Abstract
Infection with Epstein–Barr virus (EBV) has been associated with an enhanced risk of genetically susceptible individuals to develop multiple sclerosis (MS). However, an explanation for the contrast between the high EBV infection prevalence (60–90%) and the low MS prevalence (0.1%) eludes us. Here we propose a new concept for the EBV–MS association developed in the experimental autoimmune encephalomyelitis model in marmoset monkeys, which are naturally infected with the EBV-related γ1-herpesvirus CalHV3. The data indicate that the infection of B cells with a γ1-herpesvirus endows them with the capacity to activate auto-aggressive CD8+ T cells specific for myelin oligodendrocyte glycoprotein.
Keywords: MS risk, myelin oligodendrocyte glycoprotein processing, autophagy, citrullination
Introduction
Genome-wide association studies (GWAS) and the positive clinical effect of several treatments that suppress or modify immunological mechanisms support an important pathogenic role of the immune system in multiple sclerosis (MS). The trigger of the cellular and humoral autoimmune reactions against central nervous system (CNS) myelin is unknown, but may involve factors from outside the body (outside-in paradigm) or from the target organ itself (inside-out paradigm).1 The experimental autoimmune encephalomyelitis (EAE) model in common marmosets (Callithrix jacchus), a small-bodied primate, strongly supports the inside-out paradigm. In this MS pathogenic concept, autoimmunity is viewed as a dysregulated reaction of the immune system against antigens leaking from (a) primary lesion(s) in the CNS white matter.2 The nature of the primary lesion has not been defined, but could well be a small-sized pathological structure indicated as pre-active lesion,3 microglia nodule,4 or newly forming lesion.5 As reviewed elsewhere, evidence from marmoset EAE indicates that myelin oligodendrocyte glycoprotein (MOG) is the dominant trigger in CNS myelin for the development of chronic EAE.6
If MS would be the consequence of an inside-out process, the question arises how the influence of established MS risk factors from the environment can be explained, such as infection with Epstein–Barr virus (EBV).7 We believe that this question is highly relevant as MS risk factors may be connected to rate-limiting steps in the pathogenic process against which new therapies can be developed. We present here a new pathogenic mechanism that was developed in the marmoset EAE model.8
EAE in marmosets: pathogenic mechanisms
An essential difference between marmosets and frequently used inbred/specific pathogen free (SPF) laboratory mice and rats as preclinical MS model is that the marmoset immune system has been shaped by genetic diversity and lifelong exposure to similar pathogens as humans are exposed to. It was observed that especially the chronic latent infection with herpesviruses related to human cytomegalovirus (CMV) and EBV underlies the activation of a novel pathogenic mechanism that seems not to exist in SPF rodents.9
In brief, sensitization of the pathogen-educated marmoset immune system against myelin from humans or C57BL/6 mice (formulated with the strong bacterial adjuvant complete Freund’s adjuvant (CFA) elicits a chronic inflammatory/demyelinating disease that resembles MS in clinical and pathological presentation.10,11 When the experiment was repeated with myelin from a MOG-deficient B6 mouse chronic EAE did not develop.11 Further research in a model elicited with recombinant human MOG (rhMOG) showed that the initiation and progression of EAE are driven by two distinct pathogenic mechanisms.12 In the initiation phase, MHC class II/Caja-DRB*W1201-restricted T helper 1 cells against MOG epitope 24–36 as well as B cells specific for a conformational epitope at the membrane-distal part of MOG elicit inflammatory demyelination in the white matter. In this early phase mainly myelin sheaths are damaged and oligodendrocytes seem to be spared. The validity of this initiation mechanism has been corroborated by the strong clinical effect of B cell depletion with the anti-CD20 monoclonal antibody (mAb) HuMab7D8,13 or neutralization of IL-12 and IL-23 with the anti-IL-12p40 mAb ustekinumab.14 Later in the disease an unconventional type of cytotoxic T cells (CTL) is activated by another MOG epitope, residues 40–48,15 which shares sequence identity with residues 981–1003 from the UL86 open reading frame-encoded major capsid protein of CMV.16 These CTL have an atypical phenotype (CD8+CD56+CD28−) and react against the MOG40-48 epitope presented via non-classical MHC class Ib/Caja-E molecules.17 Interestingly, similar HLA-E restricted NK markers expressing CTL were found to be involved in the control of CMV infection.18 It is therefore tempting to speculate whether the autoaggressive CTL originate from the sizeable repertoire of anti-CMV effector memory T cells that expands with aging.20 In the established MS lesion, similar types of CTL have been detected in the proximity of HLA-E expressing oligodendrocytes indicating that they might kill them.19 Myelin damage via this mechanism might be more widespread as one oligodendrocyte myelinates multiple axons (up to 50) and irreversible unless the killed oligodendrocyte is replaced. We propose that the transition from Th1-mediated inflammatory pathology to the CTL-mediated degenerative pathology may underlie the transition for relapsing-remitting to secondary progressive MS.
