Abstract
Background
The World Health Organization recommends benzylpenicillin and gentamicin as antimicrobial treatment of infants with sepsis in low income settings (LICs), and ceftriaxone or cefotaxime as an alternative. In a meta-analysis from 13 LICs, Staphylococcus aureus, Klebsiella spp. and E.coli accounted for 55% of infants with sepsis. In a review of bacterial meningitis, resistance to third generation cephalosporins was >50% of all isolates, and 44% of Gram-negative isolates were gentamicin resistant. However, ceftriaxone may cause neonatal jaundice and gentamicin may cause deafness. Therefore, we compared parenteral benzylpenicillin plus gentamicin to ceftriaxone as first line treatment, assessing outcome and adverse events.
Methods
This was an open randomized trial carried out in the Queen Elizabeth Central Hospital, Blantyre, Malawi from 2010 to 2013. Infants < 60 days of age with possible severe sepsis received either benzylpenicillin and gentamicin or ceftriaxone. Adverse events and outcomes were recorded until 6 months post discharge.
Results
348 infants were included in analyses. Outcome in the benzylpenicillin and gentamicin or ceftriaxone groups was similar; deaths were 13.7% and 16.5% and sequelae 14.5% and 11.2% respectively. More infants in the penicillin/gentamicin group required phototherapy: 15% v 5%, p=0.03. Thirteen (6%) survivors had bilateral hearing loss. There was no difference between the treatment groups. By 6 months post discharge 11 more infants had died and 17 more children were found to have sequelae.
Conclusions
Ceftriaxone and gentamicin are safe for infants in our setting. Infants should receive long term follow up as many poor outcomes occurred after hospital discharge.
Keywords: neonatal sepsis, ceftriaxone, adverse events, outcome
Introduction
In an open, randomized trial of Malawian infants <60 days of age with possible severe bacterial infection, we compared parenteral benzylpenicillin plus gentamicin to ceftriaxone as first line treatment, assessing outcome and adverse events.
Background
Clinically suspected possible severe bacterial infections (pSBIs) are common in low-income settings, especially in the first month of life when mortality and morbidity are high.1 Early and appropriate therapy are critical to a good outcome. Antimicrobial therapy is guided by World Health Organisation (WHO) recommendations: first line therapy with parenteral benzylpenicillin and gentamicin; second line treatment with cefotaxime or ceftriaxone.2 Gentamicin has a potential for toxicity, especially hearing loss, but methods of monitoring blood concentrations of the drug are rarely available. There are no studies comparing the two regimens for efficacy, adverse events and outcome. Possible severe bacterial infection includes severe pneumonia, sepsis and meningitis. The WHO Young Infants sepsis study group reported that in a multicenter study in low income countries (LIC) in Asia and Africa, the three most common causes of pSBI found were Gram negative enteric bacteria, Group B Streptococcus (GBS) and Streptococcus pneumoniae.3 In a meta-analysis of reports from 13 low income settings, Staphylococcus aureus, Klebsiella spp. and E.coli accounted for 55% (39-70%) of culture positive sepsis in all infants;4 findings confirmed in a review of 21 studies, published after 2000, of neonatal invasive bacteremia in low income settings, 10 of which were in sub Saharan settings.5 In a six-country review of bacterial meningitis, resistance to second and third generation cephalosporins was present in >50% of all isolates, and 44% of Gram-negative isolates were gentamicin-resistant.6
Early onset sepsis (<7 days) is often associated with risk factors in the mother and /or delivery and the causative agents are GBS, S. aureus and Gram negative bacteria such as E. coli. Late onset infections (7-60 days) are commonly caused by bacteria such as S. pneumoniae, S. aureus, Klebsiella pneumoniae and also GBS.2
In high-income settings, first line antimicrobial treatment is usually benzylpenicillin or ampicillin and gentamicin for non-meningitis cases, and cefotaxime with ampicillin for meningitis or as second line therapy. The ampicillin is to cover Listeria monocytogenes infections.7 Ceftriaxone has been avoided in infants because of perceived safety issues, especially in infants who are jaundiced or hypoalbuminaemic,8 because ceftriaxone can cause biliary sludging, although this is reversed when treatment ceases and has no persisting sequelae.8–11 Ceftriaxone can form ceftriaxone-calcium complexes if given within 48 hours of a calcium-containing intravenous (IV) infusion. These complexes precipitate in IV fluid lines, the lungs and the kidneys, sometimes with fatal results.12–14 Some national guidelines advise against using ceftriaxone in premature babies until they attain the gestational age of 41 weeks.15 Ceftriaxone is still the drug of choice in neonatal gonorrheal ophthalmitis.16
Except in inflamed meninges, gentamicin has poor CNS penetration, achieves rather poor CSF levels and does not penetrate well into cells. The therapeutic range is narrow and gentamicin may control but fail to eradicate Gram negative infections.17 In Blantyre Listeria monocytogenes is exceptionally rare. This may be because a typical Malawian diet does not include unpasteurized dairy products or salads. Surrounding countries such as Kenya, South Africa and Zimbabwe report similar causes of pSBI as Malawi.18–20
Cephalosporins are bactericidal antibiotics and although CNS penetration is modest, higher doses safely achieve therapeutic CSF drug levels. In Malawi benzylpenicillin is appropriate for GBS infections and for most S. pneumoniae infections.21,22 Klebsiella pneumoniae and, many other Gram negative bacteria are increasingly resistant to gentamicin.23,24
The recommended WHO first line therapy may be inadequate empirical therapy where Gram negative bacteria account for half or more of all cases of SBI in infants <2 months of age. For this reason, we compared benzylpenicillin and gentamicin to ceftriaxone as first line treatment for pSBI in infants, and monitored for safety, especially jaundice, during therapy.
