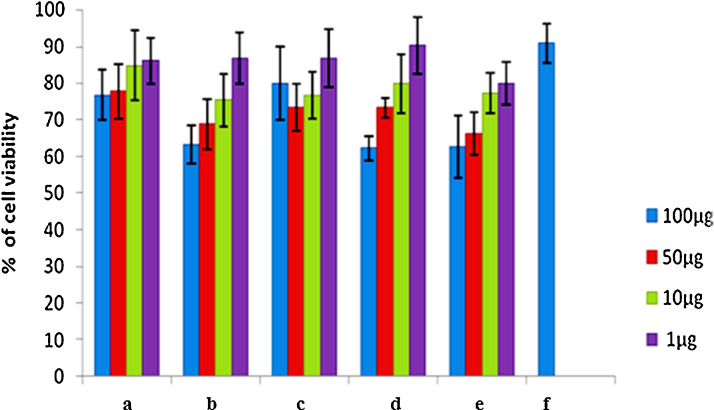
Different concentrations of AgNO3, HAuCl4, silver nanoparticles, gold nanoparticles and plant extract ranging from 1 to 100 μg/ml were used to study the viability of MDA-MB-231 cells and the toxicity was measured. Interestingly, HAuCl4, AgNO3 and A. indica leaves extract (positive control) treated cells did not show much toxic effects in all the tested concentrations; AgNO3 treated tumour cells showed more than 60% viable cells at 100 μg/ml concentration (Fig. 5). Gold nanoparticles treated MDA-MB-231 cells exhibited slightly higher toxic effects than the silver nanoparticles at 1, 10 and 50 μg/ml concentrations; whereas, at 100 μg/ml concentration, both silver and gold nanoparticles showed comparatively higher toxic effects (40%) than the other treated cells (Fig. 5). The results of this study suggest that the cytotoxicity of biologically synthesized silver and gold nanoparticles was increased with the increasing concentration of nanoparticles.
Fig. 5.
Cytotoxic effects of biosynthesized silver and gold nanoparticles. MDA-MB-231 cells were treated with HAuCl4, AgNO3, plant extract, silver nanoparticles, gold nanoparticles for 48 h and the cell viability was determined by MTT assay. Data represent mean ± SD of three independent experiments. (a) Cells exposed with HAucl4 did not show much toxic effect; (b) cells exposed with AgNO3 shows more than 60% of viable cells; (c) cells exposed with plant extract did not show much toxic effect; (d) cells exposed with silver nanoparticles; (e) and gold nanoparticles at 100 μg/ml conc. shows almost 40% of cell toxicity; (f) solvent control.
3.2.2. Morphological evidence of apoptosis by dual staining (AO/EB)
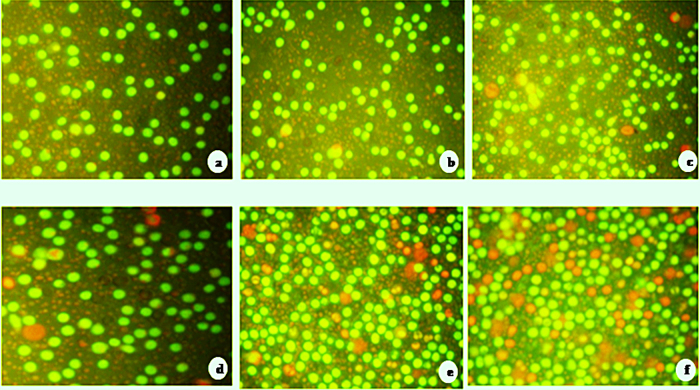
Apoptotic morphological changes caused by both silver and gold nanoparticles were studied using acridine orange/ethidium bromide differential staining method. The stained cells were characterized to viable (light green), early apoptotic (bright green fluorescence and condensed chromatin), late apoptotic (orange fluorescence) and nonviable cells (red coloured fluorescence) (Fig. 6a–f). Both silver and gold nanoparticles treated cells showed condensed nuclei, membrane blebbing and apoptotic bodies. In contrast, the control cells showed intact nuclear architecture. However, very few apoptotic bodies were noticed in AgNO3 and HAuCl4 treated cells.
Fig. 6.
Morphological evidence of apoptosis by AO/EB dual staining. Fluorescent microscopic images of MDA-MB-231 human breast cancer cells treated with (a) untreated cells; (b) cells treated with aqueous leaves extract of A. indica shows intact morphology; (c) few apoptotic cells observed in AgNO3; (d) HAuCl4 treated cells; (e) cells treated with gold nanoparticles and (f) silver nanoparticles shows apoptotic bodies and fragmented nuclei.
