Abstract
Purpose
To evaluate the role of the visual system homeobox gene 1 (VSX1) in the pathogenesis of familial keratoconus.
Methods
Families with two or more individuals with keratoconus were recruited and their members examined. The coding region and intron-exon junctions of the VSX1 gene were sequenced in affected individuals. In cases where there were possible pathogenic changes, segregation within the pedigree was analyzed. Meta analysis of reports on an association of p.D144E change with keratoconus phenotype was performed.
Results
Probands from a panel of 85 apparently unrelated keratoconus families were included. Eleven sequence variants were observed, including the previously reported c.432C>G (p.D144E) change and two novel intronic single nucleotide polymorphisms. However, these three changes did not cosegregate with the disease phenotype.
Conclusions
We excluded the c.432C>G sequence alteration as the direct cause of the disease. Lack of possibly pathogenic VSX1 sequence variants in the familial panel suggests that involvement of this gene in the pathogenesis of keratoconus is likely to be confined to a small number of pedigrees, at least in the population studied.
Keratoconus is a non-inflammatory corneal thinning disorder of unknown pathogenesis with a prevalence of 54.5 per 100,000 in the general population [1]. It is a leading cause for corneal transplantation in industrialized countries. It may be associated with other known genetic disorders [1,2], but non-syndromic disease is by far the most common [1]. Inheritance of keratoconus does not usually follow a simple Mendelian pattern, and in the majority of cases, it appears to be sporadic [2]. Studies in different populations show that between 6% and 23.5% of all patients have family histories of keratoconus [3–5]. The proportion is higher if individuals with a subclinical phenotype (forme fruste disease), recognized by videokeratopography, are included [6–8]. In familial keratoconus, the inheritance pattern is reported mainly to be autosomal dominant with variable phenotypic expression and incomplete penetrance [1,7,9]. Autosomal recessive and X-linked inheritance have also been proposed [2,10]. Linkage studies have shown that keratoconus is a genetically heterogeneous disorder, and several potential loci have been identified [11–16].
To date, the only gene reported to be implicated in keratoconus is the visual system homeobox gene (VSX1, OMIM 605020), a homeodomain containing transcription factor, also known as the retinal inner nuclear layer homeobox gene (RINX) [17]. VSX1 is involved in ocular development and is highly conserved across species [18–20]. Expression in humans was demonstrated in embryonic craniofacial, adult corneal, and adult retinal cDNA libraries [21]. In another study, VSX1 transcripts were detected during human corneal wound healing but were not present in normal corneal stroma [22]. There are two major transcript variants for this gene: the first variant (NCBI RefSeq NM_014588.4) encoding protein isoform A (NCBI RefSeq NP_055403.2) and the second (NCBI RefSeq NM_199425.1) encoding protein isoform B (NCBI RefSeq NP_955457). The latter differs in the 3' UTR and coding region and is predicted to encode a truncated protein of 126 amino acid residues compared to isoform A, which has 365 residues [23].
Posterior polymorphous corneal dystrophy (PPCD) is a bilateral autosomal dominant disorder that primarily affects the posterior corneal layers. The corneal endothelium typically transforms into an epithelial-like phenotype and Descemet’s membrane becomes irregularly thickened due to the deposition of an abnormal collagenous layer [24]. There may also be irregular corneal astigmatism [25,26], and an association between PPCD and keratoconus has been described in at least three case series [27–29]. VSX1 was selected as a candidate gene for PPCD and keratoconus because of its position within the PPCD linkage region on chromosome 20 [30]. Sequencing of this gene has revealed several variants. Some of them leading to protein changes (p.L17P, p.D144E, p.L159M, p.G160D, p.R166W, p.H244R, and p.P247R) were reported to be pathogenic or possibly pathogenic in keratoconus [17,31]. However, subsequent reports questioning the involvement of the VSX1 gene in both sporadic and familial keratoconus and PPCD pathogenesis have emerged [32–34]. To further assess the influence of VSX1 changes in familial keratoconus, we have looked for deleterious variants within this gene in probands from 85 unrelated British families with keratoconus. We also studied segregation of possible pathogenic changes within pedigrees.