Both the Th1 cells that mediate EAE initiation as well as the CTL that drive EAE progression are hyper-reactive against MOG. This is illustrated by the observation that via in vivo activation these T cell specificities atypical EAE models can be induced, respectively by immunization with rhMOG protein or MOG34-56 peptide formulated with the mineral oil incomplete Freund’s adjuvant (IFA).21,22 The models are atypical because the immunizing antigen formulations lack the normally requisite danger signals for the activation of naïve T cells, but they elicit antigen-experienced T cells. These IFA-based formulations are inadequate for EAE induction in immunologically immature SPF mice. A site where the immune response to CNS injury may take place is in the CNS draining lymphoid compartment (cervical lymph nodes, spleen), where we detected myelin-containing myeloid cells which express markers of professional APC, such as CD40, CD86, and the dendritic cell-specific C-type lectin DC-SIGN.23 Intriguingly, surgical removal of cervical and lumbar lymph nodes did not affect EAE initiation, but abrogated chronic relapsing EAE in a MOG-induced Biozzi ABH mouse model.24 In another set of experiments in the same Biozzi mouse EAE model, we could show that EAE initiation can be achieved with MOG-deficient myelin, while the evolution of chronic relapsing disease is disturbed.25 Combination of these findings underlies the concept that the activation of T cells against the immunogenic (read post-translationally modified6) form of MOG occurs in the CNS draining lymphoid compartment.
However, CNS injury happens quite frequently in human life without eliciting chronic autoimmune reactions. Hence, the question arises why this does happen in MS. We postulate here a decisive role of EBV infection.
Epstein–Barr virus (EBV)
EBV is a γ1-herpesvirus that causes usually asymptomatic infection in > 90% of the adult human population. EBV is the human-specific representative in the genus lymphocryptovirus (LCV) that comprises γ1-herpesviruses of non-human primates, such as callitrichine herpesvirus 3 (CalHV3) of marmosets, herpesvirus papio of baboons, and macaque LCV of rhesus monkeys. These viruses have in common that they infect and transform B cells.26
There is strong epidemiological evidence for a pathogenic role of EBV in MS, especially when first exposure occurs at young adult age.27 However, the mechanism(s) underlying the association are not clear. It has been proposed that EBV infection may elicit a cross-reactive T cell response against the myelin protein MBP.28 One major problem with this response-to-infection concept is the contrast of the high infection prevalence of EBV, ranging from 60% in young adults to > 90% in adults, with the low prevalence of MS (0.1%). A critical but often overlooked variable may be the fact that only very few B cells contain the virus (<0.01%).29 This finding led us to postulate that the increased MS risk by EBV-exposure may not be due to the immune response against the virus, but to pathogenic capacities that B cells acquire by infection with EBV.30 The reason why we do not all develop MS may be that the disease develops only when autoreactive B cell clones with a specificity relevant to MS are infected by the virus.