3.2.3. Caspase-3 assay
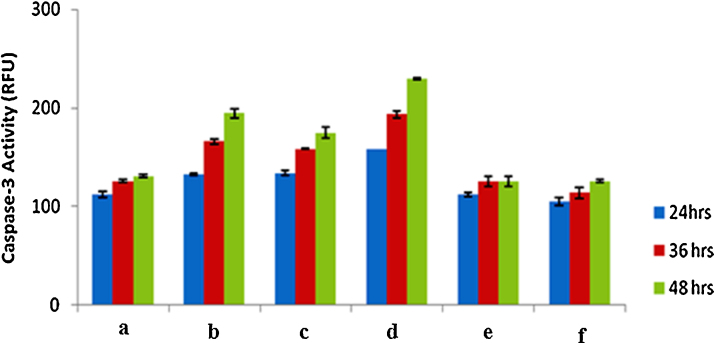
To investigate whether apoptosis is mediated by caspase-3, cell lysates treated with AgNO3, HAuCl4, silver nanoparticles, gold nanoparticles and plant extract were analysed. Levels of caspase-3 were found to be elevated in the silver nanoparticles treated tumour cells (Fig. 7). Plant extract treated cells exhibited slightly higher activity compared to gold nanoparticles treated ones. However, AgNO3, HAuCl4, treated cells showed much lower activity (Fig. 7). The elevated level of caspase-3 was, further, confirmed by measuring the proteolytic activity of the fluorogenic peptide Ac-DEVD-AMC, a caspase-3 specific substrate and its activity was found to be highest at 48 h. The increased levels of caspase-3 activation suggest that silver and gold nanoparticles induce apoptosis in MDA-MB-231 breast cancer cells in a caspase-3-dependent manner.
Fig. 7.
Metal nanoparticles induced apoptosis in MDA-MB-231 breast cancer cells via caspase-3. Cells treated with (a) untreated cells; (b) aqueous leaves extract of A.indica; (c) gold nanoparticles; (d) silver nanoparticles; (e) HAucl4; (f) AgNO3. The caspase activity was found to be highest at 48 h for silver nanoparticles and gold nanoparticles treated cells at the same time increased activity was shown in plant leaves extract treated cells due to the presence of phytochemicals and the values were represented as mean ± SD of three independent experiments of three replicates.
3.2.4. DNA fragmentation assay
To investigate whether biologically synthesized nanoparticles induced cell death via apoptosis, DNA laddering assay was performed on agarose gel. A clear fragmented DNA ladders were observed in both silver and gold nanoparticles treated MDA-MB-231 cells whereas AgNO3 and HAuCl4 treated cells did not show such clear fragmented DNA ladders (Fig. 8). In addition, the untreated (control) cells did not show any prominent DNA ladders on the agarose gel. Therefore, the data obtained from this study confirms that both silver and gold nanoparticles induced cell death through apoptosis.
Fig. 8.

Effect of metal nanoparticles on DNA fragmentation assay to investigate whether nanoparticles induced cell death via apoptosis. Cells were exposed with (a) HAucl4; (b) gold nanoparticles; (c) AgNO3; (d) silver nanoparticles; (e) untreated cells for 48 h and the isolated DNA content were separated on agarose gel.
4. Discussions
In the recent years, biosynthesis of silver nanoparticles using plant extracts is getting more popular due to the strong antibacterial action of zerovalent silver and easy reduction of silver (I) salts. In our earlier study, silver nanoparticles were biosynthesized using aqueous leaves extract of A. indica as reducing and capping agents and those results were briefly discussed here [28]. The formation of silver nanoparticles was very rapid and it was completed within 30 min. The peak at 420 nm confirmed the biogenic synthesis of silver nanoparticles from A. indica leaves extract. Similarly, Jeyaraj et al. (2013) have recently reported that Podophyllum hexandrum leaves extract effectively synthesized silver nanoparticles at 420 nm [22]. Further, High Resolution – Transmission Electron Microscopy (HR-TEM) analysis confirmed the biosynthesis and the synthesized silver nanoparticles were predominantly in spherical shape with uniform size ranging from 20–30 nm. The XRD spectrum of biosynthesized silver nanoparticles was matched well with the JCPDS file no. 04–0783, which indicates the crystalline nature of face-centred cubic silver. These results were in good agreement with the recent reports.
