Abstract
We report on the first silica encapsulation of a metazoan (Daphnia magna), with a high initial viability (96% of the population remained active 48 h after encapsulation). Moreover, the co-encapsulation of this crustacean and microalgae (Pseudokirchneriella subcapitata) was achieved, creating inside a silica monolith, the smallest microcosm developed to present. This artificial ecosystem in a greatly diminished scale isolated inside a silica nanoporous matrix could have applications in environmental monitoring, allowing ecotoxicity studies to be carried out in portable devices for on-line and in situ pollution level assessment.
Keywords: Silica co-encapsulation, Microcosm, Daphnia magna-microalgae
1. Introduction
Silica is considered to be chemically and mechanically inert, optically transparent, thermally stable and resistant to microbial attack [1] It is found in many living organisms including diatoms, bacteria and plants, as well as in higher animals, and it is also widely used for the production of goods or as additive in the food industry. The application of the sol–gel process to develop silica-based materials for cellular encapsulation has been continuously explored over the last decades due to the unique properties of silica allowing the entrapped organisms to remain accessible to external reagents through the pores of the silica matrix [2]. The sol–gel derived matrix offers a biocompatible environment with several advantages: (i) chemical stability and no swelling in biological media, (ii) no biodegradability ensuring long term confinement of living species and (iii) tunable porosity allowing a fine control of molecular transport between the external medium to the cell, as well as preventing the contact with other microorganisms. In the recent years, efforts have been mainly focused on the diversification of encapsulated cell types. As a result, the range of potential biotechnological applications of these “living materials” has enormously increased [3]. A two-step procedure [4], which includes pre-encapsulation of the biological guest in calcium alginate matrix, allows protecting living cells from cytotoxic precursors during the synthesis of the silica network, resulting in improved cellular viability and preserved biological activity [5]. Moreover, it also avoids direct contact of cells with the encapsulation matrix during operation, enabling cell growth and division inside the liquid cavities within the inorganic matrix. As a consequence, the encapsulation of an entire culture, cellular aggregates or even multicellular organisms such as filamentous fungi instead of individual cells was made possible [6], [7]. However and in spite of being a high biocompatible system, to the best of our knowledge, there are no reports on encapsulation of a metazoan in these inorganic matrices.
Along with continuous efforts to adapt materials chemistry to the conditions of life, developments to improve the matrix properties and functions are currently creating materials that fulfill the requirements of different applications. In particular, routes based on sol–gel chemistry [8] are increasingly being used for the design of biosensing platforms for environmental monitoring [9]. In the last years several devices have been proposed, mainly based on microalgae encapsulation, which allow for real time detection of toxic levels of pollutants before they cause any damage to the environment.
However, to simulate direct discharge of chemical wastes into aquatic ecosystems, the most appropriate approach is to re-create the environment in a 20–100 L volume of culture medium where several species are represented (typically autotrophs and heterotrophs) [10]. Ecosystems consist of various organisms with different physiological properties and sensitivities to toxic agents, and with complex interactions such as competition, predation and association. Ecological effects at the community level cannot therefore be deduced from the results of single-species tests. “Microcosms” are experimental ecosystems constructed in the laboratory, expected to make it possible to evaluate ecotoxicity at the community level. They have been successfully applied in prediction of ecological fate and effects of xenobiotics in different environments [11], [12]. The microalgae Pseudokirchneriella subcapitata and crustacean Daphnia magna are frequently used as models of autotroph and heterotroph organisms, respectively, in their formulation. In these systems D. magna consume algae and is benefited by the oxygen supply generated by the autotrophic organism [13].
We propose herein the co-encapsulation of a D. magna and P. subcapitata in a nanoporous silica matrix, by means of the two-step procedure, creating inside a silica monolith the smallest microcosm developed to present. In order to evaluate the feasibility of this experiment, we also tested the toxicity of materials (alginate and silica matrix) used to make the encapsulation on D. magna. This silica-encapsulated microcosm could have application in environmental monitoring, allowing ecotoxicity studies to be carried out in economical and portable devices for on-line and in situ pollution level assessment.