Our interest in a pathogenic role of LCV-infected B cells in the primate EAE model was sparked by the observation that the discrepant clinical effect in the marmoset EAE model of mAbs against CD20 versus mAbs against the B cell cytokines BlyS (B lymphocyte stimulator) and APRIL (a proliferation-inducing ligand), mirroring the opposite clinical effects of anti-CD20 mAbs and atacicept in MS,31 could be explained by the differential depletion of CalHV3.32
LCV-infected B cells as special antigen presenting cells (APC)
In classical MS concepts B cells were only held responsible for the production of autoantibodies that mediate demyelination via macrophages and complement factors. In more recent concepts, however, B cells have a broader pathogenic role, including also antigen presentation, cytokine production and the organization of ectopic lymphoid structures in the CNS.31 This prompted us to test the effect of B cell depletion in an atypical model induced with MOG34-56/IFA, in which only the EAE progression pathway is activated. Also in this model, where antibodies binding CNS myelin are not formed,22 late stage administration of anti-CD20 mAb arrested EAE development.33 The results obtained in this model led to the conclusion that B cells are requisite APC for a novel subset of strongly pathogenic CD8+CD56+IL-17+ T cells that drive EAE evolution.
B cells are excellent APC because they can capture and ingest low doses of antigen via their surface-expressed B cell antigen receptor (BCR). Following degradation of the antigen by cathepsins in the endolysosomal compartment surviving peptides of 10 to 12 residues length are presented via MHC class II molecules to CD4+ve T cells. However, antigen presentation via MHC class I to CD8+ve T cells, commonly indicated as cross-presentation, is not a specialty of B cells. Recent work shows that B cells acquire this capacity by infection with EBV.8
Using lysates of non-infected and LCV-infected CD20+ B cells from primate spleens we have unraveled molecular changes induced by the infection.8 As the transcriptome of marmosets has not been fully annotated, we used cells from rhesus monkeys for these experiments. In brief, we discovered that in non-infected B cells the MOG40-48 epitope of the core pathogenic CTL is rapidly degraded through cleavage by cathepsin (Cat) G at the arginine residues at positions 41 and 46. Comparative transcriptome analysis of non-infected and LCV-infected B cells revealed multiple molecular changes relevant for antigen cross-presentation, including enhanced expression of second signal molecules regulating T cell activation (CD40, CD70, CD86, and PD-1), assembly of immunoproteasomes, expression of peptidyl arginine deiminase (PAD) enzymes that convert arginine into citrulline, and activation of the autophagy pathway.8 Of note, inactivation of the autophagy pathway in CD8αα+ mouse DC, which are the proven specialists in antigen cross-presentation, impairs their capacity to cross-present exogenous ovalbumin via MHC class-I to OT-1 cells.34
We observed that the combination of an acidic (Ser) residue at position 42 with citrullination of the Arg46 residue in the MOG40-48 epitope protects the long peptide MOG35-51 against degradation by CatG, although it has at least seven potential CatG cleavage sites (underlined: MEVGWYRSPFSRVVHLY). The reason why the Arg46 residue is so important may be that it is part of the putative F-LIR motif FSRV via which the peptide may interact with the LC3-II docking molecule of autophagosomes.8 The putative role of the Ser42 residue is stabilization of the relatively weak interaction of the F-LIR motif with LC3-II.35