Interestingly, both silver and gold nanoparticles were formed within 30 min due to the rapid reduction of silver and chloroaurate ions by A. indica leaves extract. In contrast, Elavazhagan and Arunachalam (2011) have reported that Memecylon edule leaves extract took 1 h for the biosynthesis of gold nanoparticles while it was 3 h for silver [12]. However, in some studies, much faster rate of biosynthesis of silver and gold nanoparticles was observed. For instance, Dubey et al. (2010) have rapidly synthesized both silver and gold nanoparticles within 15 min from Sorbus aucuparia leaves extract [11]. Recently, Gangula et al. (2011) have reported that Breynia rhamnoides stem extract rapidly biosynthesized both silver and gold nanoparticles approximately 7 min and this is the much faster reduction process reported for the first time [16]. It is clear from these studies that the plant extract mediated biosynthesis is very simple, fast, low cost involvement, eco-friendly and safe for human therapeutic use [29], [19]. Thus, this biogenic method of nanoparticles synthesis has much reduced impact to the environment and is recently emerged as viable alternative to conventional physical, chemical and even microbial methods.
Silver and gold nanoparticles are being extensively synthesized using plant extracts, although the exact mechanism for this biogenic synthesis still remains to be completely unknown. However, a few hypotheses have been proposed to give some insights on the mechanical aspects of nanoparticles biosynthesis. Recent studies have shown that biomolecules such as protein, phenol and flavonoids present in the plant extract play an important role in the reduction of metals ions and capping of the nanoparticles [40]. Although the reduction of metal salts is environmentally benign, it is chemically a complex phenomenon involving an array of plant compounds such as vitamins, enzymes/proteins, organic acids such as citrates, amino acids and polysaccharides [1]. The preliminary phytochemical screening of secondary metabolites has clearly revealed the presence of glucosides, flavonoids, phenolic compounds, alkaloids and carbohydrates in the leaves extract of A. indica (data not shown). We strongly believe that glucosides may be responsible for the bio-reduction of both silver and chloroaurate ions. However, biosynthetic products or reduced cofactors may also play a key role in the reduction of respective salts to nanoparticles.
In this present study, the cytotoxicity of silver and gold nanoparticles was increased with the increasing concentration of nanoparticles. This statement is true particularly in the case of MCF-7, another human breast cancer cell, which showed 100% cell death at 50 μg/ml concentrations of silver nanoparticles [23]. On the contrary, the mushroom derived silver nanoparticles showed significant cytotoxicity against MDA-MB-231 cell lines at comparatively low concentration (6 μg/ml) [17]. The results of the present study suggest that silver and gold nanoparticles reduced the viability of MDA-MB-231 cells in a dose dependent manner. Based on these studies, it is here speculated that the cytotoxicity of nanoparticles is relied much on the nature of cell types and size of particles. Many researchers have also drawn similar conclusion [17], [33].
Apoptosis is broadly considered as a distinctive mode of programmed cell death that eliminates genetically determined cells [15]. The induction of apoptosis is confirmed by two factors, (1) reduced and shrunken cells and (2) DNA fragmentation [36]. In this study, silver and gold nanoparticles treated cells showed apoptotic features such as condensed nuclei, membrane blebbing and apoptotic bodies at 48 h and these morphological changes were evident through AO/EB dual staining. Adding strengthen to the fact, silver and gold nanoparticles treated MDA-MB-231 cells showed clear fragmented DNA ladders, suggesting that cell death is due to apoptosis.
In general, the fragmented DNA ladders indicate late apoptotic process in which caspase-3 plays a pivotal role [3], [20]. The earlier studies have demonstrated that caspase-3 cascade activation is responsible for several apoptotic mechanisms [18]. Thus, it is obvious that DNA fragmentation and caspase-3 activation mediate the apoptotic process. In this present study, silver and gold nanoparticles treated MDA-MB-231 cells showed increased levels of caspase-3, indicating that apoptosis is mediated through caspase-3 cascade. These findings were coincided with the previous reports [17]. Caspase-3 activation may be initiated either through extrinsic pathway or intrinsic pathway due to the presence of toxicants in the surrounding environment [15], [6]. In addition, caspase cascade activation is also reported to occur through the activation of granzyme B or death receptor or apoptosome [31]. In this study, although the silver nitrate caused cell toxicity was observed and the plant extract also up-regulated caspase-3 activity, however, only the gold and silver nanoparticles induced cell toxicity were specifically associated with all the observations of apoptosis including caspase-3 activity, AO/EB staining and DNA fragmentation.