2. Materials and methods
2.1. Microalgae growth
P. subcapitata was purchased from The Culture Collection of Algae and Protozoa (Cumbria, UK). Algae were maintained in a nycthemeral cycle of 16 h of illumination at 5000 lx and 8 h of darkness in the Lefebvre–Czarda medium 1 and were transplanted weekly under sterile conditions (autoclaving 20 mi, 130 °C, 1.3 bars).
2.2. D. magna growth
Daphnids (D. magna) were reared in M4 medium [14] Thirty individuals were kept in 2 L glass flasks at (20 ± 1) °C under 2000 lx (16 h/day); they were fed with a solution of P. subcapitata (106–107 cells/daphnid) added daily in the culture flasks. Neonates were collected daily and used in tests or discarded. Half of the medium was renewed once a week. Adult daphnids were discarded after 1 month and new cultures were initiated with neonates.
2.3. Daphnid acute toxicity test: mortality test
Daphnid mortality test was carried out according to the ISO standard protocol (ISO, 1995). In order to test toxicity of silica matrix, we added 0, 1, 2, 3 or 4 piece of silica matrix (volume = (100 ± 1) μL; surface area = (90 ± 3) mm2) into a glass test tube containing 10 mL of daphnids rearing medium. Then, five neonate daphnids (<24 h) were transferred into each tube. There were four tubes (20 daphnids) per tested “concentration”.
In order to test toxicity of alginate, we added sodium alginate (0, 0.1, 0.2, 0.4, 0.8, 1.6 or 3.2 mg/L) into a glass test tube containing 10 mL of daphnids rearing medium. Then, five neonate daphnids (<24 h) were transferred into each tube. There were three tubes (15 daphnids) per tested concentration.
For preventing any potential algal growth, tubes were placed in the darkness during the exposure period. After 24 h and 48 h, the number of daphnids with reduced mobility was recorded in each tube. The median effective concentration for mortality (LC50) was calculated using probit analysis [15].
2.4. Two-step encapsulation
The pre-encapsulation in alginate was performed by stirring 2 volumes of M4 medium containing daphnids neonates and P. subcapitata in suspension with 1 volume of 2.0% sodium alginate (Fluka BioChemica). Formation of alginate beads was done by dropwise addition of this cell suspension (using Pasteur pipettes) in a 0.2 M CaCl2 solution. After 3 min stirring, beads of about 8 mm diameter were easily collected by filtration. The time in contact with CaCl2 solution is not enough for complete alginate-Ca2+ crosslinking, forming liquid capsules with a ∼1 mm thick calcium alginate matrix envelope (naked-eye observation).
Alternatively, 100 μL of the M4 medium containing daphnids neonates and P. subcapitata in suspension was poured into an individual mold (disposable UV–vis cuvette), CaCO3 nanoparticles were gently placed on the surface of the liquid, 10 μL of 2.0% sodium alginate solution was poured on top and 0.2 M CaCl2 solution was immediately added in the form of a mist by means of a nebulizer machine.
The second step of the immobilization procedure consisted of a silicate (sodium silicate, Riedel-de Haën; NaOH 10%, SiO2 27%) sol–gel process in the presence of commercial silica nanoparticles (LUDOX HS-40, 40% in water, obtained from Aldrich), leading to a nanoporous monolithic structure. Monoliths were prepared at room temperature by mixing volumes of the different precursor solutions to obtain a SiO2:H2O molar relation of 0.038 with a fixed proportion of polymeric to particulate silica precursors (1:4) at constant pH 7.0, adjusted with HCl.
3. Results
As described in Section 2, daphnids and microalgae are co-immobilized in calcium alginate capsules of (8.5 ± 0.5) mm diameter and are further immersed in tubes where a mixture of sodium silicate and colloidal silica is vigorously mixed. This colloidal solution undergoes a rapid sol–gel transition, and alginate capsules are quickly covered with a nanoporous silica gel (time of gelation: 2–3 min). As a result, silica biomaterials with liquid macrocavities containing daphnids and microalgae in M4 culture medium are formed. After 20 min (necessary time for the consolidation of the silica matrix), the biomaterials are immediately rinsed with distilled water, and fresh M4 medium is added to the tube (see Fig. 1).