Concluding remarks
Studies by Weller et al. (reviewed in Laman and Weller36) have shown that immune reactions against myelin antigens draining from the brain are induced in the cervical lymph nodes. In line with these findings we observed that myelin leaking from primary EAE lesions inside the marmoset CNS accumulate in DC-like myeloid APC located within the CNS draining lymphoid compartment.23 The localization within T cell areas implies that autoaggressive T cells against MOG can be activated, which is corroborated by the observation that surgical removal of cervical and lumbar lymph nodes (CLN, LLN) impairs chronic relapsing EAE in a Biozzi mouse model.24 However, immune responses in CLN against CNS antigens are more often protective than pathogenic,37 indicating that additional pathogenic events may be involved for induction of autoimmunity against the CNS. A recent study revealed a role of MOG in the regulation of tolerance and autoimmunity. MOG derived from healthy CNS is tolerogenic by the expression of glycans that mediate binding with DC-SIGN.38 Factors generated during CNS inflammation (e.g. TNF-α) induce post-translational modifications (PTM) that make MOG immunogenic. A crucial modification is altered glycosylation, by which binding to DC-SIGN is abrogated.38 A second important PTM is citrullination of the protease sensitive MOG40-48 epitope, by which fast proteolytic degradation of the epitope is prevented. It has been well established that the immunogenicity of antigen peptides can be substantially enhanced by delay of proteolytic degradation.39
In a B6 mouse EAE model induced with MOG35-55/CFA the Arg46 residue is one of the TCR contact residues within the core MOG40-48 epitope.40 Replacement of this residue for citrulline could thus potentially affect T cell recognition of the epitope. However, accumulating evidence indicates that a high proportion of the myelin antigens in the inflamed CNS is citrullinated.41 Citrullination is particularly pathogenic when EBV-infected B cells become involved in the pathogenic process.
Summarizing concept
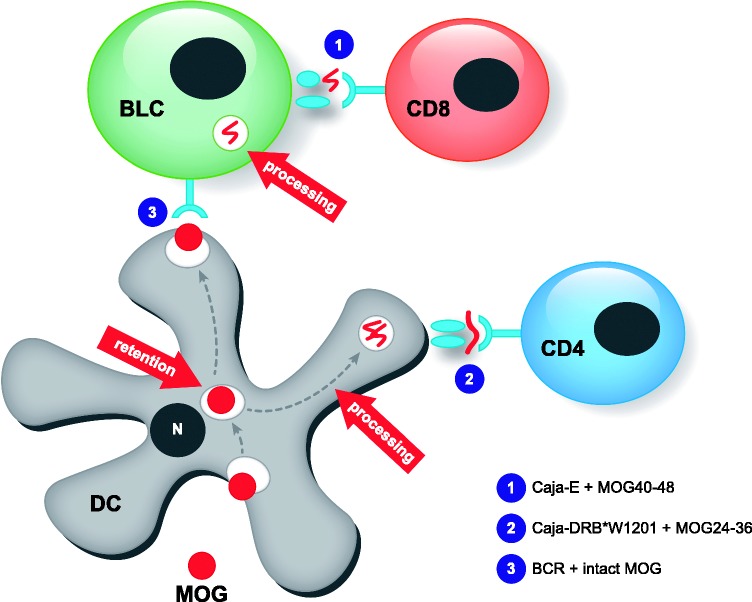
These findings have led to a concept that is graphically presented in Figure 1. Myeloid APC in CNS draining compartments capture myelin leaking from (a) primary lesion(s) within the CNS white matter and pre-process it in their endolysosomal compartment. Conceptually, a proportion of the liberated myelin antigens (MOG for example) are transferred from the myeloid APC to B cells. It has been shown that DC can retain intact antigen for longer periods of time and then release it for uptake by B cells, which subsequently process the antigen and present epitopes to T cells.42 In the B cells MOG is further processed by cathepsins in the endolysosomal compartment. When the B cell is not infected with EBV, the immunodominant MOG40-48 is rapidly degraded and activation of the highly pathogenic CD8+ CTL is avoided. However, when the B cell is infected with EBV the MOG40-48 epitope is protected against degradation and cross-presented to the autoaggressive CTL.
Figure 1.