Apoptosis inducing agents that specifically target the tumour cells might have the potential to be developed as new anti-tumour drugs since apoptotic cell death does not induce an inflammatory response. The anti-inflammatory property of A. indica leaves extract was previously well studied [35]. As expected, both silver and gold nanoparticles biosynthesized from A. indica leaves extract did not show any inflammatory response, suggesting that nanoparticles targeted only the tumour cells. Based on the results obtained from these studies, it is quite apparent that biologically synthesized silver and gold nanoparticles have better therapeutic potentials than the reported chemically synthesized nanoparticles. Therefore, it might be worthwhile to explore the biosynthesized nanoparticles as a possible source of novel anticancer drugs.
5. Conclusions
In this present study, silver and gold nanoparticles were rapidly synthesized using aqueous leaves extract of A. indica as novel source of bio-reductants. This single step procedure appears to be suitable for large scale production as it is simple, faster, cost-effective, environmentally benign and safe for clinical research. Further, the plant extract derived nanoparticles exhibited strong cytotoxic effects against MDA-MB-231 cells, which suggest that biologically synthesized silver and gold nanoparticles might be used as novel anticancer agents for the treatment of breast cancer. However, the fate, transport and accumulation of nanoparticles inside the human body must be thoroughly studied prior to the approval to use as anticancer drug.
Acknowledgements
The authors thank the Director, CAS in Botany, University of Madras for laboratory facilities. We are grateful to the Director, Centre for Biotechnology, Anna University for cell culture facilities. The authors are thankful to Dr. Udayakumar Muthulingam, Pachaiyappa’s College, Chennai for taxonomical identification of the plant sample. The Head, SAIF, IIT-Madras is gratefully acknowledged for HR-TEM analysis.
Footnotes
Available online 13 August 2014
Contributor Information
C. Krishnaraj, Email: krishnarajbio@gmail.com.
P.T. Kalaichelvan, Email: ptkalai2003@yahoo.com.
References
- 1.Ahmad N., Alam M.K., Singh V.N., Sharma S. Bio prospecting AgNPs from wild desmodium species. J. Bionanoscience. 2009;3:97–104. [Google Scholar]
- 2.Ali D.M., Thajuddin N., Jeganathan K., Gunasekaran M. Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids Surf. B. 2011;85:360–365. doi: 10.1016/j.colsurfb.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Allan R.T., Hunter W.J., III, Agrawal D.K. Morphological and biochemical characterization and analysis of apoptosis. J. Pharmacol. Toxicol. Methods. 1997;37:215. doi: 10.1016/s1056-8719(97)00033-6. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society, Breast Cancer Facts & Figures 2011–2012. Atlanta: American Cancer Society, Inc. 2011.
- 5.American Cancer Society, Breast Cancer Facts & Figures 2013–2014. Atlanta: American Cancer Society, Inc. 2013.
- 6.Asharani P.V., Hande M.P., Valiyaveettil S. Anti-proliferative activity of silver nanoparticles. BMC Cell Biol. 2009;10:65. doi: 10.1186/1471-2121-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beveridge T.J., Hughes M.N., Lee H., Leung K.T., Poole R.K., Savvaidis I., Silver S., Trevors J.T. Metal-microbe interactions: contemporary approaches. Adv. Microb. Physiol. 1997;38:178. doi: 10.1016/s0065-2911(08)60158-7. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharyya S., Kudgus R.A., Bhattacharya R., Mukherjee P. Inorganic nanoparticles in cancer therapy. Pharm. Res. 2011;28:237–259. doi: 10.1007/s11095-010-0318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandran S.P., Chaudhary M., Pasricha R., Ahmad A., Sastry M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol. Progr. 2006;22:577–583. doi: 10.1021/bp0501423. [DOI] [PubMed] [Google Scholar]