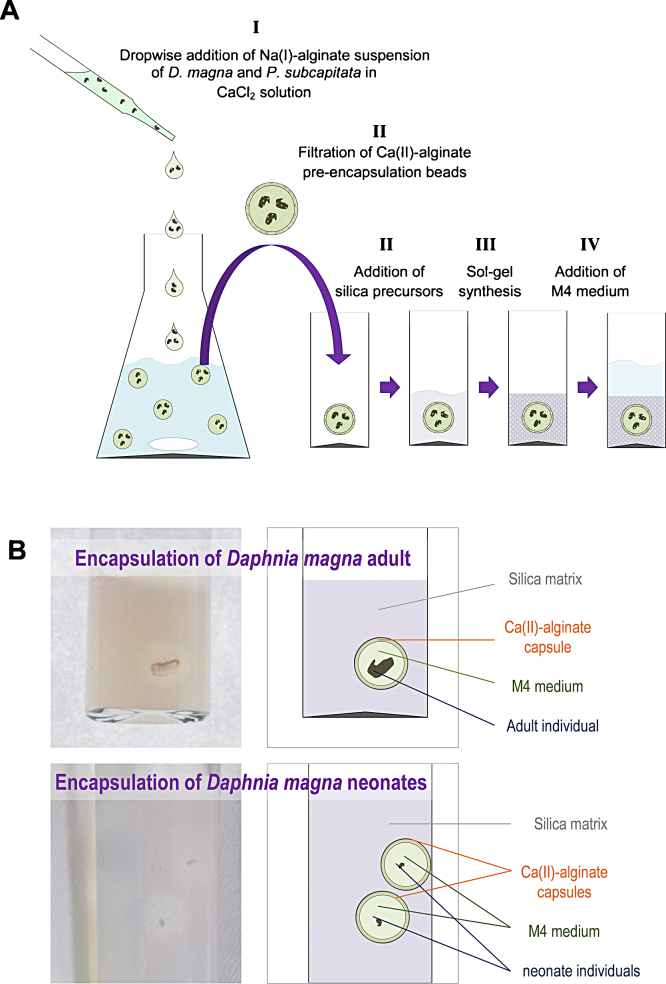
Fig. 1.
(A) Schematic representation of the co-encapsulation procedure of Daphnia magna and Pseudokirchneriella subcapitata in a silica matrix: (I) the pre-encapsulation beads are formed by dropwise addition of M4 medium/sodium alginate with containing daphnids neonates and P. subcapitata in suspension in a CaCl2 solution. (II) After 3 min of continuous stirring, liquid sodium alginate capsules of about 8 mm diameter with a ∼1 mm thick calcium alginate matrix envelope are easily collected by filtration. (III) Addition of silica precursors (sodium silicate and preformed commercial silica nanoparticles) adjusted to pH 7.0, leads to a nanoporous monolithic structure. (IV) After sol–gel synthesis, fresh M4 medium is added. (B) Photographs of Daphnia magna encapsulation in silica matrices, showing the crustacean to calcium alginate capsule volume relation in the case of adult (up) or neonate (down) encapsulation.
The high biocompatibility of this silica encapsulation procedure with P. subcapitata microalgal cells is well established [16]. In this work, only the assessment of initial viability (1 h after encapsulation) is conducted by averaging the content of 10 macrocavities. To this end, the silica hydrogel is removed and samples are exposed to 0.05% potassium citrate to solubilize the calcium alginate shell. The total number of cells inside individual cavities (2.35 × 105) was determined by counting cells in a Mallassez counting chamber; (99.2 ± 1.1)% of P. subcapitata cells remains intact, in good agreement with previous published results [16].
