Abstract
Background
Multiple sclerosis (MS) patients have central nervous system (CNS) lesions that may impede cognitive and sensorimotor function. Few rehabilitative therapies are available.
Objectives
The objective of this paper is to study effects of noninvasive tongue stimulation using the Portable Neuromodulation Stimulator (PoNS™) combined with intensive cognitive and physical rehabilitation on working memory, gait, balance and concomitant changes in the brain.
Methods
Fourteen MS patients, seven each in an active and a sham stimulation group, participated. Participants received intensive physical therapy and working memory training for 14 weeks. Functional magnetic resonance imaging (fMRI) using motor imagery and working-memory tasks were completed prior to and following therapy, as were sensory organization tests (SOT), motor performance measures, and neuropsychological assessment.
Results
On the SOT, the active group showed significant improvement from baseline. fMRI revealed significant blood oxygen level-dependent signal changes in the left primary motor cortex for the Active Group, while the sham group had increased activity in bilateral premotor cortices. All individuals improved on working-memory tasks, but only the active group showed increased dorsolateral prefrontal cortex activity.
Conclusions
In this cohort of MS patients, the results suggest that PoNS stimulation can enhance motor performance and working memory while also driving neuroplasticity. Further studies are warranted to explore these findings.
Keywords: Cognition, functional MRI, multiple sclerosis, rehabilitation
Introduction
Multiple sclerosis (MS) affects more than 500,000 patients in North America and is associated with diminished quality of life and productivity. MS patients have central nervous system (CNS) lesions that may impede cognitive and sensorimotor function.1–3 Proficient ambulation is often used as a metric of disease progression4,5 and, in this regard, as many as 40% of MS patients require walking assistance within 15 years of disease onset.6 The need for low-cost therapeutic intervention to address these effects of MS is pressing.
Functional electrical stimulation using surface electrodes has been shown to effectively improve gait, but only when the stimulator is active.7–9 Studies have shown that the tongue can be used as an effective interface for conveying electrical signals to the CNS,10–13 including sensory substitution in balance-impaired or blind people.14–17 Individuals with primary vestibular disorders using electrical stimulation of the tongue together with head-position information demonstrated sustained balance improvements beyond their final stimulation session.13,14 In the present study, we use cranial nerve noninvasive neuromodulation (CN-NINM), a form of electrotactile stimulation delivered by a portable neuromodulation stimulator device (PoNS™14) that sends a series of small electrical pulses via a tongue electrode array to try to improve balance and gait problems in patients with MS. We also investigated whether this kind of stimulation could improve working memory in these patients.
Tyler et al.14 reported on chronic MS patients with gait disturbance and demonstrated improved gait with CN-NINM training. They intimated that tongue-based neuro-stimulation may amplify the benefits of physical therapy for improving gait in such patients. Recent fMRI studies suggest that motor improvements following electrical stimulation of the tongue are likely related to modulation of neural activity within structures of the brain that control balance and movement.13,14,18,19
The present study used a multimodal neuroimaging approach to examine neuromodulation associated with CN-NINM training and to explore the beneficial effects of PoNS™ stimulation as reported by Tyler et al.14 We adapted a gait imagery functional magnetic resonance imaging (fMRI) task previously used to study gait and balance in Parkinson’s disease (PD) patients.20 In that study, PD patients showed more brain activity in the supplementary motor area (SMA) than control participants during the gait imagery task, and this increase was interpreted as compensatory in nature. Furthermore, it was suggested that higher activity in locomotor regions could be used to predict improved gait function.20 Based on these findings, but cognizant of the different lesion patterns, we hypothesized that compared to post-training scans, our baseline fMRI results in MS subjects would be characterized by greater activation in the SMA and by reduced activation in the primary motor cortex and cerebellum. We expected this pattern to be reversed in post-training follow-up scans. In addition to motor function, we speculated that working-memory rehabilitation training could also facilitate improvement in cognitive function.21–23 To address this possibility, we used a probe working-memory task to document putative functional change associated with working-memory rehabilitation training. This task has been used previously in healthy normal controls and in mild traumatic brain injury (mTBI) patients.24,25 The control group showed strong activation within the dorsolateral prefrontal cortex (DLPFC), whereas mTBI patients with post-concussive symptoms displayed reduced activation in the DLPFC.24,25 We hypothesized that MS patients would display greater DLPFC activation following rehabilitation training with the PoNS device.