- 10.Chauhan A., Zubair S., Tufail S., Sherwani A., Sajid M., Raman S.C., Azam A., Owais M. Fungus-mediated biological synthesis of gold nanoparticles: potential in detection of liver cancer. Int. J. Nanomedicine. 2011;6:2305–2319. doi: 10.2147/IJN.S23195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubey S.P., Lahtinen M., Sarkka H., Sillanpaa M. Bioprospective of Sorbus aucuparia leaf extract in development of silver and gold Nanocolloids. Colloids Surf. B. 2010;80:26–33. doi: 10.1016/j.colsurfb.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 12.Elavazhagan T., Arunachalam K.D. Memecylon edule leaf extract mediated green synthesis of silver and gold nanoparticles. Int. J. Nanomedicine. 2011;6:1265–1278. doi: 10.2147/IJN.S18347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fong J., Wood F. Nanocrystalline silver dressings in wound management: a review. Int. J. Nanomedicine. 2006;1:441–449. doi: 10.2147/nano.2006.1.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franco-Molina M.A., Mendoza-Gamboa E., Sierra-Rivera C.A. Antitumor activity of colloidal silver on MCF-7 human breast cancer cells. J. Exp. Clin. Cancer Res. 2010;29:148. doi: 10.1186/1756-9966-29-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franco J.L., Posser T., Dunkley P.R. Methyl mercury neurotoxicity is associated with inhibition of the antioxidant enzyme glutathione peroxidase. Free Radical Bio. Med. 2009;7:449–457. doi: 10.1016/j.freeradbiomed.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Gangula A., Podila R., Ramakrishna M., Karanam L., Janardhana C., Rao A.M. Catalytic reduction of 4-nitrophenol using biogenic gold and silver nanoparticles derived from Breynia rhamnoides. Langmuir. 2011;27(15):268–15274. doi: 10.1021/la2034559. [DOI] [PubMed] [Google Scholar]
- 17.Gurunathan S., Jegadeesh R., Sri Nurestri A.M., Priscilla A.J., Vikineswary S. Green synthesis of silver nanoparticles using Ganoderma neo-japonicum Imazeki: a potential cytotoxic agent against breast cancer cells. Int. J. Nanomedicine. 2013;8:4399–4413. doi: 10.2147/IJN.S51881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu R., Yong K.T., Roy I., Ding H., He S., Prasad P.N. Metallic nanostructures as localized plasmon resonance enhanced scattering probes for multiplex dark field targeted imaging of cancer cells. J. Phys. Chem. C Nanomater. Interfaces. 2009;113:2676–2684. doi: 10.1021/jp8076672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang J., Li Q., Sun D., Lu Y., Su Y., Yang X., Wang H., Wang Y., Shao W., He N., Hong J., Chen C. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology. 2007;18:105104–105114. [Google Scholar]
- 20.Janicke R.U., Sprengart M.N., Wati M.R., Porter A.G. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J. Biol. Chem. 1998;273:9357. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- 21.Jain S., Hirst D.G., O’Sullivan J.M. Gold nanoparticles as novel agents for cancer therapy. Br. J. Radiol. 2012;85:101–113. doi: 10.1259/bjr/59448833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeyaraj M., Rajesh M., Arun R., Mubarak Ali D., Sathishkumar G., Sivanandhan G., Kapildev G., Manickavasagam M., Premkumar K., Thajuddin N., Ganapathi A. An investigation on the cytotoxicity and caspase-mediated apoptotic effect of biologically synthesized silver nanoparticles using Podophyllum hexandrum on human cervical carcinoma cells. Colloids Surf. B. 2013;102:708–717. doi: 10.1016/j.colsurfb.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 23.Jeyaraj M., Sathishkumar G., Sivanandhan G., Mubarak Ali D., Rajesh M., Arun R., Kapildev G., Manickavasagam M., Thajuddin N., Premkumar K., Ganapathi A. Biogenic silver nanoparticles for cancer treatment: an experimental report. Colloids Surf. B. 2013;106:86–92. doi: 10.1016/j.colsurfb.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 24.Kalaiarasi R., Jayalakshmi N., Venkatachalam P. Phytosynthesis of nanoparticles and its applications. Plant Cell Biotechnol. Mol. Biol. 2010;11:1–16. [Google Scholar]
- 25.Karuppaiya P., Satheeshkumar E., Chao W.T., Kao Y., Chen E.C.F., Tsay H.S. Anti-metastatic activity of biologically synthesized gold nanoparticles on human fibro sarcoma cell line HT-1080. Colloids Surf. B. 2013;110:163–170. doi: 10.1016/j.colsurfb.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 26.Kim D.W., Hong G.H., Lee H.H., Choi S.H., Chun B.G., Won C.K., Hwang I.K., Won M.H. Effect of colloidal silver against the cytotoxicity of hydrogen peroxide and naphthazarin on primary cultured cortical astrocytes. Neuroscience. 2007;117:387–400. doi: 10.1080/00207450600592016. [DOI] [PubMed] [Google Scholar]