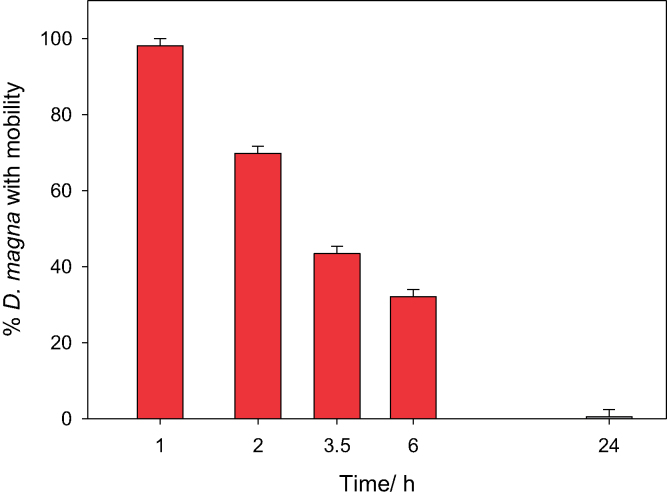
To evaluate the effect of the encapsulation procedure on D. magna, the content of each macrocavity was observed under an optical microscope (100× magnification) and the mobility of daphnids was recorded. The analysis reveals that 98% of the D. magna population (52 out of 53 total daphnids tested) remains active 1 h post-encapsulation, but this percentage drops to ∼32% only 6 h post-encapsulation (17 out of 53 total daphnids tested), and at 24 h post-encapsulation daphnids present no mobility. The complete set of results is presented in Fig. 2.
Fig. 2.
Evaluation of the effect caused by silica encapsulation on Daphnia magna. The content of each macrocavitiy containing approximately 5 daphnids neonates is observed under an optical microscope (100× magnification) and the mobility of daphnids is recorded as a function of time (24 h).
Apart from the possible deleterious effect of the confinement itself, we hypothesized that the low biocompatibility towards D. magna could arise from the direct contact with alginate polymer or from the proximity to the silica surface and/or the soluble species derived from the silica matrix synthesis. To further investigate this, we carried out acute toxicity test separately with sodium alginate and preformed silica matrix. In the last case, the inorganic matrix synthesis was done as described before except for the fact that aliquots of 100 μL of the precursor mix were poured into individual molds to obtain silica hydrogel monoliths of identical volume and shape. The different level of exposure of daphnids to silica preformed matrix was achieved by adding a different number of silica preformed pieces in each test tube. Results showed no toxic acute effect of silica hydrogel on D. magna at 48 h (maximum exposure: silica volume of 400 μL and contact surface area of 360 mm2 in a total volume of 10 mL). On the other hand, alginate polymer showed a high toxicity effect. The LC50 (lethal concentration for 50% of population) at 24 h of exposure is 1.3 mg/L of sodium alginate and the LC95 (lethal concentration for 95% of population) at 24 h is 2.5 mg/L, much lower than the alginate concentration required for the formation of calcium alginate shell capsules. Furthermore, a concentration of 0.4 mg/L of sodium alginate was lethal after 48 h of exposure.
D. magna being a planktonic crustacean, the alginate itself is not expected to cause a direct deleterious effect, and mortality could be due to the depletion of multivalent cations from the culture medium and/or the viscosity generated by the polymer chains partially crosslinked by multivalent cations. This could affect neonate daphnids by at least two mechanisms: physical exhaustion derived from moving in a higher viscosity medium and/or the obstruction of the sites of respiratory gas exchange, which takes place at the level of the integument [17].
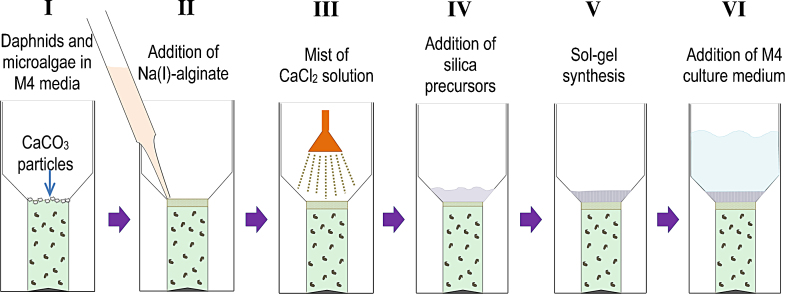
This prompted us to design a new immobilization method in order to obtain portable modular biosensors. As the contact with a silica matrix seemed to be well tolerated by these organisms and calcium alginate per se is not expected to cause toxicity, a new procedure in layers was designed, generating a liquid microenvironment inside the silica matrix. As described in Section 2, daphnids and microalgae cells in liquid M4 media are poured into a small mold and CaCO3 nanoparticles are gently placed on the surface of the liquid, a volume of sodium alginate solution added on top and CaCl2 solution added as a mist, form a calcium alginate thin layer on the surface of the liquid, which is supported by the inclined lateral walls of the mold (see Fig. 3). The second step of the immobilization procedure consists on the synthesis of the inorganic matrix above the calcium alginate layer, leading to a silica nanoporous layer of 2.0 mm width.