Methods
Patient population
Participants were randomly assigned to two groups regardless of age, gender, individual Expanded Disability Status Scale (EDSS) score, disease state, functional status, or MS chronicity: seven in a PoNS Stimulation Group (active group) and seven in a Sham PoNS™ Stimulation Group (Sham Group). Demographic information is presented in Table 1. At the end of the study, individuals assigned to the sham group were offered the opportunity to use the active device, and five individuals returned and completed the active training (rollover group).
Table 1.
Demographics.
| Active PoNS™ | Sham PoNS™ | |
|---|---|---|
| Age (years) | 47.7 | 49.7 |
| Range | 28–61 | 38–62 |
| Sex: women/men | 4/3 | 4/3 |
| Initial WASI FSIQ rating | 111.1 | 113.3 |
| range | 84–123 | 84–126 |
| Education (years) | 14.7 | 16.7 |
| Range | 12–19 | 13–19 |
| Handedness: right/left | 5/2 | 6/1 |
| Language: English/French | 5/2 | 5/2 |
| MS duration (years) | 11.2 | 22.3a |
| Range | 3–26 | 9–37 |
| Initial EDSSb | 4.2 | 4.8 |
| Range | 3–6 | 3–6 |
Disease duration for the sham group is significantly longer (F = 5.109, p ≤ 0.045).
EDSS above 6 was exclusionary.
PoNS: Portable Neuromodulation Stimulator; WASI: Wechsler Abbreviated Scale of Intelligence; FSIQ: Full-Scale Intelligence Quotient; MS: multiple sclerosis; EDSS: Expanded Disability Status Scale.
Active group members used a device providing perceivable electrical stimulation, whereas the sham group used a device providing a non-perceivable stimulus. Therapists and other research personnel were not informed as to which group an individual belonged, and participants and therapists were instructed not to discuss any details of the stimulus sensation with each other. Additionally, individuals were instructed not to adjust the stimulus intensity in the presence of therapists. Questions about device use or stimulation were to be addressed to the principal investigator (PI).
The Montreal Neurological Institute and Hospital (MNIH) and Concordia University Research Ethics Boards approved this study, and it was sanctioned by Health Canada. Participants signed an ethics board-approved written informed consent form and they provided permission for publication of images and behavioural data. All computerized patient data were denominated and patient files were stored in a locked cabinet in the PI’s office.
Procedure
Prior to the start of CN-NINM training, each participant underwent baseline evaluations including structural and fMRI, balance tests (SOT), Dynamic Gait Index (DGI), neuropsychological assessment (Handedness; Wechsler Abbreviated Scale of Intelligence (WASI) Vocabulary and Matrix Reasoning; California Verbal Learning Test (CVLT)-11; D-Kefs Trails, Color/Word and Verbal Fluency; Tower of London Second Edition; Ruff 2 and 7; Wechsler Adult Intelligence Scale Fourth Edition (WAIS-IV) Letter-Number sequencing, Coding and Symbol Search; Paced Auditory Serial Addition Test (PASAT); Leonard Tapping; and Grooved Pegboard). In addition, the MS Impairment Scale, Fatigue Impact Scale, Cognitive Function Inventory for MS, and Beck Depression and Anxiety questionnaires were completed.
Each participant was issued his or her own PoNS device version 2.2. The PI instructed individuals in use and maintenance of the device accompanied by written instructions (in English or French as appropriate). The PoNS device (see Figures 1 and 2) is held lightly in place by the lips and teeth around the neck of the tab that goes into the mouth and rests on the anterior, superior part of the tongue.14 Active group members adjusted the PoNS stimulus until felt as a moderately intense tingling sensation. The sham device required no stimulus adjustment.