Methods
This was an open randomized trial carried out in the pediatric department of the Queen Elizabeth Central Hospital (QECH) in Blantyre, Malawi from March 2010 to February 2013. QECH is an 1100-bedded public government hospital; it is also the main teaching hospital of the Malawi medical school. It serves as the referral hospital for the southern half of the country and also as the district hospital for Blantyre. The children’s department admits 28,000 children a year and about 80,000 children are seen annually in the emergency unit.
Inclusion criteria
Children ≤ 2 months of age in whom there was clinical suspicion of severe sepsis, pneumonia or meningitis were eligible for inclusion. Following WHO guidelines, pSBI (including severe pneumonia, sepsis and BM) was suspected in the presence of convulsions, bulging fontanelle, lethargy, coma, poor feeding, irritability, apneic episodes or abnormal cry.25 In infants <7 days old an extended diagnostic algorithm included fever, agitation, no spontaneous movement, cyanosis, slow capillary refill (<3 secs) and lower chest wall in-drawing.
Enrolment took place after the guardian was fully informed and written consent was given. We recorded demographic, clinical and laboratory findings, including details about the pregnancy and the delivery.
Exclusion criteria
Infants not to be enrolled were: those with clinical severe jaundice (yellow discoloration of the skin extending to the lower limbs); children with known hypersensitivity to any of the three antibiotics and those who had been hospitalized for >72 hours, to avoid enrolling nosocomial infections. Children with previous neurological abnormalities such as hydrocephalus and neural tube defects were not enrolled. Excluded patients received standard treatment of benzylpenicillin and gentamicin.
Endpoints
The primary endpoints were differences in outcome and occurrence of jaundice between the two treatment groups.
Randomization
Randomization was by computer-generated numbers in blocks of ten. Treatment allocations were sealed in consecutively numbered opaque envelopes and opened in numerical order by the recruiting clinician at enrolment. Allocation was to either to benzylpenicillin 50,000 iu/kg 8 hourly IV (100,000 iu 8 hourly IV for BM) and daily gentamicin 6 mg/kg IV (standard smaller doses for low birth weight infants and very premature babies) or ceftriaxone IV 50 -100 mg/kg od (depending on age) for 5-14 days.
Samples on admission
Laboratory investigations were carried out at the Malawi-Liverpool-Wellcome Trust Clinical Research Programme Laboratories which are externally quality controlled. A blood sample was taken for a full blood count, culture, electrolytes, glucose and an HIV antibody test (Determine®). All infants testing positive by HIV antibody test had a blood sample tested by PCR to identify active HIV infection.
Cerebral spinal fluid (CSF) was taken for biochemistry, microscopy and culture. A positive CSF was one in which a culture was positive or the white cell count was >50 cells/ul with neutrophils forming the greater proportion of the cells.
Clinical monitoring and care
All infants were monitored by study nurses every 2–4 hours and seen at least twice daily by the study team. Most infants had hearing tests and when clinically appropriate an ultrasound scan of the head. Bilirubin levels were measured twice daily with a transcutaneous bilirubinometer (Konica Minolta Drager Air Shields JM 103). MRIs were carried out when their findings might benefit the child.
We provided supportive care according to unit protocols. Calcium is not added to any infusions and serum gentamicin levels are not available.
If the infant deteriorated despite the treatment given, and after discussion with the principal investigator, an appropriate antibiotic could be added to the treatment schedule or a switch made to the antibiotic(s) in the other study arm. If the CSF or blood culture report showed that a child was receiving inappropriate antimicrobial therapy for the bacteria grown, the treatment was changed for a more suitable antibiotic.
Follow-up
Follow-up was at one and six months after hospital discharge when neurological and hearing assessments were done. Age-appropriate hearing tests were carried out by trained nurses using oto-evoked potentials (Echocheck) and distraction tests. The neurological assessment was made by a trained research clinician.
Sample size
To detect a 40% lower case fatality rate in the ceftriaxone group than in the benzylpenicillin and gentamicin group, (ie to reduce the overall case fatality rate of meningitis from 50% to 30%) with a confidence of 90% and power of 80% required 107 infants in each arm (total = 214). To detect a difference in jaundice development in the ceftriaxone group of 18% compared to 8% in the penicillin and gentamicin group, the sample size needed with a confidence of 90% and power of 80% was 158 in each arm, (total = 316). To allow for mortality (much of it early) and loss to follow up, an extra 10% were to be recruited; i.e. 174 to each group: 348 in total.