Methods
Identification of keratoconus families
The study was performed in accordance to the tenets of the Declaration of Helsinki and with the approval by the local ethics committee. Informed consent was obtained from each study participant before they were included into the study. Patients with a family history of keratoconus were identified during routine examination and pedigrees were constructed. The ethnic backgrounds of the patients was recorded. All affected and unaffected family members older than 18 years and willing to participate were enrolled in the study. For each pedigree, a history of any other hereditary disorder was obtained.
Clinical examination included best corrected visual acuity and slit-lamp examination. Videokeratopography using an Orbscan II operating under software version 3.12 (Bausch & Lomb Incorporated, Rochester, NY) was performed. The diagnosis of keratoconus was based on the characteristic clinical signs, a characteristic pattern on videokeratography [1], corneal thinning measured by Orbscan II, and/or ultrasound pachymetry. Corneal graft surgery for keratoconus was also taken as confirmation of disease. Familial keratoconus was established when at least two members from one pedigree were clinically affected. Keratoconus suspects with forme fruste disease were not used in ascertaining the familial nature of the disease.
DNA collection and sequencing
Genomic DNA was prepared from blood leukocytes using a Nucleon III BACC3 genomic DNA extraction kit (GE Healthcare, Chalfont St. Giles, UK), according to the manufacturer’s instructions.
The coding regions of the VSX1 and the exon-intron junctions of both transcripts A and B were amplified using seven sets of oligonucleotide primers (Table 1) that flank the coding region of each exon by at least 57 nucleotides.
Table 1. Primer sequences used for amplification of VSX1 coding exons.
| Variant 1 | Variant 2 | Primer sequences |
|---|---|---|
| Exon 1A | Exon 1A | 5'-CTATAAAGCTTCCTCTAAGC-3' |
| 5'-AGCCAGATCCCTGTCCTG-3' | ||
| Exon 1B | Exon 1B | 5'-TTCGCCATCACGGACCTGC-3' |
| 5'-GAACGGCACGTCCGCTAG-3' | ||
| Exon 1C | Exon 1C | 5'-TGTGGCTTCGGCACGCAG-3' |
| 5'-ATGGTCTGTGACCCCTGC-3' | ||
| Exon 2 | Exon 2 | 5'-AGTAGGCACTAAAAATGCTGGC-3' |
| 5'-CAGAATTATCTTCCCAAATGGC-3' | ||
| Exon 3 | Exon 3 | 5'-CATTCAGAGGTGGGGTGTTC-3' |
| 5'-TCTTGTGGTGCCTTCAGCTA-3' | ||
| Exon 4 | 5'-TTTCCTTCAGAAATAGCATGGG-3' | |
| 5'-CGTTGCTTTGCTTTGGAAAT-3' | ||
| Exon 5A | 5'-CTTTAAACCCCTAAGGTGGGAG-3' | |
| 5'-AACCACACATCTCAAATGATGC-3' | ||
| Exon 5B | 5'-TCAAGATAAAATGCAATGCCAC-3' | |
| 5'-AGCACCATATTTTCCCATCAAC-3' |
NCBI RefSeq NM_014588.4 (variant 1) and NCBI RefSeq NM_199425.1 (variant 2) were used as reference sequences. The primers for exon 1A-C were identical to those reported by Heon et al. [17].
Mutation screening was performed in the proband from each family using an approximately 50 ng template DNA, 50 picomoles of gene-specific primers, 12.5 μl of ReddyMix™ PCR master mix (1.5 mM MgCl2; ABgene, Epsom, UK), and water, which was added to adjust the volume to a total of 25 μl. Thermal cycling was performed in a TC-512 Gradient Thermal Cycler (Techne, Stone, UK) with the following program: initial denaturation for five min at 94 °C, 35 cycles of 95 °C for 30 s, 52 °C (exon 1A and 4), 57 °C (exon 1B, 1C, 2, and 3) and 59 °C (exon 5A and 5B) for 30 s, 72 °C for 30 s, and a final extension for five min at 72 °C. Amplified DNA fragments were purified using Montage PCR96 Cleanup Kit (Millipore, Billerica, MA) and directly sequenced using the BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA). Nucleotide sequences were read both manually and using sequence analysis software (DNASTAR, Inc., Madison, WI) and compared with the published VSX1 complementary sequences (NCBI RefSeq NM_014588.4 and NM_199425.1). If a previously reported or novel change in the coding sequence that might impair the VSX1 protein structure or function was observed, the particular sequence variant was also determined in all available family members.