Putative role of EBV-infected B cells in the response-to-injury paradigm of chronic EAE. Antigen leaking from a primary CNS lesion, MOG in particular,11 is captured by DC-SIGN+ myeloid cells (DC?) in lymphoid organs that drain the CNS (spleen, cervical, and lumbar lymph nodes). One part of the antigens is processed and the epitope 24–36 is presented via MHC class II (Caja-DRB*W1201) to CD4+ T cells, resulting in activation.43 Another part passes the DC without being processed and is transferred to B cells.42 In lymphocryptovirus-infected B cells the proteolysis-sensitive CD8 epitope (residues 40–48) escapes destructive processing,8 and is cross-presented via MHC class Ib (Caja-E) to autoaggressive CTL,17 which drive EAE progression.15 This model envisages that B cell depletion with anti-CD20 mAb abrogates EAE progression.13 The figure has been drafted after MacPherson et al.42
We thus propose a central regulatory role of B cells in the perpetuation of chronic MS. By EBV infection of the B cells the destructive processing of a critical epitope, which is essentially a tolerance mechanism, is converted into productive processing eliciting autoaggressive T cell activation, which is a pathogenic mechanism.
Acknowledgement
We would like to thank Mrs Francesca van Hasselt for the artwork.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Stys PK, Zamponi GW, van Minnen J, et al. Will the real multiple sclerosis please stand up? Nat Rev Neurosci 2012; 13: 507–514. [DOI] [PubMed] [Google Scholar]
- 2.’t Hart BA, Hintzen RQ, Laman JD. Multiple sclerosis – a response-to-damage model. Trends Mol Med 2009; 15: 235–244. [DOI] [PubMed] [Google Scholar]
- 3.Van der Valk P, De Groot CJ. Staging of multiple sclerosis (MS) lesions: pathology of the time frame of MS. Neuropathol Appl Neurobiol 2000; 26: 2–10. [DOI] [PubMed] [Google Scholar]
- 4.Singh S, Metz I, Amor S, et al. Microglial nodules in early multiple sclerosis white matter are associated with degenerating axons. Acta Neuropathol 2013; 125: 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol 2004; 55: 458–468. [DOI] [PubMed] [Google Scholar]
- 6.’t Hart BA, Weissert R. Myelin oligodendrocyte glycoprotein has a dual role in T cell autoimmunity against central nervous system myelin. Mult Scler J Exp Transl Clin 2016; 2: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belbasis L, Bellou V, Evangelou E, et al. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol 2015; 14: 263–273. [DOI] [PubMed] [Google Scholar]
- 8.Jagessar SA, Holtman IR, Hofman S, et al. Lymphocryptovirus infection of nonhuman primate B cells converts destructive into productive processing of the pathogenic CD8 T cell epitope in myelin oligodendrocyte glycoprotein. J Immunol 2016; 197: 1074–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.’t Hart BA, van Kooyk Y, Geurts JJ, et al. The primate autoimmune encephalomyelitis model; a bridge between mouse and man. Ann Clin Transl Neurol 2015; 2: 581–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.’t Hart BA, Bauer J, Muller HJ, et al. Histopathological characterization of magnetic resonance imaging-detectable brain white matter lesions in a primate model of multiple sclerosis: a correlative study in the experimental autoimmune encephalomyelitis model in common marmosets (Callithrix jacchus). Am J Pathol 1998; 153: 649–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jagessar SA, Smith PA, Blezer E, et al. Autoimmunity against myelin oligodendrocyte glycoprotein is dispensable for the initiation although essential for the progression of chronic encephalomyelitis in common marmosets. J Neuropathol Exp Neurol 2008; 67: 326–340. [DOI] [PubMed] [Google Scholar]