Statistical Analysis
Statistical analysis was per protocol, done using Stata version 14.0 StataCorp Texas 77845 USA. The difference in means of normally distributed variables was performed using an independent samples t-test. Chi-square tests were used to assess relationship between categorical variables. Univariate logistic regression model was used to assess factors associated with poor outcome to obtain unadjusted odds ratios. Multivariate logistic regression model was also fitted to identify factors that are independently associated with outcome. All statistical tests were 2 tailed. Statistical significance was declared at a value of <0.05. The 95% confidence intervals for the odds ratios were obtained and reported.
Adverse events
Severe adverse events were reported to a data safety management board (DSMB) through the clinical monitor within 48 hours of their occurrence. The main safety endpoint for ceftriaxone was a transcutaneous bilirubin level at which phototherapy would be instituted. This level depended on gestational and postnatal age according to departmental bilirubin level graphs (see tables, Supplemental Digital Content 1 and 2). If levels were reached that required phototherapy, it was commenced and 8 hourly transcutaneous bilirubin levels were measured. If bilirubin concentrations decreased or remained stable, further doses of ceftriaxone were given and monitoring continued. If bilirubin concentrations increased, no further ceftriaxone was given.
Serious events included deaths, jaundice levels at or beyond ‘phototherapy’ levels, anemia (Hb <6 g/dl) while on therapy and serious adverse drug reactions (rashes, bronchospasm, anaphylactic shock).
Any changes from one antibiotic to another were reported and the reasons for change documented. The study was to be stopped if bilirubin levels requiring a change in antibiotic therapy were found in 30% more of the children receiving ceftriaxone than of those receiving benzylpenicillin and gentamicin.
Ethical considerations
Benzylpenicillin and gentamicin are widely used to treat neonatal infections despite the theoretical complications of renal failure and hearing loss. Ceftriaxone can cause conjugated bilirubinaemia and jaundice without permanent sequelae. Ceftriaxone has been used for several years in many centers across the region as second line treatment for neonatal infections. Nevertheless because of these theoretical complications all infants were monitored closely.
All guardians gave written consent to be enrolled after being fully informed of the study. Permission was granted by the College of Medicine Research and Ethics Committee (COMREC) to undertake the study (P2010/819) and the trial was registered with clinicaltrials.gov (NCT01247909).
Results
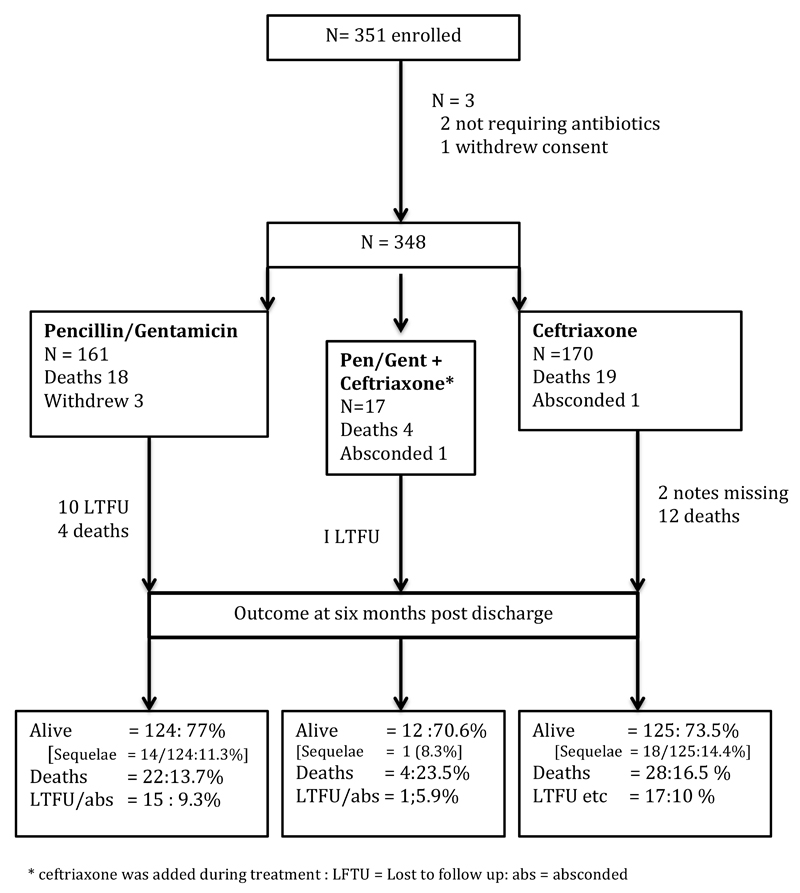
From March 2010 to Feb 2013 a total of 351 infants less than 60 days of age were enrolled; one parent withdrew consent before signing and two infants were deemed not to require antibiotics. The remaining 348 were included in analyses. (Figure 1) Of these, 161 (46.5%) were given gentamicin / benzylpenicillin and 170 received ceftriaxone: 17 received both. Baseline characteristics were similar in the two groups, except for prevalence of clinical jaundice (n= 12; 6.5% in the penicillin v 23; 14% in the ceftriaxone group p=0.02). Table 1
Figure 1.