Figure 1.
The relative size of the Portable Neuromodulation Stimulator as compared to a Canadian two-dollar coin.
Figure 2.
Portable Neuromodulation Stimulator device as held in the mouth by one of the trainers performing a 20-minute balance exercise.
Training procedure
Trainers (physiotherapists and exercise physiologists) all underwent the same intensive educational program to ensure consistent therapy was offered to participants. The CN-NINM protocol consisted of two stages: first an in-lab training program during which therapists developed subject-specific regimens appropriate to ability levels. In this stage, individuals completed twice-daily training sessions at the study center under the supervision of a CN-NINM certified trainer for two weeks (excluding weekends). The morning and afternoon sessions consisted of 90-minute physical therapy sessions and were separated by a three- to four-hour break. Therapy included: warm-up, balance, gait, motor control exercises, and breathing and awareness techniques (BAT). In addition, participants were asked to perform a BAT session using the PoNS at least one hour before bedtime. Subject exercise programs were reviewed daily during the in-lab training phase.
Stage 2 was 12 weeks of at-home training where individuals performed the same exercises as those learned during the in-lab phase. During both stages, participants completed three sessions per day: morning, afternoon and evening. Working-Memory retraining employed the COGMED package26 administered on a computer screen. Visuospatial and verbal working-memory training automatically identified the participant’s maximum threshold, and administered tasks aimed to increase that threshold (completed four times per week for approximately 10 weeks).
Participants returned every two weeks for re-evaluation and “fine tuning” of their programs during which the trainer evaluated their abilities and adjusted the intervention activities accordingly. These bi-weekly sessions also included SOT and DGI reassessments.
Following the 14 weeks of training, the tests performed at pre-training baseline were repeated, including the SOT, DGI, Neuropsychological Assessment (NA), and structural and fMRI. For the NA the same order of tests was employed as in the pre-training assessment.
Baseline and post CN-NINM training sessions
SOT
The SOT measured baseline stability under six progressively difficult conditions. For the easiest condition, participants stood on a fixed platform with their eyes open and therefore vision and somatosensory inputs were permitted. For the most difficult condition the individuals’ visual surround and the platform surface were swayed and therefore the visual surround and somatosensory inputs were conflicted – thus isolating the vestibular system.27
DGI
Tasks included: steady state walking; walking with changing speeds, with head turns horizontally and vertically, stepping over and around obstacles; pivoting, and stair climbing.28
NA
The NA was carried out by a clinical neuropsychologist and took approximately 150 minutes.
fMRI
MRI used a 3-Tesla Siemens Magnetom Trio A Tim System with a 32-channel head coil (for image acquisition see supplementary information).
Gait imagery fMRI
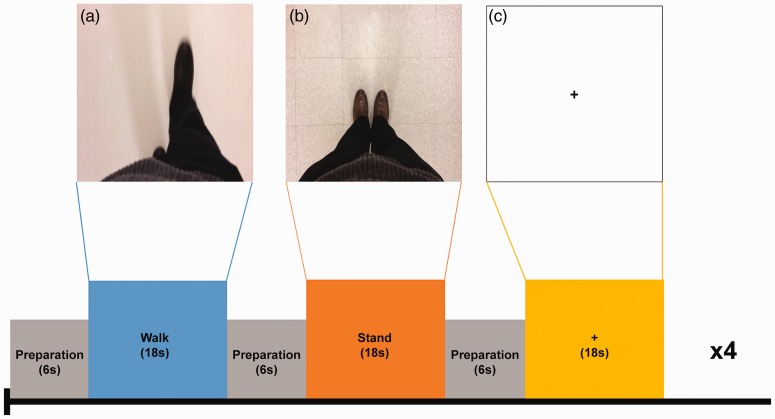
The task consisted of three gait conditions, namely STAND (S), WALK (W), and REST (R). W consisted of a video clip that showed an actor first standing and then walking in an egocentric perspective. S showed an actor standing upright with no movement. R was a fixation cross at the center of the screen. The participant is prompted to identify himself or herself as the actor and to mentally imitate the movements as viewed in the clips. Each condition (S, W, R) lasted 18 seconds, with a six-second interval between each for preparation. For the latter, a blank display was shown for 4.5 seconds followed by a 1.5-second instruction sentence. Each functional run consisted of four blocks of each condition presented pseudo-randomly, to avoid the same condition being presented consecutively, for a total of four minutes and 48 seconds (see Figure 3).