Fig. 3.
New immobilization method layer by layer to avoid the contact of daphnids with alginate. (I) Daphnids and microalgal cells in liquid M4 media are poured into a small mold and CaCO3 nanoparticles are gently placed on the surface of the liquid; (II) sodium alginate solution is added on top and (III) CaCl2 solution is added as a mist, to form a calcium alginate thin layer on the surface of the liquid, which is supported by the inclined lateral walls of the mold. (IV–V) Synthesis of the inorganic matrix above the calcium alginate layer, leading to a silica nanoporous layer of ∼2 mm width. (VI) Addition of fresh M4 culture medium.
To evaluate the biocompatibility of this immobilization procedure, the mobility of daphnids was evaluated for a 48 h period. The analysis reveals that 96% of the D. magna population (48 out of 50 total daphnids tested) remains active 1 h post-encapsulation, and is still active after 48 h inside the immobilization modules. This goes to show that the last immobilization method has a high intrinsic biocompatibility, which would allow the development of biosensing modules to perform acute toxicity test for environmental monitoring.
4. Conclusion
Employing a two-step immobilization procedure, we accomplished the co-immobilization of a crustacean (D. magna) and microalgae (P. subcapitata) in a nanoporous silica matrix. The procedure allows the organisms to remain in liquid culture during the synthesis of both the Ca-alginate and the silica matrix that would immobilize and isolate the small liquid culture from the surroundings.
This could provide a general approach for the design of modular biosensing devices, allowing ecotoxicity studies to be carried out in portable devices for in situ pollution level monitoring. Moreover, the high biocompatibility obtained suggests that this technique could be advantageously applied to many other species, allowing for different microcosms formulations in contiguous modules of a multiple sensor.
The silica matrix is mechanically stable and non-degradable by microorganisms. Additionally, its porosity can be tuned from the synthesis parameters to allow free diffusion of high molecular weight molecules but avoid microorganism contamination, assuring not only the conservation of biosensing modules but avoiding at the same time a false positive resulting from the interaction with other species present in the natural sample of water. On the other hand, its controlled porosity and the possibility of silica surface derivatization could allow for selective transport of particular pollutants, conferring different selectivity to each module in the arrangement.
Although promising, the results shown here must be complemented with further research in order to optimize the modular biosensor design. For instance, the development of automatic systems based on image processing for the analysis of both daphnids mobility and algal population growth. Work in both directions is currently in advance in our laboratories.
Acknowledgments
This work was performed in the frame of the ECOS-Sud A12B02 program and has been supported by the University of Lyon (ENTPE), CONICET GI-PIP 11220110101020, ANPCyT PICT-2013-2045, and UBACyT 20020130100048BA from Argentina. MP, MJ and SAB are Research Scientist of CONICET (Argentina).
Footnotes
Available online 11 October 2014
AFNOR, Détermination de l’inhibition de croissance de Scenedesmus subspicatus par une substance. Norme experimentale NT90–304; Association Française de Normalisation: Paris, France, 1980.