Study enrollment and outcome
Table 1.
Baseline characteristics on admission
| Characteristic | Unit of measure | Ceftriaxone N =170 | Penicillin/Gentamicin N=161 | Total * N = 331 | P value |
|---|---|---|---|---|---|
| Sex:N (%) | Female | 82/167 (49%) | 75/157 (48%) | 157/324 (48%) | 0.83 |
| Known Birth weight | Number (%) | 123 (72) | 132 (82) | 255 (77) | 0.05 |
| Admission weight Kgs | Number known (%) | 170 (100) | 160 (99) | 330 | 0.49 |
| Median [IQR] | 3.2 [1.9, 4.5] | 3.1 [1.9, 4.2] | (99.5) | ||
| Age groups: n (%) | ≤7 days | 34 (20) | 43 (27) | 77 (23) | 0.33 |
| 8days-30days | 93 (55) | 78 (48) | 171(52) | ||
| ≥ 30 days | 43 (25) | 40 (25) | 83 (25) | ||
| Mode of delivery n(%) | LSCSa/Instrumental | 16 (9) | 10 (6) | 26 (8) | 0.31 |
| SVD | 151(89) | 150 (93) | 301(91) | ||
| Unrecorded | 3 (2) | 1 (1) | 4 (1) | ||
| Parity: n (%) | Single | 152 (89) | 151 (94) | 303(92) | 0.21 |
| Twins | 16 (9.5) | 9 (5.5) | 25 (7.5) | ||
| Unrecorded | 2 (0.5) | 1 (0.5) | 3 (0.5) | ||
| Temperature: n (%) | <36.5° C | 26 (15.5) | 20 (12) | 46 (14) | 0.56 |
| 36.5- 37.5° C | 106 (62.5) | 98 (61) | 204(62) | ||
| >37.5° C | 36 (21.5) | 41 (26) | 77 (23) | ||
| Unrecorded | 2 (0.5) | 2 (1) | 4 (1) | ||
| Fever days:n (%) | ≤1 day | 85 (50) | 78 (48) | 163(49) | 0.88 |
| 1 - 2 day | 40 (24) | 42 (26) | 82 (25) | ||
| >2 days | 43 (25) | 40 (25) | 83 (25) | ||
| Unrecorded | 2 (1) | 1 (1) | 3 (1) | ||
| Sucking : n (%) | Yes | 116 (68) | 124 (79.5) | 240(72.5) | 0.10 |
| No | 52 (31) | 36 (22) | 88 (26.5) | ||
| Unrecorded | 2 (1) | 1 (0.5) | 3 (1) | ||
| Convulsions: n (%) | Yes | 22 (13) | 15 (9.5) | 37 (11.5) | 0.38 |
| No | 147 (86.5) | 145 (90) | 292(88) | ||
| Unrecorded | 1 (0.5) | 1 (0.5) | 2 (0.5) | ||
| Total bilirubinb n (%) | <1mmol/l | 158 (93.5) | 138 (86) | 296(90) | 0.02 |
| >1 mmol/l | 9 (5.5) | 21 (13) | 30 (9) | ||
| Unrecorded | 3 (1) | 2 (1) | 4 (1) | ||
| Difficult breathing: n (%) | No | 82 (48) | 80 (49.5) | 162(49.5) | 0.91 |
| Yes | 85 (50) | 80 (49.5) | 165(49.5) | ||
| Unrecorded | 3 (2) | 1 (0.5) | 4 (1) | ||
| Cough days: n (%) | ≤1 day | 119 (70) | 112 (69.5) | 231(70) | 1 |
| >1 day | 50 (29.5) | 48 (30) | 98 (29) | ||
| Unrecorded | 1 (0.5) | 1 (0.5) | 2 (0.5) | ||
| Haemoglobin | Number tested (%) | 162 (95) | 148 (92) | 310(94) | 0.26 |
| Median g/dl{range} | 12.5{3.6-39} | 12.95{1.1 -22.8} | |||
| Oxygen saturation % | ≤90% | 112 (66) | 106 (66) | 218(66) | 0.90 |
| >90% | 49 (29) | 48 (30) | 97 (29.5) | ||
| Unrecorded | 9 (5) | 7 (4) | 16 (4.5) | ||
| Total blood WBC : n(%) | ≤5000 cm3 | 16 (9) | 18 (11) | 34 (10.5) | 0.65 |
| 5 - ≤10,000 cm3 | 49 (29) | 38 (24) | 87 (26) | ||
| 10 – ≤15,000 cm3 | 52 (31) | 44 (27) | 96 (29) | ||
| >15,000cm3 | 44 (26) | 47 (29) | 91 (27.5) | ||
| Unrecorded | 9 (5) | 14 (9) | 23 (7) | ||
| Malaria Parasites on BFc or positive MRDTd n(%) | Negative | 155 (91) | 144 (89) | 299(90) | 0.71 |
| Not done | 15 (9) | 17 (11) | 32 (10) |
excluded 17 cases who received both antibiotic therapies
= lower section Caesarean section
measured transcutanously
Blood film
Malaria rapid diagnostic test
Overall inpatient mortality was 12%; (n= 41) and a further 11 died within six months of discharge (total mortality n=52;15%). Sequelae were found in 4.6% (n =16) at hospital discharge and a further 17 (total =33;12.6%) of 261 survivors at six months after discharge. (Figure 1). Causes of death after discharge could not be verified but six (18.7%) had neurological sequelae following meningitis, four had significant congenital abnormalities, two HIV positive infants had further admissions for probable Pneumocystis jirovecii pneumonia (PJP), four had been admitted with severe shock or sepsis and no cause for later death was given.