Figure 3.
Schematic representation of experimental design.
Working-memory fMRI
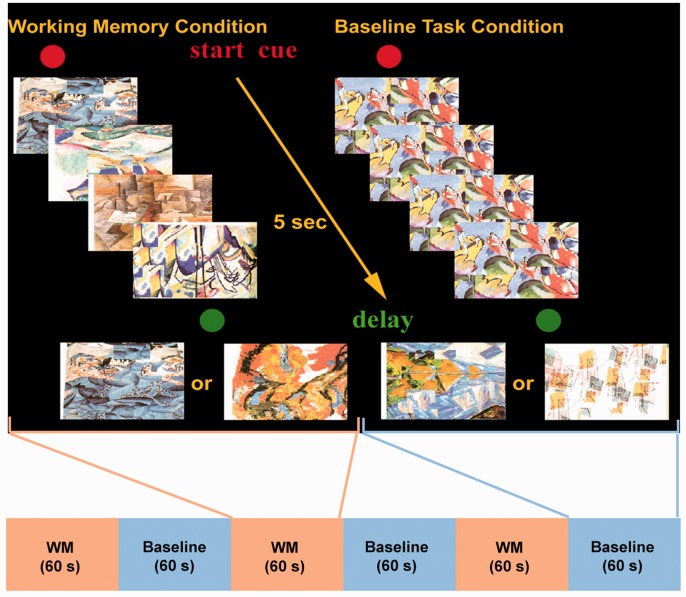
In the probe task, participants were familiarized with five abstract drawings. During each trial, four of five items were presented successively in random order at the center of a computer screen. After presentation of the fourth item, a delay of one second was introduced. A test item was then presented and the participant had to indicate within 1.5 seconds by button press whether this test item was one of the four items presented prior to the delay or whether it was an item that had not been presented. In the baseline control condition, the format and type of stimulus presentation, mode of response, and the timing of events were identical to the experimental working-memory task. During stimulus presentation in each control trial, one item was presented four times in succession at the center of the screen, followed by a delay of one second. After the delay, one of two different items associated with either a left or a right mouse button press was presented at the center of the screen and the participant had 1.5 seconds to respond (see Figure 4).
Figure 4.
Schematic diagram of externally ordered working-memory task.
Statistical analyses of behavioral measures were completed with Systat version 23.0 (SPSS Inc). The observed differences between pre- and post-training in both groups were compared using two-way analysis of variance (ANOVA) with group (active or sham) as the between-subject factor and time (pre-training and post-training) as the repeated measure. The dependent variables were the scores on the neuropsychological tests and the self-report questionnaires (Beck Depression Inventory (BDI)-II, Beck Anxiety Inventory (BAI), Modified Fatigue Scale, MS Impact Scale, and Cognitive Function). DGI and SOT were assessed at baseline and after each two-week interval and these scores were analyzed.
Results
Behavioral measures
On the MFIS, MSIS-29, and Cognitive Function questionnaires there were no significant changes in either group over time nor were there on the BDI-11 or BAI. The findings on the latter two tests are interesting in that this cohort of MS patients were neither clinically depressed nor anxious.
Neuropsychology tests
Participants completed detailed neuropsychological testing pre- and post-training and there was a trend for the active group to benefit more. Both groups demonstrated improvement on most cognitive measures, but because of multiple comparisons, we set the p value to 0.01. The following areas showed significant improvement: Symbol Search, Colour-Word Naming and Inhibition, and Trail Making Switching; except for Colour-Word naming, all have a working-memory component. Both groups demonstrated very significant improvement on their COGMED scores from baseline to post-training (p > 0.0001). The Group × Time interaction was not significant; however, there was a trend for the active group to show more improvement (p = 0.15).