- 27.Kotha A., Sekharam M., Cilenti L., Siddiquee K., Khaled A., Zervos A., Carter B., Turkson J., Jove R. Resveratrol inhibits Src and Stat3 signaling and induces the apoptosis of malignant cells containing activated Stat3 protein. Mol. Cancer Ther. 2006;5:621–629. doi: 10.1158/1535-7163.MCT-05-0268. [DOI] [PubMed] [Google Scholar]
- 28.Krishnaraj C., Jagan E.G., Rajasekar S., Selvakumar P., Kalaichelvan P.T., Mohan N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf. B. 2010;76:50–56. doi: 10.1016/j.colsurfb.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Kumar V., Yadav S.K. Plant-mediated synthesis of silver and gold nanoparticles and their applications. J. Chem. Technol. Biotechnol. 2009;84:151–157. [Google Scholar]
- 30.Li G., He D., Qian Y., Guan B., Gao S Cui Y., Yokoyama K., Wang L. Fungus-mediated green synthesis of silver nanoparticles using Aspergillus terreus. Int. J. Mol. Sci. 2012;13:466–476. doi: 10.3390/ijms13010466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moaddad S., Ahari H., Shahbazzadeh D., Motallebi A.A., Anvar A.A., Rahman-Nya J., Shokrgozar M.R. Toxicity study of nanosilver (Nanocid®) on osteoblast cancer cell line. Iran. Nano Lett. 2011;1:11. [Google Scholar]
- 32.Narayanan K.B., Sakthivel N. Coriander leaf mediated biosynthesis of gold nanoparticles. Mater. Lett. 2008;62:4588–4590. [Google Scholar]
- 33.Park M.V.D.Z., Neigh A.M., Vermeulen J.P. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials. 2011;32:9810–9817. doi: 10.1016/j.biomaterials.2011.08.085. [DOI] [PubMed] [Google Scholar]
- 34.Project on Emerging Nanotechnologies (2014). Consumer Products Inventory. Retrieved [11th July, 2014], from http://www.nanotechproject.org/cpi
- 35.Rahman M.A., Bachar S.C., Rahmatullah M. Analgesic and anti-inflammatory activity of methanolic extract of Acalypha indica Linn. Pak. J. Pharma. Sci. 2010;23:256–258. [PubMed] [Google Scholar]
- 36.Sriram M.I., Kanth S.B., Kalishwaralal K., Gurunathan S. Antitumor activity of silver nanoparticles in Dalton’s lymphoma ascites tumor model. Int. J. Nanomedicine. 2010;5:753–762. doi: 10.2147/IJN.S11727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sriram M.I., Kalishwaralal K., Gurunathan S. Biosynthesis of silver and gold nanoparticles using Bacillus licheniformis. Method Mol. Biol. 2012;906:33–43. doi: 10.1007/978-1-61779-953-2_3. [DOI] [PubMed] [Google Scholar]
- 38.Su J.M., Wang Y., Liang Y.L., Zha L. Role of cell adhesion signal molecules in hepatocellular carcinoma cell apoptosis. World J. Gastroenterol. 2005;11:4667–4673. doi: 10.3748/wjg.v11.i30.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sutter A.P., Maaser K., Barthe B., Scheru H. Ligands of the peripheral benzodiazepine receptor induce apoptosis and cell cycle arrest in oesophageal cancer cells: involvement of the p38MAPK signaling pathway. Br. J. Cancer. 2003;89:564–572. doi: 10.1038/sj.bjc.6601125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vedpriya A. Living systems: eco-friendly nanofactories. Dig. J. Nanomater. Bios. 2010;5:9–21. [Google Scholar]
- 41.Vivek R., Thangam R., Muthuchelian K., Gunasekaran P., Kaveri K., Kannan S. Green biosynthesis of silver nanoparticles from Annona squamosa leaf extract and its in vitro cytotoxic effect on MCF-7 cells. Process Biochem. 2012;47:2405–2410. [Google Scholar]
- 42.Winnicka F., Bielawski K., Bielawska A., Milty W. Apoptosis-mediated cytotoxicity of ouabain, digoxin and proscillaridin A in the estrogen independent MDA-MB-231 breast cancer cells. Arch. Pharm. Res. 2007;30:1216–1224. doi: 10.1007/BF02980262. [DOI] [PubMed] [Google Scholar]
- 43.Yeruva L., Elegbede J.A., Carper S.W. Methyl jasmonate decreases membrane fluidity and induces apoptosis via tumor necrosis factor receptor 1 in breast cancer cells. Anti-Cancer Drug. 2008;19:766–776. doi: 10.1097/CAD.0b013e32830b5894. [DOI] [PMC free article] [PubMed] [Google Scholar]