Of the 348 patients, 54 (15.5%) did not have a lumbar puncture done, 42 (14.3%) of the remaining 294 had positive CSF cultures of which 15 (36.6%) were Group B Streptococcus (GBS) and 6 (14.6 %) were Gram negative bacteria such as Acinebacter baumanni, E. coli, Klebsiella pneumoniae (Table 2)
Table 2. CSF and Blood culture findings in neonatal sepsis.
| CSF culture results | Blood Culture results | |
|---|---|---|
| Number(%) | Number(%) | |
| Not done/missing | 53 (15) | 7 (2) |
| No growth* | 254 (73) | 220 (66) |
| Group B streptococcus | 15 (4) | 16 (5) |
| Coagulase negative staphylococcus | 8 (2) | 63 (19) |
| Streptococcus pneumoniae** | 8 (2) | 5 (1) |
| Streptococcus pyogenes | 2 (1) | 0 |
| Staphylococcus aureus | 0 | 11 (3) |
| Group D streptococcus | 0 | 1 (0.5) |
| Alpha haemolytic streptococcus | 2 (1) | 3 (1) |
| Gram negativex | 6 (2) | 9 (2.5) |
| Total | 348 (100) | 335 (100) |
12 CSFs with no growth on culture had white cell counts (number of cells = 23-clumps in pus) suggestive of meningitis, 3 of these infants had positive blood cultures (1 each of Group B streptococcus, coagulase negative staphylococcus and alpha haemolytic streptococcus
1 had Gram positive diplococci on Gram stain, but was culture negative)
The Gram negative bacteria were E. coli 3, Acinebacter baumanni 2, salmonella Typhimurium 1, Enterobacter cloacae 1, Acinebacter lwolfi 1.
All diploccici, microcci, and bacilli were considered contaminants. Coagulase negative staphylococci and alpha haemolytic streptococci may have been contaminants but some of the infants from whom the samples were taken were very ill.
Blood cultures were done in 348 children; 105 (30.1%) were positive; of these 62 (59%) grew coagulase negative staphylococci, 9 (8.5%) were Gram negative bacteria, 15 (14.3%) were GBS and 11 (10.5%) were Staphylococcus aureus (Table 2). Overall more children with a positive than a negative CSF or blood culture had a poor outcome (25(44%) v 67(33%) p=0.003 and 21(41%) v 54(30%) p=0.04 respectively (see table, Supplemental Digital Content 3). Coagulase negative staphylococci and alpha hemolytic streptococci may have been contaminants but some of the infants from whom the samples were taken were very ill. Removing these bacteria from analyses made no difference to the findings. The combined outcome by CSF and blood culture results comparing no growth with growth (137 v 122) was also significant (p= 0.017) (see table, Supplemental Digital Content 3)
Outcomes were similar between the two treatment groups; inpatient mortality was 11.2% in both the benzylpenicillin and gentamicin and the ceftriaxone arms. (Table 2) At six months post discharge, deaths were 13.7% and 16.5% and sequelae in survivors were 14.5% and 11.2%. respectively, (Figure 1).
On multivariate analysis weight on admission, convulsions, not sucking, an oxygen saturation level < 90% and positive blood culture were each associated significantly with mortality and sequelae (Table 3).
Table 3.