SOT
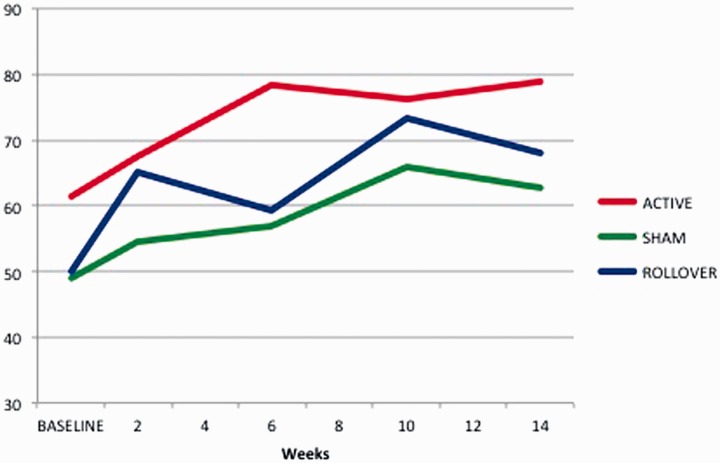
We analyzed the total composite score, and we note that both groups show a trend for improvement over time (see Figure 4), but it is more consistent for the active group. T tests comparing the week 14 score to the pre-score reveals that the improvement for the active group is significant (p < 0.001), whereas the sham group difference did not reach statistical significance (p < 0.06). For the rollover group, the results show a steady increase in mean SOT scores from initial baseline to final testing (baseline: 49.80; sham: 61.60; sham-rollover: 68.0; see Figure 5). Repeated ANOVA showed a significant effect of stimulation on SOT (F(1,4) = 16.529, p = 0.015); pairwise comparison indicated that SOT scores improved significantly from baseline to post-CN-NINM training (p = 0.046).
Figure 5.
Line graph showing change over time for Sensory Organization Tests composite scores.
DGI
Analysis of the difference scores revealed a non-significant trend in the direction of the active group showing greater improvement over time and parallel the results of Tyler et al.14 For the rollover group no difference was found in DGI scores (T1 = 13.0; T2 = 13.60 and T3 = 14.2). We note that these scores are in the impaired range.
Neuroimaging
Gait imagery fMRI
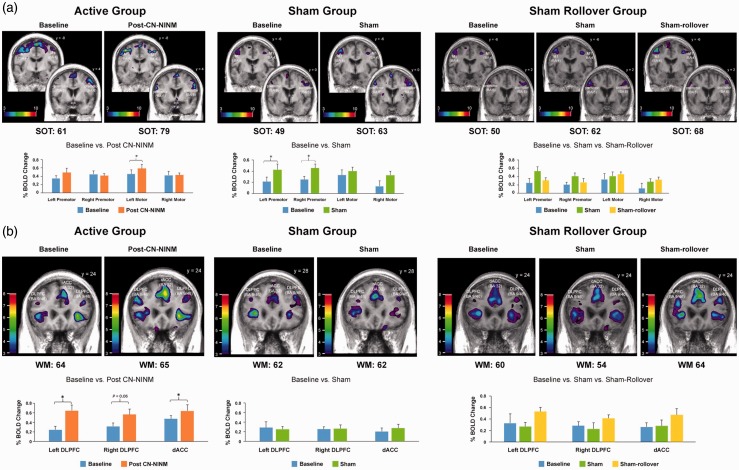
For the active group, pre- and post-CN-NINM training fMRI showed task-related activations in bilateral premotor and motor regions. Paired t-test comparing post- vs. pre-training scans revealed a significant increase in blood oxygen level-dependent (BOLD) signal in the left motor cortex (t = 3.015, p = 0.024), as we had predicted. We had hypothesized a reduction in the magnitude of activation in the premotor regions, but that is not evident. Interestingly, the left premotor cortex showed a significant increase in BOLD signal after CN-NINM training (Figure 6). For the sham group, pre- and post-training fMRI also showed task-related activations in bilateral premotor and motor regions. Paired-t test comparing post- vs. pre-PoNS scans revealed a significant increase in BOLD signal in bilateral premotor regions only (left: t = 3.067, p = 0.02; right: t = 4.167, p = 0.006; Figure 6). In the rollover group, the fMRI at the end of the active stimulation indicated further increase in activation in bilateral primary motor cortices and a reduction in activation in bilateral premotor cortices compared to sham stimulation, findings very similar to the active group; however, these were not statistically significant.