Fifty unrelated control individuals (100 chromosomes) of various ethnic backgrounds (26 white, 12 African-American, 12 Asian) without any corneal disease were also screened by direct sequencing for changes in all VSX1 coding regions.
Analysis of previously published data
To evaluate the statistical significance of the p.D144E change which was reported in keratoconus [17,31,34] but rarely present in unaffected controls [35], keratoconus probands and healthy controls from previously published studies screened for this variant were counted and genotype frequencies were compared. A Weighted Odds Ratio was calculated using the Mantel-Haenszel method [36].
Results
Eighty-five families were recruited for this study. Clinical examinations of two or more affected individuals were performed in 63 families. In 22 other families, keratoconus was present in the proband and corneal graft surgery for keratoconus had been performed in at least one other family member. Consanguinity was present in two families. Thirty-six families described themselves as white British, seven as white but not British, 24 were of Indian or Pakistani origin, 13 were Africans or African-Caribbeans, and the origin was not classified in five families. In 30 pedigrees, there were at least one affected individual in two or more successive generations, indicating an autosomal dominant mode of inheritance. In 15 pedigrees, affected individuals were present in different family branches and the disease was transmitted within the pedigree by asymptomatic relatives, suggesting also autosomal dominant inheritance but with incomplete penetrance and/or variable expressivity. However, forme fruste disease could not be excluded in the reportedly unaffected individuals. In 38 pedigrees, all affected members were siblings indicating either an autosomal recessive or autosomal dominant mode of inheritance with incomplete penetrance and/or variable expressivity. In two pedigrees, the disease was transmitted via an unaffected female, which is compatible with an X-linked or autosomal dominant inheritance mode with incomplete penetrance and/or variable expressivity.
There were no other ocular or systemic disorders that cosegregated with keratoconus. Fuch’s endothelial corneal dystrophy was present in a 53-year-old individual from one family, but there were no endothelial abnormalities in his 23-year-old daughter who also had keratoconus or in his 20-year-old unaffected son. None of the 85 probands had PPCD.
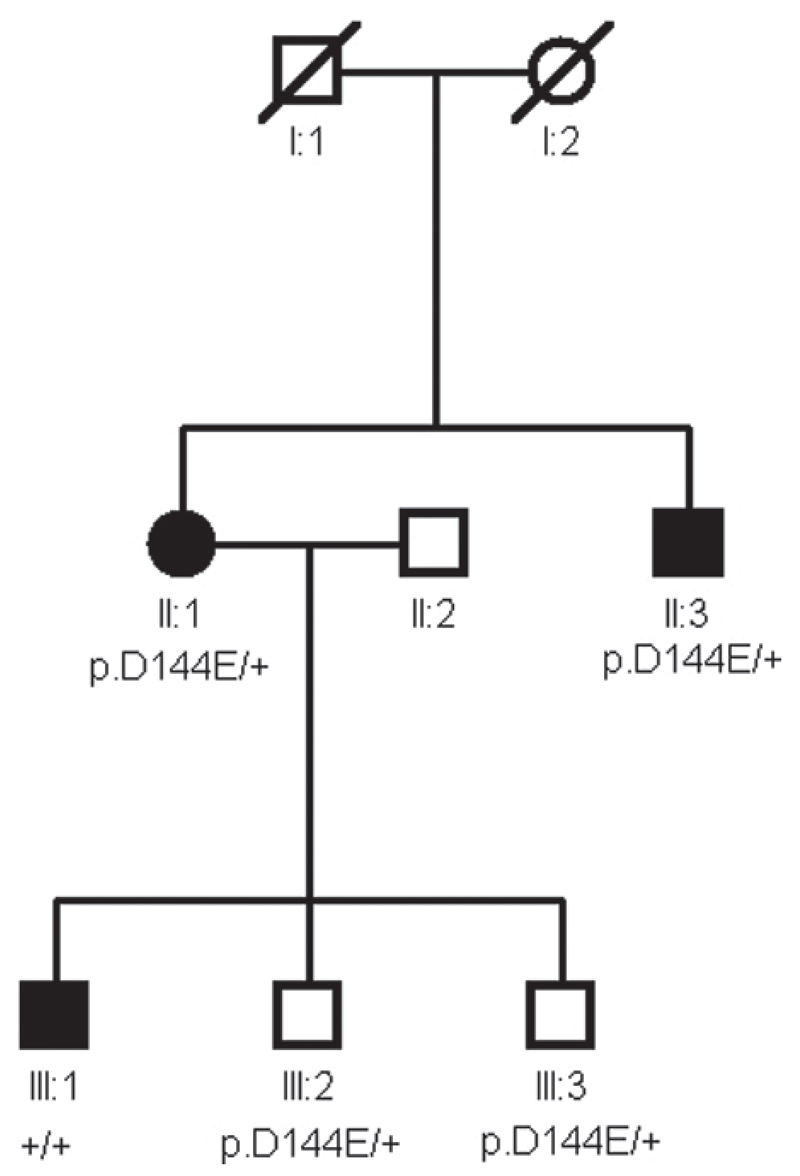