- 12.’t Hart BA, Gran B, Weissert R. EAE: imperfect but useful models of multiple sclerosis. Trends Mol Med 2011; 17: 119–125. [DOI] [PubMed] [Google Scholar]
- 13.Kap YS, van Driel N, Blezer E, et al. Late B cell depletion with a human anti-human CD20 IgG1kappa monoclonal antibody halts the development of experimental autoimmune encephalomyelitis in marmosets. J Immunol 2010; 185: 3990–4003. [DOI] [PubMed] [Google Scholar]
- 14.Brok HP, Van Meurs M, Blezer E, et al. Prevention of experimental autoimmune encephalomyelitis in common marmosets using an anti-IL-12p40 monoclonal antibody. J Immunol 2002; 169: 6554–6563. [DOI] [PubMed] [Google Scholar]
- 15.Kap YS, Smith P, Jagessar SA, et al. Fast progression of recombinant human myelin/oligodendrocyte glycoprotein (MOG)-induced experimental autoimmune encephalomyelitis in marmosets is associated with the activation of MOG34-56-specific cytotoxic T cells. J Immunol 2008; 180: 1326–1337. [DOI] [PubMed] [Google Scholar]
- 16.Brok HP, Boven L, van Meurs M, et al. The human CMV-UL86 peptide 981-1003 shares a crossreactive T-cell epitope with the encephalitogenic MOG peptide 34-56, but lacks the capacity to induce EAE in rhesus monkeys. J Neuroimmunol 2007; 182: 135–152. [DOI] [PubMed] [Google Scholar]
- 17.Jagessar SA, Heijmans N, Blezer EL, et al. Unravelling the T-cell-mediated autoimmune attack on CNS myelin in a new primate EAE model induced with MOG34-56 peptide in incomplete adjuvant. Eur J Immunol 2012; 42: 217–227. [DOI] [PubMed] [Google Scholar]
- 18.Pietra G, Romagnani C, Mazzarino P, et al. HLA-E-restricted recognition of cytomegalovirus-derived peptides by human CD8+ cytolytic T lymphocytes. Proc Natl Acad Sci USA 2003; 100: 10896–10901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaguia F, Saikali P, Ludwin S, et al. Cytotoxic NKG2C+ CD4 T cells target oligodendrocytes in multiple sclerosis. J Immunol 2013; 190: 2510–2518. [DOI] [PubMed] [Google Scholar]
- 20.Sylwester AW, Mitchell BL, Edgar JB, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med 2005; 202: 673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jagessar SA, Kap YS, Heijmans N, et al. Induction of progressive demyelinating autoimmune encephalomyelitis in common marmoset monkeys using MOG34-56 peptide in incomplete freund adjuvant. J Neuropathol Exp Neurol 2010; 69: 372–385. [DOI] [PubMed] [Google Scholar]
- 22.Jagessar SA, Heijmans N, Blezer EL, et al. Immune profile of an atypical EAE model in marmoset monkeys immunized with recombinant human myelin oligodendrocyte glycoprotein in incomplete Freund’s adjuvant. J Neuroinflammation 2015; 12: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Vos AF, van Meurs M, Brok HP, et al. Transfer of central nervous system autoantigens and presentation in secondary lymphoid organs. J Immunol 2002; 169: 5415–5423. [DOI] [PubMed] [Google Scholar]
- 24.Van Zwam M, Huizinga R, Heijmans N, et al. Surgical excision of CNS-draining lymph nodes reduces relapse severity in chronic-relapsing experimental autoimmune encephalomyelitis. J Pathol 2009; 217: 543–551. [DOI] [PubMed] [Google Scholar]
- 25.Smith PA, Heijmans N, Ouwerling B, et al. Native myelin oligodendrocyte glycoprotein promotes severe chronic neurological disease and demyelination in Biozzi ABH mice. Eur J Immunol 2005; 35: 1311–1319. [DOI] [PubMed] [Google Scholar]
- 26.Ehlers B, Spiess K, Leendertz F, et al. Lymphocryptovirus phylogeny and the origins of Epstein–Barr virus. J Gen Virol 2010; 91: 630–642. [DOI] [PubMed] [Google Scholar]