Figure 6.
Results for gait imagery and working-memory functional magnetic resonance imaging. (a) Gait Imagery fMRI and (b) Working Memory fMRI.
Working-memory fMRI
The active group baseline fMRI shows activation peaks in bilateral DLPFC and the dorsal anterior cingulate cortex (dACC), but the activation in the left DLPFC is too weak for statistical significance (Figure 6). Consistent with our hypothesis, post-CN-NINM training fMRI shows significant increases in BOLD signal in the left DLPFC (t = 3.55, p = 0.01), dACC (t = 3.057, p = 0.02) and a trend toward significance in the right DLPFC (t = 2.3, p = 0.06). For the sham group, baseline as well as post-training fMRI shows sub-threshold peaks in bilateral DLPFC and rostral anterior cingulate cortex (rACC). Paired t-tests comparing pre- and post-training scans did not reveal any significant changes (Figure 6). Of note, the rollover group showed greater activation in bilateral DLPFC following active stimulation compared to baseline and sham stimulation; this trend in increased activation was not statistically significant.
Discussion
A noteworthy finding from this pilot study is that there were significant effects of interventions across the wide range of cognitive domains both in the active and in the sham groups, albeit with a trend of greater improvement in the active group. This general improvement could be, at least in part, attributable to practice effects but are more likely the effects of the working-memory retraining in combination with intensive physical therapy.
We employed COGMED retraining to try to drive neuroplasticity in working-memory-related brain regions. Consistent with our hypothesis, post-PoNS training fMRI showed a significant increase in BOLD signal in the left DLPFC and rACC and a trend for significance in the right, whereas the sham group showed sub-threshold peaks in these regions. Notwithstanding these imaging findings, corresponding improvements in formal testing of working memory were not evidenced between groups.
For the active group, functional imaging analyses for the Gait Imagery task revealed that baseline and post 14-weeks’ training showed task-related activations in bilateral premotor and motor regions, and the BOLD signal in the left motor cortex was significantly higher, as we had hypothesized. We had predicted a reduction in the magnitude of activation in the premotor regions, but this was not apparent, though a non-significant reduction in BOLD signal in the right premotor cortex was observed. In the sham group, significant BOLD changes were documented only in the premotor regions, bilaterally. The functional significance of these findings could be related to improved motor output for the active group, especially in light of the fact that the left hemisphere is considered to be more important for skilled motor performance.29 As was the case for working memory, formal tests of motor ability including DGI, pegboards, and movement sequencing did not show differences between groups.
For exploratory purposes, we compared fMRI BOLD data from the four motor regions of interest (ROIs) to the imaging data of healthy control participants. At baseline, the active group showed task-related activations comparable to that of healthy controls in all ROIs except the left motor cortex. The magnitude of the activation in the latter became comparable to that of the healthy controls after PoNS training, and this is an interesting finding that may indicate causality. The sham group’s baseline fMRI showed significantly weaker task-related activations compared to the controls in all ROIs except the left premotor cortex. After PoNS training, activation in the left and right premotor cortex was comparable to that of controls, while activation in the left and right motor cortex remained significantly weaker. We speculate that this could be related to the presence of poor motor skills in the sham group. We went on to compare the fMRI BOLD data from the three prefrontal ROIs to our imaging data from 16 healthy controls in another study on TBI.24 We observed that at baseline, the active group had significantly weaker task-related activations compared to that of these healthy controls in all three prefrontal ROIs. The magnitude of the BOLD signal in all three ROIs became comparable to that of the healthy controls following PoNS training. In contrast, the Sham Group showed significantly weaker task-related activation in all three ROIs at baseline and it remained unchanged following PoNS training.