Table 2 summarizes all VSX1 sequence variants found in our screening panel. Direct sequencing revealed the presence of c.432C>G change which leads to a p.D144E change in both of the main protein isoforms in one white British family. This sequence variant was also found in an affected brother of the proband and the proband’s two unaffected sons, but it was not present in an affected son (Figure 1) nor in any control chromosomes. We also identified two novel intronic polymorphisms, c.504-10G>A in one further proband and c.504-24C>T in three other probands. Neither polymorphisms were found in the control individuals. Because of their proximity to the intron-exon junction therefore their possible interference with splicing mechanisms, their presence in other family members was studied. Since they did not cosegregate with keratoconus in all affected relatives (data not shown), they were not considered to be disease causing. None of the other changes previously reported as pathogenic or possibly pathogenic in keratoconus [17,31] were observed in our familial panel.
Table 2. Sequence variants detected in VSX1 in 85 probands from unrelated keratoconus families.
| Reference SNP ID reported by |
Position in the gene |
Nucleotide change |
Protein change |
Observed genotypes |
Ethnicity of probands with alleles ab or bb | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variant 1 | Variant 2 | Variant 1 | Variant 2 | Isoform A | Isoform B | aa | ab | bb | ||
| rs8123716 | Exon 1 | Exon 1 | c.18G>T | c.18G>T | p=(S6S) | p=(S6S) | 76 | 9 | 0 |
Asian (3), White (5), Unclassified (1) |
| Aldave et al. [34] | Exon 1 | Exon 1 | c.174G>T | c.174G>T | p=(P58P) | p=(P58P) | 83 | 2 | 0 | White (2) |
| rs6115023 | Exon 1 | Exon 1 | c.315C>A | c.315C>A | p.D105E | p.D105E | 83 | 2 | 0 | Black (2) |
| rs6037016 | Exon 1 | Exon 1 | c.339C>T | c.339C>T | p=(G113G) | p=(G113G) | 83 | 2 | 0 | Black (2) |
| rs6050307 | Exon 1 | Exon 1 | c.391C>A | c.391C>A | p.R131S | p.R131S | 80 | 4 | 1 | Black (5) |
| Heon et al. [17] | Exon 2 | Exon 2 | c.432C>G | c.432C>G | p.D144E | p.D144E | 84 | 1 | 0 | White (1) |
| Novel 1 | Intron 2 | Intron 2 | c.504-10G>A | c.504-10G>A | - | - | 84 | 1 | 0 | Unclassified (1) |
| Novel 2 | Intron 2 | Intron 2 | c.504-24C>T | c.504-24C>T | - | - | 82 | 3 | 0 | Asian (3) |
| rs12480307 | Exon 3 | Exon 3 | c.546A>G | c.546A>G | p=(A182A) | p=(A182A) | 32 | 44 | 9 |
Asian (15), White (24), Black (11), Unclassified (3) |
| rs6138482 | Intron 3 | Exon 3 | c.627+23G>A | c.650 G>A | - | p.R217H | 67 | 17 | 1 |
Asian (4), White (13), Black (1) |
| Bisceglia et al. [31] | Intron 3 | Exon 3 | c.627+84T>A | c.711T>A | - | p=(P237P) | 32 | 44 | 9 |
Asian (15), White (24), Black (11), Unclassified (3) |
Allele ‘a’ represents the nucleotide of the reference sequence. The nomenclature of the sequence variants follows current recommendations of the Human Genome Variation Society and was described by den Dunnen et al. [39]. The “A” of the ATG-translation initiation codon is referred as nucleotide +1. The last column shows the ethnic backgrounds of the probands. Black represents African or African-Caribbean, Asian stands for Indian or Pakistani, white for white British and white ‘others’ as self reported categories by the study participants.