- 27.Ascherio A, Munger KL, Lunemann JD. The initiation and prevention of multiple sclerosis. Nat Rev Neurol 2012; 8: 602–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lang HL, Jacobsen H, Ikemizu S, et al. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nat Immunol 2002; 3: 940–943. [DOI] [PubMed] [Google Scholar]
- 29.Khan G, Miyashita EM, Yang B, et al. Is EBV persistence in vivo a model for B cell homeostasis? Immunity.1996. 5: 173–179. [DOI] [PubMed] [Google Scholar]
- 30.’t Hart BA, Jagessar SA, Haanstra K, et al. The primate EAE model points at EBV-infected B cells as a preferential therapy target in multiple sclerosis. Front Immunol 2013; 4: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krumbholz M, Derfuss T, Hohlfeld R, et al. B cells and antibodies in multiple sclerosis pathogenesis and therapy. Nat Rev Neurol 2012; 8: 613–623. [DOI] [PubMed] [Google Scholar]
- 32.Jagessar SA, Fagrouch Z, Heijmans N, et al. The different clinical effects of anti-BLyS, anti-APRIL and anti-CD20 antibodies point at a critical pathogenic role of gamma-herpesvirus infected B cells in the marmoset EAE model. J Neuroimmune Pharmacol 2013; 8: 727–738. [DOI] [PubMed] [Google Scholar]
- 33.Jagessar SA, Heijmans N, Bauer J, et al. B-cell depletion abrogates T cell-mediated demyelination in an antibody-nondependent common marmoset experimental autoimmune encephalomyelitis model. J Neuropathol Exp Neurol 2012; 71: 716–728. [DOI] [PubMed] [Google Scholar]
- 34.Mintern JD, Macri C, Chin WJ, et al. Differential use of autophagy by primary dendritic cells specialized in cross-presentation. Autophagy 2015; 11: 906–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Birgisdottir AB, Lamark T, Johansen T. The LIR motif – crucial for selective autophagy. J Cell Sci 2013; 126: 3237–3247. [DOI] [PubMed] [Google Scholar]
- 36.Laman JD, Weller RO. Drainage of cells and soluble antigen from the CNS to regional lymph nodes. J Neuroimmune Pharmacol 2013; 8: 840–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harling-Berg CJ, Park TJ, Knopf PM. Role of the cervical lymphatics in the Th2-type hierarchy of CNS immune regulation. J Neuroimmunol 1999; 101: 111–127. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Vallejo JJ, Ilarregui JM, Kalay H, et al. CNS myelin induces regulatory functions of DC-SIGN-expressing, antigen-presenting cells via cognate interaction with MOG. J Exp Med 2014; 211: 1465–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delamarre L, Couture R, Mellman I, et al. Enhancing immunogenicity by limiting susceptibility to lysosomal proteolysis. J Exp Med 2006; 203: 2049–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carrillo-Vico A, Leech MD, Anderton SM. Contribution of myelin autoantigen citrullination to T cell autoaggression in the central nervous system. J Immunol 2010; 184: 2839–2846. [DOI] [PubMed] [Google Scholar]
- 41.Chirivi RGS, van Rosmalen JWG, Jenniskens GJ, et al. Citrullination: a target for disease intervention in multiple sclerosis and other inflammatory diseases? J Clin Cell Immunol 2013; 4: 146. [Google Scholar]
- 42.MacPherson G, Kushnir N, Wykes M. Dendritic cells, B cells and the regulation of antibody synthesis. Immunol Rev 1999; 172: 325–334. [DOI] [PubMed] [Google Scholar]
- 43.Brok HP, Uccelli A, Kerlero De, Rosbo N, et al. Myelin/oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis in common marmosets: the encephalitogenic T cell epitope pMOG24-36 is presented by a monomorphic MHC class II molecule. J Immunol 2000; 165: 1093–1101. [DOI] [PubMed] [Google Scholar]