Multivariate analyses of variables affecting outcome
| Outcome | Multivariate | ||||
|---|---|---|---|---|---|
| Variable | Number assessed | Alive, no Sequelae N= 186 | Dead or Sequelae N= 91 | OR, 95% CI | P value |
| Gentamicin/Penicillin | 134 | 96 (63) | 38 (37) | ||
| Ceftriaxone | 143 | 90 (72) | 53 (28) | 0.69 (0.34, 1.36) | 0.28 |
| CSF culture -ve | 200 | 136 (68) | 64 (32) | ||
| CSF culture +ve | 36 | 21 (58) | 15 (42) | 2.15 (0.89, 5.15) | 0.08 |
| Blood culture -ve | 181 | 128 (71) | 53 (29) | ||
| Blood culture +ve | 93 | 55 (59) | 38 (41) | 2.15 (1.07, 4.38) | 0.033 |
| Weight Kg >2.5 | 225 | 162 (72) | 63 (28) | ||
| <=2.5 | 51 | 24 (47) | 27 (53) | 2.46 (1.12, 5.42) | 0.024 |
| HIV -ve | 195 | 134 (69) | 61 (31) | ||
| HIV exposed | 56 | 42 (75) | 14 (25) | 1.15 (0.49,2.58) | |
| HIV+ve | 9 | 3 (33) | 6 (67) | 4.30 (0.84,25.2) | 0.22 |
| Convulsion none | 248 | 175 (71) | 73 (29) | ||
| Convulsions | 27 | 9 (33) | 18 (67) | 5.22 (1.82,16.7) | 0.003 |
| Sucking | 200 | 153 (77) | 47 (24) | ||
| Not sucking | 74 | 30 (41) | 44 (59) | 2.61 (1.26, 5.44) | 0.010 |
| Oxygen saturation ≥90% | 179 | 130 (73) | 49 (27) | ||
| Oxygen saturation < 90% | 83 | 46 (55) | 37 (45) | 2.34 (1.12, 4.96) | 0.025 |
| Cough≤ 1day | 195 | 126 (65) | 69 (35) | ||
| Cough > 1day | 80 | 58 (73) | 22 (28) | 0.78 (0.49, 2.58) | 0.53 |
OR =odds ratio; CI = confidence interval
More infants in the penicillin/gentamicin group were clinically jaundiced on admission and more eventually required phototherapy: n=19; 15% v 7; 5%, p=0.03. (Table 4). Fifteen infants received phototherapy for 1-2 days, 8 for 3-4 days, 6 for 5-6 days and 4 for 7-11 days. Of the infants receiving >5 days of phototherapy, two were on ceftriaxone and six were on benzylpenicillin; one received both drug treatments.
Table 4.
Transcutaneous bilirubin levels at admission and the rise in bilirubin levels with benzylpenicillin +gentamicin and ceftriaxone
| Serum bilirubin levels μmol/L | Benzylpenicilln/ Gentamicin N | Benzylpenicillin/ Gentamcin requiring phototherapy N(%) | Ceftriaxone N | Ceftriaxone requiring Phototherapy N(%) |
|---|---|---|---|---|
| <5 | 100 | 0 | 135 | 0 |
| 5 - <10 | 17 | 0 | 14 | 1 |
| 10 - <15 | 18 | 2 | 14 | 1 |
| 15 - <20 | 11 | 6 | 5 | 3 |
| >20 | 14 | 11 | 2 | 2 |
| Total | 130 | 19 (15%) | 135 | 7 (6%) p=0.03 |
| Not done* | 18 | 0 | 18 | 0 |
| Received both antibiotic regimens* | 17 | 1 | ||
| Rise in serum bilirubin level (μmol/L) during admission | ||||
| <10 | 26 | 0 | 24 | 0 |
| >10 | 0 | 0 | 1 | 0 |
| Total | 26 | 0 | 25 | 0 |
36 had no bilirubin measured or it was not measured immediately on admission
Thirteen (6%) of 216 children who were tested had bilateral hearing loss; eight (61.5%) also had neurologic sequelae suggesting that the cause was the underlying infection. In four of the 13 infants with bilateral hearing loss a lumbar puncture was not done as the infants were too sick; eight of the remaining nine infants had a positive culture of blood or CSF. There was no significant difference between the treatment groups. (Table 5)
Table 5. Hearing test results (and neurological deficits) in survivors in the benzylpenicillin/gentamicin and ceftriaxone treatment arms at 6 months follow up.
| Hearing status(neuro deficits) N | Benzylpenicillin/gentamicin | Ceftriaxone | Received both antibiotics |
|---|---|---|---|
| Bilateral Hearing Loss | 4 ( 4 with global delay) | 2(1 CP 1 blind) | 0 |
| Unilateral Hearing Loss | 5 (1 with global delay) | 5 | 0 |
| Total with hearing deificts | 9 | 5 | 0 |
| Normal Hearing | 50 (4 global delay, 1 hemiplegia (1 fine motor deficit) |
52 (4 hydrocephalus, 2 blind (5 global delay, 1 hemiplegia) |
8 (1 global delay) |
| TOTAL TESTED | 68 (11 with neuro deficits) | 64 (14 with neuro deficits) | 8 (1 with neuro deficits) |
| Children not tested or Inconclusive result |
56 | 61 | 4 |
| TOTAL survivors at 6 months | 124 | 125 | 12 |
Discussion
In this study more infants had Gram positive than Gram negative infections. When treated with either benzylpenicillin + gentamicin or ceftriaxone, the outcomes in the two treatment groups were similar. This finding resembles the results of a meta-analysis of studies that used either of these two protocols.4 In a previous review of CSF results in our own hospital, Swann et al reported that more neonatal cultures were sensitive to ceftriaxone than to benzylpenicillin and gentamicin (99.1% vs 91.8%; p=0.006), especially the Gram-negative isolates (95.1% v 86.0%; p=0.012).21 A similar review of neonatal blood cultures done over that same period of time showed that 53% of the pathogens were Gram-positive and 47% Gram-negative. The four most common pathogens were S. aureus, GBS, Salmonella Typhimurium, and E. coli.22 Klebsiella sp, Acinebacter sp and Enterobacter sp, all considered nosocomial infections, accounted for 7.3%, 3.1% and 4.6% of the Gram negative pathogens The results of our study differ because we included all cases of possible severe bacterial infection, as this reflects clinical practice; only 147 (45%) blood or CSF samples grew bacteria of which 62 (42%) were coagulase negative staphylococci that may, or may not, have been contaminants. Even if the coagulase negative staphylococci are excluded we had more Gram positive (n=36/45; 80%) than Gram negative (n= 9/45; 20%) infections. This is probably because there has been a decline in invasive non typhoidal salmonella infections in Malawi over the last decade 26 and we excluded nosocomial infections.