Figure 1.
Family segregation of the p.D144E change. Unaffected individuals III:2 and III:3 are heterozygous for the p.D144E change as well as patients II:1 and II:3. Affected family member III:1 with advanced keratoconus does not carry this change.
In controls, the c.740C>G (p.P247R) was found in a single chromosome. The individual carrying this change is the same as previously reported [37]. The rest of the controls did not exhibit any sequence variants reported to be possibly implicated in the pathogenesis of corneal disease [17,31].
After taking into account three previous studies looking at VSX1 changes in keratoconus, including the p.D144E (Table 3), calculation of the Weighted Odds Ratio for affected probands produced an Odds Ratio of 5.2 with wide confidence intervals (95% Confidence Intervals 0.7-181.8, p=0.14).
Table 3. A summary for the meta analysis of p.D144E effect on previously reported keratoconus.
| Published study | Genotype |
Keratoconus probands |
Healthy controls |
Glaucoma probands |
|---|---|---|---|---|
| Heon et al. [17] | p.D144E/+ | 1 | 0 | 1 |
| +/+ | 62 | 277 | 89 | |
| Bisceglia et al. [31] | p.D144E/+ | 2 | 0 | - |
| +/+ | 78 | 125 | - | |
| Aldave et al. [35], | p.D144E/+ | 1 | 1 | - |
| Aldave et at. [34] | +/+ | 99 | 101 | - |
Heterozygous individuals are designated as p.D144E/+, individuals not carrying the p.D144E change as +/+. To date, a total of 504 healthy controls was screened for the c.432C>G leading to the p.D144E change, which was found in one subject by Aldave et al. [35] (1/1008 chromosomes) as well as in one individual that was heterozygous for this mutation out of 90 glaucoma patients by Heon et al. [17]. In clinically affected keratoconus probands, the p.D144E change was observed in four probands out of 243 screened (4/486 chromosomes) [17,31,34].
Discussion
The role of the VSX1 gene in the pathogenesis of corneal disease has been controversial. Of the changes reported as potentially causing keratoconus or PPCD the p.D144E, p.H244R, and p.P247R have also been reported in unaffected individuals [17,35,37]. In addition, no VSX1 mutations were found in the PPCD family originally linked to chromosome 20 [17], and the VSX1 gene was excluded as a cause of PPCD by further refinement of the locus on 20p11.2 in two large Czech families [37]. Finally, expression of VSX1 was not consistently shown in normal human adult cornea [17,22] and VSX1 knockout mice do not have any histological evidence of corneal abnormalities [38].
We studied 85 pedigrees with familial keratoconus, the largest series reported to date, to look for changes in the VSX1 gene. Direct sequencing of the two major transcript variants revealed the nonsynonymous p.D144E change in only one white British family. The evidence in the literature that this variant is implicated in the pathogenesis of keratoconus is ambiguous. After the initial report observing this particular allele in two siblings with both PPCD and keratoconus as well as in one glaucoma patient [17], the p.D144E was subsequently detected in two unrelated Italian families [31]. In the first family, cosegregation of the p.D144E allele with the disease phenotype could not be proven as the other member carrying this change was not available for videokeratopography examination. In the second Italian family, three first-degree relatives carrying this sequence variant were classified as keratoconus suspects based on topographical indexes, however, none of them had clinical keratoconus [31]. In our study, the p.D144E change did not cosegregate with keratoconus in all affected family members and therefore, we think that p.D144E is unlikely to be disease causing. However, as there seems to be a relatively high frequency of the p.D144E change in keratoconus patients compared to the general population, we reviewed the three previous studies that have reported on p.D144E in relation to keratoconus to assess the overall effect of this mutation (Table 3). No significant statistical association of this change with keratoconus could be proven. Nevertheless, this approach cannot completely exclude a role for this change. For example, an epigenetic interaction or partial linkage disequilibrium with another locus could also explain inconsistencies regarding the relationship between p.D144E and keratoconus.