On the SOT, we demonstrated an improvement over time following PoNS training for both the active and for the rollover group suggesting that this training can have a positive effect on balance in patients with MS. The improvement that we document in balance associated with PoNS stimulation probably has ramifications for improved and safer locomotion. A major shortcoming of this study is the low number of participants (however, this fact is mitigated by the repeated design) in each group, and the findings suggest the need for a larger study that balances disease duration across groups.
Supplementary Material
Acknowledgements
We are very appreciative of the effort that the patients put into this pilot study and we are gratified that they felt they benefited. This study required a dedicated team without whose consistent effort nothing would have happened: Marcel Mazaltarim, Philippe Mazaltarim, and Maxime Mazaltarim, Amanda Rizk, Laura Barreto, Lisa Dyck, Jennifer Ann Scott, Daniel Aponte, Lloyd Saul, Joelle Crane, Rhonda Amsel, Marina Saunders, Jennifer Ramsay, Anna Pietromonaco, Dalinda Liazoghli, Amine Zoughlami, Line Gingras, Stephanie Saoumaa, Kiara Licursi, and Tanja Tros. Physiotherapy training, DGI and SOT were conducted at the Concordia University PERFORM Centre.
Conflicts of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was sponsored by Helius Medical Technologies Inc under a contract with McGill University and the Clinical Research Unit at the Montreal Neurological Institute.
Supplementary Material
Supplementary material for this paper can be found at http://journals.sagepub.com/doi/suppl/10.1177/2055217317690561
References
- 1.Panitch H, Applebee A. Treatment of walking impairment in multiple sclerosis: An unmet need for a disease-specific disability. Expert Opin Pharmacother 2011; 12: 1511–1521. [DOI] [PubMed] [Google Scholar]
- 2.Rao SM, Leo GJ, Bernardin L, et al. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology 1991; 41: 685–691. [DOI] [PubMed] [Google Scholar]
- 3.Benedict RH, Zivadinov R. Risk factors for and management of cognitive dysfunction in multiple sclerosis. Nat Rev Neurol 2011; 7: 322–342. [DOI] [PubMed] [Google Scholar]
- 4.Motl RW. Physical activity and irreversible disability in multiple sclerosis. Exerc Sport Sci Rev 2010; 38: 186–191. [DOI] [PubMed] [Google Scholar]
- 5.Snook EM, Motl RW. Effect of exercise training on walking mobility in multiple sclerosis: A meta-analysis. Neurorehabil Neural Repair 2009; 23: 108–116. [DOI] [PubMed] [Google Scholar]
- 6.Myhr K, Riise T, Vedeler C, et al. Disability and prognosis in multiple sclerosis: Demographic and clinical variables important for the ability to walk and awarding of disability pension. Mult Scler 2001; 7: 59–65. [DOI] [PubMed] [Google Scholar]
- 7.Kelleher KJ, Spence W, Solomonidis S, et al. Ambulatory rehabilitation in multiple sclerosis. Disabil Rehabil 2009; 31: 1625–1632. [DOI] [PubMed] [Google Scholar]
- 8.Taylor NF, Dodd KJ, Prasad D, et al. Progressive resistance exercise for people with multiple sclerosis. Disabil Rehabil 2006; 28: 1119–1126. [DOI] [PubMed] [Google Scholar]
- 9.Paul L, Rafferty D, Young S, et al. The effect of functional electrical stimulation on the physiological cost of gait in people with multiple sclerosis. Mult Scler 2008; 14: 954–961. [DOI] [PubMed] [Google Scholar]
- 10.Bach-y-Rita P, W Kercel S. Sensory substitution and the human-machine interface. Trends Cogn Sci 2003; 7: 541–546. [DOI] [PubMed] [Google Scholar]
- 11.Chebat DR, Rainville C, Kupers R, et al. Tactile ‘visual’ acuity of the tongue in early blind individuals. Neuroreport 2007; 18: 1901–1904. [DOI] [PubMed] [Google Scholar]