More infants in the benzylpenicillin/gentamicin group developed jaundice than in the ceftriaxone group. Jaundice was caused mainly by the underlying infection; only seven of 24 (30%) infants commenced phototherapy after starting antibiotics. The overall hospital mortality was 42/348 (12.1%) and 4.6% survived with sequelae. Hearing loss was related to the underlying infection and not to the treatment. The outcome was worse in culture positive pSBI than in culture-negative infants (p=0.017) and worse in infants who were HIV infected or exposed than unexposed (p= 0.008).
Forty one children died in hospital but after six months a further 11 had died. Sixteen children were identified in hospital as having sequelae but by six months 17 more children were found to have sequelae. It is clear that all infants with pSBI need follow up to ensure additional supportive care for those who need it as about half who will eventually have sequelae are likely to be missed at the time of hospital discharge.
Conclusions
Ceftriaxone is safe in infants in our setting – in particular its use was not associated with a higher frequency of jaundice in this study – and hearing was not affected by gentamicin use. In this study, which did not include infants likely to have nosocomial infections, the outcome from pSBI was similar whether infants were treated with benzylpenicillin and gentamicin or with ceftriaxone. Infants with pSBI should be followed up for at least 6 months, as many may develop sequelae that were not detected on hospital discharge.
Supplementary Material
Acknowledgements
We thank all the families who allowed us to study and care for their infants. We thank the pediatric department for their help in recruiting and caring for the children; N. Kennedy, the clinical monitor and M. Mukaka who gave wise statistical advice. Malawi Liverpool Wellcome Trust Clinical Research Programme is supported by a strategic award from the Wellcome Trust.
Footnotes
The authors declare no conflicts of interest
References
- 1.Edmond K, Zaidi A. New Approaches to Preventing, Diagnosing, and Treating Neonatal Sepsis. PLoS Med. 2010 Mar;7(3):e1000213. doi: 10.1371/journal.pmed.1000213. Published online 2010 Mar 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Pocket Book of hospital care for children Guidelines for the management of common illnesses with limited resources. 2nd edition. World Health Organisation Geneva: 2013. [Google Scholar]
- 3.The WHO young infants study group. Bacterial etiology of serious infections in young infants in developing countries: results of a multicenter study. PIDJ. 1999;18(supplement):S17–S22. doi: 10.1097/00006454-199910001-00004. [DOI] [PubMed] [Google Scholar]
- 4.Downie L, Armiento R, Subhi R, et al. Community- acquired neonatal and infant sepsis in developing countries: efficacy of WHO's currently recommended antibiotics: systematic review and meta-analysis. Arch Dis Child. 2013;98(2):146–54. doi: 10.1136/archdischild-2012-302033. [DOI] [PubMed] [Google Scholar]
- 5.Huynh B-T, Padget M, Garin B, Herindrainy P, Kermorvant-Duchemin E, Watier L, Guillemot D, Delarocque-Astagneau E. Burden of bacterial resistance among neonatal infections in low income countries: how convincing is the epidemiological evidence? BMC Inf Dis. 2015 doi: 10.1186/s12879-015-0843-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prasad K, Karlupia N, Kumar A. Treatment of bacterial meningitis: an overview of Cochrane systematic reviews. Resp Med. 2009;103:945–50. doi: 10.1016/j.rmed.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Neonatal neurology. Infection of the nervous system in the newborn. In: McIntosh N, Helms P, Smyth R, Forfar, Arneil, editors. Chapter Ed Janet Rennie. 7th edition. Churchill Livingstone Elsevier; Edinburgh: 2008. pp. 305–309. [Google Scholar]
- 8.Martin E, et al. Ceftriaxone- bilirubin – albumin interactions in the neonate: an in vivo study. Eur J Pediatr. 1993;152:530–4. doi: 10.1007/BF01955067. [DOI] [PubMed] [Google Scholar]