Although some of the more rare changes were only observed in keratoconus probands from particular ethnic groups, the study size is too small to allow us to prove whether these are true associations or the result of population structure stratification.
In conclusion, this study brings the total number of unrelated keratoconus patients, screened by direct sequencing for changes in the VSX1, to 328. Only 19 of the probands in previous studies were reported to have familial disease [17,31,33]. Our failure to identify possibly pathogenic changes in a further 85 keratoconus families suggests that mutations of the VSX1 gene could only be responsible for a very small fraction of all observed familial cases.
Acknowledgements
The study was supported by The Special Trustees of Moorfields Eye Hospital and Ministry of Education of the Czech Republic (MSM 0021620806-VZ-206100-11).
References
- 1.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 2.Edwards M, McGhee CN, Dean S. The genetics of keratoconus. Clin Experiment Ophthalmol. 2001;29:345–51. doi: 10.1046/j.1442-9071.2001.d01-16.x. [DOI] [PubMed] [Google Scholar]
- 3.Hallermann W, Wilson EJ. [Genetic aspects of keratoconus (author’s transl)] Klin Monatsbl Augenheilkd. 1977;170:906–8. [PubMed] [Google Scholar]
- 4.Ihalainen A. Clinical and epidemiological features of keratoconus genetic and external factors in the pathogenesis of the disease. Acta Ophthalmol Suppl. 1986;178:1–64. [PubMed] [Google Scholar]
- 5.Owens H, Gamble G. A profile of keratoconus in New Zealand. Cornea. 2003;22:122–5. doi: 10.1097/00003226-200303000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Rabinowitz YS, McDonnell PJ. Computer-assisted corneal topography in keratoconus. Refract Corneal Surg. 1989;5:400–8. [PubMed] [Google Scholar]
- 7.Gonzalez V, McDonnell PJ. Computer-assisted corneal topography in parents of patients with keratoconus. Arch Ophthalmol. 1992;110:1413–4. doi: 10.1001/archopht.1992.01080220074024. [DOI] [PubMed] [Google Scholar]
- 8.Nesburn AB, Bahri S, Salz J, Rabinowitz YS, Maguen E, Hofbauer J, Berlin M, Macy JI. Keratoconus detected by videokeratography in candidates for photorefractive keratectomy. J Refract Surg. 1995;11:194–201. [PubMed] [Google Scholar]
- 9.Rabinowitz YS, Maumenee IH, Lundergan MK, Puffenberger E, Zhu D, Antonarakis S, Francomano CA. Molecular genetic analysis in autosomal dominant keratoconus. Cornea. 1992;11:302–8. doi: 10.1097/00003226-199207000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Claude S, Verdier R, Arnaud B, Schmitt-Bernard CF. [Accuracy of videokeratographic quantitative criteria for detection of keratoconus suspects in families with keratoconus] J Fr Ophtalmol. 2004;27:773–8. doi: 10.1016/s0181-5512(04)96212-2. [DOI] [PubMed] [Google Scholar]
- 11.Tyynismaa H, Sistonen P, Tuupanen S, Tervo T, Dammert A, Latvala T, Alitalo T. A locus for autosomal dominant keratoconus: linkage to 16q22.3-q23.1 in Finnish families. Invest Ophthalmol Vis Sci. 2002;43:3160–4. [PubMed] [Google Scholar]
- 12.Fullerton J, Paprocki P, Foote S, Mackey DA, Williamson R, Forrest S. Identity-by-descent approach to gene localisation in eight individuals affected by keratoconus from north-west Tasmania, Australia. Hum Genet. 2002;110:462–70. doi: 10.1007/s00439-002-0705-7. [DOI] [PubMed] [Google Scholar]