- 12.Tyler M, Danilov Y, Bach-y-Rita P. Closing an open-loop control system: Vestibular substitution through the tongue. J Integr Neurosci 2003; 2: 159–164. [DOI] [PubMed] [Google Scholar]
- 13.Wildenberg JC, Tyler ME, Danilov YP, et al. Sustained cortical and subcortical neuromodulation induced by electrical tongue stimulation. Brain Imaging Behav 2010; 4: 199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tyler M, Kaczmarek K, Rust K, et al. Non-invasive neuromodulation to improve gait in chronic multiple sclerosis: A randomized double blind controlled pilot trial. J Neuroeng Rehabil 2014; 11: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wildenberg J, Tyler M, Danilov Y, et al. High-resolution fMRI detects activity and neuromodulation of individual brainstem nuclei. J Neurosurg 2011; 115: A438–A439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danilov YP, Tyler ME, Skinner KL, et al. Efficacy of electrotactile vestibular substitution in patients with peripheral and central vestibular loss. J Vestib Res 2007; 17: 119–130. [PMC free article] [PubMed] [Google Scholar]
- 17.Vuillerme N, Cuisinier R. Sensory supplementation through tongue electrotactile stimulation to preserve head stabilization in space in the absence of vision. Invest Ophthalmol Vis Sci 2009; 50: 476–481. [DOI] [PubMed] [Google Scholar]
- 18.Badke MB, Sherman J, Boyne P, et al. Tongue-based biofeedback for balance in stroke: Results of an 8-week pilot study. Arch Phys Med Rehabil 2011; 92: 1364–1370. [DOI] [PubMed] [Google Scholar]
- 19.Kaczmarek KA. The tongue display unit for electrotactile spatiotemporal pattern presentation. Scientia Iranica D 2011; 18: 1476–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wai YY, Wang JJ, Weng YH, et al. Cortical involvement in a gait-related imagery task: Comparison between Parkinson’s disease and normal aging. Parkinsonism Relat Disord 2012; 18: 537–542. [DOI] [PubMed] [Google Scholar]
- 21.Solari A, Motta A, Mendozzi L, et al. Computer-aided retraining of memory and attention in people with multiple sclerosis: A randomized, double-blind controlled trial. J Neurol Sci 2004; 222: 99–104. [DOI] [PubMed] [Google Scholar]
- 22.Plohmann AM, Kappos L, Ammann W, et al. Computer assisted retraining of attentional impairments in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 1998; 64: 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeLuca J, Chelune GJ, Tulsky DS, et al. Is speed of processing or working memory the primary information processing deficit in multiple sclerosis? J Clin Exp Neuropsychol 2004; 26: 550–562. [DOI] [PubMed] [Google Scholar]
- 24.Ptito A, Chen JK, Johnston KM. Contributions of functional magnetic resonance imaging (fMRI) to sport concussion evaluation. NeuroRehabilitation 2007; 22: 217–227. [PubMed] [Google Scholar]
- 25.Chen JK, Johnston KM, Collie A, et al. A validation of the Post-Concussion Symptom Scale in the assessment of complex concussion using cognitive testing and functional MRI. J Neurol Neurosurg Psychiatry 2007; 78: 1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klingberg T. Training and plasticity of working memory. Trends Cogn Sci 2010; 14: 317–324. [DOI] [PubMed] [Google Scholar]
- 27.Broglio S, Ferrara M, Sopiarz K, et al. Reliable change of the Sensory Organization Test. Clin J Sport Med 2008; 18: 148–154. [DOI] [PubMed] [Google Scholar]
- 28.Chiu YP, Fritz SL, Light KE, et al. Use of item response analysis to investigate measurement properties and clinical validity of data for the Dynamic Gait Index. Phys Ther 2006; 86: 778–787. [PubMed] [Google Scholar]
- 29.Kimura D. Acquisition of a motor skill after left-hemisphere damage. Brain 1977; 100: 527–542. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.