- 9.Van Reempts PJ, Van Overmere B, Mahieu LM, Vanacker KJ. Clinical experience with ceftriaxone treatment in the neonate. Chemotherapy. 1995;41:316–22. doi: 10.1159/000239361. [DOI] [PubMed] [Google Scholar]
- 10.Gulian JM, Gonad V, Delamere C, Palix C. Bilirubin displacement of ceftriaxone in neonates: evaluation by determination of free bilirubin and erythrocyte-bound bilirubin. J Soc Antimicrob chemother. 1987;19:823–829. doi: 10.1093/jac/19.6.823. [DOI] [PubMed] [Google Scholar]
- 11.Mulhall A, de Louvois J, James J. Pharmacokinetics and safety of ceftriaxone in the neonate. Eur J Pediatr. 1985;144:379–382. doi: 10.1007/BF00441782. [DOI] [PubMed] [Google Scholar]
- 12.Monhe SV, Prescott WA, Johnson KK, Kuhman L. Safety of ceftriaxone sodium at the extremes of age. Expert opinion Drug Safety. 2008;7(5):515–23. doi: 10.1517/14740338.7.5.515. [DOI] [PubMed] [Google Scholar]
- 13.Bradley JS, Wassel RT, Lee L, Nambiar S. Intravenous Ceftriaxone and Calcium in the Neonate: Assessing the Risk for Cardiopulmonary Adverse Events. Pediatrics. 2009;123:e609–613. doi: 10.1542/peds.2008-3080. [DOI] [PubMed] [Google Scholar]
- 14.FDA Alert Ceftriaxone (marketed as Rocephin) Information Sep 2007
- 15.Letter to all health professionals from AFSSAPA dated November 2006. http://www.who.int/selection_medicines/committees/subcommittee/2/Ceftriaxone.pdf.
- 16.Laga M, Naamara W, Burnam RC, et al. Single dose therapy of gonococcal opthalamia neonatorum with ceftriaxone. NEJM. 1986;315:1382–5. doi: 10.1056/NEJM198611273152203. [DOI] [PubMed] [Google Scholar]
- 17.Price EH, de Louvois J, Workman MR. Antibiotics for salmonella meninigitis in children. J Antimicrob Chemother. 2006;46:653–655. doi: 10.1093/jac/46.5.653. [DOI] [PubMed] [Google Scholar]
- 18.English M, Ngama M, Musenda C, et al. Causes and outcomes of young infant admissions to a Kenyan District Hospital. Arch Dis Child. 2003;88:438–43. doi: 10.1136/adc.88.5.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hafferjee IE, Bhana RH, Coovadia YM, Hoosen AA, Marajh AV, Gouws E. Neonatal Group B streptococcal infections in Indian (Asian) babies in South Africa. J Infect. 1991;22:225–31. doi: 10.1016/s0163-4453(05)80003-9. [DOI] [PubMed] [Google Scholar]
- 20.Nathoo KJ, Pazvakavemba I, Chided OS, Chirisa C. Neonatal meningitis in Harare Zimbabwe: a 2 year review. Ann Trop Paed. 1991;11:11–15. doi: 10.1080/02724936.1991.11747472. [DOI] [PubMed] [Google Scholar]
- 21.Swann OV, Everett DB, Furyk JS, Harrison EM, Msukwa MG, Heyderman RS, Molyneux EM. Bacterial meningitis in Malawian infants less than 2 months of age: etiology and susceptibility to World Health Organization first-line antibiotics. Pediatr Infect Dis J. 2014;33(6):560–5. doi: 10.1097/INF.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gwee A, Everett D, Molyneux EM. Bacteraemia in Malawian neonates and infants 2002-2007: A retrospective review. BMJ Open. 2012 May 15;2(3) doi: 10.1136/bmjopen-2012-000906. pil.e000906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maoulainine FM, Elidrissi NS, Chkil G, et al. Epidemiology of nosocomial bacterial infection in neonatal intensive care unit in Morocco. Arch Pediatr. 2014 Jun 30; doi: 10.1016/j.arcped.2014.04.033. pii: S0929-693X(14)00230-9. [DOI] [PubMed] [Google Scholar]
- 24.Downie L, Armiento R, Subhi R, et al. Community-acquired neonatal and infant sepsis in developing countries: efficacy of WHO's currently recommended antibiotics--systematic review and meta-analysis. Arch Dis Child. 2013;98(2):146–54. doi: 10.1136/archdischild-2012-302033. [DOI] [PubMed] [Google Scholar]
- 25.Young Infants Clinical Signs Study Group. Clinical signs to predict severe illness in children less than 2 months: a multicentre study. Lancet. 2008;371:135–42. doi: 10.1016/S0140-6736(08)60106-3. [DOI] [PubMed] [Google Scholar]
- 26.Feasey NA, Everett D, Faragher EB, Roca-Feltrer A, Kang’ombe A, Denis B, et al. Modelling the Contributions of Malaria, HIV, Malnutrition and Rainfall to the Decline in Paediatric Invasive Non-typhoidal Salmonella Disease in Malawi. PLoS Negl Trop Dis. 2015;9(7):e0003979. doi: 10.1371/journal.pntd.0003979. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.