- 13.Brancati F, Valente EM, Sarkozy A, Feher J, Castori M, Del Duca P, Mingarelli R, Pizzuti A, Dallapiccola B. A locus for autosomal dominant keratoconus maps to human chromosome 3p14-q13. J Med Genet. 2004;41:188–92. doi: 10.1136/jmg.2003.012872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutchings H, Ginisty H, Le Gallo M, Levy D, Stoesser F, Rouland JF, Arne JL, Lalaux MH, Calvas P, Roth MP, Hovnanian A, et al. Identification of a new locus for isolated familial keratoconus at 2p24. J Med Genet. 2005;42:88–94. doi: 10.1136/jmg.2004.022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang YG, Rabinowitz YS, Taylor KD, Li X, Hu M, Picornell Y, Yang H. Genomewide linkage scan in a multigeneration Caucasian pedigree identifies a novel locus for keratoconus on chromosome 5q14.3-q21.1. Genet Med. 2005;7:397–405. doi: 10.1097/01.gim.0000170772.41860.54. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Rabinowitz YS, Tang YG, Picornell Y, Taylor KD, Hu M, Yang H. Two-stage genome-wide linkage scan in keratoconus sib pair families. Invest Ophthalmol Vis Sci. 2006;47:3791–5. doi: 10.1167/iovs.06-0214. [DOI] [PubMed] [Google Scholar]
- 17.Heon E, Greenberg A, Kopp KK, Rootman D, Vincent AL, Billingsley G, Priston M, Dorval KM, Chow RL, McInnes RR, Heathcote G, et al. VSX1: a gene for posterior polymorphous dystrophy and keratoconus. Hum Mol Genet. 2002;11:1029–36. doi: 10.1093/hmg/11.9.1029. [DOI] [PubMed] [Google Scholar]
- 18.Burmeister M, Novak J, Liang MY, Basu S, Ploder L, Hawes NL, Vidgen D, Hoover F, Goldman D, Kalnins VI, Roderick TH, et al. Ocular retardation mouse caused by Chx10 homeobox null allele: impaired retinal progenitor proliferation and bipolar cell differentiation. Nat Genet. 1996;12:376–84. doi: 10.1038/ng0496-376. [DOI] [PubMed] [Google Scholar]
- 19.Chen CM, Cepko CL. Expression of Chx10 and Chx10-1 in the developing chicken retina. Mech Dev. 2000;90:293–7. doi: 10.1016/s0925-4773(99)00251-8. [DOI] [PubMed] [Google Scholar]
- 20.Chow RL, Snow B, Novak J, Looser J, Freund C, Vidgen D, Ploder L, McInnes RR. Vsx1, a rapidly evolving paired-like homeobox gene expressed in cone bipolar cells. Mech Dev. 2001;109:315–22. doi: 10.1016/s0925-4773(01)00585-8. [DOI] [PubMed] [Google Scholar]
- 21.Semina EV, Mintz-Hittner HA, Murray JC. Isolation and characterization of a novel human paired-like homeodomain-containing transcription factor gene, VSX1, expressed in ocular tissues. Genomics. 2000;63:289–93. doi: 10.1006/geno.1999.6093. [DOI] [PubMed] [Google Scholar]
- 22.Barbaro V, Di Iorio E, Ferrari S, Bisceglia L, Ruzza A, De Luca M, Pellegrini G. Expression of VSX1 in human corneal keratocytes during differentiation into myofibroblasts in response to wound healing. Invest Ophthalmol Vis Sci. 2006;47:5243–50. doi: 10.1167/iovs.06-0185. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi T, Huang J, Deeb SS. RINX(VSX1), a novel homeobox gene expressed in the inner nuclear layer of the adult retina. Genomics. 2000;67:128–39. doi: 10.1006/geno.2000.6248. [DOI] [PubMed] [Google Scholar]
- 24.Krachmer JH. Posterior polymorphous corneal dystrophy: a disease characterized by epithelial-like endothelial cells which influence management and prognosis. Trans Am Ophthalmol Soc. 1985;83:413–75. [PMC free article] [PubMed] [Google Scholar]
- 25.DeRespinis PA, Norden RA, Rispoli LC. Posterior polymorphous dystrophy associated with astigmatism and amblyopia in children. J Refract Surg. 1996;12:709–14. doi: 10.3928/1081-597X-19960901-14. [DOI] [PubMed] [Google Scholar]