References
- 1.Meunier C.F., Dandoy P., Su B.-L. Encapsulation of cells within silica matrixes: towards a new advance in the conception of living hybrid materials advance in the conception of living hybrid materials. J. Colloid Interface Sci. 2010;324(2):211–224. doi: 10.1016/j.jcis.2009.10.050. [DOI] [PubMed] [Google Scholar]
- 2.Nassif N., Bouvet O., Rager M.N., Roux C., Coradin T., Livage J. Living bacteria in silica gels. Nat. Mater. 2002;1(1):42–44. doi: 10.1038/nmat709. [DOI] [PubMed] [Google Scholar]
- 3.Blondeau M., Coradin T. Living materials from sol–gel chemistry: current challenges and perspectives. J. Mater. Chem. 2012;22:22335–22343. [Google Scholar]
- 4.Perullini M., Jobbágy M., Soller-Illia G.J.A.A., Bilmes S.A. Cellular growth at cavities created inside silica monoliths synthesized by sol–gel. Chem. Mater. 2005;17:3806–3808. [Google Scholar]
- 5.Perullini M., Jobbágy M., Bermúdez Moretti M., Correa Garcia S., Bilmes S.A. Chemi. Mater. 2008;20:3015–3021. [Google Scholar]
- 6.Perullini M., Rivero M.M., Jobbágy M., Mentaberry A., Bilmes S.A. Plant cell proliferation inside an inorganic host. J. Biotechnol. 2007;127:542–548. doi: 10.1016/j.jbiotec.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 7.Perullini M., Jobbágy M., Mouso N., Forchiassin F., Bilmes S.A. Silica–alginate–fungi biocomposites for remediation of polluted water. J. Mater. Chem. 2010;20(31):6479–6483. [Google Scholar]
- 8.Brinker C.J., Scherer G.W. Academic Press, Inc.; San Diego, CA, USA: 1990. Sol–Gel Science: The Physics and Chemistry of Sol–Gel Processing. [Google Scholar]
- 9.Depagne C., Roux C., Coradin T. How to design cell-based biosensors using the sol–gel process. Anal. Bioanal. Chem. 2011;400(4):965–976. doi: 10.1007/s00216-010-4351-y. [DOI] [PubMed] [Google Scholar]
- 10.Metcalf R.L., Lu P.-Y., Bowlus S. Degradation and environmental fate of 1-(2,6 difluorobenzoyl)-3-(4-chlorophenyl) urea. J. Agric. Food Chem. 1975;23(3):359–364. doi: 10.1021/jf60199a025. [DOI] [PubMed] [Google Scholar]
- 11.Triffault-Bouchet G., Clément B., Blake G. Ecotoxicological assessment of pollutant flux released from bottom ash reused in road construction. Aquat Ecosyst. Health Manage. 2005;8:405–414. [Google Scholar]
- 12.Clément B., Vaille G., Moretto R., Vernusc E., Abdelghafour M. Effects of a physico-chemical treatment of a dredged sediment on its ecotoxicity after discharge in laboratory gravel pit microcosms. J. Haz Mat. 2010;175:205–215. doi: 10.1016/j.jhazmat.2009.09.150. [DOI] [PubMed] [Google Scholar]
- 13.Vidal T., Gonçalves A.M.M., Pardal M.A., Azeiteiro U.M., Gonçalves F. Assessing the toxicity of betanal® on growth and sensitiveness of five freshwater planktonic species. Fresen. Environ. Bull. 2009;18(5):585–589. [Google Scholar]
- 14.Elendt B.P., Bias W.R. Trace nutrient deficiency in Daphnia magna cultured in standard medium for toxicity testing: effects of the optimization of culture conditions on life history parameters of Daphnia magna. Water Res. 1990;24:1157–1167. [Google Scholar]
- 15.Finney D.J., editor. Probit Analysis. Cambridge University Press; Cambridge, England: 1952. [Google Scholar]
- 16.Ferro Y., Perullini M., Jobbágy M., Bilmes S.A., Durrieu C. Development of a biosensor for environmental monitoring based on microalgae immobilized in silica hydrogels. Sensors. 2012;12:16879–16891. doi: 10.3390/s121216879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pirow R., Wollinger F., Paul R.J. The sites of respiratory gas exchange in the planktonic crustacean Daphnia magna: an in vivo study employing blood haemoglobin as an internal oxygen probe. J. Exp. Biol. 1999;202:3089–3099. doi: 10.1242/jeb.202.22.3089. [DOI] [PubMed] [Google Scholar]