- 26.John GR. Videokeratographic abnormalities in a family with posterior polymorphous dystrophy. Cornea. 1998;17:380–3. doi: 10.1097/00003226-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Driver PJ, Reed JW, Davis RM. Familial cases of keratoconus associated with posterior polymorphous dystrophy. Am J Ophthalmol. 1994;118:256–7. doi: 10.1016/s0002-9394(14)72911-3. [DOI] [PubMed] [Google Scholar]
- 28.Weissman BA, Ehrlich M, Levenson JE, Pettit TH. Four cases of keratoconus and posterior polymorphous corneal dystrophy. Optom Vis Sci. 1989;66:243–6. doi: 10.1097/00006324-198904000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Gasset AR, Zimmerman TJ. Posterior polymorphous dystrophy associated with keratoconus. Am J Ophthalmol. 1974;78:535–7. doi: 10.1016/0002-9394(74)90249-9. [DOI] [PubMed] [Google Scholar]
- 30.Heon E, Mathers WD, Alward WL, Weisenthal RW, Sunden SL, Fishbaugh JA, Taylor CM, Krachmer JH, Sheffield VC, Stone EM. Linkage of posterior polymorphous corneal dystrophy to 20q11. Hum Mol Genet. 1995;4:485–8. doi: 10.1093/hmg/4.3.485. [DOI] [PubMed] [Google Scholar]
- 31.Bisceglia L, Ciaschetti M, De Bonis P, Campo PA, Pizzicoli C, Scala C, Grifa M, Ciavarella P, Delle Noci N, Vaira F, Macaluso C, et al. VSX1 mutational analysis in a series of Italian patients affected by keratoconus: detection of a novel mutation. Invest Ophthalmol Vis Sci. 2005;46:39–45. doi: 10.1167/iovs.04-0533. [DOI] [PubMed] [Google Scholar]
- 32.Tang Y, Picornell Y, Li X, Yang H, Rabinowitz Y. VSX1 gene: three single nucleotide polymorphisms (L159m, R166W AND H244R) are not associated with keratoconus. ARVO Annual Meeting; 2006 April 30-May 4; Fort Lauderdale (FL). [Google Scholar]
- 33.Aldave AJ. VSX1 mutation and corneal dystrophies. Ophthalmology. 2005;112:170–1. doi: 10.1016/j.ophtha.2004.10.017. author reply171-2. [DOI] [PubMed] [Google Scholar]
- 34.Aldave AJ, Yellore VS, Salem AK, Yoo GL, Rayner SA, Yang H, Tang GY, Piconell Y, Rabinowitz YS. No VSX1 gene mutations associated with keratoconus. Invest Ophthalmol Vis Sci. 2006;47:2820–2. doi: 10.1167/iovs.05-1530. [DOI] [PubMed] [Google Scholar]
- 35.Aldave AJ, Yellore VS, Principe AH, Abedi G, Merrill K, Chalukya M, Small KW, Udar N. Candidate gene screening for posterior polymorphous dystrophy. Cornea. 2005;24:151–5. doi: 10.1097/01.ico.0000141235.26096.1d. [DOI] [PubMed] [Google Scholar]
- 36.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 37.Gwilliam R, Liskova P, Filipec M, Kmoch S, Jirsova K, Huckle EJ, Stables CL, Bhattacharya SS, Hardcastle AJ, Deloukas P, Ebenezer ND. Posterior polymorphous corneal dystrophy in Czech families maps to chromosome 20 and excludes the VSX1 gene. Invest Ophthalmol Vis Sci. 2005;46:4480–4. doi: 10.1167/iovs.05-0269. [DOI] [PubMed] [Google Scholar]
- 38.Chow RL, Volgyi B, Szilard RK, Ng D, McKerlie C, Bloomfield SA, Birch DG, McInnes RR. Control of late off-center cone bipolar cell differentiation and visual signaling by the homeobox gene Vsx1. Proc Natl Acad Sci U S A. 2004;101:1754–9. doi: 10.1073/pnas.0306520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.den Dunnen JT, Antonarakis SE. Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum Mutat. 2000;15:7–12. doi: 10.1002/(SICI)1098-1004(200001)15:1<7::AID-HUMU4>3.0.CO;2-N. Erratum in: Hum Mutat 2002; 20(5):403. [DOI] [PubMed] [Google Scholar]